Abstract
Medicinal plants are a plentiful source of bioactive molecules with much structural diversity. In cancer treatment, molecules obtained from plants represent an attractive alternative to other treatments because several plant-derived compounds have exhibited lower toxicity and higher selectivity against cancer cells. In this review, we focus on the possible application of bioactive molecules obtained from plants against more primitive cell populations in cancers, cancer stem cells. Cancer stem cells are present in several kinds of tumors and are responsible for recurrences and metastases. Common anti-cancer drugs exhibit lower effectiveness against cancer stem cells because of their biological features. However, recently discovered natural phytometabolites exert cytotoxic effects on this rare population of cells in cancers. Therefore, this review presents the latest research on promising compounds from plants that can act as antitumor drugs and that mainly affect stem cell populations in cancers.
Keywords: Natural compounds, Cancer stem cells, ABC transporters, Wnt, Notch, Hedgehog, NF-κB
1. CANCER STEM CELLS: A NEW TARGET AGAINST CANCERS
Cancer is a global disease that occurs by the accumulation of mutations in the cells of the body [1, 2]. Drug resistance, which impairs the effectiveness of anti-cancer drugs, is one of the most common problems in cancer therapy, and resistance to chemotherapeutic agents exerts a negative impact on the survival rate of cancer patients [3]. However, the causes of drug resistance are numerous and complex. Various factors result in therapeutic failure, such as genetic mutations that promote heterogeneous populations and the presence of cancer stem cells (CSCs), as reviewed elsewhere [4-6]. CSCs represent a small population of cancer cells with a low proliferation rate and a high tumorigenic potential [7]. Emerging evidence shows the presence of mutations in stem cells and progenitor cells, which might lead to tumorigenesis [7]. CSCs are more resistant to conventional chemotherapy and radiotherapy, and they are able to regenerate a whole new tumor if they remain alive during treatment [8-10]. Targeting these cells among actively dividing cancer cells may significantly contribute to solving the problem of resistance and relapse (Fig. 1 ).
Fig. (1).
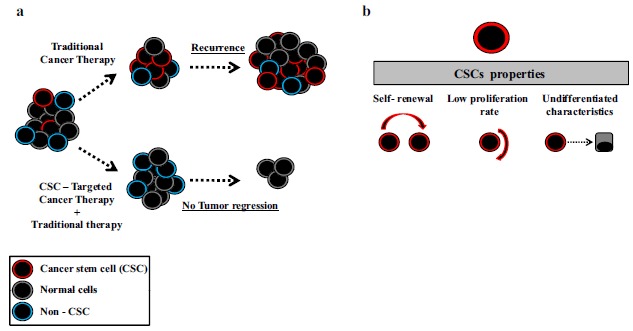
(a) Schematic illustration of CSCs sensitivity to therapy. (b) Properties of CSCs.
Analysis of tumor cell lines, animal xenograft models, and human leukemic samples, including acute myeloid leukemias (AML) and acute lymphoblastic leukemias (ALL), revealed cell populations with distinct proliferation rates. The major observations relate to the leukemic blasts from patients in which two populations showed different proliferation patterns: a proportion of cells with fast growth within 24 h and a smaller subset of cells proliferating only after weeks to months [11-13]. The conclusion of this study was that the population with a low proliferation rate could be the result of mutations of primitive hematopoietic precursors [12, 13]. However, the existence of heterogeneous tumor cell populations was only discovered in 1997. This population of cells was shown to have stem cell properties, such as self-renewal capacity, low proliferation rate, and the ability to differentiate recapitulating the phenotype of the disease observed in patients [14].
Some experiments have suggested that CSCs might arise from normal stem cells and progenitor cells of adult tissues that have accumulated malignant mutations and have lost their ability to self-regulate [13, 15, 16]. It has recently been demonstrated that CSCs are present in both hematologic malignancies and solid tumors. Recently, surface markers used to identify adult stem cells in tissues have been used to recognize CSCs such as leukemia CD34+/CD38− [17], breast cancer CD44+/ESA+/CD24− [18], brain cancer CD133+ [19],pancreatic cancer CD44+/CD24+/ESA+ [20], colon cancer CD133+ [21], liver cancer CD133+ [22], prostate cancer CD44+/CD133+ [23], lung cancer CD133+ [24], and ovarian cancer CD133+/CD44+/CD117+ [25].
Extensive studies of CSC biology have revealed some critical mechanisms of drug resistance in CSCs, such as overexpression of ATP-binding cassette (ABC) transporters [26]. Moreover, some key pathways are critical for the survival and drug resistance of CSCs, including NOTCH, WNT/β-catenin, Hedgehog (Hh) and nuclear factor kappa B (NF-κB), among others [27, 28]. Therefore, inhibition of the resistance mechanisms and intracellular pathways leading to the elimination of CSCs are important features to be considered in the search for new compounds targeting cancer. The focus of this review is to describe how natural compounds can modulate these resistance mechanisms and intracellular pathways in the fight against CSCs.
2. NATURAL COMPOUNDS IN CANCER TREATMENT
Even with knowledge of the causes of cancer and its associated risk factors, efforts to prevent its emergence have proved to be insufficient. The elucidation of the mechanisms involved in carcinogenesis and the focus on finding new therapies for the treatment of cancer are considered the main strategies for controlling cancer. Despite the development of new techniques for the discovery of new drugs, such as chemical synthesis, combinatorial chemistry, and molecular modeling, the use of natural sources, especially plants, remains one of the most importance sources for the discovery and development of new anti-cancer drugs [10, 29, 30].
The use of natural compounds in many different contexts for the treatment of several diseases is one of the oldest forms of medical practice. According to the World Health Organization (WHO), approximately 65- 80% of the world’s population in developing countries relies on the use of traditional medicine in primary health care [31]. However, the bioactive components in most of these natural compounds that have been used for centuries have not yet been fully elucidated. On the other hand, numerous new molecules have been identified and are now used in medical therapy, especially in oncology. From 1940 to 2010, approximately 175 anti-cancer molecules were launched on the market and 75% of these molecules came directly or indirectly from natural compounds [32].
In oncology, the first natural compounds to be identified were the vinca alkaloids vincristine and vinblastine, which were isolated from Catharanthus roseus, as well as cytotoxins isolated from Podophyllum sp. in the 1950s [33]. In 1964, actinomycin was approved [33], and then, in 1966, camptothecin was isolated from the Chinese plant Camptotheca acuminata, a quinoline alkaloid that acts as an inhibitor of topoisomerase I, as reviewed in [34]. Because of its unique mechanism of action, analog compounds were then synthesized and investigated for their effectiveness as cancer treatments [34]. The success of these studies led to the development of chemotherapeutic drugs that are currently in use, such as paclitaxel and docetaxol, which were approved in the 1990s for breast cancer treatment and have been effectively used for the treatment of various cancers [35]. Several other medicines were derived from these compounds, such as rapamycin, which was isolated in 1975. After its approval in 2007, it brought to market 12 derived natural compounds that were all approved for cancer treatment including ixabepilone (2007), romidepsin (2009), cabazitaxel (2010), abiraterone acetate (2011) and carfilzomib (2012), among others [32]. Additionally, in 2012, Abraxane®, an injectable paclitaxel nanoparticle and another example of the numerous derivatives based on these findings, was approved [36]. The diverse plant species and numerous identified compounds are still proving to be potent anti-cancer agents.
3. PHYTOMETABOLITES TARGETING CSCS MODULATE ABC TRANSPORTERS, KEY REGULATORS OF ANTICANCER DRUG RESISTANCE
Two important properties of adult stem cells, self-renewal and quiescence, guarantee the permanence of these cells in the undifferentiated state in tissues throughout the life of an organism [37]. This permanence is attributed to several mechanisms that block the signaling pathways associated with proliferation and differentiation and to mechanisms that protect a wide variety of compounds, ensuring that adult stem cells remain in the undifferentiated state [38, 39].
The presence of efflux transporters such as ABC transporters on the cell membrane is an important mechanism that protects the cellular homeostasis of stem cells [40]. These transporters allow the efflux of a variety of small regulatory molecules such as steroids that could trigger proliferation or differentiation [41]. For instance, the expression of these transporters could be related to the undifferentiated state because the evaluation of the expression of the ABCG2 gene, also known as the breast cancer resistance protein (BCRP) gene, showed high expression levels in the most primitive hematopoietic population (CD34+CD38-) and low expression levels in mature cells [42]. Interestingly, the same transporters are also expressed in tumor cells, particularly in CSCs [43, 44]. It is well established that the presence of efflux pumps is an important mechanism of resistance and evasion to chemotherapy treatment that is strongly associated with the recurrence of cancer [45]. The presence of these transporters in specific cell populations could easily be determined with the dye efflux assays using fluorophores by flow cytometry [46, 47].
The ABC transporter family includes a family of at least 48 genes grouped into seven families (A-G) with different functions [48]. Among the main transporters are P-glycoprotein (P-gp), encoded by the ABCB1 gene (also called as multidrug resistant protein-1, MRD1), the breast cancer resistance protein (BCRP/ABCG2), and the multidrug resistance-associated proteins (MRP1 and MRP2), encoded by the ABCC1 and ABCC2 genes, respectively [49]. All of these molecules use the energy obtained from ATP hydrolysis to translocate substances across the cell membrane against an electrochemical gradient [50]. Structurally, these transporters have two transmembrane domains formed from 6-12 α-helices, which determine the binding specificity to the substrate, and two ATP-binding domains, called nucleotide-binding folds, which hydrolyze ATP to provide energy for the efflux pump, as depicted in (Fig. 2 ), and described in [50].
Fig. (2).
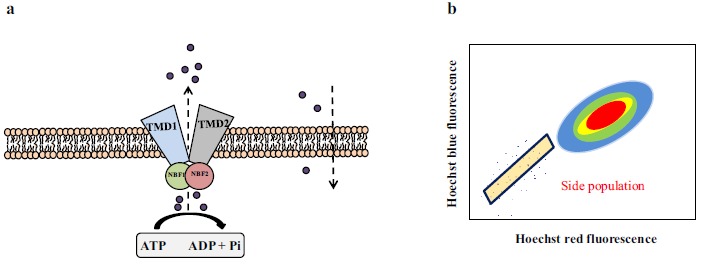
(a) ABC transporters have two transmembrane domains (TMD) and two ATP-binding domains (nucleotide-binding folds NBF). ABC transporters efflux substrates using the power provided by ATP hydrolysis. (b) Schematic illustration of a side population. Side populations are localized off to the side of the main population of cells.
Several studies have demonstrated the role of ABC transporters in the resistance of CSCs. In rat glioblastoma multiforme CD133+ cells, the presence of ABCB1 contributed to resistance to the anti-neoplastic drugs camptothecin and doxorubicin [51]. Additionally, ABCG2 protein expression was directly associated with the migration and invasion ability of U251 glioma stem-like cells [52]. Similarly, a direct association with increased ABCG2 expression was also observed in CD44+CD24- low ESA+ cells obtained from metastatic breast cancer [53]. The expression of ABCB5 was associated with tumor progression and relapse of oral cancer squamous cell carcinoma. ABCB5 expression was also associated with the presence of a putative CSC compartment in CD44+ cells [54]. Moreover, several signaling pathways that regulate self-renewal and stem cell pluripotency, such as the WNT pathway, can modulate the expression of efflux pumps in CSCs. Activation of WNT leads to overexpression of the ABCB1 gene in uterine sarcoma and breast cancer [55]. OCT-3/4 protein deregulation can also contribute to drug resistance in glioblastoma cells and can increase ABCG2 gene expression [56].
Once the overexpression of ABC transporters was recognized as a mechanism that confers the resistance of tumor cells to several drugs, many efforts were made to develop drugs that decrease the expression or functions of ABC transporters. The first drugs used for this purpose were called the first generation modulators or inhibitors of ABC transporters and included molecules such as verapamil, cyclosporine and quinine [57]. Although beneficial effects were observed in preclinical studies, few beneficial effects were observed in clinical trials [58]. Verapamil, which also acts as an inhibitor of calcium channels, induced toxicity in cardiomyocytes [58]. To overcome the limitations of the first generation modulators of ABC transporters, the specific drugs, such as valspodar (PSC-833) and ebiricodar (VX710), were specifically developed against them. These modulators were called the second generation modulators of ABC transporters [59], and showed better efficacy than the first generation modulators when used in combination with traditional chemotherapy. However, they had serious side-effects on hepatic and intestinal metabolism by inhibiting enzymes of the cytochrome P450 family and reducing the clearance of drugs [59]. The third generation modulators of ABC transporters, such as elacridar (GF120918), laniquidar (R101933), zosuquidar (LY335979) and tariquidar (XR9576), are more active and have fewer side effects compared to the other generations of modulators, reducing the expression of ABCG2 and ABCC1 genes [60].
Recently, the research goal has been to investigate natural product modulators to overcome multidrug resistance in cancer. The beneficial activity of natural modulators on ABC transporters is mainly associated with synergism with other anti-tumor drugs. Natural compounds can act as competitors of active sites of efflux pumps, reducing the chemotherapeutic efflux [61]. Among the class of secondary metabolites, flavonoids stand out as efflux pump inhibitors particularly because they inhibit P-gp ATP-ase activity by interacting with the ATP-binding sites [62, 63]. The natural product polyphenol epigallocatechin-3-gallate (EGCG), the most abundant and active phenolic compound found in green tea, exhibits antitumor properties [64-66]. Numerous studies have demonstrated that EGCG affects many signaling pathways including Janus kinase (JAK)/signal transducer and activator of transcription (STAT), mitogen-activated protein kinases (MAPK), phosphoinositide 3-kinase (PI3K/AKT), WNT, Hedgehog (Hh) and NOTCH [67, 68]. Additionally, EGCG stimulates telomere fragmentation by inhibiting telomerase activity [67]. Moreover, beyond its ability to act as a signaling modulator, EGCG also demonstrated the ability to inhibit efflux pumps. EGCG decreased the activity and expression of ABCB1(P-gp) and BRCP genes in breast cancer cell lines resistant to tamoxifen [69]. Further studies performed in glioma stem-like cells isolated from the glioblastoma cell line U87 showed that the EGCG treatment increased the sensitivity of glioma stem-like cells to temozolomide by reducing the expression of P-gp [68]. Structural studies performed have shown that EGCG most likely binds to the ATP-binding site of the ABCB1 (P-gp) transporter [70].
Curcumin is one of the most potent and studied polyphenols found in the rhizomes of Curcuma longa. Three curcuminoids are found in rhizomes, curcumin I, II, and III, and they possess a wide variety of biological effects. Antioxidant, anti-inflammatory and anti-mutagenic effects of curcumin have been reported. The combination of curcumin with a variety of chemotherapeutic agents, such as tamoxifen, cisplatin, doxorubicin, and vincristine, has been shown damaging to cancer cells in vitro [71-77]. Studies carried out in KB-V1 (cervical carcinoma) and NIH3T3 (murine fibroblast) cells, which overexpress multidrug resistance (MDR)-1, showed that the three curcuminoids were able to increase the retention of fluorophores in a dose-dependent manner by inhibiting the MDR1 pump. Among the three isoforms of curcumin, curcumin I exhibited the highest affinity for P-gp due to the presence of two methoxy groups (H3CO), one on each side of the molecule, as demonstrated by structure-activity relationship studies [78]. Moreover, curcuminoids sensitized different lineages to chemotherapeutic agents. Curcuminoids inhibit the efflux of mitoxantrone and pheophorbide in cells expressing wild type and R482T mutant of ABCG2 gene, which express ABCG2 gene at high levels [79]. Furthermore, curcumin reduced the expression of the ABCB1 gene (also known as MDR1B) mediated by the PI3K/AKT and NF-κB pathways in the multi-drug resistance leukemia L1210/Adr [80].
Apigenin is a flavonoid present in vegetables and medicinal plants, such as Salvia officinalis, Lawsonia inermis, Turnera aphrodisiaca, Ocimum basilicum and Tamarindus indica. This flavonoid has awakened great interest due to its chemopreventative, antioxidant, anti-mutagenic, anti-inflammatory, and antiviral properties [81-85]. The effects of apigenin on the ABC transporters have been evaluated in the multi-drug resistant cell line CEM/ ADR5000, an acute lymphoblastic leukemia (ALL) cell line that specifically overexpresses P-gp, and in the MDA-MB-231 cell line, a breast cancer cell line transduced with the BCRP expression vector. In both cell lines, apigenin increased the intracellular levels of doxorubicin. Additionally, apigenin increased docetaxel uptake in HEK293 cells transfected with the ABCB5 expression cassette [86]. The ability of apigenin to inhibit different ABC transporters makes this molecule an interesting prototype for the development of broad-spectrum inhibitors.
