Abstract
Gnetales comprise three unusual genera of seed plants, Ephedra, Gnetum, and Welwitschia. Their extraordinary morphological diversity suggests that they are survivors of an ancient, more diverse group. Gnetalean antiquity is also supported by fossil data. Dispersed “ephedroid” (polyplicate) pollen first appeared in the Permian >250 million years ago (Myr), and a few megafossils document the presence of gnetalean features in the early Cretaceous. The Cretaceous welwitschioid seedling Cratonia cotyledon dates the split between Gnetum and Welwitschia to before 110 Myr. Ages and character evolution of modern diversity are, however, controversial, and, based on molecular data, it has recently been suggested that Ephedra is very young, only 8–32 Myr. Here, we present data on the evolutionary history of Ephedra. Fossil seeds from Buarcos, Portugal, unequivocally link one type of Cretaceous polyplicate pollen to Ephedra and document that plants with unique characters, including the peculiar naked male gametophyte, were established already in the Early Cretaceous. Clades in our molecular phylogeny of extant species correspond to geographical regions, with African species in a basal grade/clade. The study demonstrates extremely low divergence in both molecular and morphological characters in Ephedra. Features observed in the fossils are present in all major extant clades, showing that modern species have retained unique reproductive characters for >110 million years. A recent origin of modern species of Ephedra would imply that the Cretaceous Ephedra fossils discussed here were members of widespread, now extinct sister lineage(s), and that no morphological innovations characterized the second diversification.
Keywords: molecular phylogeny, fossil record, Gnetales, molecular dating
The extraordinary form and habit of the three extant genera of Gnetales have fascinated and puzzled botanists ever since the discovery of Welwitschia (1) in the Namib desert in 1860, and the group continues to be central in ongoing discussions about seed plant phylogeny (2–8). However, we do not yet have a clear understanding of the phylogenetic position of the Gnetales, and its fossil history is poorly known. Drewria potomacensis (9) and Eoantha zherikhinii (10) can unambiguously be assigned to the Gnetales, and a welwitschioid fossil seedling, Cratonia cotyledon (11), from the Early Cretaceous of Brazil, clearly belongs to crown group Gnetales, based on the presence of an embryo feeder and a unique venation pattern, shared by the fossil and Welwitschia (11–13). These fossils show that Gnetales were more diverse in the past than they are today, and Cratonia provides a minimum age of ≈110 million years (Myr) for the split between Gnetum and Welwitschia. Morphological and molecular analyses of relationships within the Gnetales have been congruent in placing Ephedra as sister to Gnetum and Welwitschia (3, 5, 8), and the split between Ephedra and the Gnetum-Welwitschia lineage must therefore be older. This early divergence of major gnetalean lineages does not necessarily imply that modern diversity was established in the Early Cretaceous. The age of extant Ephedra was recently estimated to 8–32 Myr (14) by using molecular sequence data (rbcL) and assuming a constant rate of evolution calculated by landmark event calibration (14). The result reflects the low amount of divergence between rbcL sequences within the genus and the assumption of clock-like substitution rates.
The 35–45 modern species of Ephedra (15) are similar also in gross morphology, with decussate branching, reduced leaves, and ovulate cones, often with fleshy bracts and seeds with an inner integument and an outer envelope. The apical part of the integument is extended into a micropylar tube that serves for pollen reception. The pollen cones consist of stalked microsporangia surrounded by paired bracts. Pollen grains are distinct, with longitudinal ridges and valleys (polyplicate), due to alternation of thicker and thinner exine regions. During germination, the exine is shed, leaving the male gametophyte naked (16), and the shed exine curls up in a characteristic way, resulting in transverse striations (16). Dispersed polyplicate pollen, similar to that of Ephedra and Welwitschia, occurs in sediments ranging back to the Permian, >250 Myr (17), and is particularly common in the Early Cretaceous (18), but their presumed affinity to the Gnetales has rarely been confirmed. Eoantha zherikhinii (10), an Early Cretaceous gnetalean ovule with an extending micropylar tube, contains polyplicate pollen of Ephedripites-type w ith intact ex ines. Further, Welwitschia-type pollen was found in association (not in situ) with Drewria potomacensis (9), but in general, little has been known about the plants that produced the so called “ephedroids.”
The discovery presented here, of exceptionally well-preserved fossil seeds with unique Ephedra characters and in situ polyplicate pollen, documents that some Cretaceous polyplicate pollen was produced by Ephedra plants. The fossils and a molecular phylogeny contribute data on the evolutionary history of Ephedra.
