Abstract
Dendritic spines are small protrusions from dendritic shafts that contain the postsynaptic sites of glutamatergic synapses in the brain. Spines undergo dramatic activity-dependent structural changes that are particularly prominent during neuronal development. Although changes in spine shape or number have been proposed to contribute to forms of synaptic plasticity that underlie learning and memory, the extent to which spines remain plastic in the adult brain is unclear. We find that induction of long-term potentiation (LTP) of synaptic transmission in acute hippocampal slices of adult mice evokes a reliable, transient expansion in spines that are synaptically activated, as determined with calcium imaging. Similar to LTP, transient spine expansion requires N-methyl-d-aspartate (NMDA) receptor-mediated Ca2+ influx and actin polymerization. Moreover, like the early phase of LTP induced by the stimulation protocol, spine expansion does not require Ca2+ influx through L-type voltage-gated Ca2+ channels nor does it require protein synthesis. Thus, transient spine expansion is a characteristic feature of the initial phases of plasticity at mature synapses and so may contribute to synapse remodeling important for LTP.
Keywords: actin, hippocampus, dendrites, imaging, multiphoton
Long-term memory is thought to be mediated by an activity-dependent enhancement in synaptic transmission that relies, in part, on structural changes in synapses (1–5). The high concentration of actin in dendritic spines led to the proposal that activity-dependent changes in spine shape could modify synaptic efficacy (6, 7). During development, spines are indeed dynamic, exhibiting small movements, de novo growth, or retraction (8–10). In hippocampal cultures, synaptic activity has both stimulatory effects, inducing the appearance of new spines (11, 12) and the outgrowth of dendritic filopodia (13), as well as inhibitory effects, causing motility to stop (14) and spines to contract (15). Focal uncaging of glutamate or tetanic stimulation causes an expansion in spine size of hippocampal neurons in slice culture that is transient in large spines but can persist in small spines, where it may contribute to long-term potentiation (LTP) of synaptic transmission (16, 17).
Although it is clear that spines are dynamic during neuronal development, it is less clear whether spines with mature synapses remain plastic in the adult (18–20). Electron microscopy studies of spine structure during LTP in adult hippocampus have led to conflicting results. Studies in dentate gyrus performed nearly 30 years ago found that prolonged high-frequency synaptic stimulation induced a long-lasting increase in spine size that was thought to reflect osmotic swelling (21, 22). In contrast, a more recent study of Schaffer collateral synapses on CA1 neurons found little long-lasting increase in spine size during LTP (23). Others have reported distinct morphological changes during LTP, including increased numbers of perforated synapses (24) and presynaptic boutons that contact multiple spines (11) (but cf. ref. 25). However, studies using fixed tissue do not provide a dynamic picture of spine shape. Nor do they distinguish spines that have been activated by synaptic stimulation during the induction of LTP from the majority of spines that have not been stimulated.
Here, we have used two-photon microscopy to image both spine morphology and spine calcium levels, as an index of synaptic activity, during induction of LTP in acute slices from adult hippocampus. Similar to recent results in slice culture (16), we find that induction of LTP evokes a transient and reliable expansion in nearly all spines that are activated by synaptic transmission. However, spine expansion did not persist in the adult hippocampal slice regardless of initial spine size or shape, despite the reliable induction of LTP. Nonetheless, we found a close correlation between the conditions necessary for spine expansion and the induction of LTP. Thus, although persistent spine expansion may not be necessary for LTP in the adult, transient spine expansion is likely to represent an initial phase of synapse remodeling important for the long-term enhancement of synaptic transmission.
Methods
Electrophysiology. Transverse hippocampal slices (400 μm) were prepared from 4- to 8-week-old transgenic C57BL/6 mice (line M) expressing enhanced GFP (EGFP) (26). The Schaffer collateral pathway was stimulated with a bipolar tungsten electrode (FHC, Bowdoinham, ME) at 0.033 Hz, and field potentials were recorded in CA1 stratum radiatum (Supporting Text, which is published as supporting information on the PNAS web site). Calcium imaging was performed by using whole-cell recording. Patch pipettes were filled with 115 mM KMeSO4, 20 mM KCl, 10 mM Hepes, 4 mM MgCl2, 4 mM Na2ATP, 10 mM sodium phosphocreatine, and 0.4 mM Na2GTP, titrated to pH 7.2–7.3 with KOH (all reagents were purchased from Sigma, except KMeSO4 from ICN). Calcium Orange (Molecular Probes, 0.2 mM) or EGTA (Sigma, 0.1 mM) was added to the solution immediately before experiments. Patch pipette resistance was 3–6 MΩ; access resistance was 10–25 MΩ.
