Abstract
LIM domains-containing protein 1 (LIMD1) is encoded at chromosome 3p21.3, a region commonly deleted in many solid malignancies. However, the function of LIMD1 is unknown. Here we show that LIMD1 specifically interacts with retinoblastoma protein (pRB), inhibits E2F-mediated transcription, and suppresses the expression of the majority of genes with E2F1-responsive elements. LIMD1 blocks tumor growth in vitro and in vivo and is down-regulated in the majority of human lung cancer samples tested. Our data indicate that LIMD1 is a tumor-suppressor gene, the protein product of which functionally interacts with pRB and the loss of which promotes lung carcinogenesis.
Keywords: lung cancer, retinoblastoma
The archetypal tumor-suppressor gene (TSG) is represented by the retinoblastoma 1 (RB1) gene (1, 2). Loss of its function results in susceptibility to retinoblastoma, a sporadic or hereditary pediatric neoplasm arising from retinal cells harboring either deleted or mutated RB alleles (1, 3, 4). To identify binding partners of retinoblastoma protein (pRB), we performed a yeast two-hybrid screen using full-length pRB fused to the GAL4 DNA-binding domain as bait. We identified the LIM domains-containing protein 1 (LIMD1) sequence, which is localized within the common eliminated region 1 (C3CER1; also called CER1) on chromosome 3p21.3 (5, 6). C3CER1 is one of the putative tumor-suppressor regions identified by the “elimination test,” a functional test system that identifies regularly lost (eliminated) chromosome segments in microcell hybrid-derived severe combined immunodeficiency (SCID) tumors (7). Within chromosome 3p are several TSG regions. Nine homozygous deletion regions have been described, four of which were found in lung tumors. C3CER1 (megabases 43.32–45.74) is located between two such regions, AP20 and LUCA (8, 9). Deletions in this chromosome 3p region are a common event in solid malignancies including breast, gastric, colorectal, ovarian, and renal (6). Furthermore, C3CER1 loss of heterozygosity (LOH) exceeds 90% in lung tumors compared to the putative TSG FHIT (65%) and the TSG VHL (72%) (6), which map at 3p14.2 and 3p25.3, respectively. Therefore, because deletion in this region is a common event in lung tumors, chromosome 3p is under intense study for the identification of putative TSGs (10).
Here, we confirm the specific binding of LIMD1 to pRB and its ability to repress E2F-driven transcription and cell proliferation. Furthermore, we demonstrate that LIMD1 inhibits tumor growth in both in vitro and in vivo models. Finally, we show that LIMD1 expression is down-regulated in lung cancer.
Materials and Methods
Cell Culture, Transfection, and Reporter Assays. All cell lines were cultured in DMEM (GIBCO/BRL) supplemented with 10% FCS, 2 mM glutamine, 50 units/ml penicillin, and 50 μg/ml streptomycin. For transient transfections, 8 × 105 adherent cells were plated in a 25-cm2 flask and the following day were transfected by using FuGene (Roche Diagnostics) per the manufacturer's instructions. The reporter plasmid pGL2–3xE2F-luciferase contains three copies of the E2F DNA-binding site derived from the adenovirus E2A gene promoter and has been described (11). Luciferase activity in cell lysates was normalized to cotransfected β-galactosidase values [relative E2F-luciferase (E2F-luc) activity].
Plasmids. To create N-terminal hemagglutinin (HA)-tagged LIMD1, we performed PCR amplification on human LIMD1 cDNA (kindly provided by S.I.) by using the 5′-HA-START primer (5′-GCAGCATGTACCCATACGATGTTCCAGATTACGCTATGGATAAGTATGACGACCTGGGCCTGGAGGCC-3′) and the 3′-STOP primer (5′-GAAGTTGTGCGTGTGAAGGGCTGTAGATGAGGGTCT-3′). The PCR product was TA cloned into pcDNA3.1 Topo (Invitrogen) and sequence-verified.
pHR-CMV-HA-LIMD1-IRES-GFP was produced as follows: pcDNA3.1-HA-LIMD1 was cut with HindIII, treated with the Klenow fragment of DNA polymerase to produce blunt ends, purified and cut with XhoI, and then ligated directionally into the SmaI/XhoI-cut pHR-CMV-IRES-GFP plasmid downstream of the cytomegalovirus (CMV) promoter and upstream of the internal ribosome entry site (IRES)-GFP.
Xpress (Invitrogen)-tagged LIMD1 and deletion mutant plasmids were created as follows. LIMD1 cDNA was used as a template for PCR to create consecutive deletions in a C- to N-terminal direction. PCR amplification was performed by using the 5′-LIMD1-START primer (5′-GAGATCGAATTCGCAATGGATAAGTATGACGACCTGGGCCTG-3′) in combination with the 3′ primer, which introduced the indicated C-terminal deletion followed by a stop codon. Consecutive LIMD1 deletions in an N- to C-terminal direction were achieved by using the 3′-LIMD1-STOP primer (5′-CTCTGCAGGTCGACCTAGAAGTGGTGCTGGTGAAGGGCTGTA-3′) in combination with the indicated 5′ primer, which annealed at the indicated amino acid and introduced an ATG start codon before this residue. All PCR products produced by this PCR deletion mutagenesis method were gel-purified and TOPO cloned into pcDNA4/HisMax (Invitrogen), which resulted in an N-terminal Xpress-tagged LIMD1 (when using just the 5′-START and 3′-STOP primers) or the indicated deletion mutants. All PCR full-length and deletion mutants of LIMD1 were sequence-verified.
pGEX6P-1-LIMD1 plasmid was constructed as follows. Human LIMD1 cDNA was PCR-amplified by using primers described above to incorporate an EcoRI and SalI site. The resulting PCR product was restriction enzyme-digested with EcoRI and SalI and ligated into similarly cut pGEX6P-1 vector (Amersham Pharmacia Biotech). The plasmid with the correct insert was sequence-verified on both DNA strands.
pHR-CMV-E7-IRES-GFP plasmid was constructed by BamHI digestion of pLXSN-E7 (a gift from David Beach, Queen Mary's School of Medicine and Dentistry, London) to excise the E7 cDNA, which was then ligated into similarly cut pHR-CMV-IRES-GFP.
