Abstract
Background:
Cell cycle regulation of neural progenitor cells (NPCs) is an essential process for neurogenesis, neural development, and repair after brain trauma. Stromal cell-derived factor-1 (SDF-1, CXCL12) and its receptors CXCR4 and CXCR7 are well known in regulating the migration and survival of NPCs. The effects of CXCL12 on NPCs proliferation, cell cycle regulation, and their associated signaling pathways remain unclear. Cyclin D1 is a protein required for progression through the G1 phase of the cell cycle and a known downstream target of β-catenin. Therefore, cyclin D1 plays critical roles of cell cycle regulation, proliferation, and survival in NPCs.
Methods:
Primary mouse NPCs (mNPCs) were derived from brain tissues of wild-type, Cxcr4 knockout, or Cxcr7 knockout mice at mouse embryonic day 13.5 (E13.5). Flow cytometry was used to perform cell cycle analysis by quantitation of DNA content. Real-time PCR and Western blot were used to evaluate mRNA and protein expressions, respectively. Ki67 immunostaining and TUNEL assay were used to assess the proliferation and survival of mNPCs, respectively.
Results:
CXCL12 pretreatment led to the shortening of G0/G1 phase and lengthening of S phase, suggesting that CXCL12 regulates cell cycle progression in mNPCs. Consistently, CXCL12 treatment increased the expression of CyclinD1 and β-catenin, and promoted proliferation and survival of mNPCs. Cxcr7 knockout of mNPCs blocked CXCL12-mediated mNPCs proliferation, whereas Cxcr4 knockout mNPC did not significantly effect CXCL12- mediated mNPCs proliferation.
Conclusion:
CXCR7 plays an important role in CXCL12-mediated mNPC cell cycle regulation and proliferation.
Keywords: mNPCs, cell cycle, CyclinD1, proliferation, anti-apoptosis
Introduction
Neural stem cells (NSCs) have two fundamental physical properties, including self-renew and differentiation, and they have the ability to become both neurons and glial cells [1, 2]. During the early developmental stages of the central nervous system (CNS), NSCs are found in the early embryonic ventricular area, where they sustain neurogenesis and gliogenesis. Additionally, NSCs can produce a series of cell type-lineage progenitors in a temporal and spatial manner, leading to the generation of a heterogeneous cell population. These cells are generally referred to
NPCs (neural progenitor cells) [3]. Because of their multiple biological functions, NSCs have good prospects in clinical applications of stem cell-based cell replacement therapies for neurodegenerative diseases [4-6]. Growing areas of research on NPCs include seeking a high survival rate of NSCs, accurate control of proliferation and differentiation, as well as detecting the functions of NSCs.
In terms of the physiological characteristics, NSCs generally have four existing states, including self-renewal, quiescent condition, apoptosis, and terminal differentiation. These processes are all involved in cell cycle regulation [7]. The cell cycle mainly involves two important stages, mitotic phase (M phase) and inter-mitosis. Inter-mitosis stage include three phases: G1, S, and G2. Unidirectional movement through these phases is driven by the activity of cyclin-dependent kinases (cdks) activated by specific Cyclins [8]. CyclinD/cdk4/6 effects passage through G1 phase, while cdk phosphorylation inactivated retinoblastoma protein permits the cell to pass through the G1 “restriction point” and enter into S phase under the regulation of CyclinE/cdk2 and CyclinA/cdk2. In S phase, the cell undergoes semi-conservative DNA replication. The G2 phase, under the influence of CyclinA/cdk1 and CyclinB/cdk2, ultimately drives cells into mitotic division. Finally, degradation of the mitotic Cyclins by the Anaphase Promoting Complex (APC/C) leads to mitotic exit and re-entry into the next G1 phase [9, 10]. Recent reports showed that G1 phase plays a decisive role in the regulation of cell proliferation and differentiation [11]. Artegiani et al. demonstrated that the overexpression of CyclinD/cdk4 in the hippocampus of mice induced the constant expansion of NPCs while suppressing neurogenesis [12]. Lange et al. showed that restrained CyclinD/cdk4 lengthened the G1 phase and increased the number of neurons, while decreasing the amount of NPCs [13].
Stromal cell-derived factor-1 (SDF-1, CXCL12) belongs to an extensive family of small secreted proteins that regulate cell migration and survival during the development of the nervous system [14]. CXCR4 and CXCR7 have been identified as CXCL12 receptors. Research into malignant peripheral nerve sheath tumors and colorectal cancer has shown that CXCL12/CXCR4 could induce the proliferation and invasion of cancer cells through the activation of PI3K signaling pathway and increasing CyclinD1 expression [15]. Neviana et al. showed that CXCL12/CXCR4 promotes C-Kit+ cardiac stem/progenitor cell quiescence through the extension of the G0/G1 phase [16]. In the hematopoietic system, CXCL12 is essential for the migration and homing of primitive hematopoietic cells. Recent research has indicated that CXCL12 regulates the cell cycle and facilitates cell survival of CD34+ primitive hematopoietic cells [17]. To date, there has been no direct evidence to clarify the effects of CXCL12 on the cell cycle of neural stem cells. Here, we present findings that describe the cell cycle regulation, proliferation, and anti-apoptosis of mouse NPCs with CXCL12.