Imaging. Two-photon microscopy was carried out as described (27). Eight-bit images of 512 × 512 pixels were collected every 30sina z-series of 10–16 images taken at 0.3- to 1-μm steps, and projected and aligned by using software custom-written in Interactive Data Language (IDL, Research Systems, Boulder, CO). Methods of spine analysis are provided in Supporting Text.
Calcium transients were determined by plotting fluorescence intensity by using line scan mode (2 ms per line) and fitting the data with the subtraction of one exponential function from a second exponential function. From the fitted curve, we calculated the peak amplitude from the fluorescence increase divided by baseline fluorescence (ΔF/F), expressed as a percentage, and the time constant of rise in ms. For all expanding and stable spines, the two line scans with maximal peak amplitudes were selected so as not to contaminate calcium transients resulting from successful transmitter release with the absence of a calcium transient upon failed transmitter release.
All data are reported as mean ± SEM, unless otherwise indicated. Statistical comparisons (paired or unpaired t tests, Mann–Whitney rank sum tests) were performed by using sigmastat (SPSS, Chicago). Mann–Whitney rank sum tests were used to compare the ratio of spine area change during the second trial to change during the first trial with either drug or solvent applied on the second trial.
Results
We imaged CA1 pyramidal cells in acute hippocampal slices from adult transgenic mice (4–8 weeks old) that expressed EGFP in a subset of neurons (26) (Fig. 1A). Induction of LTP in the Schaffer collateral pathway (100-Hz tetanic stimulation for 1 s) evoked a transient expansion in a small fraction of spines (Fig. 1 B and C and Movie 1, which is published as supporting information on the PNAS web site), similar to that in slice cultures (16). Spines began expanding 30 s after stimulation, reached a maximal size within 1–2 min, and then slowly returned to their initial size in 10–20 min. The expansion was typically symmetrical, although some spines showed an asymmetric outgrowth, suggestive of branching (Fig. 1D and Movie 2, which is published as supporting information on the PNAS web site). Although basal spine morphology varied greatly, we routinely observed expansion of both large and small spines, and of spines with mushroom, thin, or stubby shapes (28). Transient spine expansion occurred on both small- and large-diameter dendritic shafts (Movie 3, which is published as supporting information on the PNAS web site). It occurred both at 25°C (our typical recording condition) and at a more physiological temperature (34°C, data not shown). Importantly, transient spine expansion was also observed in CA1 cells loaded with the fluorescent dye Alexa 594 during whole-cell recordings, indicating that transient spine expansion was not an artifact of EGFP expression or due to changes in EGFP distribution (data not shown).
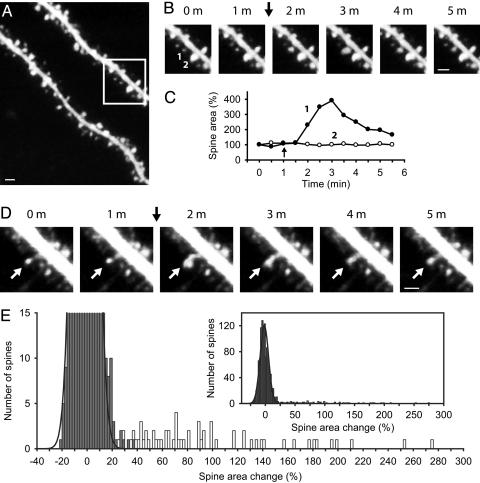
Fig. 1.
