Abstract
Cytoskeletal abnormalities and synaptic loss, typical of both familial and sporadic Alzheimer disease (AD), are induced by diverse stresses such as neuroinflammation, oxidative stress, and energetic stress, each of which may be initiated or enhanced by proinflammatory cytokines or amyloid-β (Aβ) peptides. Extracellular Aβ-containing plaques and intracellular phospho-tau-containing neurofibrillary tangles are postmortem pathologies required to confirm AD and have been the focus of most studies. However, AD brain, but not normal brain, also have increased levels of cytoplasmic rod-shaped bundles of filaments composed of ADF/cofilin-actin in a 1:1 complex (rods). Cofilin, the major ADF/cofilin isoform in mammalian neurons, severs actin filaments at low cofilin/actin ratios and stabilizes filaments at high cofilin/actin ratios. It binds cooperatively to ADP-actin subunits in F-actin. Cofilin is activated by dephosphorylation and may be oxidized in stressed neurons to form disulfide-linked dimers, required for bundling cofilin-actin filaments into stable rods. Rods form within neurites causing synaptic dysfunction by sequestering cofilin, disrupting normal actin dynamics, blocking transport, and exacerbating mitochondrial membrane potential loss. Aβ and proinflammatory cytokines induce rods through a cellular prion protein-dependent activation of NADPH oxidase and production of reactive oxygen species. Here we review recent advances in our understanding of cofilin biochemistry, rod formation, and the development of cognitive deficits. We will then discuss rod formation as a molecular pathway for synapse loss that may be common between all three prominent current AD hypotheses, thus making rods an attractive therapeutic target.
Keywords: oxidative stress, prion signaling, proinflammatory cytokines, amyloid-β, NADPH oxidase
Introduction
Alzheimer disease (AD), the major form of age-related dementia, impacts over 5 million Americans with someone developing the disease every 67 seconds [Alzheimer’s Association, 2015]. No currently approved treatments for AD target the causes of the dementia and most of the current treatments in clinical trials are immunotherapies, which are extremely expensive, rivaling the current patient health and long-term care costs. New therapeutic approaches are needed. These may require new targets.
AD is a multifactorial disease with both familial (genetic; FAD) and sporadic (SAD) forms. Although FAD is estimated to constitute less than 5% of all cases, the more we learn about SAD, the more we discover possible linkages to genes capable of conferring susceptibility to AD [Van Cauwenberghe et al., 2015]. Thus, even SAD may have as yet undetermined genetic causes or predispositions that are likely aggravated by a sedentary lifestyle and nutritional deficiencies [Creegan et al., 2015]. Because SAD accounts for the vast majority of cases and may have a variety of initiating factors, the ideal therapeutic strategy is to identify the initiating factors in each case and treat those specifically, i.e., personalized medicine [Laske, 2012; Chintamaneni and Bhaskar, 2012]. However, patients with either FAD or SAD do develop a common final pathology and thus the major approach to finding a cure has been to work backwards from the pathology to understand how they are initiated. This review will address such an approach that focuses on an understudied pathology in AD, that of cofilin-actin rod formation (Figure 1).
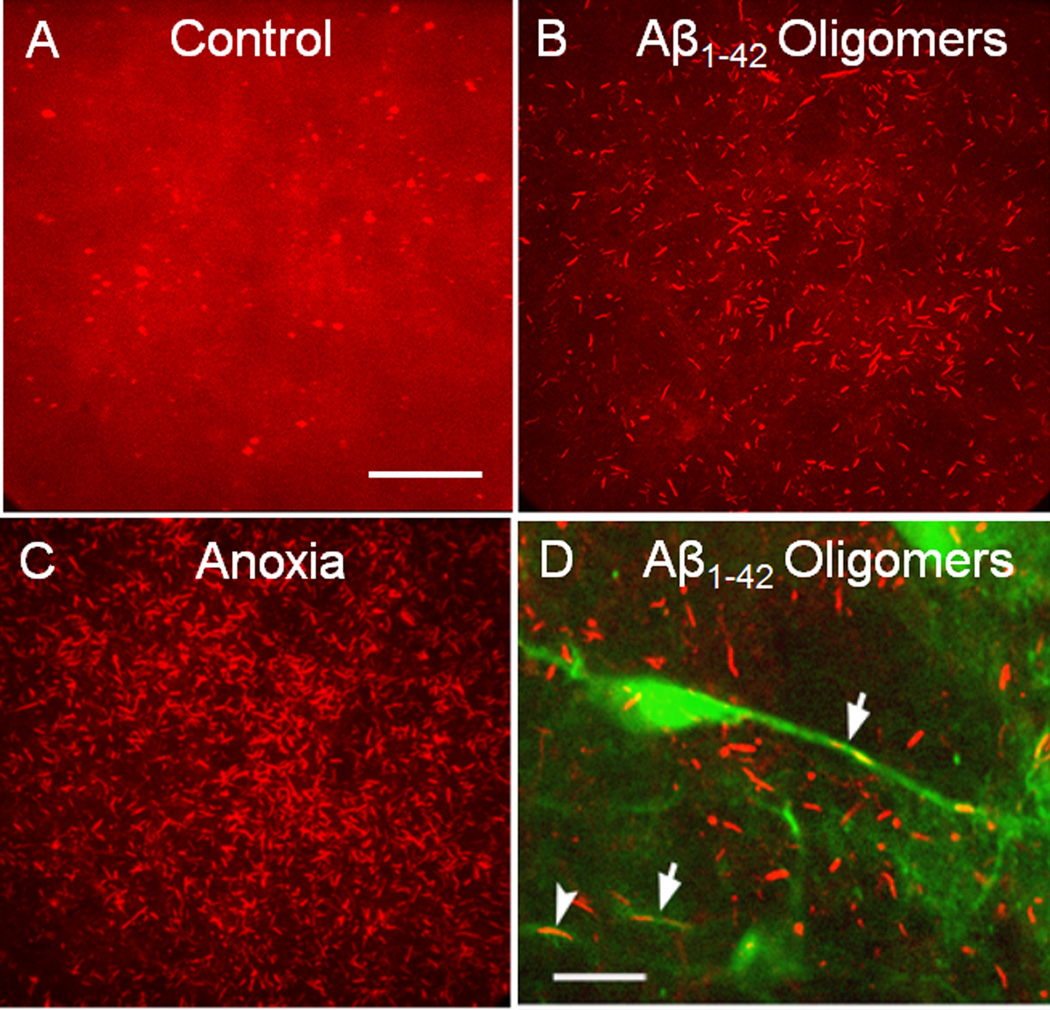
Figure 1. Cofilin-stained rods in cultured mouse hippocampal slices induced by treatment with Aβ peptides or anoxia.
Projection images of confocal stacks (30 µm) of cofilin immunostained cultured mouse hippocampal brain slices (14 d in vivo before treatment). (a) Untreated slice showing diffuse cofilin labelling and some puntate spheroid staining. (b) Slice treated for 24 h with 600 nM Aβ1–42 oligomers. (c) Brain slice subjected to one hour of anoxia before fixation. (d) Brain slice from a Thy-1-YFP-transgenic mouse treated similarly to the slice in (b). Arrows point to YFP-positive neurites with rods. Only ~10% of neurons in the hippocampus of the Thy-1-YFP mice express YFP. For all panels, slices were fixed in 4% formaldehyde, permeabilized with 100% methanol (−20° C) for 3 min, blocked and immunostained for cofilin with rabbit 1439 antibody [Shaw et al., 2004] and an Alexa 561 or 594 secondary antibody. Scale bars (a–c) and (d) 10 µm. Images modified from Davis et al., 2009.
Actin Dynamics and Development of Cofilin Pathology
The rapid assembly and disassembly of actin monomers (G-actin) into filaments (F-actin) is critical to many cell behaviors, including synaptic plasticity associated with memory and learning [Penzes and Rafalovich, 2012]. Many cellular proteins influence the dynamics of actin assembly, but the ADF (aka destrin)/cofilin family of proteins are particularly interesting because of their regulation and diverse functions, including maintaining cellular homeostasis [reviewed in Bernstein and Bamburg, 2010]. Although ADF and cofilin-1 have subtle differences in their quantitative interactions with actin in vitro [Pope et al., 2004; Chen et al., 2004] and in vivo [Tahtamouni et al., 2013; Wolfe et al., 2015], they can substitute for each other qualitatively in some but not all cellular functions [e.g. Hotulainen et al., 2005; Wiggan et al., 2012]. In neurons, for example, cofilin, but not ADF, appears to function in postsynaptic remodeling whereas both ADF and cofilin participate in presynaptic vesicle release [Wolf et al, 2015]. Mammalian neurons contain five to ten fold more cofilin than ADF [Minamide et al., 2000; Garvalov et al., 2007]. For simplification we will hereafter only refer to cofilin when discussing neuronal roles of this protein family.
Monomeric actin (G-actin) contains a bound nucleotide and can exist in the ATP, ADP-Pi or ADP form [reviewed in Bugyi and Carlier, 2010]. A few different actin monomer sequestering proteins prevent spontaneous nucleation of filaments in vivo and define unique actin monomer pools that differentially contribute to actin assembly in different cellular domains [Vitriol et al., 2015]. The nucleotide bound to G-actin is equilibrated through actin binding to profilin [Pollard and Cooper, 2009] or cyclase associated protein 1 (CAP1) [Zhou et al., 2014] and reflects the ATP:ADP ratio that is present in the cell, which under normal conditions is >9:1 [Atkinson et al., 2004] (Figure 2). When actin assembles, ATP is rapidly hydrolyzed generating ADP-Pi-actin which slowly releases the inorganic phosphate (Pi) to generate ADP-actin subunits.
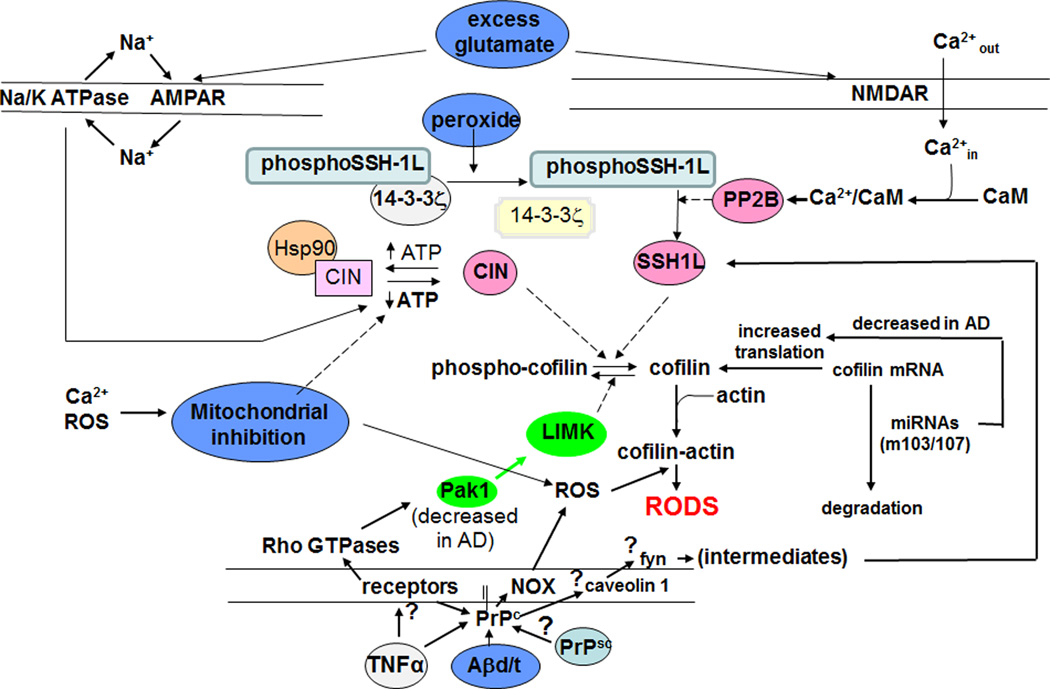
Figure 2. Schematic of likely molecular routes of rod formation from cofilin and actin pools.
Several routes of rod formation are possible, including oxidative cross-linking of cofilin before or after binding to F-actin or to actin monomers to induce rod assembly. Profilin binding to actin monomers opens the nucleotide pocket allowing the actin-bound nucleotide to equilibrate with the cellular adenine nucleotide pool, whereas cofilin binding to ADP-actin inhibits nucleotide exchange [Nishida, 1985].