Construction, Production, and Infection of Lentivirus Expressing LIMD1. To obtain pseudotyped lentivirus [recombinant HIV-1 with vesicular stomatitis virus G (VSV-G) envelope protein], which expresses HA-tagged LIMD1 or E7 protein, we used the gene delivery and production system developed by Naldini et al. (12).
Yeast Two-Hybrid Screen. The cloning of full-length pRB into the pAS2.1 bait vector has been described (13). The resulting plasmid (pAS2.1-pRB) was pretransformed into the Saccharomyces cerevisiae PJ69-4a (MATa trp1-90 leu2-3,112 ura3-52 his3-200 gal4Δgal80ΔLYS2::GAL1-HIS3 GAL2-ADE2 met2::GAL7-lacZ) (14) reporter strain by using a modified lithium acetate protocol (see Yeast Protocols Handbook, BD Biosciences Clontech). Subsequently, this strain was cotransformed with the BC3 cDNA library (produced by using the Stratagene HybriZAP cDNA library kit) in the GAL4 DNA activation domain (GAL4AD) fusion “prey” vector. Selection for positive colonies and cDNA clone isolation were as previously described (15).
Generation of Recombinant GST–LIMD1 Fusion Protein. Full-length LIMD1 was expressed as a recombinant GST fusion protein from the pGEX6P-1-LIMD1 plasmid. Protein was produced and purified as previously described for other GST fusion proteins (15).
Anti-LIMD1 mAb Production and Purification. Recombinant LIMD1 was used in the production of the 3F2/G6 anti-LIMD1 mAb. The method for this production has been described previously (15). The epitope on LIMD1 for the 3F2/G6 mAb was determined to be between amino acids 144 and 214 (data not shown).
Immunoprecipitations, Immunoblots, and Immunofluorescence. Immunoprecipitations and immunoblots were as previously described (15). Briefly, transfected cells were washed three times with ice-cold PBS, fixed with 4% paraformaldehyde for 15 min at room temperature, washed as above, and then permeabilized with 0.5% Triton X-100 for 13 min and immunostained as described (15). The primary Abs used were anti-Xpress mAb (R910-25, Invitrogen) and anti-HA mAb (12CA5, Roche Applied Science).
In Vivo Studies. The human non-small cell lung cancer (NSCLC) tumor cell line A549 was used for determining the inhibitory effects of lentivirus-LIMD1-HA on the establishment of metastatic tumors. Briefly, A549 tumor cells were infected with either lentivirus [empty, vector only (VO)] or lentivirus-LIMD1-HA at a multiplicity of infection (moi) of 100 in cell culture. Three days later, cells were harvested, washed with PBS, and resuspended in sterile PBS at a density of 1 × 106 viable cells per 200 μl. Female nude mice were injected in the tail vein with lentivirus-infected (empty) or lentivirus-LIMD1-HA-infected A549 tumor cells. Eight animals were used in each group. Two weeks after injection, animals were killed, injected intratracheally with 15% India ink, and fixed in Fekete's solution (60% ethanol, 8% formaldehyde, and 4% glacial acetic acid). Lung tumor formation was observed under a dissecting stereomicroscope, and the number of lung tumors was counted. For the A9 fibrosarcoma tumorigenicity assay in severe combined immunodeficient (SCID) mice, a P1-derived artificial chromosome (PAC) fragment, RP6-3307, that contained full-length LIMD1 gene in a blasticidin-selectable pPAC4 vector and empty pPAC4 vector was used for transfections (16). According to standard protocols, 50–70% confluent monolayer A9 cells were transfected with a mixture of 1–2 μg of PAC DNA and 10 μl of Lipofectamine (Life Technologies, Rockville, MD) per well, in six-well plates. The transfectants were selected on blasticidin (1–2 μg/ml). Two positive clones were chosen and expanded in vitro for inoculation into SCID mice. All transfectants were analyzed by fluorescent in situ hybridization (FISH) and PCR.
One million A9 and A9-LIMD1-PAC transfectants were inoculated s.c. into 6-week-old SCID mice in three sites, with a fourth site used for A9 controls. The mice were observed for tumor formation once a week up to 6 weeks. The two positive LIMD1-PAC clones were inoculated in two series into four mice per clone and three inoculation sites per mouse. A9 cells were inoculated as controls at one site per mouse. Tumor growth was monitored once a week for 6 weeks.
BrdUrd Cell Proliferation Assay. The Cell Proliferation ELISA, BrdU (Colorimetric) (Roche Applied Science) immunoassay for the quantification of cell proliferation, based on the measurement of BrdUrd incorporation during DNA synthesis, was used per the manufacturer's instructions.
Microarray Processing and Analysis. RNA preparation, microarray processing, and data analysis of the MDA-MB435 cell line (hereafter referred to as MB435) were performed as described by Wang et al. (17). Affymetrix (Santa Clara, CA) Hg-U133 Plus 2.0 chips were used in this analysis. The human orthologs for the E2F1-responsive genes were selected by using lists previously obtained by Ma et al. (18). The false discovery rate q of 0.05 assures that <5% of genes with q values <0.05 can be expected to be false positives.
Quantitative RT-PCR (qRT-PCR). Total RNA from normal and tumor samples was treated with DNase I (RNase-Free, Ambion, Oxon, U.K.) before cDNA synthesis according to the manufacturer's instructions. cDNA was generated from 1 μg of total RNA by using the SuperScript III First-Strand Synthesis System for RT-PCR (Invitrogen).