Two-photon imaging of the adult hippocampal CA1 stratum radiatum region reveals dendritic spines that transiently expand in response to tetanic synaptic stimulation. (A) Wide-field image of CA1 apical dendrites. (B) Images at 1-min intervals from the boxed region in A show spine 1 undergoing a transient expansion and spine 2 remaining stable after a 1-s, 100-Hz tetanic stimulation (arrow). (C) Measurement of the two-dimensional projected area of spine 1 (filled circles) and spine 2 (open circles) before and after the 1-s, 100-Hz stimulation (arrow). (D) Images at 1-min intervals of a spine (white arrows) exhibiting asymmetric transient expansion in response to a 1-s, 100-Hz tetanus (black arrow). (E) Histogram of the measured change in spine area after 1-s, 100-Hz stimulation for 1,155 spines from 20 slices (note truncated y axis). Gray and white bins represent, respectively, spines that were visually classified as stable (n = 1,090), with superimposed Gaussian fit, or expanding (n = 65). (Inset) Entire histogram. (Scale bars: 1 μm.)
To provide objective criteria for classifying spines as expanding or nonexpanding, we constructed a frequency histogram of percent change in spine area after tetanic stimulation for a given spine in a subset of our experiments (20 of 59). The histogram displayed a large, normally distributed peak centered near ≈0% change (–1.41 ± 7.76%, mean ± SD, n = 1,090), representing the large number of spines that failed to expand (Fig. 1E). In addition, the histogram contained a broad, skewed second peak with spine changes ranging from 25% to 275%. We therefore classified spines falling into this second peak, which displayed a change in area of >3 SD of the Gaussian peak (>22% change), as belonging to the population of expanding spines. This objective classification was in good agreement with our subjective visual identification of expanding spines (Fig. 1E, white bins) and nonexpanding spines (Fig. 1E, gray bins), which justified our using the more rapid visual classification for the ≈2,000 spines in the remaining 39 experiments.
Spine expansion was clearly related to the tetanus because spines never changed size in the absence of stimulation, even during experiments lasting up to 4 h. On average, spines that responded almost doubled in size (87.3 ± 4.1% increase, n = 172). In some instances, spine size almost quadrupled. However, all expanding spines subsequently returned to their original pretetanus size, reaching 113.7 ± 8.0% of their original size after 20 min (n = 18, P = 0.103) and 100.2 ± 3.9% of their original size after 1 h (n = 17, P = 0.965). In contrast to results in slice culture (16), we failed to observe any persistent change in spine size, regardless of the initial spine size. Thus, both large spines (area of >0.26 μm2, equivalent to volume of >0.1 μm3) and small spines (area of <0.26 μm2) returned to their initial size within 20–60 min after tetanic stimulation (Fig. 7, which is published as supporting information on the PNAS web site). A similar transient spine expansion was observed when LTP was induced by a 200-Hz tetanic stimulation or θ-burst stimulation (Fig. 8 A–D, which is published as supporting information on the PNAS web site). In contrast, induction of depotentiation by using a 15-min, 1-Hz stimulus train after induction of LTP caused no change in spine size (–0.7 ± 3.2%, n = 3, P = 0.840; Fig. 8E).
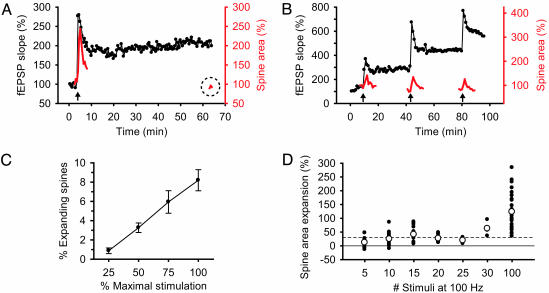
The transient nature of the spine expansion contrasts with the persistent LTP of the field excitatory postsynaptic potential (fEPSP) (60.5 ± 15.0% increase in fEPSP over baseline after 20 min, n = 9, P < 0.005; Fig. 2A). Consecutive bouts of tetanic stimulation induced reproducible, transient spine expansion, even though each tetanus caused a maintained, additive increase in synaptic strength (Fig. 2B). Thus, spine expansion is probably not responsible for the maintained expression of LTP, although it might contribute to its induction.
Fig. 2.