Methods
Morphological Studies. The fossils were collected from the Early Cretaceous Buarcos locality, situated north of Figueira da Foz, Portugal, locality details in Friis et al. (19). The plant-bearing sediments were previously assigned to the “Arenitos de Carrascal” and thought to be of preAlbian age (Barremian or Aptian) (19). The sequence is now included in the lowermost member (Calvaria Member) of the Figueira da Foz Formation established recently by Dinis (20). The age of this part of the sequence is late Aptian to early Albian, or perhaps early Albian (21). The coalified fossils were extracted from the sediment by sieving in water and cleaned with hydrochloric and hydrofluoric acid, and water. They were initially investigated by using a stereomicroscope. For light microscope studies, seeds were macerated by using nitric acid followed by ammonia or by using sodium hypochlorate. For scanning electron microscopy (SEM) studies, specimens were mounted on aluminum stubs, coated with gold in a sputter coater, and examined with a Hitachi 4300 field emission scanning electron microscope at 5 kV. Twenty-five species of extant Ephedra (Table 1) were investigated for the presence of papillae on the apical, inner surface of the outer envelope, by using a stereomicroscope.
Table 1. Presence of papillae on the apical, inner surface of the outer envelope.
| No. | Taxa | Voucher | Papillae on seed envelope | Stapf 1889 Section/“Tribe” |
|---|---|---|---|---|
| 83 | E. alata Decne. | C-303 (S) | Present | Alatae/Tropidolepides |
| 81 | E. altissima Desf. | C-620 (S) | Present | Ephedra/Scandentes |
| E. altissima Desf. | C-628-629 (S) | Present | Ephedra/Scandentes | |
| E. antisyphilitica Berl. & C. A. Mey. | C-612 (S) | Present | Ephedra/Antisyphiliticae | |
| 80 | E. aphylla Forssk. | C-7791 (S) | Present | Ephedra/Scandentes |
| E. californica S. Watson | C-602 (S) | Present | Asarca/Ascara | |
| E. campylopoda C. A. Mey. | C-7619, C-593 (S) | Present | Ephedra/Scandentes | |
| 60 | E. chilensis C. Presl. | E00130260 (E) | Present | Ephedra/ |
| 75 | E. chilensis C. Presl. | 49.0542 (UC) | Present | Ephedra/ |
| E. clokeyi Cutler | C-589-590 (S) | Present | Ascara/ | |
| E. cutleri Cutler | C-583 (S) | Present | Ascara/ | |
| E. distachya L. | C-493 (S) | Present | Ephedra/Leptocladae | |
| E. equisetina Bunge | C-460 (S) | Present | Ephedra/Leptocladae | |
| E. fasciculata A. Nelson | C-442 (S) | Present | Ascara/ | |
| E. fedtschenkoae Paulsen | — (S) | Present | Ephedra/Leptocladae | |
| 84 | E. foliata Boiss. & C. A. Mey. | C-7816 (S) | Present | Ephedra/Scandentes |
| E. funera Cov. | C-360 (S) | Present | Ascara/ | |
| E. gerardiana Wall. & Florin | C-353 (S) | Present | Ephedra/Leptocladae | |
| 66 | E. intermedia Schrenk & C. A. Mey. | C-325 (S) | Present | Ephedra/Pachycladae |
| 02 | E. likiangensis Florin | 03-926 (S) | Present | Ephedra/Leptocladae |
| E. lomatolepis Schrenk | C-287 (S) | Present | Ephedra/ | |
| 07 | E. minuta Florin | 03-930 (S) | Present | Ephedra/Leptocladae |
| E. pachyclada Boiss. | C-219 (S) | Present | Ephedra/Pachycladae | |
| 71 | E. procera C. A. Mey. | 04903487 (Mo) | Present | Ephedra/Leptocladae |
| 73 | E. rupestris Benth. | 87.1368 (UC) | Present | Ephedra/Antisyphiliticae |
| 33 | E. sinica Stapf | EtOH (S) | Present | Ephedra/Leptocladae |
| 76 | E. tweediana Fisch. & C. A. Mey. | 66.0742 (UC) | Present | Ephedra/Antisyphiliticae |
Taxon Sampling and Gene Sequencing. Taxon sampling was designed to investigate the phylogeny of Ephedra. Representatives from all major continents were selected (Table 2). We have sequenced two chloroplast genes [the ribulose-bisphosphate carboxylase large subunit (rbcL) and the small ribosomal protein 4 gene (rps4)], and three nuclear ribosomal regions [1,230 bp from the large subunit (26S), the small subunit (18S), and the internal transcribed spacer 1+2 (ITS)]. The intron and spacer of the chloroplast trnL-F region were also sequenced, but they contained no variation. Primer sequences and references are given in Table 3. The ITS primers 18SF and 26SR were designed for seed plants and have proven useful on a range of land plants. DNA was extracted, amplified, and sequenced by using standard methods. Fragments were assembled and edited by using the staden package (22).