Real-time qRT-PCR was performed on an ABI PRISM 7700 sequence detector (Applied Biosystems) by using the SYBR Green PCR Master Mix (Applied Biosystems) in duplicate, with triplicate nontemplate controls (NTCs) in a 25-μl PCR reaction. One microliter of cDNA was used in a 25-μl PCR mixture containing 1× SYBR Green PCR Master Mix and 0.9 μM LIMD1 forward primer (LIMD1 5′-TACCACAAGGTGCTGGCCCCCAAG) and 0.3 μM reverse primer (LIMD1 3′-TGGCCATCTTCATCATTGAGCTCCAGAC) (which produced a product of 163 base pairs and spans nucleotides 1765–1928 of LIMD1 cDNA) or 0.3 μM GAPDH forward and reverse primers (GAPDH 5′-GGA GTC AAC GGA TTT GGT CGT A and GAPDH 3′-GGC AAC AAT ATC CAC TTT ACC AGA GT, respectively). The comparative Ct method was used as described previously (19).
Results and Discussion
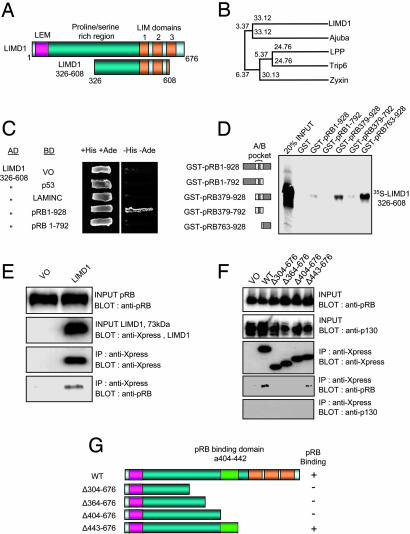
LIMD1 Binds the pRB Tumor Suppressor. To identify binding partners of pRB, we performed a yeast two-hybrid screen using full-length pRB as bait fused to the GAL4 DNA-binding domain. From this screen, LIMD1 was independently identified three times as a specific pRB-interacting protein (Fig. 1A). LIMD1 is a member of the LIM domain family of proteins (5, 20). LIM domains consist of a cysteine-rich consensus sequence containing two distinct zinc-binding subdomains that mediate protein–protein interactions (20, 21). LIM-domain-containing proteins play a role in intracellular signaling, transcriptional regulation, and cellular differentiation during development (18, 19). The predicted protein structure of the human LIMD1 gene reveals three LIM domains located at the C terminus (Fig. 1A), indicating that it belongs to group 3 of this family (Fig. 1B) (5).
Fig. 1.
pRB binds to LIMD1, a member of the zyxin LIM domain family of proteins. (A) A yeast two-hybrid screen of a HeLa cDNA library with GAL4 DNA-binding domain pRB obtained a cDNA encoding amino acids 326–608 of LIMD1. The predicted protein structure of the human LIMD1 gene reveals three LIM domains located at the C terminus and a unique N-terminal region (amino acids 1–68) that encodes a LEM domain (amino acids 18–68) as previously reported (bioinformatics analyses of both the LIMD1 gene and product can be found at www.dsi.univ-paris5.fr/genatlas and http://bioinfo.weizmann.ac.il/cards/index.shtml). (B) LIMD1 belongs to group 3 of the gene family encoding LIM motifs. Shown are phylogenetic relationships between human LIMD1 and other family members containing three LIM domains, Ajuba (24), LPP (lipoma preferred partner) (40), Trip6 (thyroid receptor interacting protein 6) (41), and zyxin (42). Numbers indicate relative distances of branches. (C). The specificity of the pRB–LIMD1 interaction in the yeast two-hybrid assay was confirmed by combining these two proteins with positive and negative controls and then assayed by prototrophy for histidine (His) and adenine (Ade). The indicated GAL4 binding domain (BD) and GAL4 activation domain (AD) fusion or VO plasmids were cotransformed into yeast strain PJ69-4a. Growth on medium lacking histidine and adenine (–His–Ade) is indicative of specific interactions. LAMINC, lamin C. (D) Amino acids 326–608 of LIMD1 interact directly with the C terminus of pRB (amino acids 763–928). [35S]Methionine-labeled LIMD1 (amino acids 326–608) was produced by using the Promega in vitro TNT kit and incubated with the indicated GST-pRB fusion proteins. GST pull-down assays were performed as described (15). (E) Endogenous pRB and transfected Xpress-tagged LIMD1 interact in vivo. IP, immunoprecipitation. (F) pRB binds to amino acids 404–442 of LIMD1 in vivo. U2OS cells were transfected with VO, full-length LIMD1 (WT), or the indicated deletion mutant (Δ). Immunoprecipitations were performed as above. Anti-human pRB mAb, clone G99-549, was from BD Biosciences Pharmingen, and anti-Xpress mAb was from Invitrogen. (G) Graphical summary of the pRB-binding domain as determined by in vivo coimmunoprecipitations.
The yeast two-hybrid interaction between LIMD1 and pRB was specific (Fig. 1C) and confirmed the use of the indicated GST–pRB fusion proteins in an in vitro pull-down assay with [S35]methionine-labeled LIMD1 (Fig. 1D). Both the yeast two-hybrid and GST pull-down interaction assays indicated that LIMD1 (amino acids 326–608) interaction depends on the pRB C-terminal 136 amino acids.
We examined the ability of these two proteins to interact in vivo. LIMD1 (73 kDa) containing an Xpress tag was transfected into U2OS (pRB+/+) cells, and endogenous pRB specifically coimmunoprecipitated with the anti-Xpress mAb only (Fig. 1E). Next, a series of Xpress-tagged LIMD1 deletion mutants was constructed and transfected into U2OS cells. This localized the pRB-binding site to amino acids 404–442 (see Fig. 1 F and G for summarization). LIMD1 did not bind the retinoblastoma family member p130 in vivo (Fig. 1F), indicating the specificity of the pRB interaction.