Cofilin has a strong affinity for ADP-actin subunits in a filament and a very weak affinity for ATP- and ADP-Pi- actin subunits [Maciver and Weeds, 1994; Carlier et al., 1997; Chen et al., 2004], which maintain a different conformation [Belmont et al., 1999]. Certain proteins, e.g., cortactin, bind preferentially to the ATP- or ADP-Pi-form and tend to stabilize this newly assembled region of the actin filament [Bryce et al., 2005]. The preferential and cooperative binding of cofilin to ADP-actin subunits [Hayden et al., 1993; Carlier et al., 1999] induces a slight rotation (~5 deg per subunit) which was identified by image reconstruction of electron micrographs [McGough et al., 1997]. Cryo-electron micrographs show the filament twist induces a major conformational change in the actin subunit that is greater than that which occurs when monomer is incorporated into a filament [Galkin et al., 2011]. Importantly, phalloidin binding to F-actin inhibits cofilin-binding and its attendant structural change that shifts both the DNase I binding loop and the hydrophobic loop of the actin subunit away from the C-terminus [Scoville et al., 2009]. As a consequence of the structural change, cofilin-saturated actin filaments do not bind to phalloidin [McGough et al., 1997], a reagent commonly used in a fluorescently modified form to label F-actin in cells. Cofilin binding stabilizes the saturated regions of the filaments and exerts structural changes that are perpetuated to their pointed ends [Ngo et al., 2015]. These changes probably account for the enhanced release of Pi [Blanchoin and Pollard, 1999] and can alter filament interactions with other proteins, such as myosin II. Indeed, cofilin competition with myosin II for F-actin binding is an important normal cellular function of cofilin, independent from actin turnover and directly affecting cell behavior [Wiggan et al., 2012].
In vitro, purified cofilin can sever actin and thus form more filament ends for either nucleation or depolymerization [Andrianantoandro and Pollard, 2006], depending upon the relative concentration of actin and actin binding proteins. The F-actin dynamizing activity of cofilin is enhanced by cyclase-associated protein (CAP) [Normoyle and Brieher, 2012; Zhou et al., 2014], coronin 1A [Kueh et al., 2008; Lin et al., 2010], which aids in cofilin recruitment [Mikati et al., 2015], and Aip1 [Okreglak and Drubin, 2010], which enhances cofilin severing and accelerates monomer loss [Chen et al., 2015; Jansen et al., 2015; Nadkarni and Breiher, 2014]. Catalytic filament severing by cofilin alone is highest per molecule at low cofilin:actin ratios where low occupancy of filaments creates boundaries between occupied and unoccupied regions [Andrianantoandro and Pollard, 2006]. Cooperative binding of cofilin to F-actin [Hayden et al., 1993; Chen et al., 2004] creates regions of filament saturation where rapid severing [Chan et al., 2009] leaves smaller pieces of stable cofilin-saturated filaments (Figure 2). Depending upon the local environment, the newly formed ends of the severed filaments may nucleate filament growth or may increase subunit dissociation and filament depolymerization [reviewed in Bernstein and Bamburg, 2010; Winterhoff and Faix, 2015].
Cofilin Regulation
Cofilin binding to actin is inhibited by phosphorylation of a single cofilin residue (ser3) [Morgan et al., 1993; Agnew et al., 1995; Moriyama et al., 1996] catalyzed by members of the LIM kinase or TES kinase families [Yang et al., 1998; Arber et al., 1998; Toshima et al. 2001]. LIM kinases themselves are regulated upstream by phosphorylation in an activation loop by members of either Rho kinase (ROCK) or p21-activated protein kinase (Pak) family which in turn are regulated by binding RhoGTP or RacGTP/cdc42GTP, respectively [Edwards et al., 1999]. Phospho-cofilin binds to the scaffolding protein 14-3-3ζ, which limits the accessibility of phospho-cofilin to more general phosphatases [Gohla and Bokoch, 2002], but not to chronophin (CIN) [Gohla et al., 2005] or to phosphatases in the slingshot family [Niwa et al., 2002; Huang et al., 2006; Eiseler et al., 2009].
Slingshot (SSH-1L) is the most abundant neuronal isoform of this cofilin phosphatase. Dephosphorylation of cofilin by SSH-1L requires SSH-1L binding to F-actin, and is inhibited by protein kinase D phosphorylation of SSH-1L at ser978 [Eiseler et al., 2009; Peterburs et al., 2009]. Furthermore, isoforms of 14-3-3 sequester the phospho-SSH-1L to prevent its translocation to sites of activation [Nagata-Ohashi et al., 2004]. SSH-1L is dephosphorylated by calcineurin, a calcium/calmodulin-dependent phosphatase [Wang et al. 2005]. However, even the dephosphorylated form of SSH-1L requires F-actin binding for significant activity [Nagata-Ohashi et al., 2004; Soosairajah et al., 2005; Kurita et al., 2008], providing a self-regulating system that localizes cofilin activation to cellular sites where F-actin levels are relatively high. SSH-1L can also dephosphorylate LIMK in the activation loop, thus inhibiting its phosphorylation of cofilin [Soosairajah et al., 2005]. More details of these phosphoregulatory pathways, additional upstream regulators, and their relevance to AD may be found in a recent review [Henriques et al., 2015].
Non-phosphoregulatory mechanisms for cofilin activity also exist. Cofilin binds to various membrane phosphatidylinositides and is inactive when so bound [Yonezawa et al., 1990]. Cell stimulation, activating phospholipase C, can release cofilin and provide a pool of cofilin for enhancing F-actin dynamics at the leading edge [van Rheenen et al., 2007]. Also of interest is a recent finding of cofilin inhibition through oxidation of cysteines 139 and 147 to sulfenic or sulfinic acid by endogenously produced peroxide in the leading edge of cells in wounded confluent monoloayers [Cameron et al., 2015]. Peroxide has been recognized for some time as a second messenger in cell migration [Forman et al., 2010], although previous work suggested that exogenous peroxide stimulated cell protrusion efficiency through increased cofilin activity and free actin barbed-end formation [Taulet et al., 2012]. Further work is needed to clarify if there are differentially regulated pools of cofilin, one of which is subjected to oxidative inhibition and the other to activation, perhaps through CIN regulated dephosphorylation [Delorme-Walker et al., 2015].
Physiological and Non-physiological Mediators of Neuronal Rod Formation
The formation of rods has been studied in mammalian neurons responding to different neurodegenerative stimuli (Figure 3), including excitotoxic levels of glutamate, mitochondrial inhibitors (e.g., antimycin A), peroxide, NO, Aβ peptides, and proinflammatory cytokines [Minamide et al., 2000; Maloney et al, 2005; Davis et al., 2011; Walsh et al., 2014]. Rods also form spontaneously in the absence of these stimuli when cofilin-FP is overexpressed [Bernstein et al., 2006; Cichon et al., 2012]. The molar ratio of ADF/cofilin:actin is about 1:5 in neurons with cofilin accounting for >80% of the total ADF/cofilin pool. Most rod formation in cultured neurons occurs within the neurites and not in the soma [Minamide et al., 2000; Minamide et al., 2010]. Although some stress agents cause only a local increase in dephosphorylated cofilin, only this active form is observed in rods. Rod inducers increase production of a variety of reactive oxygen species (ROS). Peroxide, or a more reactive chlorine derivative [Dahl et al., 2015], is the most likely of these to generate the intermolecular cofilin disulfide bond found in rods, whose formation is discussed below.
Figure 3. Schematic of abbreviated signaling pathways contributing to rod formation by some initiators of neuronal stress and factors that change during AD progression.
The role of caveolin and fyn in rod formation is hypothetical but is based on their known PrPC-mediated phosphorylation [Shi et al., 2013] and that fyn inhibitors can rescue synapse loss and established memory [Kaufman et al., 2015]. Other steps diagrammed here are discussed in the text.
Composition, Structure, and Assembly of Cofilin-actin Rods
Cytoplasmic rods isolated from either cell lines or cultured primary neurons contain ADF/cofilin:actin in a 1:1 molar ratio [Minamide et al., 2010]. Rod isolation requires mechanical cell lysis because rods are not stable in non-ionic detergents. In addition, to prevent contamination of rods by microtubule fragments, microtubules are depolymerized before lysis. Mass spectrometric analysis of rods isolated from ATP-depleted A431 cells identified cofilin, ADF, cytoplasmic β- and γ-actins, and a few other proteins. Only one of the additional proteins identified by mass spectrometry, 14-3-3ζ, could be immunostained in some rods and usually only during late stages of rod maturation, suggesting it is not a core rod component. An antibody to a phospho-tau epitope containing ser262 also immunostained rods (Whiteman et al., 2009, 2011). Rods do not immunostain with the phospho-cofilin antibody suggesting they contain only the active, dephospho-form [Maloney et al., 2005]. Supporting the idea of their limited composition is the fact that rods can be assembled in vitro from mixtures of only actin and either ADF or cofilin. This was done in hanging drops [Minamide et al., 2010] and in an earlier report under conditions of disulfide bond formation [Pfannstiel et al., 2001]. Cofilin may be oxidized in vitro to form dimers and higher oligomers (Figure 2) that will induce highly ordered actin bundles [Pfannstiel et al., 2001] that in electron micrographs resemble rods seen in vivo (Figure 4).
Figure 4. Transmission electron micrographs of a rod-containing neurite showing the ~9 nm filament components in a cultured hippocampal slice treated 24 hr with Aβ1–42 oligomers.
Slices were fixed, sectioned (250 nm) and stained as described elsewhere [Davis et al., 2009]. A rod runs the entire length of the neurite in the image shown (from lower left to upper right). Filaments of the rod surround a mitochondrion. White arrow points to 12 nm gold particle (slightly wider than the filament on which it sits), which was used as a fiduciary marker in reconstructing a tilted image stack to give the tomogram in panel (b). Scale bar = 100 nm.
Three factors essential for rod formation are elevated levels of active cofilin, elevated levels of ADP-actin, and a highly oxidative environment [Minamide et al., 2000; Bernstein et al., 2012]. Energetically stressed neurons usually meet all three requirements. The stress increases active cofilin through dephosphorylation, increases ADP-actin, and generates the ROS necessary for cofilin oxidation and rod formation (Figure 2). Reducible cofilin dimers are found in rods purified from cells lysed in the presence of free sulfhydryl blocking agent added to minimize post-lysis oxidation [Bernstein et al., 2012]. Cys39 and cys147 are the residues required for intermolecular disulfide bond formation and rod incorporation both in vitro and in vivo [Pfannstiel et al., 2001; Bernstein et al., 2012].
Instances in which stress fails to generate rods provide evidence supporting the need for all three factors enumerated above. Endogenous cofilin/actin ratios are unusually low in the pig kidney proximal tubule cell line LLCPK A4.8 and fail to produce rods; cofilin overexpression corrects this [Minamide et al., 2010]. Failure of energetic stress to produce rods in Swiss 3T3 cells is overcome by first increasing intracellular pH; stress-induced acidification reduces cofilin’s ability to enhance actin severing/turnover that is needed for rod formation [Bernstein et al., 2000]. The ability of neurons to produce rods in response to treatment with Aβ oligomers (Figure 3) is enhanced by expression of cofilin phosphatases (SSH-1L or chronophin) and is inhibited by cofilin kinase LIMK1 [Davis et al., 2011].
Treatment of some cells with various cucurbitacins or their derivatives will induce rod-like structures that immunostain for cofilin and actin [Ren et al., 2012]. These triterpenoids of traditional medicines modify cysteine thiols to induce disulfide bond formation and inhibit LIM kinase [Zhang et al., 2013; Gabrielsen et al., 2013; Sari-Hassoun et al., 2015]. This mode of induction depends on Slingshot-1L phosphatase (not chronophin) and can be reversed by the disulfide reducing agent N-acetyl cysteine, but not by ROS scavengers lacking thiols [Zhang et al., 2013].
Of more physiological relevance is the energetic stress of cells to induce rods. This pathway does appear to involve the cofilin phosphatase CIN, which is kept inactive by binding to the chaperone Hsp90 if ATP is at its normal high level [Huang et al., 2008] (Figure 3). Hsp90 aids in protein folding by binding and releasing unfolded proteins in an ATP-dependent manner. Treatment of neurons with an Hsp90 inhibitor releases CIN in an active state leading to cofilin dephosphorylation and rod formation. Silencing CIN with siRNA decreases the rate and extent of rod formation in response to both ATP-depletion and Hsp90 inhibition [Huang et al., 2008]. Thus, CIN activates cofilin when cellular ATP falls.
A decline of ATP shifts the balance of kinase/phosphatase activity toward dephospho-cofilin, increases the ADP-actin fraction of total actin, and raises ROS [Minamide et al., 2000; Bernstein et al., 2012]. These conditions favor cofilin saturating regions of F-actin cooperatively. F-actin with cofilin-saturated regions is readily severed, producing small stable fragments [Chan et al., 2009; Chen et al., 2015]. The presence of these fragments and elevated ROS in stressed cells likely promotes rod formation either from direct bundling of these fragments and intermolecular disulfide cross-linking of cofilin or from oxidized dimerization of cofilin or cofilin-actin with the dimers (or cross-linked complex) participating in filament assembly and bundling [Minamide et al., 2010; Bernstein et al., 2012] (Figure 2). Whether or not the filaments that comprise the rods have the canonical structure of cofilin-saturated F-actin remains to be determined. However, rods cannot be stained with fluorescent phalloidin, a property also seen in cofilin-saturated F-actin. Furthermore, the average filament length within a rod is about 200 nm (<100 actin subunits) as determined from electron microscopy (EM) tomograms [Minamide et al., 2010], similar to the average filament lengths generated by assembling cofilin-actin mixtures [Moriyama and Yahara, 1999; Carlier et al., 1999]. The diameter of the individual filaments within the rod is approximately 9 nm (Figure 4), also about the same as a cofilin-saturated actin filament. Thus, models for rod formation will need to be consistent with these structural details. In addition, the lack of phalloidin staining can be used to differentiate rods from other cofilin and actin containing structures.