Berberine is an isoquinoline alkaloid that is found in a wide variety of plants, such as Coptis chinensis, Hydrasis canadendis, Tinospora cordifolia and the Berberidaceae plant family. Berberine has therapeutic potential against several disorders, including type 2 diabetes, neurological disorders and cardiological disorders, and it has antibacterial, anti-inflammatory, and cytotoxicity properties [87-90]. Berberine also reduced the expression of genes related to the pluripotency of stem cells and decreased the percentage of side populations in the tumor pancreatic cells PANC-1 and MIA PaCa-2 [91]. The liposome formulation of berberine exhibited effects on the expression of ABC transporters. Berberine also reduced the expression of ABCC1 and BCRP/ABCG2 genes in human breast cancer MCF-7 cells, as described in [92].
Isoliquiritigenin is a chalcone derived from licorice and from plants, such as Spatholobus suberectus [93]. The anti-proliferative effects, suppression of metastasis, angiogenesis, and the induction of apoptosis were investigated for this chalcone [64, 94-96]. However, its effects on WNT/ CTNNB1 (β-catenin) signaling in breast CSCs have drawn attention because these features are important for the treatment of this disease [97]. Isoliquiritigenin is an effective chemosensitizer that exhibits synergistic effects with common chemotherapies, such as 5-fluorouracil, epirubicin and taxol, mainly in the breast cancer cell lines, MDA-MB-231 and BT-549. Isoliquiritigenin reduced the formation of colonies and the side population in the presence or absence of epirubicin in the breast cancer cell lines, MDA-MB-231 and MCF-7. The molecular mechanisms of this compound were investigated in CSCs from MDA-MB-231. It was shown that its effects include lower expression of β-catenin target genes (BCRP/ABCG2, cyclin D1 [CCND1], survivin [also known as baculoviral IAP repeat containing 5, BIRC5], OCT4 and c-MYC). In in vivo studies, isoliquiritigenin did not exhibit adverse effects in normal adult stem cells; but in non-obese diabetic/ severe combined immunodeficient (NOD/SCID) mice inoculated with CSCs from MDA-MB-231, isoliquiritigenin reduced the tumor size alone or when administered with epirubicin [98].
The modulators described above are just a few natural compounds that could be useful tools to reduce drug resistance in various human cancers. Moreover, additional studies are being conducted to identify novel compounds that affect drug resistance displayed by tumor cells. For instance, in a high throughput assay using a library of 69 cardiotonic steroids, a compound 6 was identified with the ability to inhibit P-gp in CEM/ADR5000 cells [99]. New 9-hydro-β-agarofuran sesquiterpenes were isolated from the leaves of Celastrus vulcanicola. Four of them were found to act as powerful chemosensitizers in NIH-3T3 cells transfected with the human ABCB1 (MDR1) gene. These new compounds have better efficacies than the classical inhibitor verapamil [100]. A number of compounds isolated from various plants, which constitute Traditional Chinese and Indian medicines, such as the lignans from Arctium lappa, and the flavonoid baicalein from Scutellaria baicalensis, as well as active compounds from Withania somnifera and Tinospora cordifolia, may also provide some promising results [101-103].
4. WNT SIGNALING IS MODULATED BY NATURAL COMPOUNDS TARGETING CANCER CELLS
The WNT/CTNNB1 (β-catenin) pathway is known to control various processes during embryonic development and to regulate the maintenance of the undifferentiated state of adult stem cells. Aberrant regulation of this signaling involves epigenetic silencing, mutations or downregulation of genes that encode inhibitors of the WNT/β-catenin pathway. Altered regulation of the WNT signaling is associated with pre-malignant conditions in different types of cancer and other diseases such as fibrosis, neurodegenerative diseases or even metabolic diseases [104]. The human WNT gene family comprises 19 members encoding glycoproteins rich in cysteine residues [105]. The existence of more than 15 receptors and co-receptors for the WNT-ligand shows how important and complex this signaling pathway is [105, 106].
Currently, three WNT signaling pathways are known: the canonical WNT pathway, non-canonical planar cell polarity (PCP), and the non-canonical WNT/Ca2+ pathway. Of these, the canonical is the better understood. The canonical WNT/CTNNB1 pathway begins its signal transduction from the Frizzled (FZD) and LRP5/6 receptors [105, 106]. The WNT ligands are secreted glycoproteins that are heavily modified prior to transport and released into the extracellular milieu. Studies have revealed that the WNT proteins are glycosylated in the endoplasmic reticulum and are also palmitolated. The porcupine protein has been shown to play an important role in the palmitoylation of the WNT [107-109].
In the absence of WNT ligands, CTNNB1 (β-catenin) is bound to a complex formed by glycogen synthase kinase 3β (GSK3β), AXIN2 (also known as conductin), casein kinase 1α (CK1α) and adenomatous polyposis coli (APC) protein, where β-catenin is phosphorylated [105, 106, 110]. The β-catenin protein is then being ubiquitinated by β-transducin repeat containing protein (β-TrCP or TRCP1) E3 ubiquitin ligase and subsequently degraded by the proteasome [110]. On the other hand, the pathway is activated by binding of WNT ligands (secreted from neighboring cells) to bind to Frizzled (FZD) and low-density lipoprotein receptor-related protein (LRP)-5/-6 receptors located on target cells. After binding to receptors, the destruction complex captures and phosphorylates β-catenin, but its ubiquitination by β-TrCP is blocked resulting in the accumulation of β-catenin [111, 112]. Additionally, after the binding of WNT to its receptor, LRP5/6 is phosphorylated by CK1α and GSK3β, which enables the membrane translocation of a key negative regulator of signaling, AXIN, which binds to a conserved sequence in the cytoplasmic tail of LRP5/6. Another important event is the recruitment of the cytoplasmic protein Dishevelled (DVL) to the plasma membrane where it interacts with FZD [113]. The interaction of AXIN with phosphorylated LRP5/6 and DVL leads to the inactivation of the degradation complex and the accumulation of β-catenin, which is translocated to the nucleus [114]. In the nucleus, β-catenin forms a transcriptionally active complex with Lymphoid enhancer factor/T-cell factor (LEF/TCF) by displacing transducin-like enhancer protein (TLE) protein (human homolog of Drosophila Groucho protein) and interacts with co-activators, such as B-cell lymphoma 9 (BCL-9), Pygopus (PYGO), CREB-binding protein (CBP), and mixed-lineage leukemia (MLL) proteins [114, 115], as depicted in (Fig. 3 ).
Fig. (3).
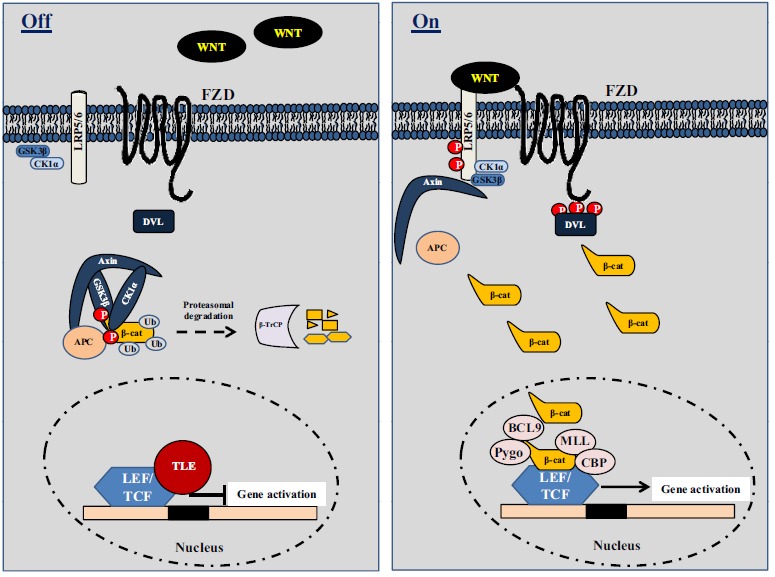
(a) “Off State”. In the absence of Wnt signals, β-catenin is captured by a degradation complex containing Gsk3 (glycogen synthase kinase 3), Axin/Conductin, CK1 (casein kinase 1) and APC (adenomatous polyposis coli) where it is first phosphorylated and then ubiquitinated on N-terminal sequences by β-TrCP (β-transducin repeat-containing protein). β-catenin is subsequently targeted for proteasomal degradation. In the nucleus, the transcriptional inhibitor TLE (human homolog of Drosophila Groucho) binds to LEF/TCF (lymphoid enhancer factor/T-cell factor) and inhibits transcription. (b) “On State”. Wnts bind to and activate FZD (Frizzled) and LRP (LDL-related receptor protein) receptors on target cells. LRP5/LRP6 are phosphorylated by CK1 and Gsk3, and DVL (Dishevelled) molecules are recruited to the plasma membrane to interact with FZD. The interaction of Axin with phosphorylated LRP5/6 and DVL leads to the inactivation of the degradation complex and the accumulation of β-catenin, which translocates to the nucleus. In the nucleus, β-catenin forms a transcriptionally active complex with LEF and TCF by displacing Groucho and interacts with co-activators such as BCL9 (B-cell lymphoma 9), Pygo (Pygopus), CBP (CREB-binding protein) and MLL (mixed-lineage leukemia).
In cancer cells, the WNT/β-catenin pathway attracted attention for its involvement in tumorigenesis and tumor aggressiveness related to intestinal adenomatous polyposis, a hereditary cancer. The discovery of the relationship between the WNT/β-catenin pathway and the tumor suppressor APC by mutational analysis was a hallmark of tumorigenesis of this type of tumor [116]. Then, further studies performed in patients with colorectal cancer demonstrated other mutations in genes that participate in the WNT/β-catenin pathway. For instance, mutations in transcription factor 7-like 2 (TCFL2) and the Wilms' tumor gene on the X chromosome (WTX), which encodes a tumor suppressor protein, were associated with carcinogenesis in colorectal cancer [117]. WTX protein was shown to participate in β-catenin degradation [118].
Other components of the pathway, such as WNT5A, which specifically acts through the non-canonical WNT/ Ca2+, are very important for the migration of stem cells during the embryonic period [119]. Additionally, CD44+CD24- CSCs from head and neck cancer exhibit overexpression of WNT5A protein; while WNT5A is knocking-down CSCs population was reduced [119, 120]. Recently, it was observed that Rspo2, an endogenous ligand, and 6-bromoindirubin-3'-oxime (BIO), a GSK3β inhibitor, produced a pronounced effect on pluripotency markers in lung adenocarcinoma cells (SPC-A1 and PC-9). The effect of this treatment stimulated the proliferation of spheres cells and enhanced the expression of stemness genes, such as NANOG, OCT4, Aldehyde dehydrogenase 1 family (ALDH1) and BCRP/ABCG2 [121]. These data indicate that the WNT pathway plays an important role in the maintenance of CSCs in lung adenocarcinoma. In stomach cancer, expression of the WNT1 protein contributes to self-renewal of CSCs and it is correlated with the increase in proliferation rate and CD44 expression in AGS cells [122]. Moreover, the WNT/β-catenin pathway may also contribute to the formation of leukemic stem cells. In samples from patients with aberrant acute myeloid leukemia, increased levels of β-catenin and other components, such as WNT1, WNT2 and LEF1 were detected in the CD34+ population, and these effects were associated with a poor prognosis in this disease [123].
Currently, natural compounds, which can modulate the WNT/β-catenin pathway, were also shown to alter the self-renewal capacity of CSCs in various tumor cellular models. In a recent study, the properties of the compounds 6-shogaol and pterostilbene were demonstrated in breast CSCs. Chemically, 6-shogaol is a derivative of 6-gingerol, a polyphenolic compound found in ginger Zinziber officinalis [124], which is generated after the dehydration of 6-gingerol. 6-shogaol is cytotoxic against colon tumor cells, reduces neuro inflammation
and improves cognitive deficit [125]. Pterostilbene is found in blueberries and grapes as an analog of resveratrol. This compound was cytotoxic to CD44+CD24-/low CSCs isolated from the human breast cancer MCF-7 cells. Moreover, pterostilbene inhibited the formation of mammospheres and decreased the expression of CD44. For instance, the surface marker CD44 is associated with the maintenance of stem cell characteristics [126]. Furthermore, 6-shogaol and pterostilbene allowed for the phosphorylation of β-catenin, thereby promoting its degradation. The expression levels of cyclin D1 (CCND1) and c-MYC proteins, which are downstream of activation of TCF/LEF by β-catenin, were also downregulated [127].
Synthetic derivatives of natural compounds are very promising venues to generate novel cancer chemotherapeutics. 8-bromo-7-methoxy chrysin (BRMC) is an example of a synthetic molecule derived from the flavonoid chrysin (5,7-dihydroxyflavone) that has been reported to possess cytotoxic activity indifferent tumor cell lines [128]. In previous studies, it was shown that BRMC can promote cell death of liver cells [129]. Additionally, BRMC was also able to inhibit self-renewal and proliferation of liver stem cells isolated from the MHCC97 cell line. This study demonstrated that BRMC reduced the expression of CD44 and CD133, important markers of non-differentiated liver CSCs, and promoted the reduction of β-catenin expression [130].
Casticin (3’, 5 dihydroxy 3,4', 6,7 tetrahydro-methoxyflavone), also known as vitexicarpin, is a component of Viticis Fructus, a traditional Chinese medicine prepared from the fruit of Vitex trifolia, and also found in Vitex agnus-cactus and Artemisia annua [131]. Regarding the properties of casticin in CSCs, it has been shown that casticin inhibits proliferation and self-renewal of liver stem cells [131]. In this study, casticin was more active against isolated CD133+ cells extracted from the MHCC97 cell line than total MHCC97 cells. Subsequently, it was shown that casticin regulates β-catenin expression and its downstream target CCND1, which have already been mentioned as targets of WNT/β-catenin signaling [131]. In these situations, casticin reduced the expression of proteins demonstrating the potential action of casticin as an antitumor drug for its selectivity in reaching the population of CSCs at low concentrations and reducing its self-renewal by inhibition of the WNT/β-catenin pathway [132]. In another study, casticin was shown to inhibit the epithelial-mesenchymal transition of liver stem cells by reducing the TWIST protein [133].
Resveratrol (3, 5, 4'-trihydroxy-trans-stilbene) is a classic example of a phytometabolite with important antitumor activities that have recently been investigated. It was first isolated in 1940 from Veratrum grandiflorum and was subsequently identified in the species such as grapes, peanuts and mulberries [134, 135]. It is noteworthy that resveratrol exhibits antioxidant, anti-inflammatory, antibacterial, and anti-tumor properties mainly against breast, prostate, gastric, colorectal, lung, leukemia, skin and pancreatic tumors [136, 137]. Additionally, resveratrol was identified as a pro-apoptotic molecule in human pancreatic CSCs. Its action on apoptosis results from the activation of caspase 3/7 and the inhibition of expression of XIAP, BCL-2 and CCND1. Resveratrol also exhibited activity in pluripotent pancreatic ESA+CD44+CD24+ CSCs, whereas reduction of the expression levels of important markers of stemness, such as NANOG, SOX2, c-MYC, OCT4 and ABCG2, were observed [138]. The actions of resveratrol against CSCs of breast cancer in an in vivo model were also described. It was observed in NOD/SCID mice that tumors generated by the transplantation of SUM159 cells in animals treated for two weeks with resveratrol were smaller compared to the control group without any apparent toxicity [139]. Moreover, the abilities of residual cells from tumors of the control group and the group treated with resveratrol were analyzed in secondary recipients. In this report, it was observed that after 30 days all six animals inoculated with cells from the control group developed tumors, whereas only one animal that received cells treated with resveratrol developed a tumor. Immunohistochemical analysis demonstrated lower expression of β-catenin and CCND1 in the groups treated with resveratrol [139]. Similar to resveratrol, the compound 3, 5, 4'-trimethoxy-stilbene, a methoxylated analog, was able to inhibit the invasiveness of breast cancer cells by downregulating the PI3K/AKT and WNT/β-catenin pathways [140].
The compound 4 methyl sulfonyl butyl isothiocyanate (also know as sulforaphane, SFN), is a substance widely found in cruciferous vegetables such as broccoli and cabbage [141]. These foods are high in isothiocyanates, which are very important natural compounds with chemopreventive properties, and are capable of modulating the signaling of WNT/β-catenin [142]. In a study performed using leukemia stem cells (CD34+CD38-) derived from chronic myeloid leukemia (CML), the compound SFN showed a synergistic effect with the main chemotherapy used for the treatment of CML, imatinib, a tyrosine kinase inhibitor of the BCR-ABL chimeric protein [143]. In this report, the authors observed that SFN exhibited synergism with imatinib in the death of CD34+CD38-cells, a population with high levels of β-catenin. Sensitivity to imatinib plus SFN was higher in the CD34+CD38+ cell population, and the expression of β-catenin and MRD1 proteins, important proteins that confer drug resistance of leukemia stem cells to imatinib, was reduced by treatment with SFN [143].