MATERIALS AND METHODS
Reagents
Recombinant mouse CXCL12 was obtained from R&D Systems (Minneapolis, MN, www.rndsystems.com ). PI/RNase Staining Buffer was obtained from BD Bioscience. In Situ Cell Death Detection Kit, TMR red was purchased from Roche. CyclinD1, β-catenin, and actin protein levels were detected using antiCyclinD1 antibody (1:1000, Cell Signaling Technologies), antiβ-catenin antibody (1:1000, BD), and anti-actin antibody (1:5000, Sigma Aldrich), respectively.
Mouse NPC Culture and Treatment
Mouse cortical NPCs were isolated from gestational day E13.5 brain tissue as previously described (Chen et al. 2015), mNPCs were cultured in mouse NeuroCult NSC Proliferation Medium (StemCell Technologies, Vancouver, BC, Canada, www.stemcell.com ) supplemented with epidermal growth factor (10 ng/ml, Sigma Aldrich) and basic fibroblast growth factor (20 ng/ml, Sigma Aldrich) for selective neurosphere cultures. Neurospheres were passaged at 3-4 day intervals, until they reached 100–150 lm in diameter. CXCL12 was dissolved in 0.1% bovine serum albumin (BSA, Life Technologies, Grand Island, NY, www.lifetechnologies.com ), and stored at –20°C until use. For CXCL12 pretreatment, cells were plated at a confluence of 60-80%, and exposed to different concentrations (0, 10, 50, 100, 250 ng/ml) of CXCL12 for 2 hours and then incubated with growth factor deprivation medium for an additional 12 to 24 hours. After CXCL12 pretreatment and growth factor deprivation, mouse NPCs were subjected to subsequent experiments.
Evaluation of Cell Cycle Progression (Flow Cytometry Assay)
Mouse NPCs were cultured in 6 well plates at a density of 0.5 million/well. For cell cycle analysis, they were digested using accutase, washed twice with cold PBS, and fixed with 70% ethanol for 24 hours at –20°C. After fixation, the cells were washed twice and resuspended in PBS and stained with PI/RNase Staining Buffer at room temperature for 30 minutes. The cell cycle distribution was acquired by FACSCanto II flowcytometer (BD Biosciences). FACS DIVA and FlowJo software were used to determine the percentage of mouse NPCs in G0, G1, and S/G2/M.
Real Time RT-PCR
Changes in gene expression were determined by quantitative real-time RT-PCR. Total RNA was isolated and purified from mouse NPCs cultured in 6 well plates using RNeasy Mini Kit (QIAGEN). The RNA concentration was measured by a Nano-Drop 2000 spectrophotometer (Thermo Scientific). mRNA was reverse-transcribed and cDNA to be used in real time PCR was prepared with SYBR Premix Ex TaqTM II (TaKaRa). Primer sequences used in qPCR were described as follows: CyclinD1, forward, GCGTACCCTGACACCAATCTC, reverse, CTCCTCTTCGCACTTCTGCTC; CyclinD2, forward, GAGTGGGAACTGGTAGTGTTG, reverse, CGCACAGAGCGATGAAGGT; CyclinD3, forward, CGAGCCTCCTACTTCCAGTG, reverse, GGACAGGTAGCGATCCAGGT; GAPDH, forward, TGGATTTGGACGCATTGGTC, reverse, TTTGCACTGGTACGTGTTGAT.
Western Blotting
After a series of treatments, mouse NPCs were lysed by M-PER Protein Extraction Buffer (Pierce, Rockford, IL, USA). Protein concentration was determined using the BCA (bicinchoninic acid) Protein Assay Kit (Pierce). An analytical 10% SDS-polyacrylamide gel electrophoresis (SDS PAGE) was prepared and then transferred to an Immuno-Blot polyvinylidene fluoride membrane (Bio-Rad, Hercules, CA, USA). After blocking in 5% fat-free milk for 1 hour, the membrane was incubated with primary antibodies for CyclinD1, actin, and β-catenin overnight at 4°C followed by horseradish peroxidase-conjugated secondary antibodies for 1 hour at room temperature. It was illuminated using a chemiluminescent substrate solution. The density of the immunoblots was determined by image lab software and analyzed using Image J program.
Immunocytochemistry
Mouse NPCs were fixed in 4% paraformaldehyde for 15 min. They were then washed with PBS three times, and coverglasses were blocked using 1% BSA for 1 hour at room temperature. Subsequently, they were incubated overnight at 4°C with primary antibodies including rabbit anti-Ki67 (1:1000; CST) and chicken anti-nestin (1:5000; Novus) for the identification of proliferating mouse NPCs. This was followed by Alexa Fluor secondary antibodies, goat-anti-rabbit IgG Alexa Fluor 488, and goat-anti-chicken IgG Alexa Fluor 568 (1:200; Life Technologies) for 1 hour at room temperature. Nuclei were counter-stained with DAPI. Coverglasses were fixed on glass slides with Mounting Medium (Sigma-Aldrich). Immunostaining was examined by a Zeiss 710 confocal laser-scanning microscope. For quantification, images were imported into Image-ProPlus version 7.0 (Media Cybernetics) to count stained cells.
In Vitro TUNEL Assay
Mouse NPCs were seeded on poly-D-lysine coated coverslips at a density of 0.1 million/well. After a series of treatments, mouse NPCs were fixed and permeablized with 0.5% triton-X 100, and the apoptotic cells were determined by In Situ Cell Death Detection Kit, TMR red Fluorescein (Roche) according to the manufacturer's protocol. Images were taken using a Zeiss fluorescent microscope. All accessed images were imported into Image-ProPlus for quantifying the number of apoptotic cells.