Relatively high levels of ADP-actin may explain the induction of cofilin-actin rods in neurons treated with the actin monomer binding agents, latrunculin A and B [Whiteman et al., 2009] (Figure 2). Both agents cause the disassembly of dynamic actin by binding ADP-actin monomers as they are released from the minus end, and both block reassembly [Morton et al., 2000]. Cofilin has about a ten-fold higher affinity for ADP-actin monomers than latrunculin and presumably displaces it [Chen et al., 2004; Bernstein et al., 2006], thus allowing the ADP-actin to incorporate into rods. Latrunculin B-induced rods were first reported in nuclei of mast cells [Pendleton et al., 2003]. Actin rods in mammalian cell nuclei were observed much earlier [Fukui and Katsumaru, 1979], as was cofilin as a nuclear rod component [Nishida et al., 1987]. Nuclear rod-like structures containing actin without cofilin have also been reported [Domazetovska et al., 2007]. Although nuclear rods are not a focus of this review, they are of significance in understanding stress responses, especially regarding the nuclear shuttling of actin reviewed elsewhere [Munsie et al., 2012; Munsie and Truant, 2012]. Nuclear trafficking of cofilin and actin has profound effects on gene regulation, chromatin architecture, and DNA repair processes that are in their infancy in being elucidated [Belin et al., 2015]. Furthermore, the formation of cytoplasmic rods may serve to decrease both the pool of ADF/cofilin available for nuclear transport and the pool of monomeric actin required for binding the transcriptional co-activator MAL, a homeostatic regulator of genes through the serum response factor (SRF) pathway [Salvany et al., 2014; Kawakami-Schultz et al., 2014; Treisman, 2013]. The SRF-cofilin-actin signaling pathway also modulates mitochondrial morphology, fragmentation, and motility in neurons [Beck et al., 2012; Tu et al., 2014], suggesting a complex interdependency between many homeostatic processes and actin pools that would likely be disrupted by rod formation.
Time-lapse imaging of rods forming in ATP-depleted HeLa cells expressing cofilin-GFP shows rods rapidly enlarging from small, quickly moving puncta and becoming stationary as they enlarge [Minamide et al., 2010]. A ready supply of actin monomer must accompany this development. Hence it is not surprising that phalloidin and jasplakinolide, two F-actin stabilizing reagents that inhibit cofilin binding to and severing of F-actin, prevent rod formation [Whiteman et al., 2009], as does cytochalasin D [Minamide et al., 2010], a barbed-end assembly inhibitor. These reagents would surely impede the rod- required remodeling of filaments and saturating-association of cofilin with actin. While overexpression of a cofilin-fluorescent protein (cofilin-FP) chimera and the stress of imaging is sufficient to drive rod formation in neurons [Bernstein et al., 2006; Cichon et al., 2012], it is not in all cells. In addition to adequate amounts of cofilin, rod formation requires appropriate temporal and spatial regulation of cofilin kinases and phosphatases and ROS production (Figure 3).
Signaling Pathways Mediating Neuronal Rod Formation
At least two independent pathways generate ROS for rod formation (Figure 3). One pathway, dependent on the cellular prion protein (PrPC) and the activity of NADPH oxidase (NOX), is activated in the same neuronal population (25% of hippocampal or cortical neurons) by both Aβ and proinflammatory cytokines (TNFα, IL-1β, IL6) [Walsh et al., 2014]. Rods form in <50% of neurites in this population. PrPC is a lipid-linked protein on the membrane outer surface that is enriched in sphingolipid membrane signaling domains [Hirsch et al., 2014]. A pathway independent of PrPC and NOX is stimulated by mitochondrial inhibitors (e.g., azide or antimycin) or excitotoxic levels of glutamate [Walsh et al, 2014]. Although NOX is the primary source of ROS generated in response to NMDA receptor activation [Brennan et al., 2009], glutamate-induced rods form via AMPA receptor activity and can be prevented by ouabain inhibition of the Na+/K+ ATPase (Figure 3) [Bernstein et al., 2012]. This explains why rods induced by glutamate form in the presence of NOX inhibitors. Furthermore, rods induced by mitochondrial inhibitors or glutamate appear in >80% of hippocampal or cortical neurons (and in most neurites), demonstrating that neither cofilin nor actin is limiting the ability of neurites to form rods. Overexpression of PrPC in neurons drives rod formation in response to Aβ or TNFα from 25% to over 40%, suggesting that the level of PrPC expression may limit rod induction by these agents (Figure 5). Surprisingly, in the absence of other rod-inducing factors, further overexpression of PrPC alone can drive NOX-dependent rod formation in 50% of hippocampal neurons [Walsh et al., 2014], suggesting that PrPC organizes membrane signaling domains for both ROS production and cofilin activation (Figure 6). This finding could help explain the age dependence of AD: PrPC accumulates increasingly in sphingolipid-containing membrane domains during aging in mouse brain [Agostini et al., 2013]. WT mice do not develop Aβ plaques or phospho-tau pathology, but they do develop age-dependent cognitive and synaptic (LTP) deficits, as well as increased gliosis in the hippocampus [Weber et al., 2015]. Although brains of aged mice have not been evaluated for rods, an age-dependent rod increase in rat hippocampus has been reported [Cichon et al., 2012].
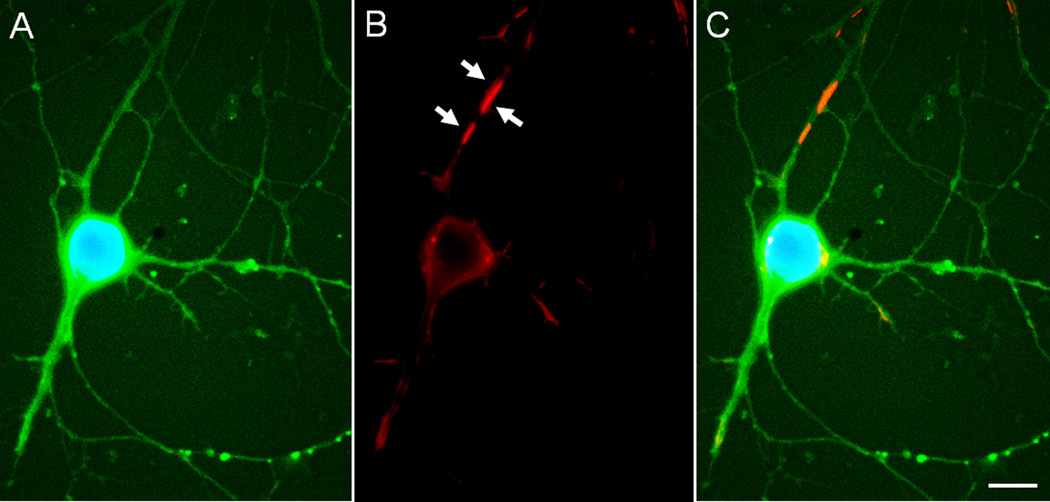
Figure 5. Overexpression of EGFP-PrPC induces rods in cultured neurons.
Cultured hippocampal rat E18 neuron (6 days in vitro) infected on day 3 with 100 moi of adenovirus for expression of EGF-PrPC (green), fixed on day 6, and immunostained for cofilin (red) and stained for DNA with DAPI (blue). (a) EGFP and DAPI stained image of neuron. (b) Cofilin immunostained cell shown in (a). White arrows point to rods. (c) Overlay of (a) and (b). Scale bar =10 µm. Images courtesy of L.S. Minamide.
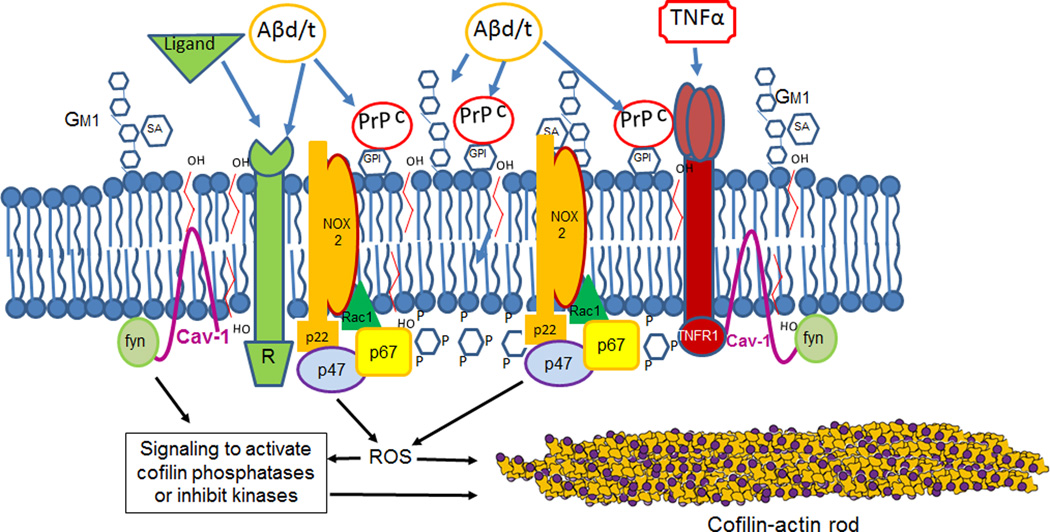
Figure 6. Schematic of a PrPC- and sphingolipid-enriched membrane signaling domain.
A number of receptors are found in these domains, some of which have been demonstrated to require PrPC as a co-receptor for Aβ-mediated rod signaling. We propose that because overexpression of PrPC alone drives rod formation through a NOX-dependent step [Walsh et al., 2014], multiple Aβ-binding components of the signaling complexes work in conjunction with PrPC to elevate the neurite ROS levels above the threshold required for rod-induction and maintenance. Other components of the signaling complex such as fyn [Ochs and Malaga-Trillo, 2014], or perhaps ROS itself (see Figure 3), activate cofilin.
Overexpression in neurons of wild type (WT) cofilin-FP chimeras induces spontaneous formation of rods in otherwise unstressed cells [Bernstein et al., 2006; Cichon et al., 2012]. Transport deficits, a decline in spine numbers, and decreased synaptic events (frequency of mEPSCs) occur within neurites that form spontaneous rods. Neurites from the same neuron without rods remain unaffected [Cichon et al., 2012], suggesting rods are having a localized effect. The irreversibility and spontaneous development of rods formed through overexpression of WT-cofilin-FPs precluded their use for investigating stress-induced rod formation and its reversal. A live cell reporter had to be developed for monitoring rod dynamics.
Various promoters were used to reduce expression of WT cofilin-FP, but even the lowest expressing of these increased spontaneous rod formation in cultured neurons. Through mutational analysis of cofilin’s non-actin binding surface, a cofilin mutant was identified that does not generate rods when overexpressed: arg21 changed to gln (R21Q) [Mi et al., 2013). Cofilin R21Q has additional behaviors appropriate for a live cell rod reporter: incorporating into rods only if endogenous cofilin is not suppressed and binding sufficiently weakly to actin to not perturb actin dynamics during neuronal growth. When expressed in hippocampal neurons, cofilin R21Q-mRFP incorporated into rods that formed within minutes in response to glutamate or its analog, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) and over hours in response to treatment with Aβ or TNFα [Mi et al., 2013]. AMPA-induced rods are reduced in area by 50% within 1 hr of adding an AMPA receptor inhibitor [Mi et al., 2013], and rods disappeared with similar half-lives after washing out Aβ [Mi et al., 2013] or TNFα [Walsh et al., 2014]. Rods that formed in response to PrPC overexpression in neurons are also completely reversed 4 hr after addition of any one of three NADPH oxidase inhibitors, demonstrating that rods induced through either the PrPC-dependent or -independent pathway are reversible. Regardless of the rod-inducing pathway, rod maintenance requires continued production of ROS [Walsh et al., 2014].
PrPC and many neuronal receptors function as co-receptors when bound to synapse-damaging Aβ dimer [Bate and Williams, 2011; Roucou, 2014], thus implicating those receptors and PrPC in AD [Benilova and De Strooper, 2013] (Figure 6). These receptors include the mGluR5 metabotropic receptor and β1-integrin [Loubet et al., 2012; Um et al., 2013; Woo et al., 2015a], neither of which has much in common functionally except for their ability to modulate cofilin activity. Indeed, all of the Aβ receptors with identified signaling pathways have a common theme, activation of pathways that modulate cytoskeletal dynamics at synapses [Geng et al., 2013; Kim et al., 2013; Kam et al., 2013; Henriques et al., 2015]. Cofilin, which regulates both pre- and postsynaptic architecture and function [Gu et al., 2010; Bosch et al., 2014; Wolf et al., 2015], may be a common target of these pathways. If so, reaching certain thresholds of cofilin activation and ROS production may generate synapse dysfunction. Elimination of any one of these Aβ receptors might reduce the levels of ROS or active cofilin to below the thresholds eliciting dysfunction. Quantification of ROS levels in WT neurites and in those from neurons in which some Aβ receptors have been silenced are needed to define the ROS threshold for rod formation.