Properties of spine expansion in response to synaptic stimulation. (A) Expansion of a dendritic spine (red trace) in response to a 1-s, 100-Hz tetanus (arrow) is transient (note dashed circle highlighting spine area 60 min after tetanus) even though potentiation of the fEPSP (black trace) is persistent. (B) Representative trace of spine expansion (red) in response to repeated bouts of 1-s, 100-Hz tetanic stimulation (arrows) given 15–20 min apart. The same dendritic spine responds to each tetanus with a transient expansion despite the maintained increase in synaptic strength (black trace). (C) The mean (± SEM) percent of spines in the field of view that respond to a 1-s, 100-Hz tetanus is linearly dependent on the stimulus strength. (D) Scatter plot of spine area expansion in response to different numbers of stimuli at 100 Hz. White circles denote the mean expansion for each stimulation. A solid line marks 0% change, and a dashed line marks the 30% change required to detect an expansion above baseline noise. Each point is one spine.
We next examined the effect of varying the intensity and duration of synaptic stimulation on spine expansion (Fig. 2 C and D). The percent of expanding spines varied directly with stimulus strength, presumably reflecting an increase in the number of presynaptic fibers that were recruited (Fig. 2C). However, even with a stimulation intensity that evoked a maximal fEPSP, no more than 10% of spines responded. Spine expansion also depended on the number of stimuli in the tetanus. Although we occasionally observed spine expansion with five stimuli, consistent spine expansion required 100 stimuli at 100 Hz, the same tetanus needed to induce LTP (Fig. 2D).
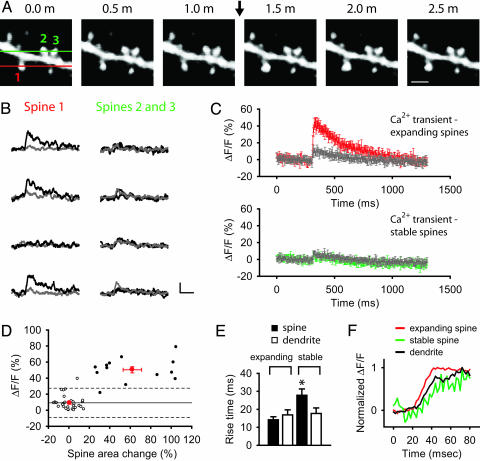
To explore why only a small fraction of spines expanded, we imaged in the same spine both expansion and postsynaptic Ca2+ levels using 0.2 mM Calcium Orange loaded by whole-cell recording to provide an index of synaptic activation (Fig. 3A). Nearly every spine that showed a significant expansion (>25%) also exhibited a large rapid Ca2+ transient in response to a single presynaptic stimulus (12 of 13 spines; Fig. 3 A and B). The average peak Ca2+ transient in expanding spines (50.4 ± 3.8%, n = 25) was significantly greater than that in the neighboring dendritic shaft (11.4 ± 1.2%, n = 19, P < 0.0001; Fig. 3 C and D), indicating that the spine itself was directly activated by synaptic input. The spine Ca2+ transients varied stochastically in an all-or-none manner in response to successive single stimuli (Fig. 3B), presumably reflecting probabilistic release of a single quantum of transmitter at an individual synaptic contact (29). Even more striking, nearly every spine that showed a large Ca2+ response (>3 SD larger than the mean dendritic shaft calcium response) also showed an expansion in response to tetanic stimulation (12 of 13 spines; Fig. 3D). Thus, spine expansion is a reliable hallmark of spines that were activated during synaptic stimulation.
Fig. 3.