Table 2. Species included in molecular analyses.
| Taxa | Voucher | rbcL | rps4 | 26S | 18S | ITS | Comments | Sec./“Tribe”* | Distrib. | |
|---|---|---|---|---|---|---|---|---|---|---|
| 83 | E. alata Decne. | C-303 (S) | AY755805 | AY755851 | AY755732 | AY755698 | AY755774 | Algeria 1980 | Alatae/Tropidolepides | Africa-Asia |
| 82 | E. altissima Desf. | C-7688 (S) | AY755804 | AY755850 | AY755731 | AY755697 | AY755773 | Tunisia 1972 | Eph./Scandentes | Africa |
| 81 | E. altissima Desf. | C-628 (S) | AY755803 | AY755849 | AY755730 | AY755696 | AY755772 | Algeria-Morocco 1936 | Eph./Scandentes | Africa |
| 25 | E. andina Poepp. ex C.A. Mey. | 10140 (K) | AY755782 | AY755821 | AY755707 | AY755670 | AY755744 | Kew 1967-25610 (cult) | Eph./Antisyphiliticae | S. America |
| 64 | E. antisyphilitica Berl. & C.A. Mey. | 04-488 (S) | AY755789 | AY755834 | AY755715 | AY755682 | AY755757 | Oklahoma 2001 | Eph./Antisyphiliticae | N. America |
| 80 | E. aphylla Forssk. | C-7791 (S) | AY755802 | AY755848 | AY755729 | AY755695 | AY755771 | Libya 1982 | Eph./Scandentes | Africa/Asia |
| 34 | E. californica S. Watson | 68-154 (O) | AY056569 | AY755827 | AY755708 | AY755676 | AY755750 | Oslo Univ Bot Gard (cult) | Asarca/Ascara | N. America |
| 87 | E. campylopoda C.A. Mey. | C-7605 (S) | AY755808 | AY755855 | AY755736 | AY755701 | AY755777 | Turkey 1971 | Eph./Scandentes | Eur-Afr-Asia |
| 60 | E. chilensis C. Presl. | E00130260 (E) | AY755786 | AY755831 | AY755712 | AY755679 | AY755754 | Talca, Chile 1980 (cult) | Eph./ | S. America |
| 75 | E. chilensis C. Presl. | 49.542 (UC) | AY755799 | AY755844 | AY755725 | AY755691 | AY755767 | Chile 1949 (cult) | Eph./ | S. America |
| 86 | E. ciliata Fisch. & C.A. Mey. | C-591 (S) | AY755807 | AY755854 | AY755735 | AY755700 | AY755776 | Afganistan 1963 | Eph./Scandentes | Africa-Asia |
| 69 | E. distachya L | S04-481 (S) | AY755793 | AY755838 | AY755719 | AY755686 | AY755761 | 68-226 (cult) | Eph./Leptocladae | Europe/Asia |
| 77 | E. distachya L | 03-684 (S) | — | AY755846 | AY755727 | AY755693 | AY755769 | Hungary 1993 | Eph./Leptocladae | Europe/Asia |
| 04 | E. equisetina Bunge | 03-928 (S) | AY755781 | AY755817 | AY755705 | AY755666 | AY755740 | Ashkhabad 1938 (cult) | Eph./Leptocladae | Asia |
| 35 | E. equisetina Bunge | 90-536 (O) | AY755783 | AY755828 | AY755709 | — | AY755751 | Oslo Univ Bot Gard (cult) | Eph./Leptocladae | Asia |
| 79 | E. equisetina Bunge | C-465 (2) (S) | AY755801 | AY755847 | AY755728 | AY755694 | AY755770 | Mangolia 1972 | Eph./Leptocladae | Asia |
| 84 | E. foliata Boiss. & C.A. Mey. | C-7816 (S) | — | AY755852 | AY755733 | — | — | Egypt 1929 | Eph./Scandentes | Africa-Asia |
| 85 | E. foliata Boiss. & C.A. Mey. | C-7808 (S) | AY755806 | AY755853 | AY755734 | AY755699 | AY755775 | Iran 1960 | Eph./Scandentes | Africa-Asia |
| 37 | E. fragilis Desf. | E00130258 (E) | AY755784 | AY755829 | AY755710 | AY755677 | AY755752 | Jerusalem, Israel 1992 (cult) | Eph./Scandentes | Europe/Africa |
| 08 | E. frustillata Miers | 04-482 (S) | AY056670 | AY755820 | AY755706 | AY755669 | AY755743 | Patagonia, Argentina 1994 | Eph./ | S. America |
| 30 | E. frustillata Miers | 10218 (K) | AY056564 | AY755825 | AY056490 | AY755674 | AY755748 | Kew 1988-8057 (cult) | Eph./ | S. America |
| 26 | E. gerardiana Wall. & Florin | 10141 (K) | AY056560 | AY755822 | AY056486 | AY755671 | AY755745 | Kew 1989-8369 (cult) | Eph./Leptocladae | Asia |
| 59 | E. gerardiana Wall. & Florin | E00130259 (E) | AY755785 | AY755830 | AY755711 | AY755678 | AY755753 | Sikkim, India 1983 (cult) | Eph./Leptocladae | Asia |