Mouse Models, Rods and Cognition
Rod pathology is rarely observed in postmortem hippocampus from human subjects with normal cognition or from WT rodents. The study of cognitive deficits due to human Aβ production has been facilitated with transgenic mouse models expressing mutations in amyloid precursor protein (APP). APP is a large single pass transmembrane protein with cleavage sites for multiple proteases. β-Secretase (BACE) always cleaves at a specific aspartate residue. Presenilins 1 and 2 [Tanzi and Bertram, 2005], components of the γ-secretase complex, cleave within the membrane spanning domain at somewhat variable sites. Together, these enzymes produce amyloid-β peptides commonly ranging in size from 39–43 amino acids [Moore et al., 2012]. Many human mutations leading to early onset, i.e. familial, AD have been found in the APP and presenilins. Results from recent studies, using an AD mouse model expressing both human APP containing the familial AD (FAD) Swedish mutation and presenilin with a familial AD exon 9 deletion (APPsw/PS1ΔE9), strongly suggest that altered cofilin function is at the heart of the Aβ-induced cognitive deficits in these mice. It is important to point out that these mice are a model system for studying the Aβ-induced synaptic deficits that are independent of tau hyperphosphorylation since no tau pathology develops.
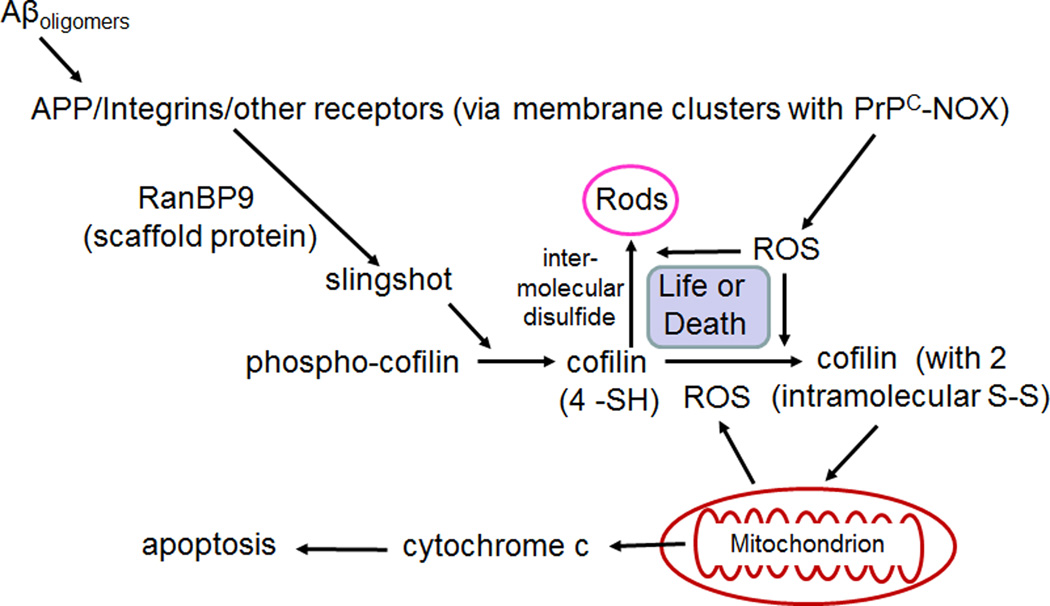
Cofilin is directly implicated in the synaptic deficits seen in APPsw/PS1ΔE9 mice. Compared to WT mice, they show significantly reduced contextual fear conditioning, a hippocampal-dependent learning paradigm, and hippocampal slices from these mutant mice show reduced long-term potentiation (LTP) following theta burst stimulation. Both deficits are completely rescued when cofilin is reduced to 50% of WT levels by crossing APPsw/PS1ΔE9 mice with cofilin hemizygous mice (APPsw/PS1ΔE9 × Cofilin+/−) [Woo et al., 2015a]. At 9 months of age, about an 8 fold increase in the number of cofilin-actin rods has been quantified in both the hippocampus and cortex of the APPsw/PS1ΔE9 mouse compared to WT [Woo et al., 2015b]. This rod pathology is likely due to hyperactivation of cofilin via dephosphorylation which can be mediated by the scaffolding protein RanBP9 [Woo et al., 2012]. RanBP9 binds both β1-integrin and SSH-1L phosphatase, locally enhancing cofilin dephosphorylation (Figure 7). Aβ oligomers bind to β1-integrins in a PrPC-dependent manner [Woo et al, 2015a]. Decreasing the levels of RanBP9 expressed in the AD mouse by 50% by crossing APPsw/PS1ΔE9 mice with the hemizygous RanBP9+/− mice reduces Aβ-induced cofilin dephosphorylation and rod formation, and restores most of the fear conditioning response as well as LTP in brain slices [Woo et al., 2012, 2015 a,b]. These findings strongly implicate the cofilin signaling pathway in these synaptic deficits. RanBP9 is also a nuclear import factor (importin 9), so its role in mediating Aβ signaling may extend beyond signaling through cofilin [Murrin and Talbot, 2007]. Nonetheless, together these results suggest that cofilin hyperactivity is essential for synaptic alterations occurring downstream of Aβ both in vitro and in vivo and that rods, if not the major cause of the deficits themselves, contribute to the defects through one of the many deleterious effects of rods enumerated above. Given these findings in APPsw/PS1ΔE9 mice, which serve as a model for only one aspect of familial AD, one needs to ask if similar cofilin dysregulation underlies cognitive decline seen in SAD for which there are no known genetic causes.
Figure 7. Schematic of pathway for Aβ oligomer-induced rods in neurons through the β1-integrin receptor and RanBP9 scaffolding protein.
We hypothesize that the formation of intermolecular disulfide bonds in cofilin (leading to rod formation) in response to ROS production is competitive with cofilin's intramolecular oxidation, which targets it to mitochondria to trigger apoptosis. Rods are reversible if REDOX pathways can reduce the ROS levels and thus rods may form and disappear many times. We hypothesize that eventually a large surge in ROS may trigger cofilin-induced apoptosis occurring later in AD progression.
In human AD brain, cofilin-actin rods were first observed by immunohistochemistry in paraffin sections but were not found in similar sections from brains of cognitively normal subjects [Minamide et al., 2000]. A more recent study quantifying cofilin immunofluorescence showed rod-like and aggregate cofilin pathology four-fold greater in number and larger in area in human AD brain sections than in age-matched controls [Rahman et al., 2014]. However, the question remains: are rods just a reporter of neurodegenerative stress or, as found in culture, a mediator of synapse loss?
Numerous studies have shown that mild cognitive impairment (MCI, a prodromal stage of AD) and early AD are characterized predominantly by loss of synapses and connectivity, which occur over the same time frame as the observed significant increase in NADPH oxidase (especially NOX2) [Ansari and Scheff, 2011]. Synaptic loss in MCI occurs prior to the wide-spread loss of neurons that characterizes mid and later stages of AD [Scheff et al., 2006; Scheff and Price, 2006]. Markers for increased oxidative stress rise during MCI [Keller et al., 2005; Ansari and Scheff, 2010] and continue to increase as AD progresses. Two other changes associated with human AD subjects also support a role for cofilin and rod formation in the disease. First, the level of cofilin mRNA in vivo is limited in part by microRNAs (miR104 and miR107), which suppress cofilin expression. These two miRs are downregulated in AD, miR107 showing a decline with the earliest stages of AD pathology [Wang W-X et al., 2008; Nelson and Wang, 2010]. When loss of miR104/107 is mimicked in cultured neurons, cofilin-actin rods form [Yao et al., 2011].
Second, numerous studies have implicated phospho-regulation of cofilin in AD. LIM kinase 1 is activated through phosphorylation by several isoforms of p21 activated kinase (Pak) [Edwards et al., 1999]. Pak1 and Pak3 levels and active (phosphorylated Pak1–3) decline relative to actin in confirmed AD brain [Zhao et al., 2006]. When loss of Pak activity is mimicked in cultured neurons, cofilin-actin rods form [Zhao et al., 2006]. In addition, overexpression of an active form of LIM kinase 1 significantly inhibits Aβ-induced rod formation in cultured neurons [Davis et al., 2011]. Persistent reduction in phospho-Pak (i.e., activated Pak) was observed as early as 2 h following treatment of dissociated rat hippocampal neurons with soluble Aβ oligomers and occurred at synthetic Aβ oligomer concentrations as low as 10 nM. Furthermore, intracerebro-ventricular injection of a Pak inhibitory peptide into WT mice induced rod-like cofilin pathology and deficits in social recognition memory [Zhao et al., 2006].
Efforts to determine changes in phospho-cofilin levels in human AD versus control brain have produced conflicting results [Heredia et al., 2006; Barone et al., 2014; Kim et al., 2013] probably due to one or more of the following uncontrolled experimental variables: 1) the large range in postmortem time intervals for sampling (2–26 hrs); 2) some sampling methods may solubilize rod proteins more effectively than others; and 3) factors causing AD in subjects studied may differ. Two additional sources exist for conflicting results in studies of phospho-cofilin levels in AD versus control brain. First, immunoblot analysis of brain extracts reflect all cell types, not just neurons in which we have seen relevant phospho-cofilin modulation. Second, PrPC-dependent rods in cultured neurons show a local decline in phospho-cofilin directly over the rods; the decline spreads slightly with time but only within the rod-containing neurite [Walsh et al., 2014]. Therefore global whole brain changes in phospho-cofilin levels may not be very informative.
Cofilin-actin Rods in Neuronal Life and Death Alternatives
Although conditions that lead to cofilin-actin rod formation contribute to synaptic dysfunction, the formation of rods actually may also have a neuroprotective function as suggested from studies of cultured neurons. Actin filament turnover in neurons utilizes a significant amount of ATP [Bernstein and Bamburg, 2003]. Cofilin sequestration by rods transiently retards the decline in mitochondrial membrane potential and helps maintain ATP levels by reducing actin turnover [Bernstein et al., 2006]. Secondly, formation of the single intermolecular disulfide between cys39 and cys147, necessary for cofilin-actin rod formation [Bernstein et al., 2012], reduces the amount of cofilin available for forming two intramolecular disulfide bonds (cys39-cys80 and cys139-cys147). The presence of these intramolecular disulfide bonds, which form under stronger oxidizing conditions, targets cofilin to mitochondria [Klamt et al., 2009], where it causes release of cytochrome c and induces apoptosis [Klempke et al, 2008; Klamt et al., 2009; Kotiadis et al., 2012; Chua et al., 2003; Wang C. et al., 2008] (Figure 7). Although not yet examined in neurons, cofilin activity (dephosphorylation) has also been linked to a necrotic cell death pathway involving protein phosphatase 2A [Tomasella et al., 2014]. Because rod formation is reversible but apoptosis and necrosis are not, one could envision a scenario in which rods appear and disappear over a prolonged period of fluctuations in neuronal stress, helping to maintain neuronal viability albeit perhaps at the expense of maintaining specific synaptic connections. If rods are not reversed by stress reduction, synapses would be lost but neurons might remain viable.
As noted earlier, SSH-1L with phosphorylated ser978 binds to 14-3-3ζ, which prevents its activation by interaction with F-actin, abundant in the cell cortex. 14-3-3ζ has two cysteine residues that form a disulfide when oxidized by ROS, reducing its affinity for SSH-1 [Kim et al., 2009] and allowing SSH-1L to be dephosphorylated by calcineurin and to increase active cofilin [Wang et al., 2005]. ROS scavenger pretreatment blocked cucurbitacin-induced rod induction due in part to blockage of SSH-1L activation [Zhang et al., 2013]. Thus, we hypothesize that the intensity of ROS production determines the extent of cofilin dephosphorylation and oxidation, whether intra- or inter-molecular disulfide bonds form, and whether rods or apoptosis ensues. Rod development would favor synaptic loss (Figure 7).
Neuronal apoptosis in response to Aβ oligomers is mediated by mitochondrial targeting of cofilin and subsequent cytochrome c release [Woo et al., 2012; Roh et al., 2013]. Silencing of cofilin expression in neuronal cell cultures protects the neurons from apoptosis induced by 18 h treatment with 1 µM Aβ oligomers [Woo et al., 2015a]. Furthermore, apoptosis in cortical neurons, induced by a 1 hr oxygen/glucose deprivation treatment with 24 h recovery, could also be largely prevented by silencing cofilin [Madineni et al., 2014], suggesting that targeting of cofilin to mitochondria is a general mechanism for apoptotic initiation in neurons.