Spines that expand exhibit a large Ca2+ transient in response to single presynaptic stimuli. (A) Spine 1 (red) undergoes transient expansion in response to a 1-s, 100-Hz tetanus (arrow), whereas the five other neighboring spines, including spines 2 and 3 (green), do not change size. Lines indicate positions of two line scans used to measure Ca2+ in spine 1 (red line) and spines 2 and 3 (green line). (Scale bar: 1 μm.) (B) Ca2+ transients in response to four single stimuli in spine 1 (black trace) and the adjoining dendritic shaft (gray trace). Data indicate three successes and one failure of synaptic transmission. Only very small Ca2+ transients were seen in spines 2 and 3 (black traces), similar in size to the Ca2+ transients in the dendritic shaft (gray trace). (Scale bar: 30% ΔF/F, 100 ms.) (C) Average Ca2+ transient (± SEM) in response to a single synaptic stimulus in expanding spines (red), stable spines (green), and their adjoining dendritic shafts (gray). (D) Ca2+ transient (y axis) plotted versus spine area change (x axis) for individual expanding spines (black circles), individual nonexpanding spines (white circles), and the population mean (± SEM) for expanding (red circle at upper right) and nonexpanding (red circle at lower left) spines. Solid line shows mean peak Ca2+ transient in the adjoining shafts (9.2%, n = 60). The two dashed lines mark 3 SDs (18.2%) above and below the mean shaft Ca2+ transient; the upper dashed line marks threshold for classifying Ca2+ transients as large or small. (E) Average rise times (± SEM) of Ca2+ transients in expanding spines, stable spines, and adjoining dendrite shafts. *, P < 0.05. (F) A single line scan reveals Ca2+ transients with a faster rise in the expanding spine (red) and a slower rise in the stable spine (green) compared with the dendrite shaft (black).
In stark contrast, spines that failed to expand showed small, relatively slow Ca2+ responses whose amplitude was similar to that observed in the neighboring dendritic shaft (Fig. 3 C and D). The amplitude of the Ca2+ transients in nonexpanding spines was 8.9 ± 1.3% (n = 59), similar to the 8.4 ± 1.0% peak amplitude in the adjoining dendritic shaft (n = 42, P = 0.497) but significantly less than the transient in nearby expanding spines (P < 0.001). In contrast to expanding spines, the Ca2+ transients in nonexpanding spines also showed little stochastic variation. The rise time of Ca2+ transients in expanding spines (14.2 ± 1.7 ms, n = 23) and their neighboring dendritic shafts (16.9 ± 2.8 ms, n = 13, P = 0.307) were similar. However, stable spines showed a significantly slower rise time (27.8 ± 3.5 ms, n = 28) compared with their neighboring dendritic shafts (17.7 ± 3.1 ms, n = 22, P < 0.05) or expanding spines (P < 0.01) (Fig. 3 E and F). The lack of variation and slow time course of the Ca2+ transients in the nonexpanding spines suggest that they arise from diffusion of Ca2+ (possibly complexed with dye) from the shaft, rather than direct activation of spine synapses (30).
These results provide two important insights. First, the spines that expand are activated during fast excitatory transmission and thus are indeed innervated by a mature synapse. Second, the relatively small fraction of expanding spines reflects the relatively small fraction of presynaptic terminals that are activated by the field stimulation. However, those spines that are directly activated by a synaptic input reliably respond to tetanic stimulation with a transient expansion.
Is the spine expansion a passive osmotic swelling response to Na+ or Ca2+ influx during synaptic transmission as previously suggested (21)? Although we reliably recorded spine expansion at the start of whole-cell recordings, spine expansion was abolished 30–60 min later (Fig. 9A, which is published as supporting information on the PNAS web site). The washout effect could be seen as soon as 10 min after the start of whole-cell recording and occurred with a relatively low concentration of Ca2+ buffer in the patch pipette (0.2 mM Calcium Orange or 0.1 mM EGTA), indicating that the loss of spine expansion was not due simply to Ca2+ buffering. Nor was the washout of spine expansion due to rundown of synaptic transmission because simultaneous Ca2+ imaging revealed an undiminished spine Ca2+ transient after 60 min of whole-cell recording (Fig. 9B). These results suggest that spine expansion is an active process that washes out during whole-cell recording, similar to the washout of LTP (31).