Curcumin is another example of an extensively studied phytometabolite with important antitumor properties [144]. Curcumin is able to inhibit this pathway and reduce the formation of mammospheres in normal and breast cancer cell lines [145]. Moreover, curcumin promoted the reduction of migration and invasion of a human osteosarcoma cell line [146]. In this study, no change of cytosolic β-catenin content was observed; however, the nuclear β-catenin level was drastically reduced. Other studies have shown that curcumin affects cell proliferation and induces apoptosis in a human hepatocellular carcinoma cell line by reducing β-catenin activity and transcription of target genes of the WNT/β-catenin pathway [147]. It is well established that reduction of the transcription factor OCT4 is important in the maintenance and regulation of pluripotency of embryonic stem cells [148, 149]. In a recent study, it was shown that curcumin promoted higher levels of GSK3β and inhibition of OCT4, which are important factors for reducing the activity of the pathway and for the induction of apoptosis in human embryonic carcinoma cells [150].
Several studies have also demonstrated the important role of WNT/β-catenin in the development of prostate cancer [151-153]. Isoflavones have also been successfully used to reduce the expression and to block transcription of genes related to pathway. One mechanism described was the increased expression of GSK3β that regulates this pathway [154]. Traditional Chinese medicine also provides several examples of compounds that act on WNT signaling. For instance, indirubin, a compound extracted from the traditional preparation called Qingdai, has been used for many years in the treatment of leukemia and is derived from the plant Indigofera suffruticosa. This component was identified as an inhibitor of GSK3β [155]. Another example is honokiol, an active component of Magnolia officinalis [156].
5. NATURAL COMPOUNDS TARGETING NOTCH SIGNALING IN CSCS
NOTCH protein signaling is another example of a signaling pathway that regulates important processes in embryonic development and supports the formation of specialized adult tissues by regulating the differentiation of stem cells. The maintenance of self-renewal in normal and cancer stem cells are also important processes regulated by NOTCH receptors [157-159]. NOTCH receptor-mediated signaling involves signal transmission induced by ligands present in the membrane of a signal-sending cell and its respective receptor on the signal-receiving cell [160]. In mammals, there are five ligands divided into two subfamilies: Delta-like (DLL1, DLL3 and DLL4) and Jagged (JAG)-1 and JAG-2 [160]. There are four NOTCH receptors (NOTCH 1 - 4), which form complex heterodimeric structures via their intracellular domains [160]. They also contain a transmembrane domain that is involved in signal transduction and an extracellular portion important for ligand recognition [160].
The NOTCH receptors are synthesized as single precursor proteins and during transport to the cell surface, undergo cleavage by the furin-like convertase enzyme in the Golgi apparatus [160]. This cleavage occurs at S1 site, which is important for receptor maturation and generates two non-covalently linked subunits. Subsequently, the NOTCH receptor is also glycosylated in the Golgi apparatus. In Drosophila, two enzymes, O-fut1 (O-fucosyltransferase) and fringe (O-fucosylpeptide β -1,3-N-acetylglucosaminyl transferase), are involved in NOTCH receptor and ligand glycosylation. In vertebrates, this process is carried out by the GDP-fucose Protein O-fucosyltransferase 1 (POFUT1) and by the fringe homologs, O-fucosylpeptide 3-beta-N-acetylglucosaminyl- transferase (LFNG, also known as Lunatic fringe), beta-1, 3-N-acetylglucosaminyltransferase manic fringe (MFNG) and beta-1, 3-N-acetylglucosaminyl-transferase radical fringe (RFNG), as reviewed elsewhere [161]. This event seems to be important for the recognition of NOTCH ligands in vertebrates. On the intracellular surface of the cellular membrane, the NOTCH receptor has a structure with several domains that are important for signal transduction. The epidermal growth factor-like portion is followed by a three cysteine-rich repeat domain that prevents signaling in the absence of a signal. Their ligands have an amino-terminal domain known as DSL (DELTA, SERRATE, and LAG2) followed by different numbers of epidermal growth factor-like domains [161].
After the interaction of NOTCH ligands with the receptor, activation occurs in two steps involving sequential cleavages. The first cleavage is mediated by a metalloprotease called Tumor Necrosis Factor-α-converting enzyme (TACE), which cleaves the extracellular receptor on the face close to the transmembrane domain (S2), as the ligands are linked to the extracellular portion and are endocytosed by the signal-sending cell. The second cleavage occurs in the transmembrane domain by the enzyme γ-secretase (S3). At this stage, the NOTCH intracellular domain is released and, in turn, is translocated to the nucleus where it heterodimerizes with the canonical C-promoter binding factor. This interaction changes the canonical C-promoter binding factor from a transcriptional repressor to an activator to move core co-repressor proteins and recruit co-activator nuclear proteins, which include proteins, such as mastermind-like (MAML1-3) proteins and CBP/p300 [162, 163], as depicted in (Fig. 4 ). For a better understanding of NOTCH pathway inhibitors, reviews are widely available [28, 164, 165].
Fig. (4).
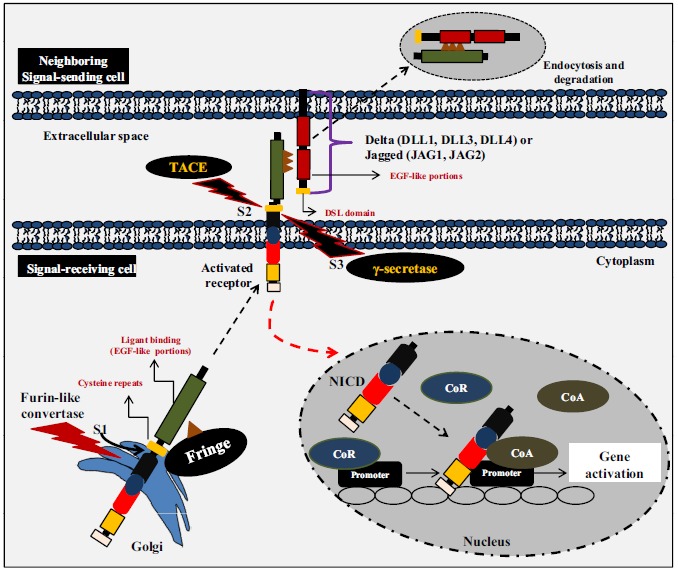
Notch signaling pathway. Notch receptors are synthesized as precursor proteins that are cleaved during transport to the cell surface where they are expressed as heterodimers. Following the binding of the ligand, placed in the surface of a neighboring cell, Notch is activated by two consecutive proteolytic cleavages that release its intracellular domain (NICD). The first proteolytic cleavage is mediated by the metalloprotease TACE, which cleaves the receptor on the extracellular side near the transmembrane domain. The second cleavage occurs within the transmembrane domain and is mediated by γ-secretase activity. This final cleavage liberates the NICD, which subsequently translocates to the nucleus where it binds to the transcription factor CBF1. This interaction converts CBF1 from a transcriptional repressor into a transcriptional activator by displacing nuclear co-repressor proteins (CoR) and by recruiting nuclear co-activator proteins (CoA).
Currently, there are extensive studies in various human disease models in which NOTCH proteins play key roles. The first examples came from the genetic studies of aberrant Notch signaling shown in patients with T-cell ALL [166, 167]. These studies have shown that 60% of patients with T-ALL have mutations in the heterodimerization domain [‘PEST’ sequence, which is a peptide sequence that is rich in proline (P), glutamic acid (E), serine (S), and threonine (T)] of NOTCH receptor that cause receptor cleavage independently of ligands and leading to a ubiquitin-mediated protein degradation of NOTCH [166, 167]. This increases the half-life of the receptor and promotes its activation in the absence of ligands [166, 167].
In one study, signaling triggered by the activation of NOTCH receptors was evaluated using a green fluorescent protein reporter gene in CSCs from human lung adenocarcinoma [168]. It was observed that cells with higher NOTCH activity showed enhanced ability to form tumor spheres, exhibited increased resistance to chemotherapeutic agents such as cisplatin and docetaxel, and exhibited high tumorigenicity in xenograft models [168]. The activation of the NOTCH receptor has also been associated with progression and poor prognosis in pancreatic cancer. It has been shown that an increase in NOTCH receptor expression contributes significantly to the acquisition of the epithelial-mesenchymal transition phenotype with high expression of mesenchymal cell markers (ZEB1, ZEB2, SNAIL2 and VIM [vimentin]), reduction of epithelial cell markers (E-cadherin, CDH1), increased growth and clonogenic capacity in the pancreatic cancer cell line-AsPC-1 [169]. High levels of NOTCH receptor expression have also been observed in prostate cancer cell lines and biopsies [170]. Higher expression levels are associated with more aggressive cancers according to the Gleason score [170]. The increase in the expression of NOTCH receptors occurs in cells surrounding vessels, suggesting a relationship between NOTCH receptors and the increase in cancer cell invasiveness [171]. Furthermore, activated NOTCH1 impairs mammary stem cell self-renewal and promotes tumor development in a high CD24+CD29+ cell population [172].
Examples of molecules extracted from plants that exert their effects on the NOTCH pathway are extensive. Honokiol, a phenolic compound that was mentioned above as an agent that acts on the WNT pathway, also has effects on colon and melanoma CSCs [173]. Honokiol was able to reduce the in vitro and in vivo growth of CSCs from colon tumors. This compound decreased the levels of various components of γ-secretase complex (needed to cleave NOTCH extracellular domain), including PSEN1 (presenilin-1), NCSTN (nicastrin), presenilin enhancer-2 (PEN2) protein and anterior pharynx-defective 1(APH1) protein, as well as the NOTCH ligand JAG-1, and NOTCH downstream target human homolog of hairy and enhancer of split-1 (HES1), which encodes a transcriptional factor HES1 [173]. Experiments performed using B16F10 and SK-MEL-28 cells, two melanoma cell lines, demonstrated that treatment with honokiol decreased the expression levels of cell primitive markers (CD271, CD166 and ABCB5) and reduced their clonogenic capacity. Honokiol also reduced the viability of these cells and increased autophagy. Additionally, treatment with honokiol decreased mainly NOTCH2 levels and also decreased levels of TNF-α converting enzyme and γ-secretase complex [174].
Atractylenolide (AT1) is a sesquiterpene lactone obtained from Rhizoma Atractylodis macrocephalae, a plant known from Traditional Chinese medicine that has anti-inflammatory, antibacterial, and antitumor effects and acts against disorders of the gastrointestinal tract [175]. Additionally, AT1 reduced the self-renewal capability of gastric CSCs enriched by sorting the CD44+ population of MGC-803 cells. MGC-803 CD44+ cells exhibit higher levels of NOTCH1 receptor than total MGC-803 cells. After treatment with AT-1, the expression of the NOTCH1 receptor and its downstream target (HES1, HEY1 and CD44) were reduced [175].
Moreover, the decoction called Xiaotan Sanjie, a preparation made using eleven different traditional Chinese medicinal herbs, is widely used against cancer in China and promotes the reduction of NOTCH1 expression in CSCs from gastric cancers. This effect was related to the anti-angiogenesis effect of Xiaotan Sanjie [176]. The compound
6-shogaol proved to be effective in reducing the self-renewal of CD44+CD24-/low breast CSCs by modulating the WNT pathway, as previously mentioned. In another study performed in the same population, 6-shogaol was confirmed as a modulator of the NOTCH pathway [177]. A reduction of spheroid formation was dependent on lower levels of NOTCH1, HES1, and CCND1. The addition of DAPT, a γ-secretase inhibitor, produced an increase in the expression of these proteins, suggesting that 6-shogaol could inhibit γ-secretase [177]. Furthermore, the EGCG compound was active in head and neck squamous cell carcinoma cell lines (K3, K4 and K5). EGCG was able to reduce self-renovation of CSCs by decreasing pluripotency markers (OCT4, SOX2, NANOG and CD44) and resistance markers (ABCC2 and ABCG2) and by inhibiting the Notch pathway [178].
6. HEDGEHOG SIGNALING AS A POTENTIAL TARGET FOR PHYTOMETABOLITES IN CSCS
Despite its critical role in embryogenesis [179, 180], abnormal activation of the Hedgehog (Hh) pathway has emerged as a driven force of tumorigenesis [181]. Furthermore, evidence implicates the Hh pathway in CSCs in a variety of cancer models, including hematological malignancies and solid tumors [182-195]. The Hedgehog signaling pathway entails two transmembrane receptors, Patched-1 (PTCH1) and Smoothened (SMO). In the absence of the ligands (Sonic Hh [Shh], Indian Hh [Ihh], or Dessert Hh [Dhh]), PTCH1 constitutively represses SMO, thus preventing its translocation to primary cilia and repressing Hh signaling. In the presence of Hh ligand, PTCH1 alleviates the repression of SMO, leading to activation and translocation of the Gli proteins to the nucleus, where they transcriptionally induce Hh target genes [196-199]. This signaling pathway may accomplish its role in cells and tissues by acting on cell proliferation, motility and adhesion, as well as cell fate. In cancer, several lines of evidence indicate that Hh signaling contributes not only to the growth and maintenance of cancer, but it is also implicated in resistance and failure of chemotherapy, thereby inducing a more aggressive phenotype. In addition, the Hh may also function as a signal to determine the CSC phenotype through the regulation of the expression of stemness-associated genes, such as NANOG, OCT4, SOX2 and polycomb complex protein BMI-1 (also known as polycomb group RING finger protein 4 [PCGF4], or RING finger protein 51 [RNF51]), as described in [183, 200-203].
Chronic myeloid leukemia (CML) is one of the best-characterized stem cell-derived malignancies and studies have demonstrated that the Hh activity is correlated with the capacity of BCR-ABL-induced leukemia stem cells [187]. In this respect, treatment of mice transplanted with BCR-ABL-infected hematopoietic stem cells with cyclopamine, a phytochemical isolated from Veratrum californicum, popularly known as corn lily, significantly inhibited the stem cell pool and further increased mice survival [185]. This steroidal alkaloid was also shown to downregulate P-glycoprotein expression in multidrug resistant Lucena-1 cells, which derived from the chemosensitive K562 cell line [204]. In line with this study, Yao and colleagues [201] revealed that cyclopamine was also able to reverse resistance in gemcitabine-resistant pancreatic cancer cells, which were demonstrated to highly express CSC markers. In ALL, cyclopamine induced eosinophilic differentiation of HL60 cells [205] and significantly diminished self-renewal capability of ALL stem cell [206]. Additionally, to leukemias, the effects of cyclopamine were demonstrated in pancreatic, colon and breast cancer, gliobastoma and multiple myeloma CSCs [192, 201, 207, 208]. Cyclopamine was shown to inhibit Hh activity by binding directly to SMO. This effect is not surprising since the natural ligands for Smo are oxysterols [209].
The green tea polyphenolic component EGCG acts through many mechanisms and it is not surprising that it is also able to modulate the Hh signaling. In pancreatic CSCs, EGCG inhibited the Hh components and GLI transcriptional activity. Moreover, it also affects the CSC self-renewal capability, either alone or in combination with quercetin, condition that had a synergistic effect through attenuation of TCF/LEF and GLI activities [210]. In the same direction, the efficacy of the polyphenol curcumin and its derivatives in targeting colorectal CSCs has been shown in several in vitro and in vivo studies [211]. Additionally, Buhrmann and colleagues [212] have recently suggested that curcumin may contribute to suppress the epithelial-mesenchymal transition (EMT) and metastasis, since in co-cultures of colorectal cancer cells with stromal fibroblasts it was observed a dramatic increase in tumor-promoting factors, CSCs survival and EMT-inducing factors, which were counteract by treatment with curcumin [212]. Genistein is an isoflavone that suppressed tumorigenicity of prostate cancer in vivo. Furthermore, this isoflavone inhibited tumorsphere and colony formation. Prostate tumorspheres were demonstrated to be reach in CSCs and treatment with genistein inhibited tumorspheres growth through downregulation of the Hh-Gli1 pathway [212]. Recently, the aqueous extract isolated from sandy beige mushroom Trametes robiniophila Murr (Huaier) was found to affect the number, viability and size of mammospheres. Treatment with Huaier aqueous extract reduced the clonogenicity of human breast cancer MCF-7 cells, with a significant reduction in the numbers of cells expressing CD44+/CD24- and a decrease in the levels of stemness markers, such as OCT4, NESTIN and NANOG. These effects were partly attributed to the inactivation of the Hh pathway [213, 214].