Statistical Analysis
Data were expressed as means ±SEM, and analyzed by one-way ANOVA followed by the Tukey's test for pairwise comparisons. Significance was considered when p < 0.05. All experiments were performed with mouse NPCs from at least three donors. Assays were performed at least three times in triplicate or quadruplicate.
RESULTS
CXCL12 Regulates Cell Cycle of Mouse NPCs
To determine whether CXCL12 pretreatment affects mouse neural stem cell functions, P13.5 mouse NPCs were incubated with different concentrations (0, 10, 50, 100, 250 ng/ml) of CXCL12 in complete growth medium for 2 hours, and then treated without growth factors for 12 hours. We analyzed the cell cycle distribution of the cells using flow cytomerical analysis. The results showed CXCL12 100 ng/ml pretreatment evidently decreased the number of cells in G1 phase, whereas the number of cells in S phase increased significantly (Fig. 1B, C). Meanwhile, we did not observe remarkable morphological changes in mouse neural stem cells after CXCL12 pretreatment and growth factors deprivation (GFD), suggesting that the cells did not undergo significant differentiation. This suggests that CXCL12 might specifically affect the proliferation or apoptosis of mouse NPCs.
Fig. (1).
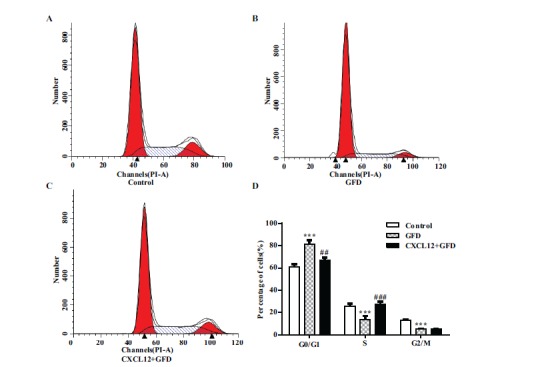
Pretreatment with CXCL12 promotes transition from G1 to S phase during growth factor deprivation (GFD). (A-C) Flow cytometry analysis shows the cell cycle phase of P13.5 mouse neural progenitor cells which were pretreated with CXCL12 for 2 hours and then treated without growth factors for 0 to 12 hours. (D) Results are presented as the mean ± SEM of three independent experiments with NPC from three mouse donors. ***P<0.001, significantly different from control group, ##P<0.01, ###P<0.001, significantly different from GFD group.
CXCL12 Increases the Gene Expression Level of Cyclin D1 and the Cyclin D1 Upstream Gene β-Catenin
There are two major genes involved in cell cycle progression. One is Cyclin and the other is Cyclin Dependent kinase (CDK). Among them, Cyclin D/CDK4,6 mainly regulates the G1 phase, and Cyclin A,E/CDK2 is a well-known regulator of the S phase. In order to further investigate whether genes related to cell cycle regulation were altered after CXCL12 pretreatment, we first examined the mRNA expression of G1 phase related genes (Cyclin D1, D2, D3) using RT-PCR assay. The expression levels of Cyclin D1 and D2 increased after CXCL12 pretreatment, while Cyclin D3 showed minimal changes (Fig. 2A). Western blot results verified that the level of Cyclin D1 protein in this group was significantly increased compared with the growth factors deprivation (Fig. 2B). It is known that the Wnt/β-catenin pathway regulates cell proliferation through the modulation of various genes related to proliferation, including c-myc and Cyclin D1. In order to determine whether Wnt signaling was altered in mouse neural stem cells, we detected the expression of β-catenin. The results of the Western Blot showed that the level of cellular β-catenin protein gradually increased in a dose-dependent manner with CXCL12 pretreatment (Fig. 2C). These data indicate that CXCL12 pretreatment increases Cyclin D1 expression to shorten the G1 phase and consequently accelerate the progression of the cell cycle. We speculate that CXCL12 might facilitate the proliferative capability of mouse neural stem cells.
Fig. (2).
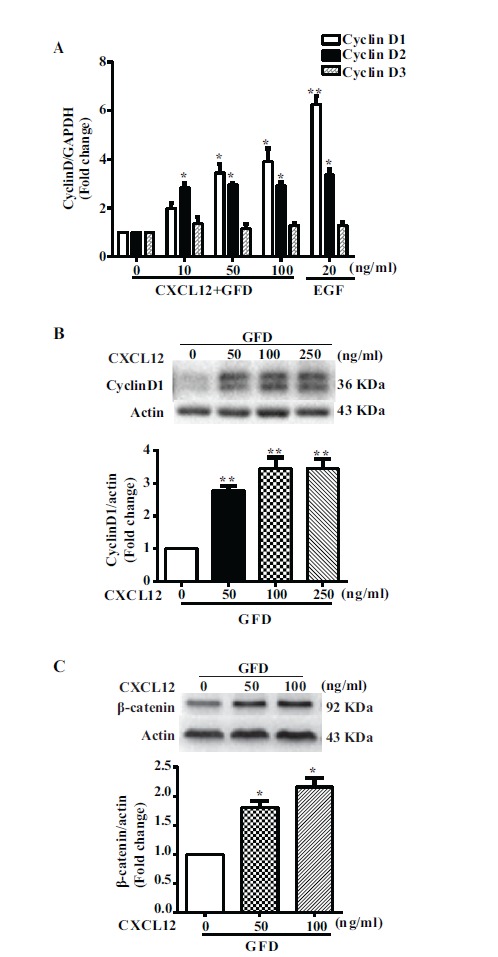
Real time PCR and Western blot analysis of CyclinD and β-catenin following CXCL12 pretreatment. (A) mNPCs pretreated with CXCL12 at different concentrations (0,10,50,100 ng/ml) for 2 hours and then treated without growth factors for 24 hours. Values are means ± SEM (n=3). (B) mNPC pretreated with CXCL12 at 0, 50, 100, 250 ng/ml for 2 hours following 24 hour starvation. Values are means ± SEM (n=3), *P<0.05, **P<0.01, significantly different from the GFD group. (C) mNPCs were pretreated with CXCL12 for 2 hours and then treated without growth factors for 24 hours. (D) Levels of β-catenin were normalized as a ratio of β-catenin to actin after densimetrical quantification of panel C and shown as fold change relative to GFD group. Results are expressed as the mean ± SEM of triplicate samples and are representative of 3 independent experiments. *P<0.05, compared with GFD.