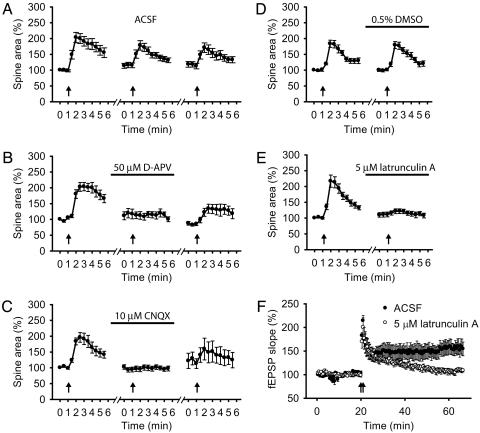
Spine expansion also resembles LTP in its requirement for the activation of both N-methyl-d-aspartate (NMDA) receptors and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors (Fig. 4 A–C). Antagonists of these receptors were tested in a protocol using repeated episodes of tetanic stimulation (100 Hz for 1 s), separated by 20-min intervals. Under control conditions (no antagonists), spines typically responded to multiple rounds of tetanic stimulation, although the responses to the second and third tetanus were somewhat reduced (Fig. 4A). However, when the second tetanus was applied in either the presence of d-2-amino-5-phosphonovaleric acid (D-APV) (50 μM) or 6-cyano-7-nitroquinoxaline-2,3-dione (CNQX) (10 μM) to block NMDA or AMPA receptors, respectively, spine expansion was abolished (Fig. 4 B and C). In a subset of experiments, we found that spine expansion partially recovered after washout of D-APV or CNQX (Fig. 4 B and C).
Fig. 4.
Transient spine expansion requires glutamatergic transmission through both N-methyl-d-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, and actin polymerization. (A–C) Average change in spine area through three repeated episodes of tetanic stimulation (arrows) delivered 20 min apart, with the second tetanus applied in the presence of artificial cerebrospinal fluid (ACSF) (A), 50 μM D-APV (bar; B), or 10 μM CNQX (bar; C). In presence of ACSF, spines expanded by 105.0 ± 17.4% with the first tetanus, and 78.1 ± 24.9% and 53.6 ± 13.0% with the second and third rounds of stimulation (n = 18). In D-APV experiments, spines initially expanded by 91.1 ± 11.5% in ACSF but showed no growth in the presence of D-APV (–3.4 ± 4.0%; n = 9; P < 0.001). In the CNQX experiments, spines initially expanded by 87.4 ± 12.1% but showed only a 1.8 ± 3.7% expansion in the presence of antagonist (n = 19, P < 0.001). After washout of D-APV or CNQX, spine expansion partially recovered, with increases in spine size of 51.6 ± 14.7% (n = 9) or 26.5 ± 16.1% (n = 6), respectively (B and C). (D and E) Measurement of spine area in response to two repetitions of tetanic stimulation (arrows) 60 min apart with the second tetanus applied in the presence of 0.5% DMSO (bar; D), or 5 μM latrunculin A (bar; E). In control experiments, spines initially expanded by 84.1 ± 12.0% and by 78.9 ± 10.3% in the presence of 0.5% DMSO, respectively (n = 17). Spines expanded by 113.2 ± 17.0% on the first trial of the latrunculin A experiments but grew by only 12.0 ± 4.7% in the presence of latrunculin A (n = 18, P < 0.001). (F) LTP induced by two trains of 1-s, 100-Hz stimulation (arrows) in the absence (ACSF, filled circles) or presence (open circles) of 5 μM latrunculin A.
A final piece of evidence that spine expansion is an active process related to LTP comes from a study of the role of actin polymerization (Fig. 4 D–F). As noted above, a striking feature of spines is their high F-actin content, which has been implicated in spontaneous, nonsynaptically driven spine motility (8, 32, 33). We found that application of latrunculin A (5 μM), an actin depolymerizing agent, virtually abolished transient spine expansion and LTP (Fig. 4 D–F), with little effect on baseline synaptic transmission (data not shown).
Discussion
Our results show that synaptic activity produces a highly reliable but transient expansion of dendritic spines in acute hippocampal slices from the adult mammalian brain. The transitory nature of this change, as well as its limitation of the small number of spines that are directly activated by synaptic transmission, may help explain the controversy as to the presence (21) or absence (23) of changes in spine size during LTP. However, although we routinely observed spine expansions in adult hippocampal slices, we failed to detect any outgrowth of filopodia from dendritic shafts (13) or appearance of new spines (12) in response to LTP-inducing stimuli. This finding contrasts with previous results in hippocampal slice cultures, suggesting that such changes may be more prominent earlier in neuronal development (34).