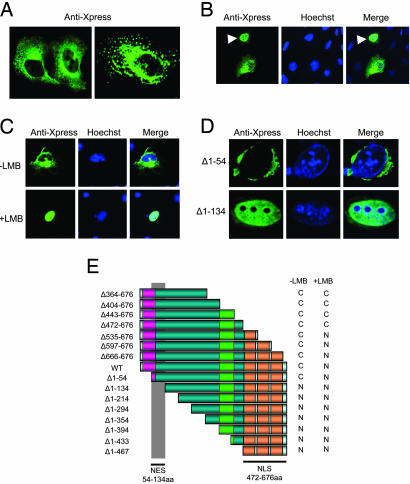
Subcellular Localization of LIMD1. Nuclear localization is a prerequisite for proteins that bind and regulate active pRB; therefore, we examined the subcellular localization of LIMD1. Indirect immunofluorescence assay (IFA) on U2OS cells transfected with Xpress-tagged LIMD1 show that LIMD1 is localized predominantly to the cytoplasm with a small but consistently specific fraction detected in the nucleus but not nucleoli (Fig. 2A Left). The staining of LIMD1 in the cytoplasm consisted of a diffuse, punctuated perinuclear, as well as cytoskeletal, pattern (Fig. 2 A Left). In addition, many cells show vesicle-like staining distributed throughout the cytoplasm (Fig. 2B Right). On average, 14% of LIMD1-expressing cells showed predominantly nuclear localized LIMD1 (Fig. 2B, arrowheads).
Fig. 2.
LIMD1 is a cytosolic protein that shuttles between the nucleus and the cytoplasm via its nuclear localizing LIM domains and its NES, respectively. (A) U2OS cells transfected with Xpress-tagged LIMD1 were grown on coverslips and 24 h posttransfection were fixed and subjected to IFA with anti-Xpress tag mAb. Images of localized proteins were obtained by using confocal laser scanning microscopy. (B) LIMD1 localizes to the nucleus. A typical example of such localization is shown (arrowheads). LIMD1 was visualized with anti-Xpress mAb, and DNA was visualized with Hoechst 33342. (C) LMB treatment of cells causes LIMD1 accumulation in the nucleus. U2OS cells previously transfected with Xpress-tagged LIMD1 were analyzed by IFA using anti-Xpress primary Ab. (Upper) Untreated U2OS cells (–LMB). (Lower) U2OS cells treated with LMB (10 ng/ml) for 4 h (+LMB). (D) LIMD1 contains a NES. A series of Xpress-tagged LIMD1 deletion mutants was constructed and transfected into U2OS cells, and the subcellular localization of these mutants was visualized with anti-Xpress mAb by IFA. Two such deletion mutants, LIMD1Δ1–54 and LIMD1Δ1–134, are shown because they show the switch between cytoplasmic/perinuclear and nuclear localization, respectively, upon deletion of amino acids 54–134. (E) Summary of localization data with the indicated LIMD1 deletion mutants. Localization in U2OS cells was determined as described above in the absence (–LMB) or presence (+LMB and data not shown) of LMB. The three LIM domain modules of LIMD1 have inherent nuclear localizing properties (NLS). C, predominant cytoplasmic localization; N, predominant nuclear localization.
The group 3 LIM domain family of proteins shares the ability to shuttle between the cytoplasm and the nucleus (20, 22–24). To determine whether LIMD1 shares this function, we treated LIMD1-transfected cells with leptomycin B (LMB), a drug that blocks nuclear export by preventing the formation of the nuclear export signal (NES)/chromosomal region maintenance 1 (CRM1)/Ran–GTP nuclear export complex (25). This resulted in nuclear accumulation of LIMD1 (Fig. 2C), suggesting that LIMD1 is actively exported and may contain a NES that facilitates cytosolic/nuclear shuttling.
Using a series of deletion mutants in IFAs in the absence of LMB, we localized the NES to a region spanning amino acids 54–134 (Fig. 2 D and E and data not shown). This NES is leucine-rich, a characteristic shared with other group 3 family NESs. However, no motifs within this NES matched those reported for other family members (data not shown). Regions important for import of LIMD1 to the nucleus were localized to the LIM domains, because LIMD1Δ472–676 in the presence of LMB is predominantly cyto-plasmic and Δ472–676 (LIM domains only) is totally nuclear (Fig. 2E). Similarly, N-terminally truncated forms of Ajuba, Trip6, paxillin, LPP, and Hic-5 containing essentially only the LIM domains localize to the nucleus (23, 26–29).
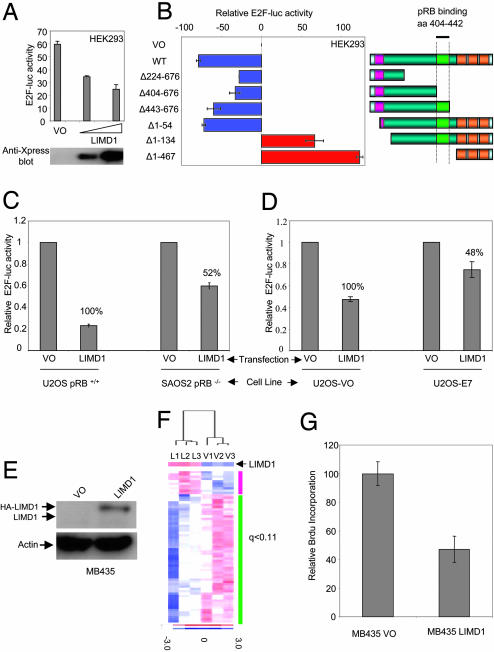
LIMD1 Regulates E2F1-Driven Transcription. pRB represses the transcriptional activity of E2F transcription factors. Because LIMD1 binds specifically to pRB in vitro and in vivo and shuttles to the nucleus, we tested the functional relevance of this interaction by performing E2F-luc reporter assays. HEK293 cells were transfected with VO or increasing concentrations of LIMD1 in the presence of a 3xE2F-luc (E2F1-luc) transcriptional reporter plasmid. LIMD1 represses E2F1-driven transcription in a concentration-dependent manner (Fig. 3A).
Fig. 3.