CXCL12 Induces the Proliferation of Mouse NPCs
To verify whether CXCL12 has a direct effect on mouse neural stem cell proliferation, we pretreated mouse neural stem cells for 2 hours with varying concentrations (10, 50, 100, 250 ng/ml) of CXCL12 followed by 24 hours of growth factor deprivation; subsequently, we stained the samples with Ki67. Quantification data showed that CXCL12 increased the total number of cells, as well as the number of Ki67 positive cell (Fig. 3G, H). These data indicate that CXCL12 pretreatment increased mouse neural stem cell proliferation compared with the growth factor deprivation only group.
Fig. (3).
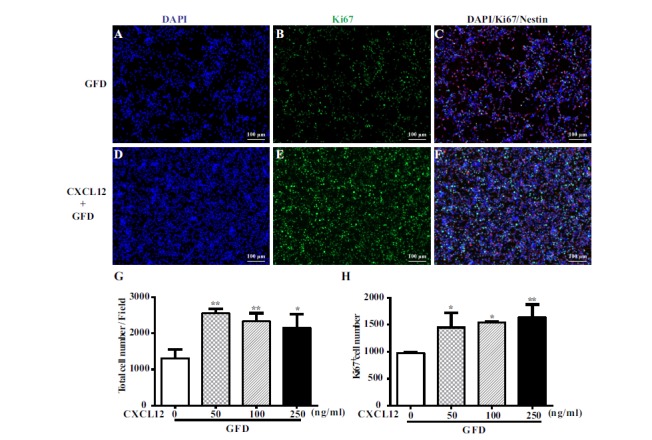
Proliferation effect of CXCL12 on mouse NPC by Ki67 staining. CXCL12-mediated proliferation of mouse NPC is determined by Ki67 staining after pretreatment of increasing concentrations of CXCL12 stimulation for 2 hours followed by the deprivation of growth factors for 24 hours. Cell proliferation was assessed by immuno-staining for Ki67 positive in mNPC in GFD group (A,B,C) and with CXCL12 pretreatment group (D,E,F). The percentage of Ki67-positive proliferating mNPCs were determined for each treatment with different concentrations of CXCL12 by counting positive cells per microscopic field in 30 pictures per condition (G,H). Results are presented as the mean ± SEM of three independent experiments with NPC from three mouse donors. *P<0.05, **P<0.01, in comparison to GFD group, scale bar=100 μm.
CXCL12 Enhances Mouse NPC Survival During Growth Factor Deprivation
The aforementioned data (Fig. 4) showed that CXCL12 could induce the proliferation of mouse neural stem cells. In this study we use growth factor deprivation to investigate the role of CXCL12 pretreatment; in particular, cells were deprived of EGF, bFGF and neural stem cell supplement medium to explore whether CXCL12 can promote mouse neural stem cell proliferation. We also sought to determine whether CXCL12 promotes mouse NPCs anti-apoptosis ability. To determine the function of CXCL12 on the survival of mouse neural stem cells, we pre-treated mouse neural stem cells with CXCL12 for 2 hours and then treated with a growth factor deprivation medium for additional 24 hours. Growth factor deprivation dramatically increased the number of TUNEL-positive mouse neural stem cells compared with the untreated control (Fig. 4A, B). CXCL12 alleviated apoptosis induced by growth factor deprivation in a dose-dependent manner (10-100 ng/ml) (Fig. 4C, D, E). To confirm the anti-apoptosis ability of CXCL12, we also detected the level of cleaved PARP using western blotting at similar experimental settings. The result showed cleaved PARP decreased after CXCL12 pretreatment in a dose-dependent manner (Fig. 4F, G). In summary, these data suggest that CXCL12 protects against apoptosis and increases the survival of mouse NPCs during growth factor deprivation.
Fig. (4).