Cofilin-actin Rods in Context: Hypotheses for AD Progression
AD pathology is characterized by progressive loss of basal forebrain cholinergic neurons that innervate the cortex and hippocampus and by two hallmarks, the extracellular senile plaques containing Aβ and the intracellular neurofibrillary tangles (NFTs) and neuropil threads containing hyperphosphorylated tau [Ubhi and Masliah, 2012]. In addition, brains of AD subjects show clear signs of oxidative stress [Ansari and Scheff, 2011] along with a potent neuroinflammatory response [Verri et al., 2012]. Each of the cytoskeletal pathologies, seen in both FAD and SAD, has suggested different hypotheses concerning the underlying cause of synaptic loss and neurodegeneration. These are often considered mechanistically exclusive. The following discussion contends that they are not.
Senile plaques and the amyloid hypothesis
The “amyloid hypothesis” states that increasing cerebral accumulation of Aβ over years to decades exacerbates cognitive decline, neurodegeneration, and senile plaque deposition associated with AD. Increased amounts of Aβ peptides, most commonly Aβ1–40 and Aβ1–42, are found in brains of subjects with AD versus those from cognitively normal subjects [Moore et al., 2012]. Furthermore, individuals with Down syndrome (DS; aka trisomy 21) living to age 40 or beyond develop all of the pathological characteristics of AD [Glenner and Wong, 1984b]. Chromosome 21 carries genes for APP, and DS patients have increased APP expression [Cataldo et al., 2003], which combined with the increase in BACE-1 activity occurring in DS patients, contributes to excess Aβ production [Sun et al., 2006a,b]. Mutations that lead to increased production of the amyloidogenic Aβ1–42 [Chartier-Harlin, et al., 1991a,b; Goate, et al., 1991; Murrell, et al., 1991; Price et al., 1995], or polymorphisms that lead to reduced clearance of Aβ peptides [Verghese et al., 2011; Wildsmith et al., 2013], are linked to early onset familial AD with high penetrance.
In sporadic AD, which accounts for the majority of AD cases, the role of genetics is less clear. Certainly specific polymorphisms (allelic variants) working in concert with each other and with non-genetic risk factors such as diet and brain trauma, can greatly impact disease susceptibility [Bertram and Tanzi, 2012]. Nevertheless, the role of Aβ in sporadic AD is a subject of great debate. In support of its causative role is the finding that individuals with the single A673T mutation in APP (the residue within the Aβ peptide adjacent to the aspartyl protease (BACE) cleavage site) have a 40% reduction in amyloidogenic peptides and are strongly protected against AD [Jonsson et al, 2012]. Most Aβ is known to be generated following plasma membrane endocytosis of APP [Cirrito et al., 2008], but much of the endosomal cargo is trafficked to lysosomes [Lorenzen et al., 2010], where cathepsin B also cleaves APP at the BACE cleavage site [Hook et al., 2012]. Interestingly, memory deficits in a transgenic AD mouse model are improved by deletion of cathepsin B [Kindy et al., 2012].
Individuals who express a common polymorphism (ε4) of apolipoprotein E (ApoE) are more at risk for early-onset AD [Schmechel et al., 1993; Strittmatter et al., 1993; Nunomura et al., 1996; Xu et al., 2014]. ApoE is a lipid carrier that is important for cholesterol homeostasis in the brain. The ε4 isoform is most prone to proteolysis, generation of toxic fragments, as well as decreased Aβ binding and decreased Aβ clearing [Jones et al, 2011], all likely detrimental effects [Verghese et al., 2011; Wildsmith et al., 2013]. Several recent reports indicate that ApoE ε4 also may increase the risk of AD either by abnormally raising ROS production, if one carries a polymorphism in the NOX p22PHOX subunit [Fu et al., 2015], or by abnormally stimulating release of proinflammatory cytokines [Keene et al., 2011; Tai et al., 2015] (discussed in the cytokine hypothesis).
Extracellular amyloid plaques (aka senile plaques) are composed mainly of fibrils formed from the self-assembly of Aβ peptides, and, although a pathological hallmark of AD [Glenner and Wong, 1984a; Hardy and Selkoe, 2002; Mattson, 2004; Price, et al., 1995; Sisodia and Price, 1995; Tanzi and Bertram, 2005], plaques have been found in brains from subjects who had no signs of cognitive impairment at death. Additionally a Japanese cohort with progressive Alzheimer-type dementia has an Aβ variant missing glu22 (E22Δ) and has no senile plaques but does show enhanced Aβ assembly into small oligomers [Tomiyama et al., 2008]. Thus, plaques per se are not responsible for the wide spread synaptic loss and neurodegeneration of AD, but specific soluble forms of Aβ may be important in mediating synaptic loss associated with cognitive impairment (Figure 8).
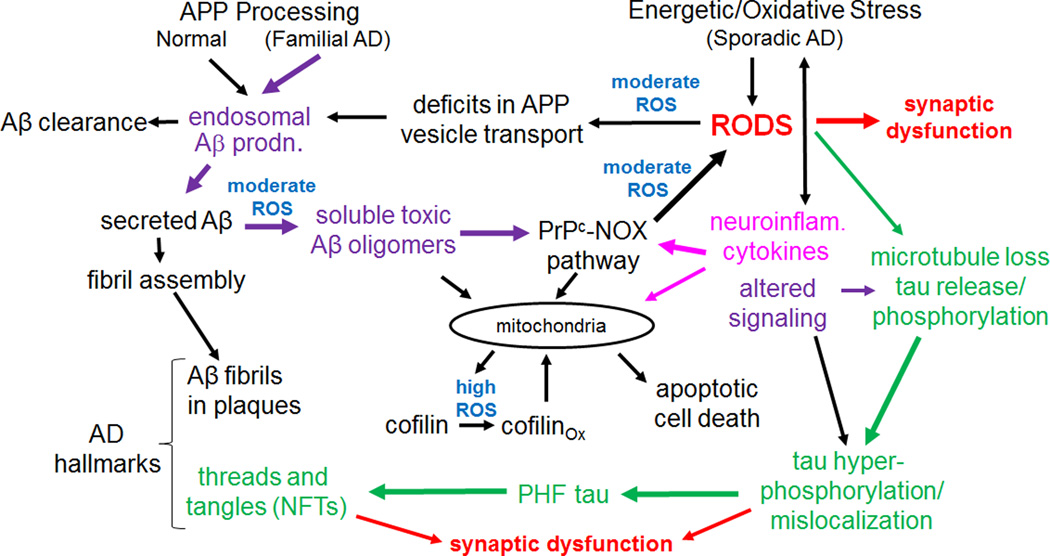
Figure 8. A hypothetical model in which rods play a central role in mediating AD progression.
Intermediates or initiators for the three major hypotheses for AD are shown in color: purple for the amyloid hypothesis, green for the tauopathy hypothesis and fuscia for the neuroinflammatory/cytokine hypothesis. Sites where reactive oxygen species are thought to be directly involved are in blue. In cognitively normal individuals, Aβ produced in endosomes is cleared or it may form fibrils and be sequestered into extracellular plaques in the brain. Although found in familial and sporadic AD, plaques may also be found in cognitively normal subjects and thus by themselves do not cause dementia. Changes in the form of soluble Aβ, arising from oxidation or other modifications, produce a more rod-inducing and synaptotoxic form. Other initiators of sporadic AD, such as neuroinflammatory cytokines, initiate a moderate ROS response that leads to rods and greater amounts of Aβ production in a feed-forward pathway. We hypothesize that rod-induced microtubule disruption releases tau for phosphorylation and initiation of PHF-tau formation, although this could be directly mediated by altered signaling. Mitochondrial dysfunction resulting from many possible sources eventually produces high ROS levels that contribute to cofilin oxidation (two internal disulfide bonds) that target it to mitochondria and cause release of cytochrome c, initiating apoptosis. Since rods are reversible, cofilin-induced apoptosis may gradually increase during cycles of rod reversal and acute high ROS levels.
Hence the focus has turned to the effects of the soluble Aβ pool which contains many species including globular oligomers [Chromy et al., 2003; Tu et al., 2014]. Characterization of diverse Aβ processing and the functional significance of the resultant products have received much attention without yielding firm conclusions as to the nature of the basic building blocks [Benilova et al., 2014; Schilling et al, 2006; Wirths et al., 2009]. However, it is highly likely that the propensity of certain individuals to produce soluble synaptotoxic forms makes them more susceptible to AD. Moreover, comparative studies of human and rodent Aβ suggest that the phenolic hydroxyl group of the single tyrosine residue (tyr10) is critical for synaptoxicity. The lab rodent Aβ does not assemble into neurotoxic or synaptotoxic species nor is it able to generate the SDS-stable dityrosine cross-linked forms that are found in human Aβ when it is subjected to oxidation in the presence of physiological levels of Cu2+ [Atwood et al, 2004; Davis et al., 2011]. Multiple forms of oxidized Aβ1–40 and Aβ1–42 have been analyzed with mass spectrometry and immunoblotting. The precise oxidized forms responsible for dementia severity, perturbation of neuronal signal processing, LTP and LTD impairment, ROS and rod production and abnormal morphology are still being evaluated [Butterfield and Boyd-Kimball, 2005; Schilling et al., 2006; Shankar et al., 2008; Lauren et al., 2009; Wirths et al., 2009; Mc Donald et al., 2010; Frier et al., 2010; Moore et al., 2012; Kostylev et al., 2015].
A potent form of soluble Aβ was isolated by gel filtration from the culture medium of cells (7PA2 cells) expressing the Swedish human AβPP mutant [Cleary et al., 2005]. The synaptic deficits produced when it is applied to neurons at its secreted concentration of ~250 pM, were blocked by a specific Aβ monoclonal antibody (6E10). This fraction of SDS-stable Aβ dimers and trimers (Aβd/t), when injected intraventricularly into adult rat brain, caused transient (24 h) memory and learning deficits [Cleary et al., 2005]. Fractions of soluble Aβd/t extracted from human AD brain also bring about similar cognitive deficits [Shankar et al., 2008]. Aβd/t at ~250 pM is optimal for inducing cofilin-actin rods in about 20% of hippocampal neurons. These rods are reversed by 24 h after washout [Davis et al., 2011]. Importantly, synthetic Aβ1–42 assembled as oligomers contains very little SDS-stable dimer, but its oxidation in vitro, forming Aβ dityrosine dimers [Atwood et al., 2004], increases by 600 fold its rod-inducing activity [Davis et al., 2011]. Taken together, these results suggest that the oxidizing conditions present in aging human brain [Ansari and Scheff, 2010], may contribute to the development of synaptotoxic forms of soluble Aβ, which characterize progression toward AD rather than deposition into plaques (Figure 8). The findings are consistent with our hypothesis that cofilin-actin rods, induced by oxidized Aβ, may mediate the rapid but transient cognitive deficits caused by microinjection of Aβd/t into rat brain.
Two other rodent models have been studied to gain insight into both behavioral and pathogenic changes during aging: the naked mole rat and the Octodon degus (O. degus). The naked mole rat is the longest-lived rodent (34 year average life span) [Andziak et al., 2006; Andziak and Buffenstein, 2006] and no age-related cognitive deficits or AD-type brain pathology has been identified in spite of very high levels of Aβ and Aβ aggregates in its brain even at a young age [Edrey et al., 2013]. Very surprisingly, the naked mole rat brain has higher oxidative stress and a poorer antioxidant capacity than normal rat brain (Andziak et al., 2006; Edrey et al, 2014), but naked mole rat Aβ appears more stable and resistant to oxidative stress than Aβ from the laboratory mouse [Perez et al., 2009]. O. degus, a Chilean rodent with a life span of 7–9 years [Lee, 2004], may develop age-dependent cognitive impairment. It is the only natural rodent model that shows age-dependent human-type pathology (plaques and tangles) [Schliebs and Arendt, 2011; Inestrosa et al., 2005]. Surprisingly, these two rodents have an identical Aβ sequence, differing from human Aβ at only a single residue (his13 to arg) [Rodriguez et al., 2014; Groen et al., 2011]. However, this Aβ1–42 has the same neurotoxicity as human Aβ1–42 although it is somewhat less prone to aggregation [Edrey et al, 2013]. Understanding factors in the aging brain milieu of these two rodents that alter their Aβ species and/or assembled aggregates may reveal the triggers for generation of synaptotoxic species. The brains of aging O. degus have higher levels of oxidative stress markers than young animals along with an up-regulation of a compensatory mechanism to overcome Aβ-induced damage [Inestrosa et al., 2014]. Further studies in these unique rodents may well reveal what dictates synaptotoxicity of Aβ peptides.