LIMD1 is a transcriptional regulator that represses E2F-driven transcription. (A Upper) HEK293 cells were transiently transfected with VO or LIMD1 expression vectors. Luciferase activity was determined 48 h later. (Lower) Cell extracts were probed for LIMD1 expression with anti-Xpress mAb. (B) The ability of LIMD1 to repress E2F-luc-driven transcription depends on the presence of the pRB-binding domain. HEK293 cells were transfected with the indicated LIMD1 deletion mutants and the E2F-luc reporter. E2F-luc transcription is shown relative to VO, which is normalized to zero. Blue bars indicate LIMD1 and its mutants that localized predominantly to the cytosol; red bars indicate LIMD1 deletion mutants that localized to the nucleus. (C) The ability of LIMD1 to repress E2F-driven transcription depends partly on the presence of pRB. U2OS (pRB+/+) and SAOS2 (pRB–/–) were transiently transfected with VO or LIMD1, and the E2F-luc levels were determined. (D) Inhibition of pRb function by E7 reduces the transcriptional repressive ability of LIMD1. U2OS cell lines previously transduced with lentivirus VO or lentivirus E7 were transfected with the indicated plasmid in the presence of the E2F-luc reporter. Relative E2F-luc activity was determined 48 h posttransfection. (E) LIMD1 is not expressed in MB435 cells. HA-LIMD1 was transduced into these cells by using lentivirus HA-LIMD1 or lentivirus only (VO). Expression of endogenous LIMD1 and HA-LIMD1 was detected by using the anti-LIMD1 mAb (3F2/G6). (F) E2F1-responsive genes are down-regulated by LIMD1. cDNA microarray analysis of MB435 cells transduced with LIMD1-expressing lentivirus (L1–3) or lentivirus only (V1–3). Heat map of 64 E2F1-responsive genes with down-regulated genes shown in blue and up-regulated genes shown in red. The green bar indicates down-regulated genes upon LIMD1 expression; the purple bar indicates up-regulated genes upon LIMD1 expression. Heat map color scale (at right) displays units of standard deviation from the mean of each gene. (G) LIMD1 inhibits cell proliferation. A BrdUrd ELISA was performed on the LIMD1-negative cell line MB435 transduced with lentivirus, with or without LIMD1 cDNA. Cells were plated 48 h posttransduction into a 96-well plate at 3 × 103 cells per well. Cells were incubated with media containing BrdUrd for 1 h before the anti-BrdUrd ELISA was performed.
Different deletion mutants of LIMD1 were examined for their ability to repress E2F-driven transcription (Fig. 3B). Deletion mutants without the pRB-binding region have a >50% reduction in their ability to repress E2F-mediated transcription (Fig. 3B, compare WT with Δ224–676 and Δ404–676). Whereas addition of the pRB-binding region significantly increases repression of E2F-transcription (Fig. 3B, compare Δ404–676 with Δ443–676). Deletion of the NES caused activation of E2F-transcription (Fig. 3B). The activation domain localized to the three LIM domains at the C terminus (Fig. 3B, Δ1–467). LIMD1 deletion mutants (Δ1–134 and Δ1–467) can therefore act in a dominant negative fashion by activating E2F-mediated transcription (Fig. 3B). It remains to be determined whether this ability is due to the loss of a transcriptional repressive domain that overlaps with the NES or to the disruption of endogenous LIMD1/pRB/E2F repressive complexes upon nuclear retention of these mutants. These findings are similar to those for the group 3 family member Trip6, which has transcriptional activation and repressive functions that overlap with its NES (23).
LIMD1 Has pRB-Dependent and -Independent Transcriptional Repressor Activities. The deletion of the pRB-binding site significantly reduced, but did not completely abolish, LIMD1-mediated repression (Fig. 3B). To address whether LIMD1 repression of E2F-driven transcription depends on pRB, we examined E2F-luc reporter activity in pRB-positive (U2OS and pRB+/+) and -negative (SAOS2 and pRB–/–) cell lines (30). The ability of LIMD1 to repress E2F-driven transcription in SAOS2 cells is reduced by 48% compared with LIMD1 repression in U2OS cells (100%) (Fig. 3C), suggesting that ≈50% of the repressive ability of LIMD1 depends partly on the presence of pRB. We also tested E2F repression by LIMD1 in the presence or absence of human papillomavirus (HPV) E7 (which abrogates the function of pRB family members). In the presence of E7, the ability of LIMD1 to repress E2F-driven transcription was reduced by 52% (Fig. 3D). This is consistent with the ≈50% loss of repression seen between pRB-positive and -negative cell lines (Fig. 3C). The pRB-independent mechanisms of LIMD1 repression remain to be elucidated. However, within amino acids 18–68 of LIMD1 is a LEM domain (Fig. 1 A). This is an ≈40 amino acid residue sequence that can specifically bind barrier-to-autointegration (BAF), a conserved chromatin protein that is a component of the SWI/SNF chromatin-remodeling complex (31–33). Therefore, LIMD1 may also induce repression by way of recruiting chromatin-remodeling proteins.
LIMD1 Expression Reduces Transcription of E2F1-Responsive Genes. The LIMD1-negative cell line MB435 [as determined by RT-PCR (data not shown) and Western blot analysis (Fig. 3E)] was transduced with lentivirus expressing HA-LIMD1 (or no cDNA insert as control). Cells were harvested and processed 48 h postinfection for gene expression microarray (GEM) analysis (Fig. 3F); 3,102 genes were significantly (q <0.11) (17) differentially expressed between LIMD1-transduced cells and control cells. Of these significantly expressed genes, we analyzed 64 E2F1-responsive genes previously reported to be activated by E2F1 (18). We found that 55 of 64 (85.9%) E2F1-responsive genes were down-regulated by LIMD1 expression (Fig. 3F). We also examined 28 genes that were not activated by E2F1 (18). Of these genes, 19 of 28 (67%) were down-regulated, indicating that LIMD1 does not have a global transcription-repressive effect, but that the effect on E2F1-responsive genes is specific. (For the gene list, see Tables 1 and 2, which are published as supporting information on the PNAS web site.)