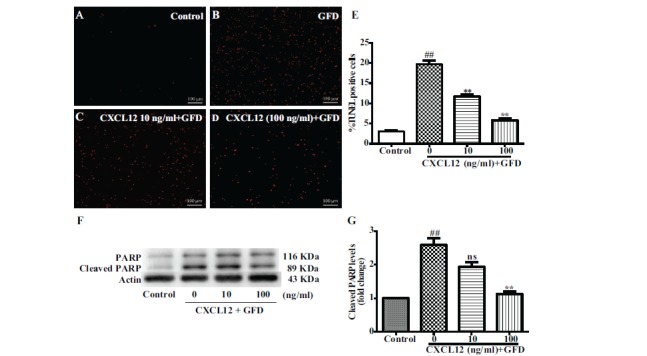
CXCL12 pretreatment protected mNPC against apoptosis induced by the deprivation of growth factors. mNPCs were pretreated with CXCL12 for 2 hours and then deprived of growth factors for 24 hours. mNPCs apoptosis was determined by TUNEL assay (A-D) or PARP cleavage assay (F). (A-D) Representative pictures from TUNEL assay are shown. (E) Quantification data of TUNEL assay were determined as a percentage of TUNEL positive cells against the total cell number. (F) Cleaved PARP protein levels were determined by Western blotting. (G) Levels of cleaved PARP were normalized as a ratio of cleaved PARP to actin after densimetrical quantification of panel F and shown as fold change relative to control. Results are expressed as the mean ± SEM of triplicate samples and are representative of 3 independent experiments. ##P<0.01 compared with control; *P<0.05, **P<0.01 compared with growth factors deprivation group without CXCL12 pretreatment. Scale bar=100 μm.
CXCR7 may Play an Important Role on the Anti-Apoptosis Function of CXCL12 in Mouse NPCs
Previous studies have found that CXCL12 has two receptors (CXCR4 and CXCR7), which play a key role in the function of CXCL12 in NPCs [18-20]. We utilized CXCR4 and CXCR7 knockout mouse NPCs to explore the potential mechanisms involved in CXCL12 induced anti-apoptosis functions during deprivation of growth factors. As with previous experimental conditions, we pretreated the two types of cells with different concentrations of CXCL12 for 2 hours. This was followed by additional growth factor deprivation for 24 hours. The DAPI staining result showed that in control wild type mNPCs, the percentage change of proliferation was 99.2%±17.63% after CXCL12 pretreatment, as compared to its own GFD group (Fig. 5E). In CXCR4 knockout mNPCs, the percentage change of proliferation was 71.4%±23.50%, a mild decrease of changes as compared with wild type mNPC. There was no significant difference between these two groups (Fig. 5E). In CXCR7 knockout mNPCs, the percentage change of proliferation was 23.4%±5.27% after CXCL12 pretreatment, which was a significant decrease of change as compared to the wild type mNPCs (Fig. 5E).
Fig. (5).
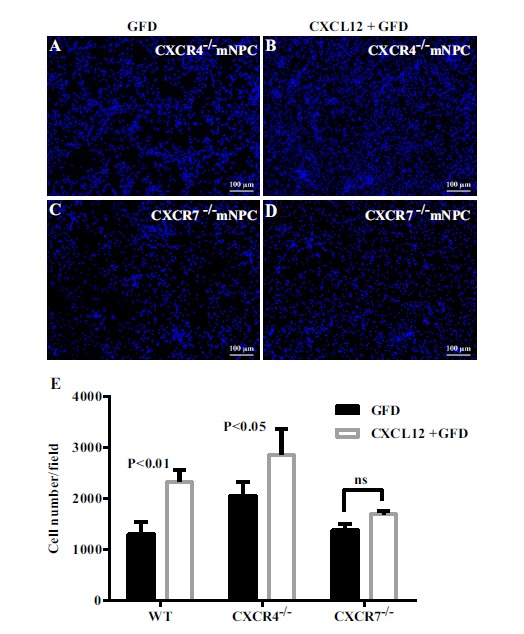
CXCR7 is required for the proliferation function of CXCL12 in mNPCs. (A-D) immuno-staining for DAPI in CXCR4 knock out and CXCR7 knock out mNPCs in the starvation group (A,C) and with the CXCL12 pretreatment group (B,D). The total cell numbers were determined for each treatment with CXCL12 by counting positive cells per microscopic field in 30 pictures per condition (E). Results are presented as the mean ± SEM of three independent experiments with NPC from three mouse donors. ns>0.05 in comparison to starvation. Scale bar=100 μm.
DISCUSSION
We demonstrated that CXCL12 pretreatment facilitated cell proliferation and promoted cell cycle progression at the G0/G1 phase transition to the S phase of mouse NPCs in vitro. Cell cycle progression is vital for proper neurogenesis, and cell cycle regulation is an important mechanism by which stem cells preserve self-renewal and multi-directional differentiation. Research has shown that cell cycle regulation of neural progenitors determined the size of the cerebral cortex development [21]. Several studies have demonstrated SDF1-CXCR4 regulation of HSC and C-Kit+ Cardiac Stem/Progenitor Cell quiescence [16, 17]. During the cell cycle, the successful transition of G0/G1 phase to the S phase is crucial for proliferation. Cyclin D/cdk4 complex plays a key role in the process, especially Cyclin D1. In our study, CXCL12 pretreatment increased Cyclin D1, and facilitated G0/G1 phase transfer to S phase in mouse NPCs. These results indicate that CXCL12 may enhance the proliferation of mouse NPCs to boost neurogenesis. Consistent with the result of cell cycle analysis we analyzed proliferation marker Ki67 of mouse NPCs using cell immunofluorescence staining of Ki67 and found that CXCL12 significantly increased total cell numbers and Ki67-positive cell numbers compared with those of growth factor deprivation. These data suggest that CXCL12 promotes the proliferation of mouse NPCs.