Neurofibrillary tangles (NFTs) and tauopathies
Tau, a microtubule binding protein, has a core structure that is capable of assembling into paired helical filaments (PHF tau) (Figure 8). NFTs are formed intracellularly from the self-assembly of PHF-tau, but the majority of PHF tau forms in the neurites where it presents as neuropil threads [Velasco et al., 1998]. PHF tau assembly is enhanced by phosphorylation on many sites by a number of different kinases [Wang JZ et al., 2013]. Although many sites of phosphorylation are accessible on tau when it is microtubule bound, tau is released from microtubules in early stages of AD [Miyasaka et al., 2010]. Aβ neurotoxicity appears to be mediated early in AD by phosphorylation of tau ser262, a residue in one of the microtubule binding domains [Qureshi et al., 2013], which is likely to be less accessible when tau is bound to microtubules.
In contrast to Aβ senile plaques, the abundance of neuropil threads does correlate with dementia severity [Giannakopoulos et al., 2007]. Aggregates of PHF collect in cell bodies to form the larger NFTs, which have a characteristic flame-like structure as they extend into neurites [Wang JZ et al., 2013]. These structures are readily observed by histochemistry and thus have become a hallmark used by pathologists to confirm the diagnosis of AD.
Mutations in tau can enhance the formation of tau inclusions in the absence of amyloid plaques, leading to non-AD types of dementias, such as frontal-temporal dementia (FTD) [Lee and Leugers, 2012]. Because of these tauopathies, an alternate hypothesis for AD was proposed: a change in tau conformation causes synaptic loss and neurodegeneration. Although a role for Aβ in changing tau is consistent with this hypothesis, it is not a required factor since there are multiple tauopathies arising from tau mutations without excess Aβ production or plaque formation.
Tau neuropil threads and cofilin pathology are both present in AD brain, but their immunostaining at high magnification does not overlap [Rahman et al., 2014]. Because rods disrupt axonal microtubules [Minamide et al., 2000; Cichon et al., 2012], we suggest the release of tau following this disruption might be the source of tau initiating the hyperphosphorylation cascade (Figure 8), which starts within tau’s microtubule binding domains [Miyasaka et al., 2010; Qureshi et al., 2013]. Tau hyperphosphorylation can lead to neuropil thread formation (Figure 8). In this regard it is interesting for several reasons to note that the scaffolding protein 14-3-3ζ associates with rods during their later stages of maturation [Minamide et al., 2010]. Genetic evidence suggests 14-3-3ζ is an effector of tau phosphorylation on the microtubule binding domain epitopes [Hashiguchi et al., 2000], especially ser262 [Qureshi et al., 2013], as well as an enhancer of tau aggregation [Sluchanko and Gusev, 2011] and NFT development [Hernandez et al., 2004; Umahara et al., 2004]. Microtubule binding domain epitopes have been found associated with rods [Whiteman et al., 2009; 2011]. Polymorphisms in 14-3-3ζ and tau genes can interactively decrease AD risk [Mateo et al., 2008], also suggesting 14-3-3ζ may enhance PHF tau development.
Tau can induce cofilin-containing actin bundles through multiple domains [Moraga et al., 1993; He et al, 2009]. These bundles differ from rods by binding phalloidin. Expression in mice of a mutant (pro301 to leu) tau associated with FTDP-17 tauopathy, led to the development within many neuronal soma of cofilin- and actin-containing rod-like structures whose formation is enhanced by Aβ [Fulga et al., 2007]. Many actin-related cytoskeletal changes are associated with tauopathies reviewed in [Frost et al., 2015], suggesting that sequestering cofilin and actin into these structures might play a role in the neurodegenerative cascade in non-Aβ-dependent dementias. How these structures form within the cell body and their relationship to cofilin-actin rods in neurites are unknown but worthy of investigation.
Hyperphosphorylated tau was originally viewed as an intracellular toxic aggregate; however, abnormalities in neuronal structure and function in response to tau hyperphosphorylation may arise from its secretion [Tang et al., 2015]. Extracellular levels of both total tau and phospho-tau are significantly higher in cerebrospinal fluid (CSF) from AD patients than from cognitively normal controls [Green et al, 1999; Jensen et al, 1995]. Extracellular tau is toxic to neuronal cells [Gomez-Ramos et al, 2006, 2008] and has been proposed to spread tau pathology through cell-to-cell transmission [Mohamed et al., 2013]. Tau secretion is mediated by the mTor pathway [Tang et al., 2015], a pathway that is modulated by Aβ and is also proposed to alter dendritic spine morphology at least in part through cofilin activity [Pozueta et al., 2013; Bosch et al., 2014].
Chronic Neuroinflammation and the Cytokine Hypothesis
The cytokine hypothesis of AD states that in response to stress from a variety of risk factors, both genetic and environmental, microglia and astrocytes become activated and produce excess amounts of the immune-modulating cytokines, such as interleukin-1 (IL-1β), and the neuritogenic cytokine S100B [Eikelenboom et al., 2011; Griffin and Barger, 2010; Yirmiya and Goshen, 2011]. This induction of reactive glia parallels the formation of neurofibrillary tangles during the progression of AD [Serrano-Pozo et al., 2011]. Through their release of proinflammatory cytokines, both microglia and astrocytes are mediators of Aβ-neurotoxicity and tau phosphorylation [Garwood et al., 2011]. Recent work suggests that these changes in cytokines precede rather than follow the AD-related pathology, appearing early in brains of subjects with Down syndrome [Griffin and Barger, 2010], all of whom eventually develop characteristic AD pathology. The S100B may lead to the growth of dystrophic neurites, and IL-1β induces expression of APP, which likely favors Aβ production. Together these findings suggest that cytokines may lead to plaque formation, which, as discussed previously, by itself does not cause dementia.
The proinflammatory cytokine IL-1β is primarily produced and released in the CNS by microglia [Yirmiya and Goshen, 2011]. It then stimulates astrocytes to release other cytokines, among which is another proinflammatory cytokine TNFα, which has potent effects on neurons. Release of GABA and glutamate from neurons stimulates microglia to release more IL-1β in a feed-forward response [Yirmiya and Goshen, 2011]. This response has led to a corollary for the cytokine hypothesis which states that regardless of the primary cause of the neuronal insult, the result will be chronic glial activation, which in turn results in further cytokine expression, reduced production of neurotrophins, and greater neuronal injury.
Proinflammatory cytokines (IL-1β, IL6 and TNFα) induce cofilin-actin rods in the same subpopulation of hippocampal neurons as Aβd/t and with the same time-course of formation. Although each of these cytokines has a unique receptor, they all induce rods via the PrPC- and NOX-dependent pathway used by Aβd/t [Walsh et al., 2014]. Taken together with all of the previous data that relates rod formation to changes accompanying AD progression, the entirety of findings suggest that local cofilin activation and sequestration into rods is a common pathway of Aβ and inflammatory cytokine signaling that leads to synaptic loss under a wide variety of neuronal stress conditions. It remains an open question as to whether rods can initiate tau hyperphosphorylation.
Conclusions and Perspectives
The generation of cofilin-actin rods and their deleterious effects when sustained provide an attractive molecular explanation for the development of AD pathology. It is attractive in part because it bridges and unites all three hypotheses previously described. In the case of the amyloid hypothesis, rods may mediate the synapse dysfunction induced by the soluble, most neurotoxic forms of Aβ: 1. Rods are generated by these forms at Aβ concentrations well within the physiological range known to be harmful, and 2. In sporadic AD, rods, generated through age-related oxidative stress, may stall APP-vesicle transport [Maloney et al., 2005], thus elevating Aβ production in a feed-forward cycle that links the production of Aβ to metabolic or environmental inducers of SAD. In this regard, a 2–3 fold increase in rat Aβ secretion occurs in cultured dissociated rat neurons or hippocampal slices treated with human Aβd/t at 250 pM [Marsden et al., 2011] although it is unknown how much of this increase arises because of induced rods. Rod-induced loss of microtubules may provide a mechanism for initiating or enhancing PHF tau formation and may initiate and/or promote the tau pathology that develops in AD. Cofilin-actin rod formation also provides an attractive molecular mechanism for the chronic neuroinflammatory hypothesis of AD in which Aβ has only a secondary role. In all three cases, rods exacerbate the oxidative cascade of neurodegeneration by accelerating mitochondrial decline and ATP depletion and disrupting essential actin dynamics.
The evidence presented here overwhelmingly supports the sufficiency of rods to cause synapse loss such as occurs in AD progression. However, neurites of neurons in culture can degenerate without rods ever forming, so evidence for the necessity of rods for synapse loss in vivo remains to be determined. Such evidence might be obtained if it were possible to generate a mouse expressing a mutant form of cofilin that maintains WT cofilin activities but never forms rods in the absence of WT cofilin. Because formation of rods transiently protects mitochondria in stressed neurites and is competitive with intramolecular oxidation of cofilin that triggers apoptosis and necrosis, such a mouse model also could be used to investigate the role of rods in neuronal survival. If rod formation does not play a significant role in protecting neurons from apoptotic or necrotic degeneration, they could be an excellent therapeutic target for treatment of AD. Decreasing either cofilin dephosphorylation or total levels of cofilin expression have already proven to be effective in reducing the cognitive deficits in the Aβ-overproducing mouse [Woo et al., 2015a,b], so reagents inhibiting slingshot phosphatase or activating LIM kinase would be likely starting points. However, many other approaches, especially those mediating the PrPC and NOX-dependent pathway, are likely to be fruitful, especially given the broad role of oxidative stress in neurodegeneration.
Acknowledgments
The authors are grateful for comments, suggestions and figures contributed by Alisa Shaw, Laurie Minamide, and O’Neil Wiggan and for our wonderful collaborators around the world without whom much of the work reviewed here could never have been accomplished. Supported in part by funding from NIH grants AG044812, AG049668, Camille and Henry Dreyfus Sr. Scientist Mentor Award, and a CSU Core Infrastructure Grant for Microscope Imaging.
Footnotes
The authors have no conflict of interest to declare.