We also failed to detect any persistent increase in spine size after induction of LTP, in contrast to the persistent increase in ≈50% of small spines reported in hippocampal slice cultures (16). This discrepancy may reflect the developmental differences between the preparations used in the two studies. One limitation of our study is that we cannot determine whether the transiently expanding spines that we imaged underwent LTP. However, we have imaged several thousand spines, using both EGFP expression and Alexa dye, from slices that routinely demonstrated robust LTP and have documented transient, nonpersistent changes in >175 spines. It seems highly unlikely that we would have failed to sample any spines with persistent size changes if such changes were necessary for the expression of LTP.
If, as our findings indicate, at least some forms of LTP do not require a permanent increase in spine size, what might be the function of the transient expansion? One possibility is that transient spine expansion may be important for synapse remodeling that is required for the enhancement of synaptic transmission during LTP. Indeed, transient spine expansion and the LTP induction process share many similarities. Thus, the same stimulation patterns required to induce LTP are needed to produce reliable spine expansion. Similar to LTP, spine expansion is associated with a significant postsynaptic calcium transient, requires N-methyl-d-aspartate (NMDA) receptor activation, washes out during whole-cell recording, and requires actin polymerization. Although further experiments will be required to determine the precise relation between spine expansion and LTP, our results clearly indicate that transient structural changes in dendritic spines are a prominent feature during the induction of long-term synaptic plasticity in the adult brain.
Supplementary Material
Acknowledgments
We thank Joshua Sanes (Harvard University, Cambridge, MA) for providing EGFP-M mice and Rafael Yuste for helpful comments on the manuscript. This work was supported by a grant from the National Institutes of Health (to S.A.S. and E.R.K.), a Howard Hughes Medical Institute predoctoral fellowship (to C.L.), and the Charles E. Culpeper Biomedical Pilot Initiative from the Rockefeller Brothers Fund (to S.S.Z.).
Author contributions: C.L., S.A.S., and S.S.Z. designed research; C.L. and S.S.Z. performed research; E.R.K., A.B., L.Z., and S.A.S. contributed new reagents/analytic tools; C.L. and S.S.Z. analyzed data; and C.L. and S.A.S. wrote the paper.
Abbreviations: LTP, long-term potentiation; fEPSP, field excitatory postsynaptic potential; EGFP, enhanced GFP; D-APV, d-2-amino-5-phosphonovaleric acid; CNQX, 6-cyano-7-nitroquinoxaline-2,3-dione; ACSF, artificial cerebrospinal fluid.
References
- 1.Bailey, C. H. & Kandel, E. R. (1993) Annu. Rev. Physiol. 55, 397–426. [DOI] [PubMed] [Google Scholar]
- 2.Yuste, R. & Bonhoeffer, T. (2001) Annu. Rev. Neurosci. 24, 1071–1089. [DOI] [PubMed] [Google Scholar]
- 3.Bonhoeffer, T. & Yuste, R. (2002) Neuron 35, 1019–1027. [DOI] [PubMed] [Google Scholar]
- 4.Nimchinsky, E. A., Sabatini, B. L. & Svoboda, K. (2002) Annu. Rev. Physiol. 64, 313–353. [DOI] [PubMed] [Google Scholar]
- 5.Kasai, H., Matsuzaki, M., Noguchi, J., Yasumatsu, N. & Nakahara, H. (2003) Trends Neurosci. 26, 360–368. [DOI] [PubMed] [Google Scholar]