LIMD1 Inhibits Cell Proliferation. Because the pRB/E2F1 complex can regulate cell cycle progression (transition from G1 to S phase) by the regulation of E2F-responsive genes necessary for S phase progression and thus proliferation, we examined the effects of LIMD1 expression on cell proliferation in the LIMD1-negative cancer cell line MB435 (Fig. 3E). DNA synthesis was determined by measuring BrdUrd incorporation. A significant inhibition of BrdUrd incorporation in MB435 cells was observed in the presence of WT LIMD1 relative to vector control (Fig. 3G), indicating that LIMD1 expression inhibits cell proliferation.
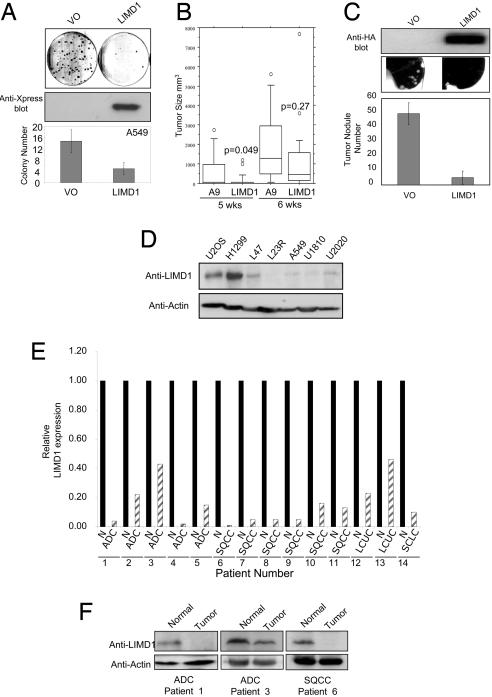
LIMD1 Represses Colony Formation. To test whether the transcriptional and proliferate suppressive effects of LIMD1 translate to a tumor-suppressive phenotype, we tested the growth-suppressive effects of ectopically expressed LIMD1 on transformed cell lines. Colony formation assays were performed by selecting for the Zeocin-resistance gene carried by our LIMD1 expression plasmids. Plasmids containing the full ORF of LIMD1 and the VO control were transfected into non-small cell lung cancer (NSCLC) A549 and HEK293 cells. The number of Zeocin-resistant colonies after LIMD1 transfection was reduced by >80% in both cell types (Fig. 4A and data not shown). LIMD1 mutants unable to interact with pRB but expressed at comparable levels did not inhibit colony formation (data not shown).
Fig. 4.
LIMD1 causes growth suppression in vitro and in vivo.(A Top) LIMD1 inhibits colony formation and growth when transiently expressed in A549 cells. (Middle) Expression of HA-LIMD1 is shown 24 h posttransfection. This experiment was repeated at least three times in triplicate with A549 (Bottom) and HEK293 (data not shown) cells. (B) LIMD1 reduces growth rate in the mouse A9 fibrosarcoma model. Shown are cumulative results of tumor size of mouse fibrosarcoma A9 and A9-LIMD1 P1-derived artificial chromosome (PAC) transfectants (LIMD1) versus weeks postinjection. Circles represent outliers. (C) LIMD1 significantly (P < 0.05) reduces development of A549 experimental lung metastases in nu/nu mice. Lentivirus expressing either LIMD1 or VO control was transduced into A549 cells and injected via tail veins into mice. (Middle) Metastatic colonies (tumor nodules) on the surface of the lung were counted without knowledge of the treatment group. (D) The expression of endogenous LIMD1 is reduced in lung cancer cell lines. Using an anti-LIMD1 mAb (Upper), we examined the expression of endogenous LIMD1 in six lung cancer cell lines equalized for protein concentration (Lower, anti-actin) and compared with a positive control cell line (U2OS). (E) mRNA levels for LIMD1 are reduced in all lung tumors tested compared with adjacent normal lung. Total RNA was extracted from paired lung tumor and adjacent normal tissue and subjected to qRT-PCR. All LIMD1 mRNA levels in tumor samples (hatched bars) are shown relative to the patient-paired normal tissue (filled bars), normalized to one. The tumors analyzed included five adenocarcinomas (ADCs), six squamous cell carcinomas (SQCCs), two large cell undifferentiated carcinomas (LCUCs), and one small cell lung carcinoma (SCLC). (F) Protein levels of LIMD1 are reduced in lung tumors. Two adenocarcinomas (ADCs) and one squamous cell carcinoma (SQCC), together with their matched normal tissue controls, were picked from those in E, and protein extracts were normalized and blotted for the expression of LIMD1. Anti-actin blotting confirmed comparable loading of protein.
LIMD1 A9 Fibrosarcoma Mouse Model. To further evaluate the growth-inhibitory effects of LIMD1 expression in vivo, we used the A9 mouse fibrosarcoma cells that form xenografts and were used to obtain the initial functional evidence, demonstrating that chromosome 3p contains tumor-suppressive activity (34). In these previous experiments (34), the in vivo growth of A9 cells in nude mice was inhibited by transfection with a 2-megabase piece of human chromosome 3 encompassing 3p21–22. Five weeks postinjection (p.i.), LIMD1 expression caused a significant (P < 0.05) decrease in tumor size (Fig. 4B). This same effect on tumor growth was observed 6 weeks p.i., although this was not statistically significant (Fig. 4B). These data indicate that LIMD1 suppresses or delays the growth of tumor cells in an experimental model.
LIMD1 Inhibits Development of Experimental Lung Metastases in Vivo. We evaluated the efficacy of LIMD1 in suppressing in vivo tumor growth and metastases. A549 cells were transduced with a lentivirus expressing HA-LIMD1 or lentivirus only. More than 95% of the cells consistently expressed LIMD1, as determined by IFA for the HA tag and by fluorescent activated cell sorting (FACS) for GFP (see Materials and Methods). Once expression was confirmed, cells were grown for a further 24 h, and an equal number of viable cells was injected into the tail veins of athymic nude mice. LIMD1 expression led to a significantly reduced incidence of lung metastases (Fig. 4C Middle and Bottom).