Moreover, arsenic trioxide has also been shown effective in antagonizing the Hh pathway in CSCs. Han and colleagues [214] reported the potential of this compound to inhibit the viability of pancreatic CSCs by binding to SHH/GLI protein complex in vitro and in vivo [215]. In a cell free condition, these authors demonstrated that arsenic trioxide was able to change the structure of recombinant GLI1 zinc finger peptides and its binding to GLI1 was confirmed in cultures of pancreatic cancer cells [215]. Since the recent approval of the drug vismodegib (Erivedge) by the US Federal Drug Administration (FDA) in 2012, it is expected a greater interest in research into new drugs that inhibit the Hh activity. Indeed, remarkable progress has been made in the identification of compounds from natural sources that target this signaling pathway, and most importantly in CSC, which may result in the development of new leaders for drug discovery in this area.
7. NF-κB AND APOPTOSIS MODULATORS AGAINST CSCS
Tumor formation or even the development of cancer cell drug resistance can be initiated by dysregulated apoptosis. NF-κB is a transcription factor for a large group of genes that are involved in different pathways. These pathways play important roles in tumorigenesis and suppression of these pathways is essential for the induction of tumor cell apoptosis [216]. Similarly, NF-κB influences cellular development, induces inflammatory responses, participates in wound repair, regulates cell survival and is constitutively activated in different types of cancer [217, 218]. By blocking the apoptotic event to save cells that might otherwise be killed, NF-κB can be dangerous and can lead to the maintenance of cancerous cells. In cancer cells, this anti-apoptotic activity and promotion of proliferation could lead to the development of safe inhibitors of the NF-κB pathway [219].
The NF-κB family of transcription factors consists of five members, p65, RELB, c-REL, p50 and p52, all of which contain a REL-homology domain that is responsible for DNA binding, dimerization, nuclear translocation and endogenous inhibition of NF-κB binding [217-221].
Nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor alpha (IĸBα) inhibits NF-κB activity by masking the nuclear localization signals of NF-κB proteins and keeping them sequestered in an inactive state in the cytoplasm [217-221]. In addition, IκBα blocks the ability of NF-κB transcription factors to bind to DNA, which is required for NF-κB's proper functioning. The IκB kinase (IKK) is an enzyme complex that is part of the upstream NF-κB signal transduction cascade. IKK specifically, phosphorylates the inhibitory IκBα protein [220, 221]. This phosphorylation results in the dissociation of IκBα from NF-κB. NF-κB, which is now free migrates into the nucleus and activates the expression of over 500 genes involved in immunoregulation, growth regulation, inflammation and apoptosis [220, 221].
The induction of anti-apoptotic genes such as BCL-xL, MCL-1, cFLIP and cIAPs by NF-κB contributes to the downregulation of the activity of the caspase cascade that forms the core of the apoptotic pathway [222]. Controlling multiple genes and being involved in a variety of human diseases makes NF-κB an interesting target for anti-cancer therapy. The inhibition of NF-κB enhanced the apoptosis induced by TNF-α related apoptosis-inducing ligand (TRAIL) in various tumor cells [223]. The same signal led to
the activation of two parallel but opposing pathways, the pro-apoptotic and the anti-apoptotic pathways [224]. However, the mechanisms behind the pro-apoptotic role of NF-κB remain elusive. They may involve the recruitment of caspase 8 and enhanced expression levels of the TRAIL1 and TRAIL2 receptors by upregulation of c-REL, as described in [220].
NF-κB has various levels of regulation and, given its importance in human diseases, this pathway could be targeted at different levels, including protein kinases, phosphatases, nuclear translocation and DNA binding for selectively inducing apoptotic cell death. Moreover, in some cancer stem cells, NF-κB is aberrantly expressed, and its inhibition rapidly leads to cell death [225]. Several natural compounds have been suggested to be potent anti-cancer agents, and some of them act on CSCs. Curcumin and EGCG are examples of phytometabolites that have been described as potent NF-κB inhibitors acting as anti-cancer agents [226]. The function of both compounds is also related to their ability to suppress breast CSCs by specifically inhibiting STAT3 phosphorylation and retaining the STAT3/NF-κB interaction [227]. Parthenolide, a sesquiterpene lactone, is an example of a natural product that acts on leukemia stem cells and mammary breast cancer stem cells by inhibiting NF-κB activity [225]. Genistein, an isoflavonoid, is another pythometabolite that significantly inhibits NF-κB DNA-binding activity [228]. To date, hundreds of different NF-κB inhibitors have been reported, but so far no drugs that inhibit NF-κB have been approved.
CONCLUSION
Several latest reviews highlight the possibility of selective targeting CSCs using a plethora of natural compounds [229-236]. We also summarized the information about the compounds reviewed here in (Table 1). Since CSCc are able to impair the effectiveness of anti-cancer drugs, they might underline the resistance of tumors to chemotherapeutic agents, thereby exerting poor survival rates for cancer patients. Therefore, a search for novel anti-cancer compounds in general and from natural sources in particular is important quest for cancer biologist and clinicians alike. The notion that natural compounds form plants, fungi and marine life forms might display lesser side effects on normal adjacent cells and tissues than the synthetic drugs would facilitate the basic and translational research in the field. On the practical note, new anti-cancer drug cocktails containing the natural compounds should target all cancer cell populations, including CSCs. Natural compounds found throughout an entire planet categorized into several classes, such as terpenes, flavonoids, isoflavones, lignans, neolignans, glycosides, coumarin, chromones, quinones, phytosterols and alkaloids, among others, attest to the exceptional structural diversity present in plants, thereby making natural compounds, especially medicinal plants, an inexhaustible source of original models. Keeping in mind that Brazil, for example, hosts between 15 and 20% of our planet's biodiversity presenting the greatest diversity of endemic species in the different kingdoms. In this context, it is believed that among 250 thousands of cataloged plant species worldwide, approximately 120 thousands of them grow in Federative Republic of Brazil, making Brazilian biodiversity an important source of raw material for developing new anti-cancer drugs.
Table 1.
Natural compounds and cancer stem cells.
| Source | Compound | Origin of CSCs | Targets | Refs. | |||
|---|---|---|---|---|---|---|---|
| Camellia sinensis | EGCG | Breast, colon, pancreatic | CCND1, BCL-xL, HBP1, AKT, HSP90 | [67-69, 229, 230] | |||
| Zinziber officinalis | 6-Gingerol | Colon | WNT/CTTNB1, TNF, NF-κB, AP1 | [229, 230] | |||
| Adansonia, Aralia, Moringa, Morus, and Toona species | β-carotene | Neuroblastoma | HIF-1α, OCT3/4. DLK1 | [229, 230] | |||
| Scutellaria baicalensis | Baicalein | Bone marrow, CML | ABCG | [229] | |||
| Curcuma longa | Curcumin | Breast, brain, colon, pancreatic | WNT/CTTNB1, Hh, NOTCH, ABCB1, ABCC1, ABCG1 | [78-80, 148-150, 229, 230] | |||
| Thysanoptera genus | Cyclopamine | Breast, bone marrow, CML | Hh, SMO | [229, 230] | |||
| Vaccinium genus, Rubus genus | Delphinidin | Neuroblastoma | WNT/CTTNB1, Hh, NOTCH | [229] | |||
| Glycine max (L.) Merr. | Genistein | Breast, ovarian, kidney, melanoma | WNT/CTTNB1, Hh, NOTCH | [228-230] | |||
| Gossypium genus | Gossypol | Prostate | DNA damage, p53 reactivation, apoptosis | [229, 230] | |||
| Cruciferaceae sp. | Isothiocyanates (SFN) |
Prostate, pancreatic, cervical | NF-κB, PKB/AKT, Let-7 | [141-143, 229, 230] | |||
| Mentha piperita, Mentha spicata, Salvia dorrii, Hyptis crenata | Linalool | AML | NF-κB, p53, cell cycle arrest | [229, 230] | |||
| Solanum lycopersicum, Citrus × paradise | Lycopene | Breast | WNT/CTTNB1, Hh, NOTCH | [229] | |||
| Tanacetum parthenium | Parthenolide | Breast, AML, lung | NF-κB, TNFRSF10B, PMAIP1 | [225, 229] | |||
| Piper nigrum | Piperine | Breast | WNT/CTTNB1 | [229] | |||
| Platycodon grandiflorum | Platycodon saponin | Prostate | PI3K/AKT/ERK1, SMAD | [229, 230] | |||
| Psoralea corylifolia | Psoralidin | Prostate | PI3K/AKT, NOTCH1 | [229] | |||
| Capparis spinosa, Piper genus | Quercetin | Breast, oral, pancreatic, colon | WNT/CTTNB1, Hh, BCL/ BAX, MAPK, Let-7, KRAS | [229, 230] | |||
| Vitis vinifera, Prunus genus | Resveratrol | Mammospheres, medulloblastoma, colon | Apoptosis, DAPK, BNIP3, FASN | [138-140, 229, 230] | |||
| Streptomyces albus | Salinomycin | Breast, cervical, prostate, colon | WNT/CTTNB1, mTOR, CD133 | [229, 230] | |||
| Silybum marianum | Silibinin | Breast, lung, prostate, colon | WNT/CTTNB1, NOTCH1, Hh, CD133 | [229, 230] | |||
| Thymus vulgaris, Ocimum basilicum, Origanum genus | Ursolic acid | Breast, colon, prostate | WNT/CTTNB1, NOTCH1, Hh, PI3K/AKT | [229, 230] | |||
| Withania somnifera | Withaferin A | Breast | NOTCH1 | [101-103, 229] | |||
| Salvia officinalis, Lawsonia inermis, Turnera aphrodisiaca, Ocimum basilicum, Tamarindus indica | Apigenin | ALL, breast | ABC transporters | [86] | |||
| Coptis chinensis, Hydrasis canadendis, Tinospora cordifolia, Berberidaceae | Berberin | Breast, pancreatic | ABC transporters, ABCC1, BCRP/ABCG2 | [91, 92] | |||
| Spatholobus suberectus | Isoliquiritigenin | Breast | WNT/CTNNB1, CCND1, BCRP/ABCG2, OCT4, c-MYC, BIRC5 | [97, 98] | |||
| Vaccinium genus | Pterostilbene | Breast | CD44, WNT/CTNNB1 | [125-127] | |||
| Vitex trifolia, Vitex agnus-cactus, Artemisia annua | Casticin | Liver | WNT/CTNNB1, TWIST | [131-133] | |||
| Magnolia sp. | Honokiol | Melanoma | WNT/CTNNB1, NOTCH2, CD271, CD166, ABCB5, PSEN1, NCSTN, PEN2, APH1, JAG1 | [173, 174] | |||
| Atractylodis macrocephalae | Atractylenolide | Gastric | NOTCH1, HES1, HEY1, CD44 | [175] | |||
| Zinziber officinalis | 6-shogaol | Breast | NOTCH1, HES1, CCND1 | [177] | |||
| Trametes robiniophila Murr | Huaier | Breast | OCT4, NESTIN, NANOG | [213, 214] | |||
ACKNOWLEDGEMENTS
This work was supported by grants from Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP n° 2013/09068-7 to EJP-G) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq n° 473797/ 2013). H.F.V.T. was supported by a PhD fellowship from Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank Prof. Edward A. Yates for English editorial assistance.
LIST OF ABBREVIATIONS
- ALL
Acute Lymphoblastic Leukemias
- AML
Acute Myeloid Leukemias
- APC
Adenomatous Polyposis Coli
- ALDH1
Aldehyde Dehydrogenase 1 Family
- ABC
ATP-binding Cassette
- AT1
Atractylenolide
- BIRC5
Baculoviral IAP Repeat Containing 5
- BCRP
Breast Cancer Resistance Protein
- BRMC
8-bromo-7-methoxy Chrysin
- BCL-9
B-cell Lymphoma 9
- CSCs
Cancer Stem Cells
- CK1α
Casein Kinase 1α
- CML
Chronic Myeloid Leukemia
- CBP
CREB-binding Protein
- EGCG
Epigallocatechin-3-gallate
- EMT
Epithelial-mesenchymal Transition
- FDA
Federal Drug Administration
- GSK3β
Glycogen Synthase Kinase 3β
- HES1
Hairy and Enhancer of split-1
- Hh
Hedgehog
- JAK
Janus Kinase
- IKK
IκB Kinase
- LRP
Low-density Lipoprotein Receptor-related Protein
- LEF/TCF
Lymphoid enhancer factor/T-cell Factor
- MAML
Mastermind-like
- MAPK
Mitogen-activated Protein Kinase
- MLL
Mixed-lineage Leukemia
- MRD1
Multidrug Resistant Protein-1
- NOD/SCID
Non-obese Diabetic/severe Combined Immunodeficient
- NF-κB
Nuclear Factor Kappa B
- IĸBα
Nuclear Factor of Kappa Light Polypeptide gene enhancer in B-cells Inhibitor Alpha
- NBF
Nucleotide-binding Folds
- PI3K
Phosphoinositide 3-kinase
- POFUT1
Protein O-fucosyltransferase 1
- STAT
Signal Transducer and Activator of Transcription
- SMO
Smoothened
- SFN
Sulforaphane
- TCFL2
Transcription Factor 7-like 2
- β-TrCP or TRCP1
β-transducin Repeat Containing Protein
- TLE
Transducin-like Enhancer Protein
- TMD
Transmembrane Domains
- TNF-α
Tumor Necrosis Factor-α
- TACE
Tumor Necrosis Factor-α-converting enzyme
- TRAIL
TNF-α related Apoptosis-inducing Ligand
- WTX
Wilms’ tumor Gene on the X Chromosome
- WHO
World Health Organization
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
REFERENCES
- 1.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A. Jr., Kinzler, K.W. Cancer genome landscapes. Science. 2013;339(6127):1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Holohan C., Van Schaeybroeck S., Longley D.B., Johnston P.G. Cancer drug resistance: an evolving paradigm. Nat. Rev. Cancer. 2013;13(10):714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 4.O'Connor M.L., Xiang D., Shigdar S., Macdonald J., Li Y., Wang T., Pu C., Wang Z., Qiao L., Duan W. Cancer stem cells: A contentious hypothesis now moving forward. Cancer Lett. 2014;344(2):180–187. doi: 10.1016/j.canlet.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Martinez-Outschoorn U.E., Lin Z., Ko Y.H., Goldberg A.F., Flomenberg N., Wang C., Pavlides S., Pestell R.G., Howell A., Sotgia F., Lisanti M.P. Understanding the metabolic basis of drug resistance: therapeutic induction of the Warburg effect kills cancer cells. Cell Cycle. 2011;10(15):2521–2528. doi: 10.4161/cc.10.15.16584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garraway L.A., Janne P.A. Circumventing cancer drug resistance in the era of personalized medicine. Cancer Discov. 2012;2(3):214–226. doi: 10.1158/2159-8290.CD-12-0012. [DOI] [PubMed] [Google Scholar]
- 7.Vinogradov S., Wei X. Cancer stem cells and drug resistance: the potential of nanomedicine. Nanomedicine (Lond.) 2012;7(4):597–615. doi: 10.2217/nnm.12.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dean M., Fojo T., Bates S. Tumour stem cells and drug resistance. Nat. Rev. Cancer. 2005;5(4):275–284. doi: 10.1038/nrc1590. [DOI] [PubMed] [Google Scholar]
- 9.Insan M.B., Jaitak V. New approaches to target cancer stem cells: current scenario. Mini Rev. Med. Chem. 2014;14(1):20–34. doi: 10.2174/13895575113136660107. [DOI] [PubMed] [Google Scholar]
- 10.Paredes-Gamero E.J., Nogueira-Pedro A., Miranda A., Justo G.Z. Hematopoietic modulators as potential agents for the treatment of leukemia. Front. Biosci. (Elite Ed.) 2013;5:130–140. doi: 10.2741/e602. [DOI] [PubMed] [Google Scholar]
- 11.Clarkson B.D. Review of recent studies of cellular proliferation in acute leukemia. Natl. Cancer Inst. Monogr. 1969;30:81–120. [PubMed] [Google Scholar]