References
- Agnew BJ, Minamide LS, Bamburg JR. Reactivation of phosphorylated actin depolymerizing factor and identification of the regulatory site. J Biol Chem. 1995;270:17582–17587. doi: 10.1074/jbc.270.29.17582. [DOI] [PubMed] [Google Scholar]
- Agostini F, Dotti CG, Perez-Canamas A, Ledesma MD, Benetti F, Legname G. Prion protein accumulation in lipid rafts of mouse aging brain. PLOS ONE. 2013;8:e74244. doi: 10.1371/journal.pone.0074244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association. 2015 Alzheimer’s disease facts and figures. Alzheimer’s & Dementia. 2015;11:332–384. doi: 10.1016/j.jalz.2015.02.003. [DOI] [PubMed] [Google Scholar]
- Andrianantoandro E, Pollard TD. Mechanism of actin filament turnover by severing and nucleation at different concentrations of ADF/cofilin. Mol Cell. 2006;24:13–23. doi: 10.1016/j.molcel.2006.08.006. [DOI] [PubMed] [Google Scholar]
- Andziak B, Buffenstein R. Disparate patterns of age-related changes in lipid peroxidation in long-lived naked mole-rats and shorter lived mice. Aging Cell. 2006;5:525–532. doi: 10.1111/j.1474-9726.2006.00246.x. [DOI] [PubMed] [Google Scholar]
- Andziak B, O’Connor TP, Qi W, DeWaal EM, Pierce A, Chaudhuri AR, Van Remmen H, Buffenstein R. High oxidative damage levels in the longest-living rodent, the naked mole-rat. Aging Cell. 2006;5:463–471. doi: 10.1111/j.1474-9726.2006.00237.x. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer’s disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansari MA, Scheff SW. NADPH oxidase activation and cognition in Alzheimer disease progression. Free Radic Biol Med. 2011;51:171–178. doi: 10.1016/j.freeradbiomed.2011.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arber S, Barbayannis FA, Hanser H, Schneider C, Stanyon CA, Bernard O, Caroni P. Regulation of actin dynamics through phosphorylation of cofilin by LIM-kinase. Nature. 1998;393:805–809. doi: 10.1038/31729. [DOI] [PubMed] [Google Scholar]
- Atkinson SJ, Hosford MA, Molitoris BA. Mechanism of actin polymerization in cellular ATP depletion. J Biol Chem. 2004;279:5194–5199. doi: 10.1074/jbc.M306973200. [DOI] [PubMed] [Google Scholar]
- Atwood CS, Perry G, Zeng H, Kato Y, Jones WD, Ling K-Q, Huang X, Moir RD, Wang D, Sayre LM, et al. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry. 2004;43:560–568. doi: 10.1021/bi0358824. [DOI] [PubMed] [Google Scholar]
- Barone E, Mosser S, Fraering PC. Inactivation of brain cofilin-1 by age, Alzheimer’s disease and γ-secretase. Biochim Biophys Acta. 2014;1842:2500–2509. doi: 10.1016/j.bbadis.2014.10.004. [DOI] [PubMed] [Google Scholar]
- Bate C, Williams A. Amyloid-β-induced synapse damage is mediated via cross-linking of cellular prion proteins. J Biol Chem. 2011;286:37955–37963. doi: 10.1074/jbc.M111.248724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck H, Flynn K, Lindenberg KS, Schwarz H, Bradke F, Di Giovanni S, Knöll B. Serum response factor (SRF)-cofilin-actin signaling axis modulates mitochondrial dynamics. Proc Nat Acad Sci USA. 2012;109:E2523–E2532. doi: 10.1073/pnas.1208141109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin BJ, Lee T, Mullins RD. DNA damage induces nuclear actin filament assembly by formin-2 and spire-1/2 that promotes efficient DNA repair. eLIFE. 2015;2015 doi: 10.7554/eLife.07735. 10.7554/eLife.07735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belmont LD, Orlova A, Drubin DG, Egelman EH. A change in actin conformation associated with filament instability after Pi release. Proc Natl Acad Sci USA. 1999;96:29–34. doi: 10.1073/pnas.96.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benilova I, De Strooper B. Neuroscience. Promiscuous Alzheimer amyloid: yet another binding partner. Science. 2013;341:1354–1355. doi: 10.1126/science.1244166. [DOI] [PubMed] [Google Scholar]
- Benilova I, Gallardo R, Ungureanu A-A, Cano VC, Snellix A, Ramakers M, Bartic C, Rousseau F, Schymkowitz J, De Strooper B. The Alzheimer disease protective mutation A2T modulates kinetic and thermodynamic properties of amyoid-β (Aβ) aggregation. J Biol Chem. 2014;289:30977–30989. doi: 10.1074/jbc.M114.599027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. Actin-ATP hydrolysis is a major energy drain for neurons. J Neurosci. 2003;23:1–6. doi: 10.1523/JNEUROSCI.23-01-00002.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Bamburg JR. ADF/cofilin: a functional node in cell biology. Trends Cell Biol. 2010;20:187–195. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernstein BW, Painter WB, Chen H, Minamide LS, Abe H, Bamburg JR. Intracellular pH modulation of ADF/cofilin proteins. Cell Motil Cytoskeleton. 2000;47:319–336. doi: 10.1002/1097-0169(200012)47:4<319::AID-CM6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Chen H, Boyle JA, Bamburg JR. Formation of actin-ADF/cofilin rods transiently retards decline of mitochondrial potential and ATP in stressed neurons. Am J Physiol Cell Physiol. 2006;291:C828–C839. doi: 10.1152/ajpcell.00066.2006. [DOI] [PubMed] [Google Scholar]
- Bernstein BW, Shaw AE, Minamide LS, Pak CW, Bamburg JR. Incorporation of cofilin into rods depends on disulfide intermolecular bonds: implications for actin regulation and neurodegenerative disease. J Neurosci. 2012;32:6670–6681. doi: 10.1523/JNEUROSCI.6020-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertram L, Tanzi RE. The genetics of Alzheimer’s disease. Prog Mol Biol Transl Sci. 2012;107:79–100. doi: 10.1016/B978-0-12-385883-2.00008-4. [DOI] [PubMed] [Google Scholar]
- Blanchoin L, Pollard TD. Mechanism of interaction of Acanthamoeba actophorin (ADF/cofilin) with actin filaments. J Biol Chem. 1999;274:15538–15546. doi: 10.1074/jbc.274.22.15538. [DOI] [PubMed] [Google Scholar]
- Bosch M, Castro J, Saneyoshi T, Matsuno H, Sur M, Hayashi Y. Structural and molecular remodeling of dendritic spine substructures during long-term potentiation. Neuron. 2014;82:444–458. doi: 10.1016/j.neuron.2014.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryce NS, Clark ES, Leysath JL, Currie JD, Webb DJ, Weaver AM. Cortactin promotes cell motility by enhancing lamellipodial persistence. Curr Biol. 2005;15:1276–1285. doi: 10.1016/j.cub.2005.06.043. [DOI] [PubMed] [Google Scholar]
- Bugyi B, Carlier MF. Control of actin filament treadmilling in cell motility. Annu Rev Biophys. 2010;39:449–470. doi: 10.1146/annurev-biophys-051309-103849. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Boyd-Kimball D. The critical role of methionine 35 in Alzheimer’s amyloid beta-peptide (1–42)-induced oxidative stress and neurotoxicity. Biochim Biophys Acta. 2005;1703:149–156. doi: 10.1016/j.bbapap.2004.10.014. [DOI] [PubMed] [Google Scholar]
- Cameron JM, Gabrielsen M, Chim YH, Munro J, McGhee EJ, Sumpton D, Eaton P, Anderson KI, Yin H, Olson MF. Polarized cell motility induces hydrogen peroxide to inhibit cofilin via cysteine oxidation. Curr Biol. 2015;25:1520–1525. doi: 10.1016/j.cub.2015.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Laurent V, Santolini J, Melki R, Didry D, Xia GX, Hong Y, Chua NH, Pantaloni D. Actin depolymerizing factor (ADF/cofilin) enhances the rate of filament turnover: implication in actin-based motility. J Cell Biol. 1997;136:1307–1322. doi: 10.1083/jcb.136.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier MF, Ressad F, Pantaloni D. Control of actin dynamics in cell motility. Role of ADF/cofilin. J Biol Chem. 1999;274:33827–33830. doi: 10.1074/jbc.274.48.33827. [DOI] [PubMed] [Google Scholar]
- Cataldo AM, Petanceska S, Peterhoff CM, Terio NB, Epstein CJ, Villar A, Carlson EJ, Staufenbiel M, Nixon RA. APP gene dosage modulates endosomal abnormalities of Alzheimer's disease in a segmental trisomy 16 mouse model of down syndrome. J Neurosci. 2003;23:6788–6792. doi: 10.1523/JNEUROSCI.23-17-06788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan C, Beltzner CC, Pollard TD. Cofilin dissociates Arp2/3 complex and branches from actin filaments. Curr Biol. 2009;19:537–545. doi: 10.1016/j.cub.2009.02.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Hamandi K, Mullan M, Goate A, Hardy J, Backhovens H, Martin JJ, Broeckhoven CV. Screening for the beta-amyloid precursor protein mutation (APP717: Val----Ile) in extended pedigrees with early onset Alzheimer's disease. Neurosci Lett. 1991a;129:134–135. doi: 10.1016/0304-3940(91)90738-f. [DOI] [PubMed] [Google Scholar]
- Chartier-Harlin MC, Crawford F, Houlden H, Warren A, Hughes D, Fidani L, Goate A, Rossor M, Roques P, Hardy J, et al. Early-onset Alzheimer's disease caused by mutations at codon 717 of the beta-amyloid precursor protein gene. Nature. 1991b;353:844–846. doi: 10.1038/353844a0. [DOI] [PubMed] [Google Scholar]
- Chen H, Bernstein BW, Sneider JM, Boyle JA, Minamide LS, Bamburg JR. In vitro activity differences between proteins of the ADF/cofilin family define two distinct subgroups. Biochemistry. 2004;43:7127–7142. doi: 10.1021/bi049797n. [DOI] [PubMed] [Google Scholar]
- Chen Q, Courtemanche N, Pollard TD. Aip1 promotes actin filament severing by cofilin and regulates constriction of the cytokinetic contractile ring. J Biol Chem. 2015;290:2289–2300. doi: 10.1074/jbc.M114.612978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintamaneni M, Bhaskar M. Biomarkers in Alzheimer’s disease: a review. ISRN Pharmacol 2012. 2012;2012:984786. doi: 10.5402/2012/984786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chromy BA, Nowak RJ, Lambert MP, Viola KL, Chang L, Velasco PT, Jones BW, Fernandez SJ, Lacor PN, Horowitz P, et al. Self-assembly of Aβ1–42 into globular neurotoxins. Biochemistry. 2003;42:12749–12760. doi: 10.1021/bi030029q. [DOI] [PubMed] [Google Scholar]
- Chua BT, Volbracht C, Tan KO, Li R, Yu VC, Li P. Mitochondrial translocation of cofilin is an early step in apoptosis induction. Nat Cell Biol. 2003;5:1083–1089. doi: 10.1038/ncb1070. [DOI] [PubMed] [Google Scholar]
- Cichon J, Sun C, Chen B, Jiang M, Chen XA, Sun Y, Wang Y, Chen G. Cofilin aggregation blocks intracellular trafficking and induces synaptic loss in hippocampal neurons. J Biol Chem. 2012;287:3919–3929. doi: 10.1074/jbc.M111.301911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirrito JR, Kang JE, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- Creegan R, Hunt W, McManus A, Rainey-Smith SR. Diet, nutrients and metabolism: cogs in the wheel driving Alzheimer’s disease pathology? Br J Nut. 2015;113:1499–1517. doi: 10.1017/S0007114515000926. [DOI] [PubMed] [Google Scholar]
- Dahl J-U, Gray MJ, Jakob U. Protein quality control under oxidative stress conditions. J Mol Biol. 2015;427:1549–1563. doi: 10.1016/j.jmb.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RC, Maloney MT, Minamide LS, Flynn KC, Stonebraker MA, Bamburg JR. Mapping cofilin-actin rods in stressed hippocampal slices and the role of cdc42 in amyloid-beta-induced rods. J Alzheimers Dis. 2009;18:35–50. doi: 10.3233/JAD-2009-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RC, Marsden IT, Maloney MT, Minamide LS, Podlisny M, Selkoe DJ, Bamburg JR. Amyloid beta dimers/trimers potently induce cofilin-actin rods that are inhibited by maintaining cofilin phosphorylation. Mol Neurodegen. 2011;6:10. doi: 10.1186/1750-1326-6-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delorme-Walker V, Seo J-Y, Gohla A, Fowler B, Bohl B, DerMardirossian C. Chronophin coordinates cell leading edge dynamics by controlling active cofilin levels. Proc Nat Acad Sci USA. 2015;112:E5150–E5159. doi: 10.1073/pnas.1510945112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domazetovska A, Ilkovski B, Cooper ST, Ghoddusi M, Hardeman EC, Minamide LS, Gunning PW, Bamburg JR, North KN. Mechanisms underlying intranuclear rod formation. Brain. 2007;130:3275–3284. doi: 10.1093/brain/awm247. [DOI] [PubMed] [Google Scholar]
- Edrey YH, Medina DX, Gaczynska M, Osmulski PA, Oddo S, Caccamo A, Buffenstein R. Amyloid beta and the longest-lived rodent: the naked mole-rat as a model for natural protection from Alzheimer’s disease. Neurobiol Aging. 2013;34:2352–2360. doi: 10.1016/j.neurobiolaging.2013.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edrey YH, Oddo S, Cornelius C, Caccamo A, Calabrese V, Buffenstein R. Oxidative damage and amyloid-β metabolism in brain regions of the longest-lived rodents. J Neurosci Res. 2014;92:195–205. doi: 10.1002/jnr.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards DC, Sanders LC, Bokoch GM, Gill GN. Activation of LIM-kinase by Pak1 couples Rac/Cdc42 GTPase signaling to actin cytoskeletal dynamics. Nat Cell Biol. 1999;1:253–259. doi: 10.1038/12963. [DOI] [PubMed] [Google Scholar]
- Eikelenboom P, Veerhuis R, van Exel E, Hoozemans JJ, Rozemuller AJ, van Gool WA. The early involvement of innate immunity in the pathogenesis of late-onset Alzheimer’s disease: neuropathological, epidemiological and genetic evidence. Curr Alzheimers Res. 2011;8:142–150. doi: 10.2174/156720511795256080. [DOI] [PubMed] [Google Scholar]