Overexpression of E2F1 is associated with increased tumor cell growth and metastatic progression (35, 36). Therefore, our data showing that LIMD1 induced down-regulation of E2F1-responsive genes (Fig. 3F) concur with the tumor growth and metastatic inhibitory phenotype induced by LIMD1 in vitro and in vivo (Fig. 4 A, B, and C, respectively).
LIMD1 Expression Is Reduced in Lung Tumors. Finally, we tested whether LIMD1 expression is reduced in human lung cancer cell lines and lung cancers. Five of the six (83%) cell lines had reduced expression of LIMD1 protein compared with U2OS cells when equalized for protein concentration and actin expression (Fig. 4D). We next tested LIMD1 transcript levels in 14 paired malignant versus adjacent normal lung tissue samples using real-time qRT-PCR (Fig. 4E). All of the tumor samples had significantly decreased LIMD1 mRNA levels compared with their adjacent normal lung tissue (Fig. 4F). LIMD1 protein levels were determined in three of the matched samples and confirmed to be decreased in the tumors compared with normal tissue (Fig. 4F).
Chromosome 3p is known to possess tumor-suppressive activity (34). Of the 3p regions analyzed, the highest deletion frequency in solid tumors occurs at the C3CER1 region within 3p21.3 (6). Furthermore, in the majority of tumors analyzed, including lung cancer, LOH at the C3CER1 region (containing LIMD1) is more frequent than LOH at either the FHIT or the VHL TSG regions (6). The functional role in tumor formation of the majority of the 19 active genes encoded within C3CER1 is unknown (37). The lacto-transferrin gene has been shown to suppress growth in fibrosarcoma cells (38) and, like LIMD1, inhibits v-ras transformation of NIH 3T3 cells (unpublished work). LIMD1's effect on cell proliferation and tumor growth (Figs. 3 and 4), linked to its ability to bind pRB and repress E2F-driven transcription (Figs. 1 and 3), suggests that LIMD1 is a principal TSG candidate within C3CER1. In addition, reduced expression of LIMD1 in all lung cancers tested (Fig. 4 E and F) supports the finding that LOH at the C3CER1 region represents an early and recurrent event in the development of lung cancer (6).
Because pRB mutation, or loss, does not occur early in lung cancer development (39), early LIMD1 LOH (through 3p/C3CER1 deletion) may indirectly represent a loss of pRB regulation and be a critical early step in the development of lung cancer.
Supplementary Material
Acknowledgments
We thank D. Lagos, H. Laman, H. Ye, and H. Kiss for helpful discussions; the Department of Thoracic Surgery, Royal Brompton Hospital, led by Professor P. Goldstraw, for help in collection of samples of lung tumors; and J. Timms (University College London, London) for providing the MB435 cell line. This work was supported by funds from Cancer Research UK (to T.V.S., D.B., and C.B.) and The Wellcome Trust (to H.-W.W.).
Author contributions: G.K., T.V.S., and C.B. formulated the hypothesis on which the paper is based; T.V.S. designed the research; T.V.S., M.C., and A.G.N. contributed new reagents/analytic tools; T.V.S., F.M., D.B., N.P., E.D., D.N.B., and S.I. performed the research; T.V.S., H.-W.W., and G.K. analyzed data; and T.V.S., G.K., and C.B. wrote the paper.
Abbreviations: LIMD1, LIM domains-containing protein 1; pRB, retinoblastoma protein; TSG, tumor suppressor gene; C3CER1, common eliminated region 1; LOH, loss of heterozygosity; HA, hemagglutinin; VO, vector only; qRT-PCR, quantitative RT-PCR; LMB, leptomycin B; NES, nuclear export signal.
References
- 1.Knudson, A. G., Jr. (1971) Proc. Natl. Acad. Sci. USA 68, 820–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knudson, A. G., Jr. (1978) Semin. Oncol. 5, 57–60. [PubMed] [Google Scholar]
- 3.Knudson, A. G. (2001) Nat. Rev. Cancer 1, 157–162. [DOI] [PubMed] [Google Scholar]
- 4.Hinds, P. W. & Weinberg, R. A. (1994) Curr. Opin. Genet. Dev. 4, 135–141. [DOI] [PubMed] [Google Scholar]
- 5.Kiss, H., Kedra, D., Yang, Y., Kost-Alimova, M., Kiss, C., O'Brien, K. P., Fransson, I., Klein, G., Imreh, S. & Dumanski, J. P. (1999) Hum. Genet. 105, 552–559. [DOI] [PubMed] [Google Scholar]
- 6.Petursdottir, T. E., Thorsteinsdottir, U., Jonasson, J. G., Moller, P. H., Huiping, C., Bjornsson, J., Egilsson, V., Imreh, S. & Ingvarsson, S. (2004) Genes Chromosomes Cancer 41, 232–242. [DOI] [PubMed] [Google Scholar]
- 7.Imreh, S., Kholodnyuk, I., Allikmetts, R., Stanbridge, E. J., Zabarovsky, E. R. & Klein, G. (1994) Genes Chromosomes Cancer 11, 237–245. [DOI] [PubMed] [Google Scholar]
- 8.Zabarovsky, E. R., Lerman, M. I. & Minna, J. D. (2002) Oncogene 21, 6915–6935. [DOI] [PubMed] [Google Scholar]
- 9.Imreh, S., Klein, G. & Zabarovsky, E. R. (2003) Genes Chromosomes Cancer 38, 307–321. [DOI] [PubMed] [Google Scholar]