- 12.Dick J.E. Stem cell concepts renew cancer research. Blood. 2008;112(13):4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 13.Horton S.J., Huntly B.J. Recent advances in acute myeloid leukemia stem cell biology. Haematologica. 2012;97(7):966–974. doi: 10.3324/haematol.2011.054734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonnet D., Dick J.E. Human acute myeloid leukemia is organized as a hierarchy that originates from a primitive hematopoietic cell. Nat. Med. 1997;3(7):730–737. doi: 10.1038/nm0797-730. [DOI] [PubMed] [Google Scholar]
- 15.Wicha M.S., Liu S., Dontu G. Cancer stem cells: an old idea - a paradigm shift. Cancer Res. 2006;66(4):1883–1890. doi: 10.1158/0008-5472.CAN-05-3153. [DOI] [PubMed] [Google Scholar]
- 16.Shackleton M. Normal stem cells and cancer stem cells: similar and different. Semin. Cancer Biol. 2010;20(2):85–92. doi: 10.1016/j.semcancer.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 17.Bhatia M., Wang J.C., Kapp U., Bonnet D., Dick J.E. Purification of primitive human hematopoietic cells capable of repopulating immune-deficient mice. Proc. Natl. Acad. Sci. USA. 1997;94(10):5320–5325. doi: 10.1073/pnas.94.10.5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Hajj M., Wicha M.S., Benito-Hernandez A., Morrison S.J., Clarke M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi S.A., Wang K.C., Phi J.H., Lee J.Y., Park C.K., Park S.H., Kim S.K. A distinct subpopulation within CD133 positive brain tumor cells shares characteristics with endothelial progenitor cells. Cancer Lett. 2012;324(2):221–230. doi: 10.1016/j.canlet.2012.05.026. [DOI] [PubMed] [Google Scholar]
- 20.Wei H.J., Yin T., Zhu Z., Shi P.F., Tian Y., Wang C.Y. Expression of CD44, CD24 and ESA in pancreatic adenocarcinoma cell lines varies with local microenvironment. Hepatobiliary Pancreat. Dis. Int. 2011;10(14):428–434. doi: 10.1016/s1499-3872(11)60073-8. [DOI] [PubMed] [Google Scholar]
- 21.Ren F., Sheng W.Q., Du X. CD133: a cancer stem cells marker, is used in colorectal cancers. World J. Gastroenterol. 2013;19(17):2603–2611. doi: 10.3748/wjg.v19.i17.2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Piao L.S., Hur W., Kim T.K., Hong S.W., Kim S.W., Choi J.E., Sung P.S., Song M.J., Lee B.C., Hwang D., Yoon S.K. CD133+ liver cancer stem cells modulate radioresistance in human hepatocellular carcinoma. Cancer Lett. 2012;315(2):129–137. doi: 10.1016/j.canlet.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Soner B.C., Aktug H., Acikgoz E., Duzagac F., Guven U., Ayla S., Cal C., Oktem G. Induced growth inhibition, cell cycle arrest and apoptosis in CD133+/ CD44+ prostate cancer stem cells by flavopiridol. Int. J. Mol. Med. 2014;34(5):1249–1256. doi: 10.3892/ijmm.2014.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sarvi S., Mackinnon A.C., Avlonitis N., Bradley M., Rintoul R.C., Rassl D.M., Wang W., Forbes S.J., Gregory C.D., Sethi T. CD133+ cancer stem-like cells in small cell lung cancer are highly tumorigenic and chemoresistant but sensitive to a novel neuropeptide antagonist. Cancer Res. 2014;74(5):1554–1565. doi: 10.1158/0008-5472.CAN-13-1541. [DOI] [PubMed] [Google Scholar]
- 25.Walters Haygood C.L., Arend R.C., Straughn J.M., Buchsbaum D.J. Ovarian cancer stem cells: Can targeted therapy lead to improved progression-free survival? World J. Stem Cells. 2014;6(4):441–447. doi: 10.4252/wjsc.v6.i4.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zinzi L., Contino M., Cantore M., Capparelli E., Leopoldo M., Colabufo N.A. ABC transporters in CSCs membranes as a novel target for treating tumor relapse. Front. Pharmacol. 2014;5:163. doi: 10.3389/fphar.2014.00163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamamoto M., Taguchi Y., Ito-Kureha T., Semba K., Yamaguchi N., Inoue J. NF-kappaB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nat. Commun. 2013;4:2299. doi: 10.1038/ncomms3299. [DOI] [PubMed] [Google Scholar]
- 28.Takebe N., Miele L., Harris P.J., Jeong W., Bando H., Kahn M., Yang S.X., Ivy S.P. Targeting Notch, Hedgehog, and Wnt pathways in cancer stem cells: clinical update. Nat. Rev. Clin. Oncol. 2015;12(8):445–464. doi: 10.1038/nrclinonc.2015.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Butler M.S. The role of natural product chemistry in drug discovery. J. Nat. Prod. 2004;67(12):2141–2153. doi: 10.1021/np040106y. [DOI] [PubMed] [Google Scholar]
- 30.Newman D.J., Cragg G.M., Snader K.M. The influence of natural products upon drug discovery. Nat. Prod. Rep. 2000;17(3):215–234. doi: 10.1039/a902202c. [DOI] [PubMed] [Google Scholar]
- 31.Calixto J.B. Twenty-five years of research on medicinal plants in Latin America: a personal view. J. Ethnopharmacol. 2005;100(1-2):131–134. doi: 10.1016/j.jep.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 32.Newman D.J., Cragg G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012;75(3):311–335. doi: 10.1021/np200906s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Basmadjian C., Zhao Q., Bentouhami E., Djehal A., Nebigil C.G., Johnson R.A., Serova M., de Gramont A., Faivre S., Raymond E., Desaubry L.G. Cancer wars: natural products strike back. Front Chem. 2014;2:20. doi: 10.3389/fchem.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thomas C.J., Rahier N.J., Hecht S.M. Camptothecin: current perspectives. Bioorg. Med. Chem. 2004;12(7):1585–1604. doi: 10.1016/j.bmc.2003.11.036. [DOI] [PubMed] [Google Scholar]
- 35.Dias D.A., Urban S., Roessner U. A historical overview of natural products in drug discovery. Metabolites. 2012;2(2):303–336. doi: 10.3390/metabo2020303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruttala H.B., Ko Y.T. Liposome encapsulated albumin-paclitaxel nanoparticle for enhanced antitumor efficacy. Pharm. Res. 2015;32(3):1002–1016. doi: 10.1007/s11095-014-1512-2. [DOI] [PubMed] [Google Scholar]
- 37.Borst P. Cancer drug pan-resistance: pumps, cancer stem cells, quiescence, epithelial to mesenchymal transition, blocked cell death pathways, persisters or what? Open Biol. 2012;2(5):120066. doi: 10.1098/rsob.120066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Efthymiou A.G., Chen G., Rao M., Boehm M. Self-renewal and cell lineage differentiation strategies in human embryonic stem cells and induced pluripotent stem cells. Expert Opin. Biol. Ther. 2014;14(9):1333–1344. doi: 10.1517/14712598.2014.922533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoo Y.D., Kwon Y.T. Molecular mechanisms controlling asymmetric and symmetric self-renewal of cancer stem cells. J. Anal. Sci. Technol. 2015;6(1):28. doi: 10.1186/s40543-015-0071-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moitra K., Lou H., Dean M. Multidrug efflux pumps and cancer stem cells: insights into multidrug resistance and therapeutic development. Clin. Pharmacol. Ther. 2011;89(4):491–502. doi: 10.1038/clpt.2011.14. [DOI] [PubMed] [Google Scholar]
- 41.Heo H.R., Chen L., An B., Kim K.S., Ji J., Hong S.H. Hormonal regulation of hematopoietic stem cells and their niche: a focus on estrogen. Int. J. Stem Cells. 2015;8(1):18–23. doi: 10.15283/ijsc.2015.8.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Scharenberg C.W., Harkey M.A., Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99(2):507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 43.Dean M. ABC transporters, drug resistance, and cancer stem cells. J. Mammary Gland Biol. Neoplasia. 2009;14(1):3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 44.Gottesman M.M., Fojo T., Bates S.E. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat. Rev. Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 45.Fletcher J.I., Haber M., Henderson M.J., Norris M.D. ABC transporters in cancer: more than just drug efflux pumps. Nat. Rev. Cancer. 2010;10(2):147–156. doi: 10.1038/nrc2789. [DOI] [PubMed] [Google Scholar]
- 46.Goodell M.A., Brose K., Paradis G., Conner A.S., Mulligan R.C. Isolation and functional properties of murine hematopoietic stem cells that are replicating in vivo. J. Exp. Med. 1996;183(4):1797–1806. doi: 10.1084/jem.183.4.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Petriz J. 2013. [Google Scholar]
- 48.Kaminski W.E., Piehler A., Wenzel J.J. ABC A-subfamily transporters: structure, function and disease. Biochim. Biophys. Acta. 2006;1762(5):510–524. doi: 10.1016/j.bbadis.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 49.Gatti L., Beretta G.L., Cossa G., Zunino F., Perego P. ABC transporters as potential targets for modulation of drug resistance. Mini Rev. Med. Chem. 2009;9(9):1102–1112. doi: 10.2174/138955709788922656. [DOI] [PubMed] [Google Scholar]
- 50.Dean M., Rzhetsky A., Allikmets R. The human ATP-binding cassette (ABC) transporter superfamily. Genome Res. 2001;11(7):1156–1166. doi: 10.1101/gr.184901. [DOI] [PubMed] [Google Scholar]
- 51.Angelastro J.M., Lame M.W. Overexpression of CD133 promotes drug resistance in C6 glioma cells. Mol. Cancer Res. 2010;8(8):1105–1115. doi: 10.1158/1541-7786.MCR-09-0383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gong W., Wang Z., Wan Y., Shi L., Zhou Y. Downregulation of ABCG2 protein inhibits migration and invasion in U251 glioma stem cells. Neuroreport. 2014;25(8):625–632. doi: 10.1097/WNR.0000000000000161. [DOI] [PubMed] [Google Scholar]
- 53.Leccia F., Del Vecchio L., Mariotti E., Di Noto R., Morel A.P., Puisieux A., Salvatore F., Ansieau S. ABCG2, a novel antigen to sort luminal progenitors of BRCA1- breast cancer cells. Mol. Cancer. 2014;13:213. doi: 10.1186/1476-4598-13-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grimm M., Krimmel M., Polligkeit J., Alexander D., Munz A., Kluba S., Keutel C., Hoffmann J., Reinert S., Hoefert S. ABCB5 expression and cancer stem cell hypothesis in oral squamous cell carcinoma. Eur. J. Cancer. 2012;48(17):3186–3197. doi: 10.1016/j.ejca.2012.05.027. [DOI] [PubMed] [Google Scholar]
- 55.Hung T.H., Hsu S.C., Cheng C.Y., Choo K.B., Tseng C.P., Chen T.C., Lan Y.W., Huang T.T., Lai H.C., Chen C.M., Chong K.Y. Wnt5A regulates ABCB1 expression in multidrug-resistant cancer cells through activation of the non-canonical PKA/beta-catenin pathway. Oncotarget. 2014;5(23):12273–12290. doi: 10.18632/oncotarget.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hosokawa Y., Takahashi H., Inoue A., Kawabe Y., Funahashi Y., Kameda K., Sugimoto K., Yano H., Harada H., Kohno S., Ohue S., Ohnishi T., Tanaka J. Oct-3/4 modulates the drug-resistant phenotype of glioblastoma cells through expression of ATP binding cassette transporter G2. Biochim. Biophys. Acta. 2015;1850(6):1197–1205. doi: 10.1016/j.bbagen.2015.01.017. [DOI] [PubMed] [Google Scholar]
- 57.Tan B., Piwnica-Worms D., Ratner L. Multidrug resistance transporters and modulation. Curr. Opin. Oncol. 2000;12(5):450–458. doi: 10.1097/00001622-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 58.Shukla S., Ohnuma S., Ambudkar S.V. Improving cancer chemotherapy with modulators of ABC drug transporters. Curr. Drug Targets. 2011;12(5):621–630. doi: 10.2174/138945011795378540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kathawala R.J., Gupta P., Ashby C.R. Jr., Chen, Z.S. The modulation of ABC transporter-mediated multidrug resistance in cancer: a review of the past decade. Drug Resist. Updat. 2015;18:1–17. doi: 10.1016/j.drup.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 60.Wu C.P., Calcagno A.M., Ambudkar S.V. Reversal of ABC drug transporter-mediated multidrug resistance in cancer cells: evaluation of current strategies. Curr. Mol. Pharmacol. 2008;1(2):93–105. doi: 10.2174/1874467210801020093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cort A., Ozben T. Natural product modulators to overcome multidrug resistance in cancer. Nutr. Cancer. 2015;67(3):411–423. doi: 10.1080/01635581.2015.1002624. [DOI] [PubMed] [Google Scholar]
- 62.Tran V.H., Marks D., Duke R.K., Bebawy M., Duke C.C., Roufogalis B.D. Modulation of P-glycoprotein-mediated anticancer drug accumulation, cytotoxicity, and ATPase activity by flavonoid interactions. Nutr. Cancer. 2011;63(3):435–443. doi: 10.1080/01635581.2011.535959. [DOI] [PubMed] [Google Scholar]
- 63.Bansal T., Jaggi M., Khar R.K., Talegaonkar S. Emerging significance of flavonoids as P-glycoprotein inhibitors in cancer chemotherapy. J. Pharm. Pharm. Sci. 2009;12(1):46–78. doi: 10.18433/j3rc77. [DOI] [PubMed] [Google Scholar]
- 64.de Pace R.C., Liu X., Sun M., Nie S., Zhang J., Cai Q., Gao W., Pan X., Fan Z., Wang S. Anticancer activities of (-)-epigallocatechin-3-gallate encapsulated nanoliposomes in MCF7 breast cancer cells. J. Liposome Res. 2013;23(3):187–196. doi: 10.3109/08982104.2013.788023. [DOI] [PubMed] [Google Scholar]
- 65.Jiang L., Tao C., He A., He X. Overexpression of miR-126 sensitizes osteosarcoma cells to apoptosis induced by epigallocatechin-3-gallate. World J. Surg. Oncol. 2014;12:383. doi: 10.1186/1477-7819-12-383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen X., Zhang Y., Feng Y., Zhang L., Li J., Xie Y.A., Luo X. Epigallocatechin-3-gallate inhibits cell growth, induces apoptosis and causes S phase arrest in hepatocellular carcinoma by suppressing the AKT pathway. Int. J. Oncol. 2014;44(3):791–796. doi: 10.3892/ijo.2014.2251. [DOI] [PubMed] [Google Scholar]
- 67.Singh B.N., Shankar S., Srivastava R.K. Green tea catechin, epigallocatechin-3-gallate (EGCG): mechanisms, perspectives and clinical applications. Biochem. Pharmacol. 2011;82(12):1807–1821. doi: 10.1016/j.bcp.2011.07.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., Wang S.X., Ma J.W., Li H.Y., Ye J.C., Xie S.M., Du B., Zhong X.Y. EGCG inhibits properties of glioma stem-like cells and synergizes with temozolomide through downregulation of P-glycoprotein inhibition. J. Neurooncol. 2015;121(1):41–52. doi: 10.1007/s11060-014-1604-1. [DOI] [PubMed] [Google Scholar]
- 69.Farabegoli F., Papi A., Bartolini G., Ostan R., Orlandi M. (-)-Epigallocatechin-3-gallate downregulates Pg-P and BCRP in a tamoxifen resistant MCF-7 cell line. Phytomedicine. 2010;17(5):356–362. doi: 10.1016/j.phymed.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 70.Qian F., Wei D., Zhang Q., Yang S. Modulation of P-glycoprotein function and reversal of multidrug resistance by (-)-epigallocatechin gallate in human cancer cells. Biomed. Pharmacother. 2005;59(3):64–69. doi: 10.1016/j.biopha.2005.01.002. [DOI] [PubMed] [Google Scholar]
- 71.Sreejayan Rao M.N. Nitric oxide scavenging by curcuminoids. J. Pharm. Pharmacol. 1997;49(1):105–107. doi: 10.1111/j.2042-7158.1997.tb06761.x. [DOI] [PubMed] [Google Scholar]
- 72.Nagabhushan M., Amonkar A.J., Bhide S.V. In vitro antimutagenicity of curcumin against environmental mutagens. Food Chem. Toxicol. 1987;25(7):545–547. doi: 10.1016/0278-6915(87)90207-9. [DOI] [PubMed] [Google Scholar]