| 68 | E. gerardiana Wall. & Florin | 74-460 (O) | AY755792 | AY755837 | AY755718 | AY755685 | AY755760 | Oslo Univ Bot Gard (cult) | Eph./Leptocladae | Asia |
| 06 | E. intermedia Schrenk & C.A. Mey. | 03-925 (S) | AY056566 | AY755818 | AY056492 | AY755667 | AY755741 | Tien-Shan 1971 (cult) | Eph./Pachycladae | Asia |
| 66 | E. intermedia Schrenk & C.A. Mey. | 04-483 (S) | AY755790 | AY755835 | AY755716 | AY755683 | AY755758 | Tien-Shan 1971 (cult) | Eph./Pachycladae | Asia |
| 02 | E. likiangensis Florin | 03-926 (S) | AY755780 | AY755816 | AY056485 | AY755665 | AY755739 | Denver Bot Gard 1988 (cult) | Eph./Leptocladae | Asia |
| 74 | E. likiangensis Florin | 94.0389 (UC) | AY755798 | AY755843 | AY755724 | AY755690 | AY755766 | Yunnan, China 1994 (cult) | Eph./Leptocladae | Asia |
| 88 | E. major Host | 03-164 (S) | AY755809 | AY755856 | AY755737 | AY755702 | AY755778 | Andalusia, Spain 1995 | Eph./Leptocladae | Eur-Asia-Afr |
| 07 | E. minuta Florin | 03-930 (S) | AY056567 | AY755819 | AY056493 | AY755668 | AY755742 | China, Sikang 1934 (cult) | Eph./Leptocladae | Asia |
| 61 | E. minuta Florin | 04-485 (S) | AY755787 | AY755832 | AY755713 | AY755680 | AY755755 | China, Sikang 1934 (cult) | Eph./Leptocladae | Asia |
| 63 | E. minuta Florin | 04-486 (S) | AY755788 | AY755833 | AY755714 | AY755681 | AY755756 | Univ of Stockholm (cult) | Eph./Leptocladae | Asia |
| 27 | E. monosperma C.A. Mey. | 10142 (K) | AY056561 | AY755823 | AY056487 | AY755672 | AY755746 | Kew 1998-499 (cult) | Eph./Leptocladae | Asia |
| 72 | E. nevadensis S. Watson | 66.1033 (UC) | AY755796 | AY755841 | AY755722 | AY755688 | AY755764 | California 1966 (cult) | Eph./Antisyphiliticae | N. America |
| 89 | E. pachyclada Boiss | C-7844 (S) | AY755810 | AY755857 | AY755738 | AY755703 | AY755779 | Sinai 1974 | Eph./Pachycladae | Asia |
| 71 | E. procera C.A. Mey. | 04903487 (Mo) | AY755795 | AY755840 | AY755721 | — | AY755763 | Tiblisi Georgia 1999 (cult) | Eph./Leptocladae | Europe-Asia |
| 73 | E. rupestris Benth. | 87.1368 (UC) | AY755797 | AY755842 | AY755723 | AY755689 | AY755765 | Ecuador 1987 (cult) | Eph./Antisyphiliticae | S. America |
| 28 | E. sinica Stapf | 10143 (K) | AY056562 | AY755824 | AY056488 | AY755673 | AY755747 | Kew 1991-154 (cult) | Eph./Leptocladae | Asia |
| 33 | E. sinica Stapf | EtOH (S) | AY056565 | AY755826 | AY056491 | AY755675 | AY755749 | Hebei, China 2000 | Eph./Leptocladae | Asia |
| 67 | E. torreyana S. Watson | 04-487 (S) | AY755791 | AY755836 | AY755717 | AY755684 | AY755759 | New Mexico 2001 | Alatae/Habrolepides | N. America |
| 70 | E. trifurca Torr. | 04630447 (Mo) | AY755794 | AY755839 | AY755720 | AY755687 | AY755762 | Arizona 1994 (cult) | Alatae/Habrolepides | N. America |
| 76 | E. tweediana Fisch. C.A. Mey. | 66.0742 (UC) | AY755800 | AY755845 | AY755726 | AY755692 | AY755768 | Buenos Aires, Argentina | Eph./Antisyphiliticae | S. America |
| 01 | G. gnemon L | 03-926 (S) | L12680 | AY755811 | AF036488 | AY755660 | — | Fidji 2000 | — | |
| 31 | G. castatum K. Sch. | 10219 (K) | AY056576 | AY755812 | AY056497 | AY755661 | — | Kew 1964-47701 (cult) | — | |
| 32 | G. parvifolium (Warb.) W.C. Cheng | EtOH (S) | AY056577 | AY755813 | AY755704 | AY755662 | — | Namping, Xiyc. gorje 2000 | — | |
| 38 | G. indicum Merr. | E00130257 (E) | AY056574 | AY755814 | AY056495 | AY755663 | — | Hong Kong 1955 (cult) | — | |
| 39 | G. montanum Markgr. | E00130261 (E) | AY056575 | AY755815 | AY056496 | AY755664 | — | Hong Kong 1979 (cult) | — | |
| 36 | W. mirabilis Hook. f | 67-1177 (O) | AJ235814 | AY188246 | AY056484 | AF207059 | — | Oslo Univ Bot Gard (cult) | — |
Sec., section; Distrib., distribution; Eph., Ephedra.