In this study, we pretreated mouse NPCs for 2 hours with CXCL12 in the presence of growth factor EGF and bFGF to test whether CXCL12 could promote mouse NPCs proliferation followed by growth factor deprivation. We discovered that CXCL12-treated mouse NPCs without pretreatment of CXCL12 did not proliferate after they were deprived of growth factors for 24 hours (data not shown). This indicates that CXCL12 alone cannot promote cell cycle progression and induce proliferation of mouse NPCs in an environment deprived of growth factors [22]. Neural progenitor cells’ proliferation and self-renewal is affected by many factors such as extracellular factor [23]. Among these factors, EGF [24, 25], bFGF [26, 27] and IGF-1 [28, 29] played important roles during the process of NPCs proliferation and differentiation. A previous study demonstrated that blocking CXCR4 with a specific antagonist impaired the growth factor-induced progression of NPCs through the cell cycle [22]. EGF is an important mitosis-promoting factor (MPF), and plays an important role in embryonic and adult neural progenitor cell survival, migration and differentiation [30-33]. A recent study reported that CXCL12 can induce different responses, promoting NPC migration at low concentrations while favoring cell adhesion via EGF and the alpha 6 integrin at high CXCL12 concentrations [34]. Some published literature states that CXCL12 alone will not increase the proliferation of mouse cerebellum granule cells, and blocking CXCR4 with a specific antagonist impairs the growth factor-induced progression of NPCs through the cell cycle [35]. It is unclear if in the process of CXCL12 pretreatment CXCL12 coordinates with growth factor EGF or bFGF to promote cell cycle transformation and induces proliferation of mouse NPCs [36]. This needs to be explored in further research.
As a Chemokine, CXCL12 has two receptors (CXCR4 and CXCR7). Previous studies have shown that CXCR4 contributes to the quiescence of a primitive hematopoietic stem cell, and CXCR4-/- can lead to an abnormal proliferation of hematopoietic stem cells and disturb hematopoietic system steady state [37]. Early in vivo research indicated that in CXCR4-defective mice, the number of NPC in neurosphere outgrowth was two-fold less than in wild-type (WT) mice; NPC radial cell migration was also decreased [38]. CXCR7 as another receptor for CXCL12 has been studied in recently years. It has been shown that knockdown of CXCR7 in HBMECs resulted in significantly reduced HBMEC proliferation, tube formation, and migration, as well as adhesion to matrigel and tumor cells [39]. Furthermore, CXCR7 could provide a means to promote oligodendroglial differentiation facilitating endogenous remyelination activities [40]. In our study, we specifically used CXCR4 and CXCR7 gene knockout approaches to investigate the role of CXCL12 pretreatment on mouse NPCs. After CXCL12 pretreatment, CXCR4-/- mouse NPCs did not increase as much as wild type mNPC (71.4% increase as compared with 99.2% increase with wild type NPC), while CXCR7-/- mouse NPCs significantly differed from wild type mNPC (23.4% increase as compared with 99.2% increase with wild type NPC, Fig. 5E). These results indicate that CXCL12-CXCR7 interaction might be more critical for mouse NPCs undergoing pretreatment of growth factor deprivation.
Growth factor deprivation is a starvation condition, which induces the apoptosis of mouse NPCs. Neural stem cells surviving in hostile environments act as they would during human brain trauma or neurodegenerative disorders. In the repair process of trauma or a disease, normal neural stem cells migrate to the inflammation of the damaged area under the guidance of chemokines; as a chemokine, CXCL12 plays a crucial role in this process [41-43]. CXCL12 has been proposed to be a chemokine that mediates the migration of transplanted stem cells [44, 45]. One critical question is how to maintain neural stem cells properties in their initial state and during migrating? Our study provides evidence that CXCL12 promotes the proliferation and anti-apoptosis of mouse NPCs, and that CXCR7 may play an important role in these processes.
ACKNOWLEDGEMENTS
We would like to thank Drs. Zhengliang Gao and Dongsheng Xu for valuable suggestions, Dr. Matthew Mitchell for manuscript editing and Ms. Yanyan Zhang and Yueju Li for technical support. This work was partly supported by research grants by National Basic Research Program of China (973 Program Grant No. 2014CB965001), National Natural Science Foundation of China Innovative Research Groups of the National Natural Science Foundation of China (#81221001), and Joint Research Fund for Overseas Chinese, Hong Kong and Macao Young Scientists of the National Natural Science Foundation of China (#81329002); National Institutes of Health: R01 NS41858-01, 2R56NS041858-15A1 (JZ), and R03 NS094071-01 (YH). Julie Ditter, Lenal Bottoms, Johna Belling, and Robin Taylor provided outstanding administrative and secretarial support.
CONFLICT OF INTEREST
The authors confirm that this article content has no conflicts of interest.