- 73.Jurenka J.S. Anti-inflammatory properties of curcumin, a major constituent of Curcuma longa: a review of preclinical and clinical research. Altern. Med. Rev. 2009;14(2):141–153. [PubMed] [Google Scholar]
- 74.Jiang M., Huang O., Zhang X., Xie Z., Shen A., Liu H., Geng M., Shen K. Curcumin induces cell death and restores tamoxifen sensitivity in the antiestrogen-resistant breast cancer cell lines MCF-7/LCC2 and MCF-7/LCC9. Molecules. 2013;18(1):701–720. doi: 10.3390/molecules18010701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Duarte V.M., Han E., Veena M.S., Salvado A., Suh J.D., Liang L.J., Faull K.F., Srivatsan E.S., Wang M.B. Curcumin enhances the effect of cisplatin in suppression of head and neck squamous cell carcinoma via inhibition of IKKbeta protein of the NF-kappaB pathway. Mol. Cancer Ther. 2010;9(10):2665–2675. doi: 10.1158/1535-7163.MCT-10-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sadzuka Y., Nagamine M., Toyooka T., Ibuki Y., Sonobe T. Beneficial effects of curcumin on antitumor activity and adverse reactions of doxorubicin. Int. J. Pharm. 2012;432(1-2):42–49. doi: 10.1016/j.ijpharm.2012.04.062. [DOI] [PubMed] [Google Scholar]
- 77.Everett P.C., Meyers J.A., Makkinje A., Rabbi M., Lerner A. Preclinical assessment of curcumin as a potential therapy for B-CLL. Am. J. Hematol. 2007;82(1):23–30. doi: 10.1002/ajh.20757. [DOI] [PubMed] [Google Scholar]
- 78.Chearwae W., Anuchapreeda S., Nandigama K., Ambudkar S.V., Limtrakul P. Biochemical mechanism of modulation of human P-glycoprotein (ABCB1) by curcumin I, II, and III purified from Turmeric powder. Biochem. Pharmacol. 2004;68(10):2043–2052. doi: 10.1016/j.bcp.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 79.Chearwae W., Shukla S., Limtrakul P., Ambudkar S.V. Modulation of the function of the multidrug resistance-linked ATP-binding cassette transporter ABCG2 by the cancer chemopreventive agent curcumin. Mol. Cancer Ther. 2006;5(8):1995–2006. doi: 10.1158/1535-7163.MCT-06-0087. [DOI] [PubMed] [Google Scholar]
- 80.Choi B.H., Kim C.G., Lim Y., Shin S.Y., Lee Y.H. Curcumin down-regulates the multidrug-resistance mdr1b gene by inhibiting the PI3K/Akt/NF kappa B pathway. Cancer Lett. 2008;259(1):111–118. doi: 10.1016/j.canlet.2007.10.003. [DOI] [PubMed] [Google Scholar]
- 81.Patil R.H., Babu R.L., Naveen Kumar M., Kiran Kumar K.M., Hegde S.M., Nagesh R., Ramesh G.T., Sharma S.C. Anti-Inflammatory Effect of Apigenin on LPS-Induced Pro-Inflammatory Mediators and AP-1 Factors in Human Lung Epithelial Cells. Inflammation. 2015 doi: 10.1007/s10753-015-0232-z. In Press. [DOI] [PubMed] [Google Scholar]
- 82.Patil R.H., Babu R.L., Naveen Kumar M., Kiran Kumar K.M., Hegde S.M., Ramesh G.T., Chidananda Sharma S. Apigenin inhibits PMA-induced expression of pro-inflammatory cytokines and AP-1 factors in A549 cells. Mol. Cell. Biochem. 2015;403(1-2):95–106. doi: 10.1007/s11010-015-2340-3. [DOI] [PubMed] [Google Scholar]
- 83.Romanova D., Vachalkova A., Cipak L., Ovesna Z., Rauko P. Study of antioxidant effect of apigenin, luteolin and quercetin by DNA protective method. Neoplasma. 2001;48(2):104–107. [PubMed] [Google Scholar]
- 84.Patel D., Shukla S., Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise. Int. J. Oncol. 2007;30(1):233–245. [review]. [PubMed] [Google Scholar]
- 85.Hashemi M., Long N. M.; Entezari, M.; Nafisi, S.; Nowroozii, H. Anti-mutagenic and pro-apoptotic effects of apigenin on human chronic lymphocytic leukemia cells. Acta Med. Iran. 2010;48(5):283–288. [PubMed] [Google Scholar]
- 86.Saeed M., Kadioglu O., Khalid H., Sugimoto Y., Efferth T. Activity of the dietary flavonoid, apigenin, against multidrug-resistant tumor cells as determined by pharmacogenomics and molecular docking. J. Nutr. Biochem. 2015;26(1):44–56. doi: 10.1016/j.jnutbio.2014.09.008. [DOI] [PubMed] [Google Scholar]
- 87.Pang B., Zhao L.H., Zhou Q., Zhao T.Y., Wang H., Gu C.J., Tong X.L. Application of berberine on treating type 2 diabetes mellitus. 2015. [DOI] [PMC free article] [PubMed]
- 88.Ahmed T., Gilani A.U., Abdollahi M., Daglia M., Nabavi S.F., Nabavi S.M. Berberine and neurodegeneration: A review of literature. Pharmacol. Rep. 2015;67(5):970–979. doi: 10.1016/j.pharep.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 89.Kumar A. Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015;761:288–297. doi: 10.1016/j.ejphar.2015.05.068. [DOI] [PubMed] [Google Scholar]
- 90.Lu B., Hu M., Liu K., Peng J. Cytotoxicity of berberine on human cervical carcinoma HeLa cells through mitochondria, death receptor and MAPK pathways, and in-silico drug-target prediction. Toxicol. In Vitro. 2010;24(6):1482–1490. doi: 10.1016/j.tiv.2010.07.017. [DOI] [PubMed] [Google Scholar]
- 91.Park S.H., Sung J.H., Chung N. Berberine diminishes side population and down-regulates stem cell-associated genes in the pancreatic cancer cell lines PANC-1 and MIA PaCa-2. Mol. Cell. Biochem. 2014;394(1-2):209–215. doi: 10.1007/s11010-014-2096-1. [DOI] [PubMed] [Google Scholar]
- 92.Ma X., Zhou J., Zhang C.X., Li X.Y., Li N., Ju R.J., Shi J.F., Sun M.G., Zhao W.Y., Mu L.M., Yan Y., Lu W.L. Modulation of drug-resistant membrane and apoptosis proteins of breast cancer stem cells by targeting berberine liposomes. Biomaterials. 2013;34(18):4452–4465. doi: 10.1016/j.biomaterials.2013.02.066. [DOI] [PubMed] [Google Scholar]
- 93.Shim S.H. 20S proteasome inhibitory activity of flavonoids isolated from Spatholobus suberectus. Phytother. Res. 2011;25(4):615–618. doi: 10.1002/ptr.3342. [DOI] [PubMed] [Google Scholar]
- 94.Jung S.K., Lee M.H. Lim do, Y.; Kim, J.E.; Singh, P.; Lee, S.Y.; Jeong, C.H.; Lim, T.G.; Chen, H.; Chi, Y.I.; Kundu, J.K.; Lee, N.H.; Lee, C.C.; Cho, Y.Y.; Bode, A.M.; Lee, K.W.; Dong, Z. Isoliquiritigenin induces apoptosis and inhibits xenograft tumor growth of human lung cancer cells by targeting both wild type and L858R/T790M mutant EGFR. J. Biol. Chem. 2014;289(52):35839–35848. doi: 10.1074/jbc.M114.585513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fessler E., Dijkgraaf F.E., De Sousa E., Melo F., Medema J.P. Cancer stem cell dynamics in tumor progression and metastasis: Is the microenvironment to blame? Cancer Lett. 2013;341(1):97–104. doi: 10.1016/j.canlet.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 96.Zheng H., Li Y., Wang Y., Zhao H., Zhang J., Chai H., Tang T., Yue J., Guo A.M., Yang J. Downregulation of COX-2 and CYP 4A signaling by isoliquiritigenin inhibits human breast cancer metastasis through preventing anoikis resistance, migration and invasion. Toxicol. Appl. Pharmacol. 2014;280(1):10–20. doi: 10.1016/j.taap.2014.07.018. [DOI] [PubMed] [Google Scholar]
- 97.Khramtsov A.I., Khramtsova G.F., Tretiakova M., Huo D., Olopade O.I., Goss K.H. Wnt/beta-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 2010;176(6):2911–2920. doi: 10.2353/ajpath.2010.091125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang J., Xiao L.D., He G.P., Ullah S., De Bellis A. Factors contributing to caregiver burden in dementia in a country without formal caregiver support. Aging Ment. Health. 2014;18(8):986–996. doi: 10.1080/13607863.2014.899976. [DOI] [PubMed] [Google Scholar]
- 99.Zeino M., Paulsen M.S., Zehl M., Urban E., Kopp B., Efferth T. Identification of new P-glycoprotein inhibitors derived from cardiotonic steroids. Biochem. Pharmacol. 2015;93(1):11–24. doi: 10.1016/j.bcp.2014.10.009. [DOI] [PubMed] [Google Scholar]
- 100.Callies O., Sanchez-Canete M.P., Gamarro F., Jimenez I.A., Castanys S., Bazzocchi I.L. Restoration of Chemosensitivity in P-Glycoprotein-Dependent Multidrug-Resistant Cells by Dihydro-beta-agarofuran Sesquiterpenes from Celastrus vulcanicola. J. Nat. Prod. 2015;78(4):736–745. doi: 10.1021/np500903a. [DOI] [PubMed] [Google Scholar]
- 101.Su S., Cheng X., Wink M. Natural lignans from Arctium lappa modulate P-glycoprotein efflux function in multidrug resistant cancer cells. Phytomedicine. 2015;22(2):301–307. doi: 10.1016/j.phymed.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 102.Gu Y.Y., Liu L.P., Qin J., Zhang M., Chen Y., Wang D., Li Z., Tang J.Z., Mo S.L. Baicalein decreases side population proportion via inhibition of ABCG2 in multiple myeloma cell line RPMI 8226 in vitro. Fitoterapia. 2014;94:21–28. doi: 10.1016/j.fitote.2014.01.019. [DOI] [PubMed] [Google Scholar]
- 103.Maliyakkal N., Appadath Beeran A., Balaji S.A., Udupa N., Ranganath Pai S., Rangarajan A. Effects of Withania somnifera and Tinospora cordifolia extracts on the side population phenotype of human epithelial cancer cells: toward targeting multidrug resistance in cancer. Integr. Cancer Ther. 2015;14(2):156–171. doi: 10.1177/1534735414564423. [DOI] [PubMed] [Google Scholar]
- 104.Kahn M. Can we safely target the WNT pathway? Nat. Rev. Drug Discov. 2014;13(7):513–532. doi: 10.1038/nrd4233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13(12):767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 106.Holland J.D., Klaus A., Garratt A.N., Birchmeier W. Wnt signaling in stem and cancer stem cells. Curr. Opin. Cell Biol. 2013;25(2):254–264. doi: 10.1016/j.ceb.2013.01.004. [DOI] [PubMed] [Google Scholar]
- 107.Willert K., Brown J.D., Danenberg E., Duncan A.W., Weissman I.L., Reya T., Yates J.R. 3rd, Nusse, R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423(6938):448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- 108.Banziger C., Soldini D., Schutt C., Zipperlen P., Hausmann G., Basler K. Wntless, a conserved membrane protein dedicated to the secretion of Wnt proteins from signaling cells. Cell. 2006;125(3):509–522. doi: 10.1016/j.cell.2006.02.049. [DOI] [PubMed] [Google Scholar]
- 109.Hofmann K. A superfamily of membrane-bound O-acyltransferases with implications for wnt signaling. Trends Biochem. Sci. 2000;25(3):111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 110.Liu C., Li Y., Semenov M., Han C., Baeg G.H., Tan Y., Zhang Z., Lin X., He X. Control of beta-catenin phosphorylation/degradation by a dual-kinase mechanism. Cell. 2002;108(6):837–847. doi: 10.1016/s0092-8674(02)00685-2. [DOI] [PubMed] [Google Scholar]
- 111.Clevers H. Wnt/beta-catenin signaling in development and disease. Cell. 2006;127(3):469–480. doi: 10.1016/j.cell.2006.10.018. [DOI] [PubMed] [Google Scholar]
- 112.Clevers H., Nusse R. Wnt/beta-catenin signaling and disease. Cell. 2012;149(6):1192–1205. doi: 10.1016/j.cell.2012.05.012. [DOI] [PubMed] [Google Scholar]
- 113.Gao C., Chen Y.G. Dishevelled: The hub of Wnt signaling. Cell. Signal. 2010;22(5):717–727. doi: 10.1016/j.cellsig.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 114.Wend P., Holland J.D., Ziebold U., Birchmeier W. Wnt signaling in stem and cancer stem cells. Semin. Cell Dev. Biol. 2010;21(8):855–863. doi: 10.1016/j.semcdb.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 115.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: components, mechanisms, and diseases. Dev. Cell. 2009;17(1):9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Polakis P. Drugging Wnt signalling in cancer. EMBO J. 2012;31(12):2737–2746. doi: 10.1038/emboj.2012.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scheel S.K., Porzner M., Pfeiffer S., Ormanns S., Kirchner T., Jung A. Mutations in the WTX-gene are found in some high-grade microsatellite instable (MSI-H) colorectal cancers. BMC Cancer. 2010;10:413. doi: 10.1186/1471-2407-10-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Major M.B., Camp N.D., Berndt J.D., Yi X., Goldenberg S.J., Hubbert C., Biechele T.L., Gingras A.C., Zheng N., Maccoss M.J., Angers S., Moon R.T. Wilms tumor suppressor WTX negatively regulates WNT/beta-catenin signaling. Science. 2007;316(5827):1043–1046. doi: 10.1126/science/1141515. [DOI] [PubMed] [Google Scholar]
- 119.Moyes K.W., Sip C.G., Obenza W., Yang E., Horst C., Welikson R.E., Hauschka S.D., Folch A., Laflamme M.A. Human embryonic stem cell-derived cardiomyocytes migrate in response to gradients of fibronectin and Wnt5a. Stem Cells Dev. 2013;22(16):2315–2325. doi: 10.1089/scd.2012.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qin L., Yin Y.T., Zheng F.J., Peng L.X., Yang C.F., Bao Y.N., Liang Y.Y., Li X.J., Xiang Y.Q., Sun R., Li A.H., Zou R.H., Pei X.Q., Huang B.J., Kang T.B., Liao D.F., Zeng Y.X., Williams B.O., Qian C.N. WNT5A promotes stemness characteristics in nasopharyngeal carcinoma cells leading to metastasis and tumorigenesis. Oncotarget. 2015;6(12):10239–10252. doi: 10.18632/oncotarget.3518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhang Y., Zhang X., Huang J., Dong Q. Wnt signaling regulation of stem-like properties in human lung adenocarcinoma cell lines. Med. Oncol. 2015;32(5):157. doi: 10.1007/s12032-015-0596-9. [DOI] [PubMed] [Google Scholar]
- 122.Mao J., Fan S., Ma W., Fan P., Wang B., Zhang J., Wang H., Tang B., Zhang Q., Yu X., Wang L., Song B., Li L. Roles of Wnt/beta-catenin signaling in the gastric cancer stem cells proliferation and salinomycin treatment. Cell Death Dis. 2014;5:e1039. doi: 10.1038/cddis.2013.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ashihara E., Takada T., Maekawa T. Targeting the canonical Wnt/beta-catenin pathway in hematological malignancies. Cancer Sci. 2015;106(6):665–671. doi: 10.1111/cas.12655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mahady G.B., Pendland S.L., Yun G.S., Lu Z.Z., Stoia A. Ginger (Zingiber officinale Roscoe) and the gingerols inhibit the growth of Cag A+ strains of Helicobacter pylori. Anticancer Res. 2003;23(5A):3699–3702. [PMC free article] [PubMed] [Google Scholar]
- 125.Moon M., Kim H.G., Choi J.G., Oh H., Lee P.K., Ha S.K., Kim S.Y., Park Y., Huh Y., Oh M.S. 6-Shogaol, an active constituent of ginger, attenuates neuroinflammation and cognitive deficits in animal models of dementia. Biochem. Biophys. Res. Commun. 2014;449(1):8–13. doi: 10.1016/j.bbrc.2014.04.121. [DOI] [PubMed] [Google Scholar]
- 126.Shigeishi H., Biddle A., Gammon L., Emich H., Rodini C.O., Gemenetzidis E., Fazil B., Sugiyama M., Kamata N., Mackenzie I.C. Maintenance of stem cell self-renewal in head and neck cancers requires actions of GSK3beta influenced by CD44 and RHAMM. Stem Cells. 2013;31(10):2073–2083. doi: 10.1002/stem.1418. [DOI] [PubMed] [Google Scholar]
- 127.Wu C.H., Hong B.H., Ho C.T., Yen G.C. Targeting cancer stem cells in breast cancer: potential anticancer properties of 6-shogaol and pterostilbene. J. Agric. Food Chem. 2015;63(9):2432–2441. doi: 10.1021/acs.jafc.5b00002. [DOI] [PubMed] [Google Scholar]