According to Stapf (1889) (29).
Table 3. Primer sequences.
| Primer description (name) | Sequence | Reference |
|---|---|---|
| rbcL forward (rbcL 5′) | ATG TCA CCA CAA ACA GAG AC | 34 |
| rbcL reversed (rbcL 3′) | TCA AAT TCA AAC TTG ATT TCT TTC CA | 35 |
| rps4 forward (rps4Fb) | CGA TCT TCT CGA CCC TGG TGG | C.R., 2003* |
| rps4 reversed (rps4Rb) | CCG TCG AGA ATA ATA TTC TAT | C.R, 2003* |
| 18S forward (18S1) | GCT TGT CTC AAA GAT TAA GCC | C.R., 2003* |
| 18S reversed (18Srev) | CCT TCC TCT AAA CGA TAA GGT TC | C.R., 2003* |
| 26S forward (26S1) | CGA CCC CAG GTC AGG CG | 36 |
| 26S reversed (1229R) | ACT TCC ATG ACC ACC GTC CT | 36 |
| ITS forward (ITS-18SF) | GAA CCT TAT CGT TTA GAG GAA GG | C.R., 2000* |
| ITS reversed (ITS-26SR) | CCG CCA GAT TTT CAC GCT GGG C | C.R., 2000* |
Previously unpublished.
Alignment and Analyses. All sequences were aligned by using computer software bioedit (23). No insertion/deletion events were found in rbcL. A 6-bp deletion was inferred in all rps4 sequences of Gnetum and Welwitschia, and an additional 3-bp deletion for Welwitschia. Insertion/deletion events in the nuclear 18S, ITS, and 26S rDNA sequences were inferred by eye. Gaps were treated as missing data in the alignment and added as binomial characters (absent or present) at the end of the matrix. All trees were rooted on the sister group of Ephedra, the Gnetum-Welwitschia clade (8).
Bayesian analyses were performed with mrbayes 3.0 (24) by using the general time reversible (GTR) model with γ distributed rates and a proportion of invariable sites. The data set was partitioned into six data partitions (18S+26S; ITS; rbcL+rps4 1st codon positions; rbcL+rps4 2nd pos; rbcL+rps4 3rd pos; and indels). Each partition was allowed to have its own unique GTR plus gamma plus proportion of invariable sites model. The indels were treated as a morphological data partition. We ran 600,000 generations, with a sample frequency of 100, four parallel chains, and all other options at their default values. The majority rule consensus of trees from the last 100,000 generations was calculated in paup* 4.0B10 (25). Most parsimonious trees were calculated by using the heuristic search option in paup*, 500 random sequence additions, tree bisection reconnection branch swapping, and multrees off. Support values were obtained by using bootstrap in paup*, performing 1,000 replicates with 10 random sequence additions.
Results and Discussion
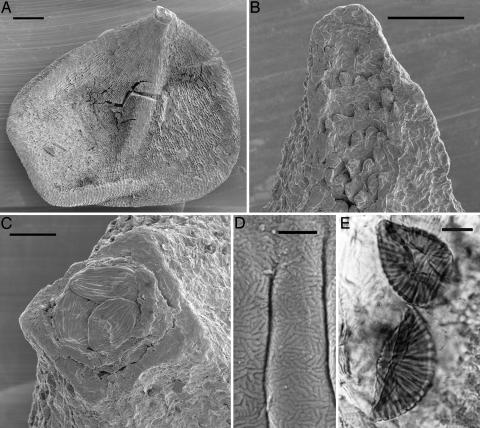
The seeds, discovered from the Early Cretaceous Buarcos locality in Portugal, are ovoid, 0.85–1.2 mm long, rounded at the base, and provided with an acuminate micropyle (Fig. 1A). The seeds have two tissues: an inner, membranous integument and an outer envelope of sclerenchymatic cells. The outer envelope often splits apically into four valves, exposing distinct papillae (Fig. 1B) on their inner surface. The papillae are identical in shape and position to those described for extant Ephedra distachya and Ephedra altissima (26), where they are thought to provide support for the micropylar tube, and to close the gap between the micropylar tube and the outer envelope (26). Polyplicate pollen occurs in situ in the micropylar tube (Fig. 1C). The pollen grains are ribbed with 10–15 visible ribs. The surface of the pollen wall is indistinctly rugulate (Fig. 1D), a feature also observed for Ephedra foliata (27). Maceration of the seeds revealed shed, upcurled exines inside the integument (Fig. 1E), matching completely the unusual shed exines of extant Ephedra (16). The shed exines indicate that the pollen grains had germinated in the ovule, leaving the male gametophyte naked in the same peculiar way described for extant Ephedra (16).