- 6.Blomberg, F., Cohen, R. S. & Siekevitz, P. (1977) J. Cell Biol. 74, 204–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crick, F. (1982) Trends Neurosci. 5, 44–46. [Google Scholar]
- 8.Fischer, M., Kaech, S., Knutti, D. & Matus, A. (1998) Neuron 20, 847–854. [DOI] [PubMed] [Google Scholar]
- 9.Lendvai, B., Stern, E. A., Chen, B. & Svoboda, K. (2000) Nature 404, 876–881. [DOI] [PubMed] [Google Scholar]
- 10.Dunaevsky, A., Blazeski, R., Yuste, R. & Mason, C. (2001) Nat. Neurosci. 4, 685–686. [DOI] [PubMed] [Google Scholar]
- 11.Toni, N., Buchs, P. A., Nikonenko, I., Bron, C. R. & Muller, D. (1999) Nature 402, 421–425. [DOI] [PubMed] [Google Scholar]
- 12.Engert, F. & Bonhoeffer, T. (1999) Nature 399, 66–70. [DOI] [PubMed] [Google Scholar]
- 13.Maletic-Savatic, M., Malinow, R. & Svoboda, K. (1999) Science 283, 1923–1927. [DOI] [PubMed] [Google Scholar]
- 14.Fischer, M., Kaech, S., Wagner, U., Brinkhaus, H. & Matus, A. (2000) Nat. Neurosci. 3, 887–894. [DOI] [PubMed] [Google Scholar]
- 15.Korkotian, E. & Segal, M. (2001) Neuron 30, 751–758. [DOI] [PubMed] [Google Scholar]
- 16.Matsuzaki, M., Honkura, N., Ellis-Davies, G. C. & Kasai, H. (2004) Nature 429, 761–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Okamoto, K., Nagai, T., Miyawaki, A. & Hayashi, Y. (2004) Nat. Neurosci. 7, 1104–1112. [DOI] [PubMed] [Google Scholar]
- 18.Trachtenberg, J. T., Chen, B. E., Knott, G. W., Feng, G., Sanes, J. R., Welker, E. & Svoboda, K. (2002) Nature 420, 788–794. [DOI] [PubMed] [Google Scholar]
- 19.Grutzendler, J., Kasthuri, N. & Gan, W. B. (2002) Nature 420, 812–816. [DOI] [PubMed] [Google Scholar]
- 20.Mizrahi, A., Crowley, J. C., Shtoyerman, E. & Katz, L. C. (2004) J. Neurosci. 24, 3147–3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Harreveld, A. & Fifkova, E. (1975) Exp. Neurol. 49, 736–749. [DOI] [PubMed] [Google Scholar]
- 22.Fifkova, E. & Van Harreveld, A. (1977) J. Neurocytol. 6, 211–230. [DOI] [PubMed] [Google Scholar]
- 23.Sorra, K. E. & Harris, K. M. (1998) J. Neurosci. 18, 658–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Geinisman, Y., DeToledo-Morrell, L. & Morrell, F. (1991) Brain Res. 566, 77–88. [DOI] [PubMed] [Google Scholar]
- 25.Harris, K. M., Fiala, J. C. & Ostroff, L. (2003) Philos. Trans R. Soc. London B 358, 745–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Feng, G., Mellor, R. H., Bernstein, M., Keller-Peck, C., Nguyen, Q. T., Wallace, M., Nerbonne, J. M., Lichtman, J. W. & Sanes, J. R. (2000) Neuron 28, 41–51. [DOI] [PubMed] [Google Scholar]
- 27.Zakharenko, S. S., Zablow, L. & Siegelbaum, S. A. (2001) Nat. Neurosci. 4, 711–717. [DOI] [PubMed] [Google Scholar]
- 28.Harris, K. M. (1999) Curr. Opin. Neurobiol. 9, 343–348. [DOI] [PubMed] [Google Scholar]
- 29.Yuste, R. & Denk, W. (1995) Nature 375, 682–684. [DOI] [PubMed] [Google Scholar]
- 30.Sabatini, B. L., Oertner, T. G. & Svoboda, K. (2002) Neuron 33, 439–452. [DOI] [PubMed] [Google Scholar]
- 31.Malinow, R. & Tsien, R. W. (1990) Nature 346, 177–180. [DOI] [PubMed] [Google Scholar]
- 32.Matus, A. (2000) Science 290, 754–758. [DOI] [PubMed] [Google Scholar]
- 33.Kim, C. H. & Lisman, J. E. (1999) J. Neurosci. 19, 4314–4324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ziv, N. E. & Smith, S. J. (1996) Neuron 17, 91–102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.