- 128.Khoo B.Y., Chua S.L., Balaram P. Apoptotic effects of chrysin in human cancer cell lines. Int. J. Mol. Sci. 2010;11(5):2188–2199. doi: 10.3390/ijms11052188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Yang X.H., Zheng X., Cao J.G., Xiang H.L., Liu F., Lv Y. 8-Bromo-7-methoxychrysin-induced apoptosis of hepatocellular carcinoma cells involves ROS and JNK. World J. Gastroenterol. 2010;16(27):3385–3393. doi: 10.3748/wjg.v16.i27.3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Quan M.F., Xiao L.H., Liu Z.H., Guo H., Ren K.Q., Liu F., Cao J.G., Deng X.Y. 8-bromo-7-methoxychrysin inhibits properties of liver cancer stem cells via downregulation of beta-catenin. World J. Gastroenterol. 2013;19(43):7680–7695. doi: 10.3748/wjg.v19.i43.7680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Shtutman M., Zhurinsky J., Simcha I., Albanese C., D'Amico M., Pestell R., Ben-Ze'ev A. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc. Natl. Acad. Sci. USA. 1999;96(10):5522–5527. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.He G., Cao X., He M., Sheng X., Wu Y., Ai X. Casticin inhibits self-renewal of liver cancer stem cells from the MHCC97 cell line. Oncol. Lett. 2014;7(6):2023–2028. doi: 10.3892/ol.2014.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.He M., Cao X.C., He G.C., Sheng X.F., Ai X.H., Wu Y.H. Casticin inhibits epithelial-mesenchymal transition of liver cancer stem cells of the SMMC-7721 cell line through downregulating Twist. Oncol. Lett. 2014;7(5):1625–1631. doi: 10.3892/ol.2014.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Das S., Das D.K. Resveratrol: a therapeutic promise for cardiovascular diseases. Recent Patents Cardiovasc. Drug Discov. 2007;2(2):133–138. doi: 10.2174/157489007780832560. [DOI] [PubMed] [Google Scholar]
- 135.Lekli I., Ray D., Das D.K. Longevity nutrients resveratrol, wines and grapes. Genes Nutr. 2010;5(1):55–60. doi: 10.1007/s12263-009-0145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Athar M., Back J.H., Tang X., Kim K.H., Kopelovich L., Bickers D.R., Kim A.L. Resveratrol: a review of preclinical studies for human cancer prevention. Toxicol. Appl. Pharmacol. 2007;224(3):274–283. doi: 10.1016/j.taap.2006.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chan M.M. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem. Pharmacol. 2002;63(2):99–104. doi: 10.1016/s0006-2952(01)00886-3. [DOI] [PubMed] [Google Scholar]
- 138.Shankar S., Nall D., Tang S.N., Meeker D., Passarini J., Sharma J., Srivastava R.K. Resveratrol inhibits pancreatic cancer stem cell characteristics in human and KrasG12D transgenic mice by inhibiting pluripotency maintaining factors and epithelial-mesenchymal transition. PLoS One. 2011;6(1):e16530. doi: 10.1371/journal.pone.0016530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fu Y., Chang H., Peng X., Bai Q., Yi L., Zhou Y., Zhu J., Mi M. Resveratrol inhibits breast cancer stem-like cells and induces autophagy via suppressing Wnt/beta-catenin signaling pathway. PLoS One. 2014;9(7):e102535. doi: 10.1371/journal.pone.0102535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsai J.H., Hsu L.S., Lin C.L., Hong H.M., Pan M.H., Way T.D., Chen W.J. 3,5,4'-Trimethoxystilbene, a natural methoxylated analog of resveratrol, inhibits breast cancer cell invasiveness by downregulation of PI3K/Akt and Wnt/beta-catenin signaling cascades and reversal of epithelial-mesenchymal transition. Toxicol. Appl. Pharmacol. 2013;272(3):746–756. doi: 10.1016/j.taap.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 141.Boddupalli S., Mein J.R., Lakkanna S., James D.R. Induction of phase 2 antioxidant enzymes by broccoli sulforaphane: perspectives in maintaining the antioxidant activity of vitamins A, C, and E. Front. Genet. 2012;3:7. doi: 10.3389/fgene.2012.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Abe N., Hou D.X., Munemasa S., Murata Y., Nakamura Y. Nuclear factor-kappaB sensitizes to benzyl isothiocyanate-induced antiproliferation in p53-deficient colorectal cancer cells. 2014;3 doi: 10.1038/cddis.2014.495. e1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Lin L.C., Yeh C.T., Kuo C.C., Lee C.M., Yen G.C., Wang L.S., Wu C.H., Yang W.C., Wu A.T. Sulforaphane potentiates the efficacy of imatinib against chronic leukemia cancer stem cells through enhanced abrogation of Wnt/beta-catenin function. J. Agric. Food Chem. 2012;60(28):7031–7039. doi: 10.1021/jf301981n. [DOI] [PubMed] [Google Scholar]
- 144.Wilken R., Veena M.S., Wang M.B., Srivatsan E.S. Curcumin: A review of anti-cancer properties and therapeutic activity in head and neck squamous cell carcinoma. Mol. Cancer. 2011;10:12. doi: 10.1186/1476-4598-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Kakarala M., Brenner D.E., Korkaya H., Cheng C., Tazi K., Ginestier C., Liu S., Dontu G., Wicha M.S. Targeting breast stem cells with the cancer preventive compounds curcumin and piperine. Breast Cancer Res. Treat. 2010;122(3):777–785. doi: 10.1007/s10549-009-0612-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Leow P.C., Tian Q., Ong Z.Y., Yang Z., Ee P.L. Antitumor activity of natural compounds, curcumin and PKF118-310, as Wnt/beta-catenin antagonists against human osteosarcoma cells. Invest. New Drugs. 2010;28(6):766–782. doi: 10.1007/s10637-009-9311-z. [DOI] [PubMed] [Google Scholar]
- 147.Xu M.X., Zhao L., Deng C., Yang L., Wang Y., Guo T., Li L., Lin J., Zhang L. Curcumin suppresses proliferation and induces apoptosis of human hepatocellular carcinoma cells via the wnt signaling pathway. Int. J. Oncol. 2013;43(6):1951–1959. doi: 10.3892/ijo.2013.2107. [DOI] [PubMed] [Google Scholar]
- 148.Rosner M.H., Vigano M.A., Ozato K., Timmons P.M., Poirier F., Rigby P.W., Staudt L.M. A POU-domain transcription factor in early stem cells and germ cells of the mammalian embryo. Nature. 1990;345(6277):686–692. doi: 10.1038/345686a0. [DOI] [PubMed] [Google Scholar]
- 149.Zhang Z.N., Chung S.K., Xu Z., Xu Y. Oct4 maintains the pluripotency of human embryonic stem cells by inactivating p53 through Sirt1-mediated deacetylation. Stem Cells. 2014;32(1):157–165. doi: 10.1002/stem.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yun J.H., Park Y.G., Lee K.M., Kim J., Nho C.W. Curcumin induces apoptotic cell death via Oct4 inhibition and GSK-3beta activation in NCCIT cells. Mol. Nutr. Food Res. 2015;59(6):1053–1062. doi: 10.1002/mnfr.201400739. [DOI] [PubMed] [Google Scholar]
- 151.Chesire D.R., Isaacs W.B. Beta-catenin signaling in prostate cancer: an early perspective. Endocr. Relat. Cancer. 2003;10(4):537–560. doi: 10.1677/erc.0.0100537. [DOI] [PubMed] [Google Scholar]
- 152.Verras M., Sun Z. Roles and regulation of Wnt signaling and beta-catenin in prostate cancer. Cancer Lett. 2006;237(1):22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 153.Kypta R.M., Waxman J. Wnt/beta-catenin signalling in prostate cancer. Nat. Rev. Urol. 2012;9(8):418–428. doi: 10.1038/nrurol.2012.116. [DOI] [PubMed] [Google Scholar]
- 154.Mahmoud A.M., Yang W., Bosland M.C. Soy isoflavones and prostate cancer: a review of molecular mechanisms. J. Steroid Biochem. Mol. Biol. 2014;140:116–132. doi: 10.1016/j.jsbmb.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.An S.M., Ding Q.P., Li L.S. Stem cell signaling as a target for novel drug discovery: recent progress in the WNT and Hedgehog pathways. Acta Pharmacol. Sin. 2013;34(6):777–783. doi: 10.1038/aps.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yao C.J., Lai G.M., Yeh C.T., Lai M.T., Shih P.H., Chao W.J., Whang-Peng J., Chuang S.E., Lai T.Y. Accompanied with Suppression of Wnt/ beta -Catenin Signaling and Apoptosis Induction. EvidBased Complement. Alternat. Med. 2013. 146136. [DOI] [PMC free article] [PubMed]
- 157.Liu J., Sato C., Cerletti M., Wagers A. Notch signaling in the regulation of stem cell self-renewal and differentiation. Curr. Top. Dev. Biol. 2010;92:367–409. doi: 10.1016/S0070-2153(10)92012-7. [DOI] [PubMed] [Google Scholar]
- 158.Schwanbeck R. The role of epigenetic mechanisms in Notch signaling during development. J. Cell. Physiol. 2015;230(5):969–981. doi: 10.1002/jcp.24851. [DOI] [PubMed] [Google Scholar]
- 159.Wang J., Sullenger B.A., Rich J.N. Notch signaling in cancer stem cells. Adv. Exp. Med. Biol. 2012;727:174–185. doi: 10.1007/978-1-4614-0899-4_13. [DOI] [PubMed] [Google Scholar]
- 160.Koch U., Radtke F. Notch and cancer: a double-edged sword. Cell. Mol. Life Sci. 2007;64(21):2746–2762. doi: 10.1007/s00018-007-7164-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Bolos V., Blanco M., Medina V., Aparicio G., Diaz-Prado S., Grande E. Notch signalling in cancer stem cells. Clin. Transl. Oncol. 2009;11(1):11–19. doi: 10.1007/s12094-009-0305-2. [DOI] [PubMed] [Google Scholar]
- 162.Wang H., Zang C., Liu X.S., Aster J.C. The role of Notch receptors in transcriptional regulation. J. Cell. Physiol. 2015;230(5):982–988. doi: 10.1002/jcp.24872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pursglove S.E., Mackay J.P. CSL: a notch above the rest. Int. J. Biochem. Cell Biol. 2005;37(12):2472–2477. doi: 10.1016/j.biocel.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 164.Espinoza I., Miele L. Notch inhibitors for cancer treatment. Pharmacol. Ther. 2013;139(2):95–110. doi: 10.1016/j.pharmthera.2013.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Andersson E.R., Lendahl U. Therapeutic modulation of Notch signalling--are we there yet? Nat. Rev. Drug Discov. 2014;13(5):357–378. doi: 10.1038/nrd4252. [DOI] [PubMed] [Google Scholar]
- 166.Gupta-Rossi N., Le Bail O., Gonen H., Brou C., Logeat F., Six E., Ciechanover A., Israel A. Functional interaction between SEL-10, an F-box protein, and the nuclear form of activated Notch1 receptor. J. Biol. Chem. 2001;276(37):34371–34378. doi: 10.1074/jbc.M101343200. [DOI] [PubMed] [Google Scholar]
- 167.Tosello V., Ferrando A.A. The NOTCH signaling pathway: role in the pathogenesis of T-cell acute lymphoblastic leukemia and implication for therapy. Ther. Adv. Hematol. 2013;4(3):199–210. doi: 10.1177/2040620712471368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hassan K.A., Wang L., Korkaya H., Chen G., Maillard I., Beer D.G., Kalemkerian G.P., Wicha M.S. Notch pathway activity identifies cells with cancer stem cell-like properties and correlates with worse survival in lung adenocarcinoma. Clin. Cancer Res. 2013;19(8):1972–1980. doi: 10.1158/1078-0432.CCR-12-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bao B., Wang Z., Ali S., Kong D., Li Y., Ahmad A., Banerjee S., Azmi A.S., Miele L., Sarkar F.H. Notch-1 induces epithelial-mesenchymal transition consistent with cancer stem cell phenotype in pancreatic cancer cells. Cancer Lett. 2011;307(1):26–36. doi: 10.1016/j.canlet.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 170.Humphrey P.A. Gleason grading and prognostic factors in carcinoma of the prostate. Mod. Pathol. 2004;17(3):292–306. doi: 10.1038/modpathol.3800054. [DOI] [PubMed] [Google Scholar]
- 171.Carvalho F.L., Simons B.W., Eberhart C.G., Berman D.M. Notch signaling in prostate cancer: a moving target. Prostate. 2014;74(9):933–945. doi: 10.1002/pros.22811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ling H., Sylvestre J.R., Jolicoeur P. Notch1-induced mammary tumor development is cyclin D1-dependent and correlates with expansion of pre-malignant multipotent duct-limited progenitors. Oncogene. 2010;29(32):4543–4554. doi: 10.1038/onc.2010.186. [DOI] [PubMed] [Google Scholar]
- 173.Ponnurangam S., Mammen J.M., Ramalingam S., He Z., Zhang Y., Umar S., Subramaniam D., Anant S. Honokiol in combination with radiation targets notch signaling to inhibit colon cancer stem cells. Mol. Cancer Ther. 2012;11(4):963–972. doi: 10.1158/1535-7163.MCT-11-0999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kaushik G., Venugopal A., Ramamoorthy P., Standing D., Subramaniam D., Umar S., Jensen R.A., Anant S., Mammen J.M. Honokiol inhibits melanoma stem cells by targeting notch signaling. Mol. Carcinog. 2015;54(12):1710–1721. doi: 10.1002/mc.22242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ma L., Mao R., Shen K., Zheng Y., Li Y., Liu J., Ni L. Atractylenolide I-mediated Notch pathway inhibition attenuates gastric cancer stem cell traits. Biochem. Biophys. Res. Commun. 2014;450(1):353–359. doi: 10.1016/j.bbrc.2014.05.110. [DOI] [PubMed] [Google Scholar]
- 176.Yan B., Liu L., Zhao Y., Xiu L.J., Sun D.Z., Liu X., Lu Y., Shi J., Zhang Y.C., Li Y.J., Wang X.W., Zhou Y.Q., Feng S.H., Lv C., Wei P.K., Qin Z.F. Xiaotan Sanjie decoction attenuates tumor angiogenesis by manipulating Notch-1-regulated proliferation of gastric cancer stem-like cells. World J. Gastroenterol. 2014;20(36):13105–13118. doi: 10.3748/wjg.v20.i36.13105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Ray A., Vasudevan S., Sengupta S. 6-Shogaol Inhibits Breast Cancer Cells and Stem Cell-Like Spheroids by Modulation of Notch Signaling Pathway and Induction of Autophagic Cell Death. PLoS One. 2015;10(9):e0137614. doi: 10.1371/journal.pone.0137614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lee S.H., Nam H.J., Kang H.J., Kwon H.W., Lim Y.C. Epigallocatechin-3-gallate attenuates head and neck cancer stem cell traits through suppression of Notch pathway. Eur. J. Cancer. 2013;49(15):3210–3218. doi: 10.1016/j.ejca.2013.06.025. [DOI] [PubMed] [Google Scholar]
- 179.McMahon A.P., Ingham P.W., Tabin C.J. Developmental roles and clinical significance of hedgehog signaling. Curr. Top. Dev. Biol. 2003;53:1–114. doi: 10.1016/s0070-2153(03)53002-2. [DOI] [PubMed] [Google Scholar]
- 180.Van Den Brink G.R., Peppelenbosch M.P. Expression of hedgehog pathway components in the adult colon. Gastroenterology. 2006;130(2):619. doi: 10.1053/j.gastro.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 181.Wicking C., McGlinn E. The role of hedgehog signalling in tumorigenesis. Cancer Lett. 2001;173(1):1–7. doi: 10.1016/s0304-3835(01)00676-0. [DOI] [PubMed] [Google Scholar]
- 182.Ehtesham M., Sarangi A., Valadez J.G., Chanthaphaychith S., Becher M.W., Abel T.W., Thompson R.C., Cooper M.K. Ligand-dependent activation of the hedgehog pathway in glioma progenitor cells. Oncogene. 2007;26(39):5752–5761. doi: 10.1038/sj.onc.1210359. [DOI] [PubMed] [Google Scholar]