REFERENCES
- 1.Alvarez-Buylla A., Seri B., Doetsch F. Identification of neural stem cells in the adult vertebrate brain. Brain Res. Bull. 2002;57(6):751–758. doi: 10.1016/s0361-9230(01)00770-5. [DOI] [PubMed] [Google Scholar]
- 2.Bian J., Zheng J., Li S., Luo L., Ding F. Sequential Differentiation of Embryonic Stem Cells into Neural Epithelial-Like Stem Cells and Oligodendrocyte Progenitor Cells. PLoS One. 2016;11(5):e0155227. doi: 10.1371/journal.pone.0155227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gal J.S., Morozov Y.M., Ayoub A.E., Chatterjee M., Rakic P., Haydar T.F. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J. Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.De Feo D., Merlini A., Laterza C., et al. Neural stem cell transplantation in central nervous system disorders: from cell replacement to neuroprotection. Curr. Opin. Neurol. 2012;25(3):322–333. doi: 10.1097/WCO.0b013e328352ec45. [DOI] [PubMed] [Google Scholar]
- 5.Christie K.J., Turnley A.M. Regulation of endogenous neural stem/progenitor cells for neural repair-factors that promote neurogenesis and gliogenesis in the normal and damaged brain. Front. Cell. Neurosci. 2013;6:70. doi: 10.3389/fncel.2012.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H., Xie Z., Wei L., et al. Human umbilical cord mesenchymal stem cell-derived neuron-like cells rescue memory deficits and reduce amyloid-beta deposition in an AbetaPP/PS1 transgenic mouse model. Stem Cell Res. Ther. 2013;4(4):76. doi: 10.1186/scrt227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pauklin S., Vallier L. The cell-cycle state of stem cells determines cell fate propensity. Cell. 2013;155(1):135–147. doi: 10.1016/j.cell.2013.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hardwick L.J., Ali F.R., Azzarelli R., Philpott A. Cell cycle regulation of proliferation versus differentiation in the central nervous system. Cell Tissue Res. 2015;359(1):187–200. doi: 10.1007/s00441-014-1895-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hydbring P., Malumbres M., Sicinski P. Non-canonical functions of cell cycle cyclins and cyclin-dependent kinases. Nat. Rev. Mol. Cell Biol. 2016;17(5):280–292. doi: 10.1038/nrm.2016.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu D., Pattabiraman V., Bacanamwo M., et al. Iroquois Homeobox Transcription Factor (Irx5) Promotes G1/S-Phase Transition in Vascular Smooth Muscle Cells by CDK2-dependent Activation. Am. J. Physiol. Cell Physiol. 2016;311(2):C179–C189. doi: 10.1152/ajpcell.00293.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hindley C., Philpott A. Co-ordination of cell cycle and differentiation in the developing nervous system. Biochem. J. 2012;444(3):375–382. doi: 10.1042/BJ20112040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Artegiani B., Lindemann D., Calegari F. Overexpression of cdk4 and cyclinD1 triggers greater expansion of neural stem cells in the adult mouse brain. J. Exp. Med. 2011;208(5):937–948. doi: 10.1084/jem.20102167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lange C., Huttner W.B., Calegari F. Cdk4/cyclinD1 overexpression in neural stem cells shortens G1, delays neurogenesis, and promotes the generation and expansion of basal progenitors. Cell Stem Cell. 2009;5(3):320–331. doi: 10.1016/j.stem.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 14.Li M., Ransohoff R.M. Multiple roles of chemokine CXCL12 in the central nervous system: A migration from immunology to neurobiology. Prog. Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mo W., Chen J., Patel A., et al. CXCR4/CXCL12 mediate autocrine cell- cycle progression in NF1-associated malignant peripheral nerve sheath tumors. Cell. 2013;152(5):1077–1090. doi: 10.1016/j.cell.2013.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dimova N., Wysoczynski M., Rokosh G. Stromal cell derived factor-1alpha promotes C-Kit+ cardiac stem/progenitor cell quiescence through casein kinase 1alpha and GSK3β. Stem Cells. 2014;32(2):487–499. doi: 10.1002/stem.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Torossian F., Anginot A., Chabanon A., et al. CXCR7 participates in CXCL12-induced CD34+ cell cycling through beta-arrestin-dependent Akt activation. Blood. 2014;123(2):191–202. doi: 10.1182/blood-2013-05-500496. [DOI] [PubMed] [Google Scholar]
- 18.Chen Q., Zhang M., Li Y.J., et al. CXCR7 Mediates Neural Progenitor Cells Migration to CXCL12 Independent of CXCR4. Stem Cells. 2015;33(8):2574–2585. doi: 10.1002/stem.2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wu Y.M., Peng H., Cui M., et al. CXCL12 increases human neural progenitor cell proliferation through Akt-1/FOXO3a signaling pathway. Neurochem. 2009;109(4):1157–1167. doi: 10.1111/j.1471-4159.2009.06043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhu B., Xu D.S., Deng X.B., et al. CXCL12 enhances human neural progenitor cell survival through a CXCR7- and CXCR4-mediated endocytotic signaling pathway. Stem Cells. 2012;30(11):2571–2583. doi: 10.1002/stem.1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chenn A., Walsh C.A. Regulation of cerebral cortical size by control of cell cycle exit in neural precursors. Science. 2002;297(5580):365–369. doi: 10.1126/science.1074192. [DOI] [PubMed] [Google Scholar]
- 22.Li M., Chang C.J., Lathia J.D., et al. Chemokine receptor CXCR4 signaling modulates the growth factor-induced cell cycle of self-renewing and multipotent neural progenitor cells. Glia. 2011;59(1):108–118. doi: 10.1002/glia.21080. [DOI] [PubMed] [Google Scholar]