Fig. 1.
Early Cretaceous Ephedra seeds from Buarcos, Portugal. (A) Overview of one of the fossil seeds (S-107680). (B) Details of papillae on the inner surface of the outer envelope (S-107685). (C) Pollen grains in the micropylar region. Note the inner circular integument and the outer squared envelope (S-107680). (D) Rugulate surface of the pollen grains (S-107680). (E) Macerated seed, exposing two shed pollen exines inside the micropyle (S-136808). [Scale bars: 100 μm(A); 50 μm(B); 25 μm(C); 1 μm(D); and 10 μm(E).] (A–D) Scanning electron micrographs. (E) Transmitted light micrograph.
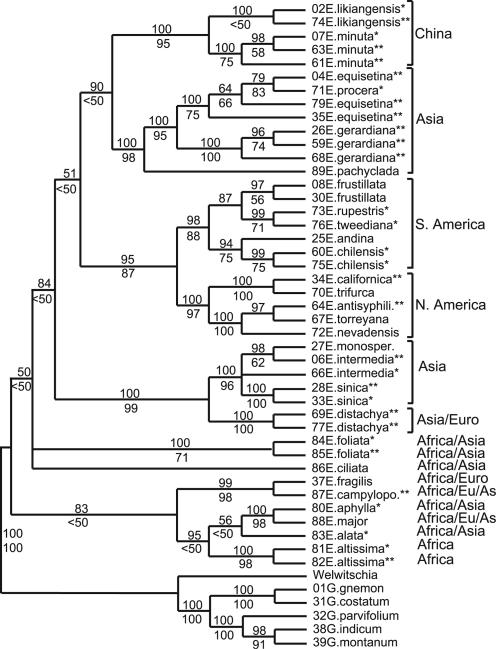
To investigate whether the fossils could be assigned to any particular group within Ephedra, we studied the seed characters in a phylogenetic context. Attempts have been made previously to resolve the phylogeny of extant Ephedra (28), but the paucity of information in most gene regions makes it difficult to get resolved results. The phylogenetic framework used here was established by using Bayesian (Fig. 2) and parsimony analyses of five gene regions from the chloroplast and nuclear genomes (the chloroplast genes rbcL and rps4, and 18S, 26S, and ITS from the nuclear ribosomal DNA). Based on the resulting phylogeny, we conclude that Stapf's widely used classification system from 1889 (29), founded on the morphology of ovulate cone bracts, is artificial. Major well supported groups correspond to geographical regions, and dry membranous bracts are present in several clades. African species constitute a basal grade or clade within Ephedra. Some of the basal species are restricted to Africa (e.g., E. altissima); others have a broader distribution in the Old World, extending from Africa into Asia or southern Europe. Ephedra alata, E. altissima, Ephedra aphylla, Ephedra campylopoda, Ephedra fragilis, and Ephedra major share a 26-bp deletion in ITS (pos. 860–886 from the end of the primer 18SF), supporting their status as a clade. Ephedra ciliata and E. foliata do not have this deletion, and their systematic position is unclear. In Bayesian analyses, the basal (African) species form a grade (Fig. 2); in the most parsimonious trees they are monophyletic. Neither of these alternatives is well supported, and further studies are needed.
Fig. 2.
Cladogram of recent Ephedra based on Bayesian analysis of five regions from the nuclear and chloroplast genomes (18S, 26S, ITS, rbcL, and rps4). Bayesian posterior probabilities are given above branches, parsimony bootstrap values below branches. Species marked * and ** were examined for papillae on the inner surface of apical part of the outer envelope. For species marked *, the DNA voucher material was investigated. For species marked **, other material has been used.
All non-African species belong to a clade that probably originated in Asia. Here, Bayesian and parsimony analyses produced congruent topologies. Of the four investigated European species, three are present also in Africa (E. major, E. fragilis, and E. campylopoda) and they belong to the basal grade/clade of African species. The European species Ephedra distachya has a broad distribution extending from Spain to Russia and China, but is absent in Africa. The two representatives of E. distachya included in this study are highly supported within one of the Asian clades. According to our results, Ephedra procera, which is generally considered a subspecies of E. major, is instead related to Ephedra equisetina. This finding should be further investigated with a more extensive species sampling. New World species are monophyletic and may have originated from within the Asian clade. They comprise two well-supported groups: a South American and a North American clade. Dry membranous bracts occur in the North American clade but also in one of the basal African species. The character differs in detailed morphology between species and has probably evolved several times. The Cretaceous flora probably contained species with dry bracts, as well as species with fleshy bracts, but this result has not been thoroughly studied yet.