- 10.Kaye, F. J. (2001) Lung Cancer 34, Suppl. 2, S35–S41. [DOI] [PubMed] [Google Scholar]
- 11.Zamanian, M. & La Thangue, N. B. (1992) EMBO J. 11, 2603–2610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naldini, L., Blomer, U., Gallay, P., Ory, D., Mulligan, R., Gage, F. H., Verma, I. M. & Trono, D. (1996) Science 272, 263–267. [DOI] [PubMed] [Google Scholar]
- 13.Radkov, S. A., Kellam, P. & Boshoff, C. (2000) Nat. Med. 6, 1121–1127. [DOI] [PubMed] [Google Scholar]
- 14.James, P., Halladay, J. & Craig, E. A. (1996) Genetics 144, 1425–1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharp, T. V., Wang, H. W., Koumi, A., Hollyman, D., Endo, Y., Ye, H., Du, M. Q. & Boshoff, C. (2002) J. Virol. 76, 802–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ioannou, P. A., Amemiya, C. T., Garnes, J., Kroisel, P. M., Shizuya, H., Chen, C., Batzer, M. A. & de Jong, P. J. (1994) Nat. Genet. 6, 84–89. [DOI] [PubMed] [Google Scholar]
- 17.Wang, H. W., Trotter, M. W., Lagos, D., Bourboulia, D., Henderson, S., Makinen, T., Elliman, S., Flanagan, A. M., Alitalo, K. & Boshoff, C. (2004) Nat. Genet. 36, 687–693. [DOI] [PubMed] [Google Scholar]
- 18.Ma, Y., Croxton, R., Moorer, R. L., Jr., & Cress, W. D. (2002) Arch. Biochem. Biophys. 399, 212–224. [DOI] [PubMed] [Google Scholar]
- 19.Heid, C. A., Stevens, J., Livak, K. J. & Williams, P. M. (1996) Genome Res. 6, 986–994. [DOI] [PubMed] [Google Scholar]
- 20.Bach, I. (2000) Mech. Dev. 91, 5–17. [DOI] [PubMed] [Google Scholar]
- 21.Dawid, I. B., Breen, J. J. & Toyama, R. (1998) Trends Genet. 14, 156–162. [DOI] [PubMed] [Google Scholar]
- 22.Wang, Y. & Gilmore, T. D. (2003) Biochim. Biophys. Acta 1593, 115–120. [DOI] [PubMed] [Google Scholar]
- 23.Wang, Y. & Gilmore, T. D. (2001) Biochim. Biophys. Acta 1538, 260–272. [DOI] [PubMed] [Google Scholar]
- 24.Goyal, R. K., Lin, P., Kanungo, J., Payne, A. S., Muslin, A. J. & Longmore, G. D. (1999) Mol. Cell. Biol. 19, 4379–4389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fornerod, M., Ohno, M., Yoshida, M. & Mattaj, I. W. (1997) Cell 90, 1051–1060. [DOI] [PubMed] [Google Scholar]
- 26.Yang, L., Guerrero, J., Hong, H., DeFranco, D. B. & Stallcup, M. R. (2000) Mol. Biol. Cell 11, 2007–2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kanungo, J., Pratt, S. J., Marie, H. & Longmore, G. D. (2000) Mol. Biol. Cell 11, 3299–3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fujita, H., Kamiguchi, K., Cho, D., Shibanuma, M., Morimoto, C. & Tachibana, K. (1998) J. Biol. Chem. 273, 26516–26521. [DOI] [PubMed] [Google Scholar]
- 29.Thomas, S. M., Hagel, M. & Turner, C. E. (1999) J. Cell Sci. 112, 181–190. [DOI] [PubMed] [Google Scholar]
- 30.Ewen, M. E., Sluss, H. K., Sherr, C. J., Matsushime, H., Kato, J. & Livingston, D. M. (1993) Cell 73, 487–497. [DOI] [PubMed] [Google Scholar]
- 31.Wang, W., Cote, J., Xue, Y., Zhou, S., Khavari, P. A., Biggar, S. R., Muchardt, C., Kalpana, G. V., Goff, S. P., Yaniv, M., et al. (1996) EMBO J. 15, 5370–5382. [PMC free article] [PubMed] [Google Scholar]
- 32.Bruder, C. E., Dumanski, J. P. & Kedra, D. (1999) Biochem. Biophys. Res. Commun. 257, 886–890. [DOI] [PubMed] [Google Scholar]
- 33.Guidi, C. J., Sands, A. T., Zambrowicz, B. P., Turner, T. K., Demers, D. A., Webster, W., Smith, T. W., Imbalzano, A. N. & Jones, S. N. (2001) Mol. Cell. Biol. 21, 3598–3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Killary, A. M., Wolf, M. E., Giambernardi, T. A. & Naylor, S. L. (1992) Proc. Natl. Acad. Sci. USA 89, 10877–10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang, S. Y., Liu, S. C., Johnson, D. G. & Klein-Szanto, A. J. (2000) Cancer Res. 60, 5972–5976. [PubMed] [Google Scholar]
- 36.Banerjee, D., Gorlick, R., Liefshitz, A., Danenberg, K., Danenberg, P. C., Danenberg, P. V., Klimstra, D., Jhanwar, S., Cordon-Cardo, C., Fong, Y., et al. (2000) Cancer Res. 60, 2365–2367. [PubMed] [Google Scholar]
- 37.Kiss, H., Yang, Y., Kiss, C., Andersson, K., Klein, G., Imreh, S. & Dumanski, J. P. (2002) Eur. J. Hum. Genet. 10, 52–61. [DOI] [PubMed] [Google Scholar]
- 38.Bezault, J., Bhimani, R., Wiprovnick, J. & Furmanski, P. (1994) Cancer Res. 54, 2310–2312. [PubMed] [Google Scholar]
- 39.Wistuba, I. I., Berry, J., Behrens, C., Maitra, A., Shivapurkar, N., Milchgrub, S., Mackay, B., Minna, J. D. & Gazdar, A. F. (2000) Clin. Cancer Res. 6, 2604–2610. [PMC free article] [PubMed] [Google Scholar]
- 40.Petit, M. M., Mols, R., Schoenmakers, E. F., Mandahl, N. & Van de Ven, W. J. (1996) Genomics 36, 118–129. [DOI] [PubMed] [Google Scholar]
- 41.Yi, J. & Beckerle, M. C. (1998) Genomics. 49, 314–316. [DOI] [PubMed] [Google Scholar]
- 42.Beckerle, M. C. (1997) Bioessays 19, 949–957. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.