- 183.Clement V., Sanchez P., de Tribolet N., Radovanovic I., Ruiz i Altaba A. Hedgehog-Gli1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 2007;17(2):165–172. doi: 10.1016/j.cub.2006.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Peacock C.D., Wang Q., Gesell G.S., Corcoran-Schwartz I.M., Jones E., Kim J., Devereux W.L., Rhodes J.T., Huff C.A., Beachy P.A., Watkins D.N., Matsui W. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc. Natl. Acad. Sci. USA. 2007;104(10):4048–4053. doi: 10.1073/pnas.0611682104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Dierks C., Beigi R., Guo G.R., Zirlik K., Stegert M.R., Manley P., Trussell C., Schmitt-Graeff A., Landwerlin K., Veelken H., Warmuth M. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell. 2008;14(3):238–249. doi: 10.1016/j.ccr.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 186.Varnat F., Duquet A., Malerba M., Zbinden M., Mas C., Gervaz P., Ruiz i Altaba A. Human colon cancer epithelial cells harbour active Hedgehog-Gli signalling that is essential for tumour growth, recurrence, metastasis and stem cell survival and expansion. EMBO Mol. Med. 2009;1(6-7):338–351. doi: 10.1002/emmm.200900039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Zhao C., Chen A., Jamieson C.H., Fereshteh M., Abrahamsson A., Blum J., Kwon H.Y., Kim J., Chute J.P., Rizzieri D., Munchhof M., VanArsdale T., Beachy P.A., Reya T. Hedgehog signalling is essential for maintenance of cancer stem cells in myeloid leukaemia. Nature. 2009;458(7239):776–779. doi: 10.1038/nature07737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Song Z., Yue W., Wei B., Wang N., Li T., Guan L., Shi S., Zeng Q., Pei X., Chen L. Sonic hedgehog pathway is essential for maintenance of cancer stem-like cells in human gastric cancer. PLoS One. 2011;6(3):e17687. doi: 10.1371/journal.pone.0017687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Takebe N., Harris P.J., Warren R.Q., Ivy S.P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 2011;8(2):97–106. doi: 10.1038/nrclinonc.2010.196. [DOI] [PubMed] [Google Scholar]
- 190.Takebe N., Warren R.Q., Ivy S.P. Breast cancer growth and metastasis: interplay between cancer stem cells, embryonic signaling pathways and epithelial-to-mesenchymal transition. Breast Cancer Res. 2011;13(3):211. doi: 10.1186/bcr2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Babashah S., Sadeghizadeh M., Hajifathali A., Tavirani M.R., Zomorod M.S., Ghadiani M., Soleimani M. Targeting of the signal transducer Smo links microRNA-326 to the oncogenic Hedgehog pathway in CD34+ CML stem/progenitor cells. Int. J. Cancer. 2013;133(3):579–589. doi: 10.1002/ijc.28043. [DOI] [PubMed] [Google Scholar]
- 192.Batsaikhan B.E., Yoshikawa K., Kurita N., Iwata T., Takasu C., Kashihara H., Shimada M. Cyclopamine decreased the expression of Sonic Hedgehog and its downstream genes in colon cancer stem cells. Anticancer Res. 2014;34(11):6339–6344. [PubMed] [Google Scholar]
- 193.Memmi E.M., Sanarico A.G., Giacobbe A., Peschiaroli A., Frezza V., Cicalese A., Pisati F., Tosoni D., Zhou H., Tonon G., Antonov A., Melino G., Pelicci P.G., Bernassola F. p63 Sustains self-renewal of mammary cancer stem cells through regulation of Sonic Hedgehog signaling. Proc. Natl. Acad. Sci. USA. 2015;112(1):3499–3504. doi: 10.1073/pnas.1500762112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Sadarangani A., Pineda G., Lennon K.M., Chun H.J., Shih A., Schairer A.E., Court A.C., Goff D.J., Prashad S.L., Geron I., Wall R., McPherson J.D., Moore R.A., Pu M., Bao L., Jackson-Fisher A., Munchhof M., VanArsdale T., Reya T., Morris S.R., Minden M.D., Messer K., Mikkola H.K., Marra M.A., Hudson T.J., Jamieson C.H. GLI2 inhibition abrogates human leukemia stem cell dormancy. J. Transl. Med. 2015;13:98. doi: 10.1186/s12967-015-0453-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Cochrane C.R., Szczepny A., Watkins D.N., Cain J.E. Hedgehog Signaling in the Maintenance of Cancer Stem Cells. Cancers (Basel) 2015;7(3):1554–1585. doi: 10.3390/cancers7030851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhao Y., Tong C., Jiang J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature. 2007;450(7167):252–258. doi: 10.1038/nature06225. [DOI] [PubMed] [Google Scholar]
- 197.Goetz S.C., Anderson K.V. The primary cilium: a signalling centre during vertebrate development. Nat. Rev. Genet. 2010;11(5):331–344. doi: 10.1038/nrg2774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Robbins D.J., Fei D.L., Riobo N.A. The Hedgehog signal transduction network. Sci. Signal. 2012;5(246):re6. doi: 10.1126/scisignal.2002906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Briscoe J., Therond P.P. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat. Rev. Mol. Cell Biol. 2013;14(7):416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 200.Po A., Ferretti E., Miele E., De Smaele E., Paganelli A., Canettieri G., Coni S., Di Marcotullio L., Biffoni M., Massimi L., Di Rocco C., Screpanti I., Gulino A. Hedgehog controls neural stem cells through p53-independent regulation of Nanog. EMBO J. 2010;29(15):2646–2658. doi: 10.1038/emboj.2010.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yao J., An Y., Wie J.S., Ji Z.L., Lu Z.P., Wu J.L., Jiang K.R., Chen P., Xu Z.K., Miao Y. Cyclopamine reverts acquired chemoresistance and down-regulates cancer stem cell markers in pancreatic cancer cell lines. Swiss Med. Wkly. 2011;141:w13208. doi: 10.4414/smw.2011.13208. [DOI] [PubMed] [Google Scholar]
- 202.Santini R., Pietrobono S., Pandolfi S., Montagnani V., D'Amico M., Penachioni J.Y., Vinci M.C., Borgognoni L., Stecca B. SOX2 regulates self-renewal and tumorigenicity of human melanoma-initiating cells. Oncogene. 2014;33(38):4697–4708. doi: 10.1038/onc.2014.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Yoon C. Park do, J.; Schmidt, B.; Thomas, N.J.; Lee, H.J.; Kim, T.S.; Janjigian, Y.Y.; Cohen, D.J.; Yoon, S.S. CD44 expression denotes a subpopulation of gastric cancer cells in which Hedgehog signaling promotes chemotherapy resistance. Clin. Cancer Res. 2014;20(15):3974–3988. doi: 10.1158/1078-0432.CCR-14-0011. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 204.Queiroz K.C., Ruela-de-Sousa R.R., Fuhler G.M., Aberson H.L., Ferreira C.V., Peppelenbosch M.P., Spek C.A. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene. 2010;29(48):6314–6322. doi: 10.1038/onc.2010.375. [DOI] [PubMed] [Google Scholar]
- 205.Takahashi T., Kawakami K., Mishima S., Akimoto M., Takenaga K., Suzumiya J., Honma Y. Cyclopamine induces eosinophilic differentiation and upregulates CD44 expression in myeloid leukemia cells. Leuk. Res. 2011;35(5):638–645. doi: 10.1016/j.leukres.2010.09.022. [DOI] [PubMed] [Google Scholar]
- 206.Lin T.L., Wang Q.H., Brown P., Peacock C., Merchant A.A., Brennan S., Jones E., McGovern K., Watkins D.N., Sakamoto K.M., Matsui W. Self-renewal of acute lymphocytic leukemia cells is limited by the Hedgehog pathway inhibitors cyclopamine and IPI-926. PLoS One. 2010;5(12):e15262. doi: 10.1371/journal.pone.0015262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Liu S., Dontu G., Mantle I.D., Patel S., Ahn N.S., Jackson K.W., Suri P., Wicha M.S. Hedgehog signaling and Bmi-1 regulate self-renewal of normal and malignant human mammary stem cells. Cancer Res. 2006;66(12):6063–6071. doi: 10.1158/0008-5472.CAN-06-0054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Bar E.E., Chaudhry A., Lin A., Fan X., Schreck K., Matsui W., Piccirillo S., Vescovi A.L., DiMeco F., Olivi A., Eberhart C.G. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 2007;25(10):2524–2533. doi: 10.1634/stemcells.2007-0166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Corcoran R.B., Scott M.P. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl. Acad. Sci. USA. 2006;103(22):8408–8413. doi: 10.1073/pnas.0602852103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Tang S.N., Fu J., Nall D., Rodova M., Shankar S., Srivastava R.K. Inhibition of sonic hedgehog pathway and pluripotency maintaining factors regulate human pancreatic cancer stem cell characteristics. Int. J. Cancer. 2012;131(1):30–40. doi: 10.1002/ijc.26323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Ramasamy T.S., Ayob A.Z., Myint H.H., Thiagarajah S., Amini F. Targeting colorectal cancer stem cells using curcumin and curcumin analogues: insights into the mechanism of the therapeutic efficacy. Cancer Cell Int. 2015;15:96. doi: 10.1186/s12935-015-0241-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Buhrmann C., Kraehe P., Lueders C., Shayan P., Goel A., Shakibaei M. Curcumin suppresses crosstalk between colon cancer stem cells and stromal fibroblasts in the tumor microenvironment: potential role of EMT. PLoS One. 2014;9(9):e107514. doi: 10.1371/journal.pone.0107514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhang L., Li L., Jiao M., Wu D., Wu K., Li X., Zhu G., Yang L., Wang X., Hsieh J.T., He D. Genistein inhibits the stemness properties of prostate cancer cells through targeting Hedgehog-Gli1 pathway. Cancer Lett. 2012;323(1):48–57. doi: 10.1016/j.canlet.2012.03.037. [DOI] [PubMed] [Google Scholar]
- 214.Wang X., Zhang N., Huo Q., Sun M., Dong L., Zhang Y., Xu G., Yang Q. Huaier aqueous extract inhibits stem-like characteristics of MCF7 breast cancer cells via inactivation of hedgehog pathway. Tumour Biol. 2014;35(11):10805–10813. doi: 10.1007/s13277-014-2390-2. [DOI] [PubMed] [Google Scholar]
- 215.Han J.B., Sang F., Chang J.J., Hua Y.Q., Shi W.D., Tang L.H., Liu L.M. Arsenic trioxide inhibits viability of pancreatic cancer stem cells in culture and in a xenograft model via binding to SHH-Gli. Onco Targets Ther. 2013;6:1129–1138. doi: 10.2147/OTT.S49148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Karin M., Lin A. NF-kappaB at the crossroads of life and death. Nat. Immunol. 2002;3(3):221–227. doi: 10.1038/ni0302-221. [DOI] [PubMed] [Google Scholar]
- 217.Orlowski R.Z., Baldwin A.S., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol. Med. 2002;8(8):385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- 218.Yang H., Dou Q.P. Targeting apoptosis pathway with natural terpenoids: implications for treatment of breast and prostate cancer. Curr. Drug Targets. 2010;11(6):733–744. doi: 10.2174/138945010791170842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Baltimore D. NF-kappaB is 25. Nat. Immunol. 2011;12(8):683–685. doi: 10.1038/ni.2072. [DOI] [PubMed] [Google Scholar]
- 220.Dai X., Zhang J., Arfuso F., Chinnathambi A., Zayed M.E., Alharbi S.A., Kumar A.P., Ahn K.S., Sethi G. Targeting TNF-related apoptosis-inducing ligand (TRAIL) receptor by natural products as a potential therapeutic approach for cancer therapy. Exp. Biol. Med. (Maywood) 2015;240(6):760–773. doi: 10.1177/1535370215579167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Gupta S.C., Sundaram C., Reuter S., Aggarwal B.B. Inhibiting NF-kappaB activation by small molecules as a therapeutic strategy. Biochim. Biophys. Acta. 2010;1799(10-12):775–787. doi: 10.1016/j.bbagrm.2010.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Kaltschmidt B., Kaltschmidt C., Hofmann T.G., Hehner S.P., Droge W., Schmitz M.L. The pro- or anti-apoptotic function of NF-kappaB is determined by the nature of the apoptotic stimulus. Eur. J. Biochem. 2000;267(12):3828–3835. doi: 10.1046/j.1432-1327.2000.01421.x. [DOI] [PubMed] [Google Scholar]
- 223.Ehrhardt H., Fulda S., Schmid I., Hiscott J., Debatin K.M., Jeremias I. TRAIL induced survival and proliferation in cancer cells resistant towards TRAIL-induced apoptosis mediated by NF-kappaB. Oncogene. 2003;22(25):3842–3852. doi: 10.1038/sj.onc.1206520. [DOI] [PubMed] [Google Scholar]
- 224.Jennewein C., Karl S., Baumann B., Micheau O., Debatin K.M., Fulda S. Identification of a novel pro-apoptotic role of NF-kappaB in the regulation of TRAIL- and CD95-mediated apoptosis of glioblastoma cells. Oncogene. 2012;31(11):1468–1474. doi: 10.1038/onc.2011.333. [DOI] [PubMed] [Google Scholar]
- 225.Guzman M.L., Rossi R.M., Karnischky L., Li X., Peterson D.R., Howard D.S., Jordan C.T. The sesquiterpene lactone parthenolide induces apoptosis of human acute myelogenous leukemia stem and progenitor cells. Blood. 2005;105(11):4163–4169. doi: 10.1182/blood-2004-10-4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Lin Y.G., Kunnumakkara A.B., Nair A., Merritt W.M., Han L.Y., Armaiz-Pena G.N., Kamat A.A., Spannuth W.A., Gershenson D.M., Lutgendorf S.K., Aggarwal B.B., Sood A.K. Curcumin inhibits tumor growth and angiogenesis in ovarian carcinoma by targeting the nuclear factor-kappaB pathway. Clin. Cancer Res. 2007;13(11):3423–3430. doi: 10.1158/1078-0432.CCR-06-3072. [DOI] [PubMed] [Google Scholar]
- 227.Chung S.S., Vadgama J.V. Curcumin and epigallocatechin gallate inhibit the cancer stem cell phenotype via down-regulation of STAT3-NFkappaB signaling. Anticancer Res. 2015;35(1):39–46. [PMC free article] [PubMed] [Google Scholar]
- 228.Li Y., Ellis K.L., Ali S., El-Rayes B.F., Nedeljkovic-Kurepa A., Kucuk O., Philip P.A., Sarkar F.H. Apoptosis-inducing effect of chemotherapeutic agents is potentiated by soy isoflavone genistein, a natural inhibitor of NF-kappaB in BxPC-3 pancreatic cancer cell line. Pancreas. 2004;28(4):e90–e95. doi: 10.1097/00006676-200405000-00020. [DOI] [PubMed] [Google Scholar]
- 229.Moselhy J., Srinivasan S., Ankem M.K., Damodaran C. Natural Products That Target Cancer Stem Cells. Anticancer Res. 2015;35(11):5773–5788. [PMC free article] [PubMed] [Google Scholar]
- 230.Marucci C., Fumagalli G., Calogero F., Silvani A., Christodoulou M.S., Martinet N., Passarella D. Natural Products and Cancer Stem Cells. Curr. Pharm. Des. 2015;21(38):5547–5557. doi: 10.2174/1381612821666151002113114. [DOI] [PubMed] [Google Scholar]
- 231.Vira D., Basak S.K., Veena M.S., Wang M.B., Batra R.K., Srivatsan E.S. Cancer stem cells, microRNAs, and therapeutic strategies including natural products. Cancer Metastasis Rev. 2012;31(3-4):733–751. doi: 10.1007/s10555-012-9382-8. [DOI] [PubMed] [Google Scholar]
- 232.Kim Y.S., Farrar W., Colburn N.H., Milner J.A. Cancer stem cells: potential target for bioactive food components. J. Nutr. Biochem. 2012;23(7):691–698. doi: 10.1016/j.jnutbio.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Burnett J., Newman B., Sun D. Targeting cancer stem cells with natural products. Curr. Drug Targets. 2012;13(8):1054–1064. doi: 10.2174/138945012802009062. [DOI] [PubMed] [Google Scholar]
- 234.Weber D.A., Wheat J.M., Currie G.M. Cancer stem cells and the impact of Chinese herbs, isolates and other complementary medical botanicals: a review. Zhong Xi Yi Jie He Xue Bao. 2012;10(5):493–503. doi: 10.3736/jcim20120503. [DOI] [PubMed] [Google Scholar]
- 235.Efferth T. Stem cells, cancer stem-like cells, and natural products. Planta Med. 2012;78(10):935–942. doi: 10.1055/s-0031-1298540. [DOI] [PubMed] [Google Scholar]
- 236.McCubrey J.A., Steelman L.S., Abrams S.L., Misaghian N., Chappell W.H., Basecke J., Nicoletti F., Libra M., Ligresti G., Stivala F., Maksimovic-Ivanic D., Mijatovic S., Montalto G., Cervello M., Laidler P., Bonati A., Evangelisti C., Cocco L., Martelli A.M. Targeting the cancer initiating cell: the ultimate target for cancer therapy. Curr. Pharm. Des. 2012;18(13):1784–1795. doi: 10.2174/138161212799859701. [DOI] [PubMed] [Google Scholar]