From the correspondence between clades and geographical regions, it is clear that major groups originated after the final rifting of the Gondwana continent. A possible origin of Ephedra in Africa is interesting because the diversity of ephedroid pollen grains is particularly high in Early Cretaceous palaeoequatorial regions of Africa-South America (30). However, this connection is only relevant if the molecular dating of extant Ephedra to 8–32 Myr (14) is incorrect. The Cretaceous seeds presented here share a number of unique characters with extant Ephedra. Polyplicate pollen lacking a colpus and seeds with an outer envelope with apical papillae constitute obvious derived characters shared by the fossils and extant Ephedra. Shedding and upcurling of the pollen exine during germination represent another synapomorphy unique to Ephedra. The polyplicate pollen grains of Welwitschia have a distinct colpus through which germination occurs and the exine is not shed (C.R. and E.M.F., unpublished results). All characters of the fossils are present in all extant groups, and it was not possible to include the seeds in any particular subgroup. Papillae were found on the apical, inner surface of the outer envelope in all investigated specimens (representatives from all major extant clades, species marked * in Fig. 2, and other species; see Table 1). Our analyses indicate that there is very little variation in key reproductive structures in fossil and extant Ephedra. The same is true for molecular data where we have investigated three nuclear and three chloroplast sequences. They contained few informative characters, and one sequence, the trnL-F region, was nearly invariable.
Concluding Remarks. The co-occurrence of dispersed ephedroid (polyplicate) pollen and megafossils with distinct gnetalean vegetative morphology, for example in the Araripe Group (11, 31, 32) and in the Potomac Group (9), has strongly suggested that these Early Cretaceous ephedroid grains were produced by gnetalean plants. The new discovery of Ephedra seeds with polyplicate pollen in situ provides direct proof for the association. The fossils document that plants with unique Ephedra characters were already present in the Early Cretaceous, characters such as the apical papillae on the seed envelope and the peculiar shedding of the pollen exine, which leaves the male gametophyte naked. Ephedra may even have been diverse and widespread at that time. Dispersed polyplicate pollen occurs frequently in Cretaceous sediments from low palaeolatitudes (18, 30). Our results support the idea that Ephedra-plants produced at least some of these grains. Further, Early Cretaceous fossils from the Crato Formation in Brazil (B. Mohr, personal communication) and the Potomac Group, zone 1, USA (P. Crane, personal communication) have a morphology very similar to extant Ephedra. The same has also been reported from China (33) and now from Portugal. Together, these findings indicate that both reproductive and vegetative features characterizing extant Ephedra were fully established and widespread in the Early Cretaceous flora, and suggest that crown group Ephedra might be of Mesozoic origin. The alternative hypothesis inferred from molecular dating (14), that the crown group is young (8–32 Myr), needs more research. We have tested the proposed age for Ephedra with commonly used methods for molecular age estimates, but found that without a calibration point within Ephedra, which may be difficult (impossible?) to attain due to the conserved morphology of the genus, the results of penalized likelihood analyses are dubious. A late differentiation of crown group Ephedra would imply that the lineage experienced two major radiation events, one in the Early Cretaceous resulting in widespread, but now extinct stem group(s), and a second radiation in the late Cenozoic resulting in the modern diversity. The implication of this hypothesis is that all characters of modern Ephedra have remained unchanged for >110 million years, through the second major diversification. This scenario seems incompatible with the hypothesis of constant substitution rates within the lineage (14), but clearly further testing and development of methods for molecular dating is needed to clarify conflicts between molecular signals and the fossil record.
Acknowledgments
We thank David Cantrill, Petra Korall, Mari Källersjö, Hervé Sauquet, and Niklas Wikström for valuable comments on the manuscript, and Ron Hoggard (University of Oklahoma, Norman) for sending plant material. This study was supported by grants from the Swedish Research Council (to E.M.F.) and from the Carlsberg Foundation (to K.R.P.).
Abbreviations: Myr, million years (ago); rbcL, ribulose-bisphosphate carboxylase large subunit; rps4, small ribosomal protein 4 gene; ITS, internal transcribed spacer.
Data deposition: The DNA sequences reported in this paper have been deposited in the GenBank database (accession nos. AY755660–AY755857).