- 23.Faigle R., Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim. Biophys. Acta. 2013;1830(2):2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds B.A., Tetzlaff W., Weiss S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992;12(11):4565–4574. doi: 10.1523/JNEUROSCI.12-11-04565.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aguirre A., Rubio M.E., Gallo V. Notch and EGFR pathway interaction regulates neural stem cell number and self-renewal. Nature. 2010;467(7313):323–327. doi: 10.1038/nature09347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vescovi A.L., Reynolds B.A., Fraser D.D., Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11(5):951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 27.Gritti A., Parati E.A., Cova L., et al. Multipotential stem cells from the adult mouse brain proliferate and self-renew in response to basic fibroblast growth factor. J. Neurosci. 1996;16(3):1091–1100. doi: 10.1523/JNEUROSCI.16-03-01091.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Arsenijevic Y., Weiss S., Schneider B., Aebischer P. Insulin-like growth factor-I is necessary for neural stem cell proliferation and demonstrates distinct actions of epidermal growth factor and fibroblast growth factor-2. J. Neurosci. 2001;21(18):7194–7202. doi: 10.1523/JNEUROSCI.21-18-07194.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Supeno N.E., Pati S., Hadi R.A., et al. IGF-1 acts as controlling switch for long-term proliferation and maintenance of EGF/FGF-responsive striatal neural stem cells. Int. J. Med. Sci. 2013;10(5):522–531. doi: 10.7150/ijms.5325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Aguirre A., Dupree J.L., Mangin J.M., Gallo V. A functional role for EGFR signaling in myelination and remyelination. Nat. Neurosci. 2007;10(8):990–1002. doi: 10.1038/nn1938. [DOI] [PubMed] [Google Scholar]
- 31.Hu Q., Zhang L., Wen J., et al. The EGF receptor-sox2-EGF receptor feedback loop positively regulates the self-renewal of neural precursor cells. Stem Cells. 2010;28(2):279–286. doi: 10.1002/stem.246. [DOI] [PubMed] [Google Scholar]
- 32.Sinor-Anderson A., Lillien L. Akt1 interacts with epidermal growth factor receptors and hedgehog signaling to increase stem/transit amplifying cells in the embryonic mouse cortex. Dev. Neurobiol. 2011;71(9):759–771. doi: 10.1002/dneu.20878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gonzalez-Perez O., Alvarez-Buylla A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res. Brain Res. Rev. 2011;67(1-2):147–156. doi: 10.1016/j.brainresrev.2011.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Merino J.J., Bellver L.V., Oset-Gasque M.J., et al. CXCR4/ CXCR7 molecular involvement in neuronal and neural progenitor migration: focus in CNS repair. J. Cell. Physiol. 2015;230(1):27–42. doi: 10.1002/jcp.24695. [DOI] [PubMed] [Google Scholar]
- 35.Klein R.S., Rubin J.B., Gibson H.D., et al. SDF-1 alpha induces chemotaxis and enhances Sonic hedgehog-induced proliferation of cerebellar granule cells. Development. 2001;128(11):1971–1981. doi: 10.1242/dev.128.11.1971. [DOI] [PubMed] [Google Scholar]
- 36.Kuhn H.G., Winkler J., Kempermann G., et al. Epidermal growth factor and fibroblast growth factor-2 have different effects on neural progenitors in the adult rat brain. J. Neurosci. 1997;17(15):5820–5829. doi: 10.1523/JNEUROSCI.17-15-05820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nie Y., Han Y.C., Zou Y.R. CXCR4 is required for the quiescence of primitive hematopoietic cells. J. Exp. Med. 2008;205(4):777–783. doi: 10.1084/jem.20072513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dziembowska M., Tham T.N., Lau P., Vitry S., Lazarini F., Dubois-Dalcq M. A role for CXCR4 signaling in survival and migration of neural and oligodenrocyyte precursors. Glia. 2005;50(3):258–269. doi: 10.1002/glia.20170. [DOI] [PubMed] [Google Scholar]
- 39.Liu Y., Carson-Walter E., Walter K.A. Chemokine receptor CXCR7 is a functional receptor for CXCL12 in brain endothelial cells. PLoS One. 2014;9(8):e103938. doi: 10.1371/journal.pone.0103938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kremer D., Cui Q.L., Kuhlmann T., et al. CXCR7 Is Involved in Human Oligodendroglial Precursor Cell Maturation. PLoS One. 2016;11(1):e0146503. doi: 10.1371/journal.pone.0146503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Krathwohl M.D., Kaiser J.L. Chemokines promote quiescence and survival of human neural progenitor cells. Stem Cells. 2004;22(1):109–118. doi: 10.1634/stemcells.22-1-109. [DOI] [PubMed] [Google Scholar]
- 42.Virgintino D., Errede M., Rizzi M., et al. The CXCL12/CXCR4/ CXCR7 ligand-receptor system regulates neuro-glio-vascular interactions and vessel growth during human brain development. J. Inherit. Metab. Dis. 2013;36(3):455–466. doi: 10.1007/s10545-012-9574-y. [DOI] [PubMed] [Google Scholar]
- 43.Xue L., Wang J., Wang W., et al. The effect of stromal cell-derived factor 1 in the migration of neural stem cells. Cell Biochem. Biophys. 2014;70(3):1609–1616. doi: 10.1007/s12013-014-0103-5. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y., Chen Q., Peng H., et al. Directed migration of human neural progenitor cells to interleukin-1beta is promoted by chemokines stromal cell-derived factor-1 and monocyte chemotactic factor-1 in mouse brains. Transl. Neurodegener. 2012;1(1):15. doi: 10.1186/2047-9158-1-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carbajal K.S., Schaumburg C., Strieter R., Kane J., Lane T.E. Migration of engrafted neural stem cells is mediated by CXCL12 signaling through CXCR4 in a viral model of multiple sclerosis. Proc. Natl. Acad. Sci. USA. 2010;107(24):11068–11073. doi: 10.1073/pnas.1006375107. [DOI] [PMC free article] [PubMed] [Google Scholar]