References
- 1.Welwitsch, F. (1861) J. Proc. Linn. Soc. Bot. 5, 182–187. [Google Scholar]
- 2.Doyle, J. A. (1996) Int. J. Plant Sci. 157, Suppl., S3–S39. [Google Scholar]
- 3.Crane, P. R. (1985) Ann. Mo. Bot. Gard. 72, 716–793. [Google Scholar]
- 4.Bowe, L. M., Coat, G. & de Pamphilis, C. W. (2000) Proc. Natl. Acad. Sci. USA 97, 4092–4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chaw, S.-M., Parkinson, C. L., Cheng, Y., Vincent, T. M. & Palmer, J. D. (2000) Proc. Natl. Acad. Sci. USA 97, 4086–4091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Donoghue, M. J. & Doyle, J. A. (2000) Curr. Biol. 10, R106–R109. [DOI] [PubMed] [Google Scholar]
- 7.Magallón, S. & Sanderson, M. J. (2002) Am. J. Bot. 89, 1991–2006. [DOI] [PubMed] [Google Scholar]
- 8.Rydin, C., Källersjö, M. & Friis, E. M. (2002) Int. J. Plant Sci. 163, 197–214. [Google Scholar]
- 9.Crane, P. R. & Upchurch, G. R. (1987) Am. J. Bot. 74, 1722–1736. [Google Scholar]
- 10.Krassilov, V. A. (1986) Rev. Palaeobot. Palynol. 47, 9–16. [DOI] [PubMed] [Google Scholar]
- 11.Rydin, C., Mohr, B. & Friis, E. M. (2003) Biol. Lett. R. Soc. London 270, 29–32. [Google Scholar]
- 12.Rodin, R. J. (1953) Am. J. Bot. 40, 371–378. [Google Scholar]
- 13.Rodin, R. J. (1958) Am. J. Bot. 45, 90–95. [Google Scholar]
- 14.Huang, J. & Price, R. A. (2003) Mol. Biol. Evol. 20, 435–440. [DOI] [PubMed] [Google Scholar]
- 15.Kubitzki, K. (1998) The Families and Genera of Vascular Plants (Springer, Berlin), Vol. 1.
- 16.El-Ghazaly, G., Rowley, J. R. & Hesse, H. (1998) Plant Syst. Evol. 213, 217–231. [Google Scholar]
- 17.Wilson, L. R. (1962) Okla. Geol. Surv. Bull. 49, 5–50. [Google Scholar]
- 18.Crane, P. R. & Lidgard, S. (1989) Science 246, 675–678. [DOI] [PubMed] [Google Scholar]
- 19.Friis, E. M., Crane, P. R. & Pedersen, K. R. (1997) Grana 36, 225–244. [Google Scholar]
- 20.Dinis, J. L. (2001) Comun. Inst. Geol. e Mineiro 88, 127–160. [Google Scholar]
- 21.Heimhofer, U., Hochuli, P. A. & Weissert, H. (2004) Polen 14, 178–179. [Google Scholar]
- 22.Staden, R. (1996) Mol. Biotechnol. 5, 233–241. [DOI] [PubMed] [Google Scholar]
- 23.Hall, T. (1997) Bioedit (North Carolina State University, Raleigh).
- 24.Huelsenbeck, J. P. & Ronquist, F. R. (2001) Bioinformatics 17, 754–755. [DOI] [PubMed] [Google Scholar]
- 25.Swofford, D. L. (1998) paup*. Phylogenetic Analysis Using Parsimony (*and Other Methods) (Sinauer, Sunderland, MA).
- 26.Thoday, M. G. & Berridge, E. M. (1912) Ann. Bot. 26, 953–985. [Google Scholar]
- 27.El-Ghazaly, G. & Rowley, J. R. (1997) Palynology 21, 7–18. [Google Scholar]
- 28.Huang, J. (2000) Ph.D. dissertation (University of Georgia, Athens).
- 29.Stapf, O. (1889) Denkschr. Math-Nat. wiss. Cla. Kaiserl. Akad. Wiss. Wien 56, 1–112. [Google Scholar]
- 30.Crane, P. R. (1996) Int. J. Plant Sci. 157, Suppl., S50–S57. [Google Scholar]
- 31.Martill, D. M., Brito, P. M., Wenz, S. & Wilby, P. R. (1993) in Palaeontological Association Field Guides to Fossils Series 5, ed. Jarzembowski, E. A. (Palaeontolgical Association, London), pp. 1–159.
- 32.Osborn, J. M., Taylor, T. N. & de Lima, M. R. (1993) Rev. Palaeobot. Palynol. 77, 171–184. [Google Scholar]
- 33.Guo, S.-X. & Wu, X.-W. (2000) Acta Palaeont. Sin. 39, 81–91. [Google Scholar]
- 34.Zurawski, G. & Clegg, M. T. (1987) Annu. Rev. Plant Physiol. 38, 391–418. [Google Scholar]
- 35.Wikström, N. & Kenrick, P. (1997) Int. J. Plant Sci. 158, 862–871. [Google Scholar]
- 36.Kuzoff, R. K., Sweere, J. A., Soltis, D. E., Soltis, P. S. & Zimmer, E. A. (1998) Mol. Biol. Evol. 15, 251–263. [DOI] [PubMed] [Google Scholar]