- Eiseler T, Doppler H, Yan IK, Kitatani K, Mizuno K, Storz P. Protein kinase D1 regulates cofilin-mediated F-actin reorganization and cell motility through slingshot. Nat Cell Biol. 2009;11:545–556. doi: 10.1038/ncb1861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman HJ, Maiorino M, Ursini F. Signaling functions of reactive oxygen species. Biochemistry. 2010;49:835–842. doi: 10.1021/bi9020378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM. Aβ oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiol Aging. 2010;32:2211–2218. doi: 10.1016/j.neurobiolaging.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost B, Gotz J, Feany MB. Connecting the dots between tau dysfunction and neurodegeneration. Trends Cell Biol. 2015;25:46–53. doi: 10.1016/j.tcb.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Q, Qi F, Tian F, Ma G, Che F, Du Y, Gao N. Association of p22phox polymorphism C242T with risk of late-onset Alzheimer’s disease in a northern Han Chineses population. Int J Neurosci. 2015 doi: 10.3109/00207454.2015.1052877. doi.org/10.3109/00207454.215.1052877. [DOI] [PubMed] [Google Scholar]
- Fukui Y, Katsumaru H. Nuclear actin bundles in Amoeba, Dictyostelium and human HeLa cells induced by dimethyl sulfoxide. Exp Cell Res. 1979;120:451–455. doi: 10.1016/0014-4827(79)90412-9. [DOI] [PubMed] [Google Scholar]
- Fulga TA, Elson-Schwab I, Khurana V, Steinhilb ML, Spires TL, Hyman BT, Feany MB. Abnormal bundling and accumulation of F-actin mediates tau-induced neuronal degeneration in vivo. Nat Cell Biol. 2007;9:139–148. doi: 10.1038/ncb1528. [DOI] [PubMed] [Google Scholar]
- Gabrielsen M, Schuldt M, Munro J, Borucka D, Cameron J, Baugh M, Mleczak A, Lilla S, Morrice N, Olson MF. Cucurbitacin covalent bonding to cysteine thiols: the filamentous-actin severing protein Cofilin-1 as an exempliary target. Cell Commun Signal. 2013;11:58. doi: 10.1186/1478-811X-11-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galkin VE, Orlova A, Kudryashov DS, Solodukhin A, Reisler E, Schroeder GF, Egelman EH. Remodeling of actin filaments by ADF/cofilin proteins. Proc Natl Acad Sci USA. 2011;108:20568–20572. doi: 10.1073/pnas.1110109108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garvalov BK, Flynn KC, Neukirchen D, Meyn L, Teusch N, Brakebusch C, Bamburg JR, Bradke F. Cdc42 regulates cofilin during the establishment of neuronal polarity. J Neurosci. 2007;27:13117–13129. doi: 10.1523/JNEUROSCI.3322-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwood CJ, Pooler AM, Atherton J, Hanger DP, Noble W. Astrocytes are important mediators of Aβ-induced neurotoxicity and tau phosphorylation in primary culture. Cell Death Dis. 2011;2:e167. doi: 10.1038/cddis.2011.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng D, Kang L, Su Y, Jia J, Ma J, Du SLJ, Cui H. Protective effects of Eph2B on Aβ1–42 oligomer-induced neurotoxicity and synaptic NMDA receptor signaling in hippocampal neurons. Neurochem Int. 2013;63:283–290. doi: 10.1016/j.neuint.2013.06.016. [DOI] [PubMed] [Google Scholar]
- Giannakopoulos P, von Gunten A, Kovari E, Gold G, Herrman FR, Hof PR, Bouras C. Stereological analysis of neuropil threads in the hippocampal formation: relationships with Alzheimer's disease neuronal pathology and cognition. Neuropath Appl Neuro. 2007;33:334–343. doi: 10.1111/j.1365-2990.2007.00827.x. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer's disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984a;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- Glenner GG, Wong CW. Alzheimer’s disease and Down’s syndrome: sharing of a unique cerebrovascular amyloid fibril protein. Biochem Biophys Res Commun. 1984b;122:1131–1135. doi: 10.1016/0006-291x(84)91209-9. [DOI] [PubMed] [Google Scholar]
- Goate A, Chartier-Harlin MC, Mullan M, Brown J, Crawford F, Fidani L, Giuffra L, Haynes A, Irving N, James L, et al. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991;349:704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Gohla A, Bokoch GM. 14-3-3 regulates actin dynamics by stabilizing phosphorylated cofilin. Curr Biol. 2002;12:1704–1710. doi: 10.1016/s0960-9822(02)01184-3. [DOI] [PubMed] [Google Scholar]
- Gohla A, Birkenfeld J, Bokoch GM. Chronophin, a novel HAD-type serine protein phosphatase, regulates cofilin-dependent actin dynamics. Nat Cell Biol. 2005;7:21–29. doi: 10.1038/ncb1201. [DOI] [PubMed] [Google Scholar]
- Gomez-Ramos A, Diaz-Hernandez M, Cuadros R, Hernandez F, Avila J. Extracellular tau is toxic to neuronal cells. FEBS Lett. 2006;580:4842–4850. doi: 10.1016/j.febslet.2006.07.078. [DOI] [PubMed] [Google Scholar]
- Gomez-Ramos A, Diaz-Hernandez M, Rubio A, Miras-Portugal MT, Avila J. Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells. Mol Cell Neurosci. 2008;37:673–681. doi: 10.1016/j.mcn.2007.12.010. [DOI] [PubMed] [Google Scholar]
- Green AJ, Harvey RJ, Thompson EJ, Rossor MN. Increased tau in the cerebrospinal fluid of patients with frontotemporal dementia and Alzheimer's disease. Neurosci Lett. 1999;259:133–135. doi: 10.1016/s0304-3940(98)00904-5. [DOI] [PubMed] [Google Scholar]
- Griffin WST, Barger SW. Neuroinflammatory cytokines- the common thread in Alzheimer’s pathogenesis. US Neurol. 2010;6:19–27. [PMC free article] [PubMed] [Google Scholar]
- Groen TV, Kadish I, et al. Age-related brain pathology in Octodon degu: Blood vessel, white matter and Alzheimer-like pathology. Neurobiol Aging. 2011;32:1651–1661. doi: 10.1016/j.neurobiolaging.2009.10.008. [DOI] [PubMed] [Google Scholar]
- Gu J, Lee CW, Fan Y, Komols D, Tang X, Sun C, Chen G, Yu K, Hartzell HC, Bamburg JR, et al. ADF/cofilin-mediated actin dynamics regulate AMPA receptor trafficking during synaptic plasticity. Nat Neurosci. 2010;13:1208–1215. doi: 10.1038/nn.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- Hashiguchi M, Sobue K, Paudel HK. 14-3-3ζ is an effector of tau protein phosphorylation. J Biol Chem. 2000;275:25247–25254. doi: 10.1074/jbc.M003738200. [DOI] [PubMed] [Google Scholar]
- Hayden SM, Miller PS, Brauweiler A, Bamburg JR. Analysis of the interactions of actin depolymerizing factor with G- and F-actin. Biochemistry. 1993;32:9994–10004. doi: 10.1021/bi00089a015. [DOI] [PubMed] [Google Scholar]
- He HJ, Wang XS, Pan R, Wang DL, Liu MN, He RQ. The proline-rich domain of tau plays a role in interactions with actin. BMC Cell Biol. 2009;10:81. doi: 10.1186/1471-2121-10-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques AG, Oliveira JM, Carvalho LP, da Cruz e Silva OAB. Aβ influences cytoskeletal signaling cascades with consequences to Alzheimer's disease. Mol Neurobiol. 2015;52:1391–1407. doi: 10.1007/s12035-014-8913-4. [DOI] [PubMed] [Google Scholar]
- Heredia L, Helguera P, de Olmos S, Kedikian G, Sola Vigo F, LaFerla F, Staufenbiel M, de Olmos J, Busciglio J, Caceres A, et al. Phosphorylation of actin-depolymerizing factor/cofilin by LIM-kinase mediates amyloid beta-induced degeneration: a potential mechanism of neuronal dystrophy in Alzheimer's disease. J Neurosci. 2006;26:6533–6542. doi: 10.1523/JNEUROSCI.5567-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez F, Cuadros R, Avila J. Zeta 14-3-3 protein favours the formation of human tau fibrillar polymers. Neurosci Lett. 2004;357:143–146. doi: 10.1016/j.neulet.2003.12.049. [DOI] [PubMed] [Google Scholar]
- Hirsch TZ, Hernandez-Rapp J, Martin-lanneree S, Launay JM, Mouillet-Richard S. PrPc signalling in neurons: from basics to clinical challenges. Biochemie. 2014;104:2–11. doi: 10.1016/j.biochi.2014.06.009. [DOI] [PubMed] [Google Scholar]
- Hook V, Funkelstein L, Wegrzyn J, Bark S, Kindy M, Hook G. Cysteine cathepsins in the secretory vesicle produce active peptides: Cathepsin L generates peptide neurotransmitters and cathepsin B produces beta-amyloid of Alzheimer disease. Biochim Biophys Acta. 2012;1824:89–104. doi: 10.1016/j.bbapap.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotulainen P, Paunola E, Vartiainen MK, Lappalainen P. Actin-depolymerizing factor and cofilin-1 play overlapping roles in promoting rapid F-actin depolymerization in mammalian nonmuscle cells. Mol Biol Cell. 2005;16:649–664. doi: 10.1091/mbc.E04-07-0555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang TY, Der Mardirossian C, Bokoch GM. Cofilin phosphatases and regulation of actin dynamics. Curr Opin Cell Biol. 2006;18:26–31. doi: 10.1016/j.ceb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Huang TY, Minamide LS, Bamburg JR, Bokoch GM. Chronophin mediates an ATP-sensing mechanism for cofilin dephosphorylation and neuronal cofilin-actin rod formation. Dev Cell. 2008;15:691–703. doi: 10.1016/j.devcel.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inestrosa NC, Reyes AE, Chacón MA, Cerpa W, Villalon A, Montiel J, Merabachvili G, Aldunate R, Bozinovic F, Abolitiz F. Human-like rodent amyloid-beta-peptide determines Alzheimer pathology in aged wild-type Octodon degu. Neurobiol Aging. 2005;26:1023–1028. doi: 10.1016/j.neurobiolaging.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Inestrosa NC, Ríos JA, Cisternas P, Tapia-Rojas C, Rivera DS, Braidy N, Zolezzi JM, Godoy JA, Carvajal FJ, Ardiles AO, et al. Age progression of neuropathological markers in the brain of the Chilean rodent Octodon degus, a natural model of Alzheimer's disease. Brain Pathology. 2014;25:679–691. doi: 10.1111/bpa.12226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen S, Collins A, Chin SM, Ydenberg CA, Gelles J, Goode BL. Single-molecule imaging of a three-component ordered actin disassembly mechanism. Nat Commun. 2015;6:7202. doi: 10.1038/ncomms8202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen M, Basun H, Lannfelt L. Increased cerebrospinal fluid tau in patients wityh Alzheimer's disease. Neurosci Lett. 1995;186:189–191. doi: 10.1016/0304-3940(95)11297-a. [DOI] [PubMed] [Google Scholar]
- Jones PB, Adams KW, Rozkalne A, Spire-Jones TL, Hshieh TT, Hashimoto T, von Armin CA, Mielke M, Bacskai BJ, Hyman BT. Apolipoprotein E: isoform specific differences in tertiary structure and interaction with amyloid-beta in human Alzheimer brain. PLoS One. 2011;6:e14586. doi: 10.1371/journal.pone.0014586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson T, Atwal JK, Steinberg S, Snaedal J, Jonsson PV, Bjornsson S, Stefansson H, Sulem P, Gudbjartsson D, Maloney J, et al. A mutation in APP protects against Alzheimer’s disease and age-related cognitive decline. Nature. 2012;488:96–99. doi: 10.1038/nature11283. [DOI] [PubMed] [Google Scholar]
- Kam T-I, Song S, Gwon Y, Park H, Yan J-J, Im I, Choi J-W, Choi T-Y, Kim J, Song D-K, et al. FcγRIIb mediates amyloid-β neurotoxicity and memory impairment in Alzheimer's disease. J Clin Invest. 2013;123:2791–2802. doi: 10.1172/JCI66827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman AC, Salazar SV, Haas LT, Yang J, Kostylev MA, Jeng AT, Robinson SA, Gunther EC, van Dyck CH, Nygaard HB, et al. Fyn inhibition rescues established memory and synapse loss in Alzheimer mice. Ann Neurol. 2015;77:953–971. doi: 10.1002/ana.24394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawakami-Schultz SV, Verdoni AM, Sattler SG, Jessen E, Kao WW, Ikeda A, Ikeda S. Serum response factor: positive and negative regulation of an epithelial gene expression nertwork in the destrin mutant cornea. Physiol Genomics. 2014;46:277–289. doi: 10.1152/physiolgenomics.00126.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keene CD, Cudaback E, Li X, Montine KS, Montine TJ. Apolipoproteins E isoforms and regulation of the innate immune response in brain of patients with Alzheimer’s disease. Curr Op Neurobiol. 2011;21:920–928. doi: 10.1016/j.conb.2011.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller JN, Schmitt FA, Scheff SW, Ding Q, Chen Q, Butterfield DA, Markesbery WR. Evidence of increased oxidative damage in subjects with mild cognitive impairment. Neurology. 2005;64:1152–1156. doi: 10.1212/01.WNL.0000156156.13641.BA. [DOI] [PubMed] [Google Scholar]
- Kim JS, Huang TY, Bokoch GM. Reactive oxygen species regulate a slingshot-cofilin activation pathway. Mol Biol Cell. 2009;20:2650–2660. doi: 10.1091/mbc.E09-02-0131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T, Vidal GS, Djurisic M, William CM, Birnbaum ME, Garcia KC, Hyman BT, Schatz CJ. Human LilrB2 is a β-amyloid receptor and its murine homolog PirB regulates synaptic plasticity in an Alzheimer's model. Science. 2013;341:1399–1404. doi: 10.1126/science.1242077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindy MS, Yu J, Zhu H, El-Amouri SS, Hook V, Hook GR. Deletion of the cathepsin B gene improves memory deficits in a transgenic Alzheimer’s disease mouse model expressing AβPP containing the wild-type β-secretase site sequence. J Alzheimers Dis. 2012;29:827–840. doi: 10.3233/JAD-2012-111604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klamt F, Zdanov S, Levine RL, Pariser A, Zhang Y, Zhang B, Yu LR, Veenstra TD, Shacter E. Nat Cell Biol. 2009;11:1241–1246. doi: 10.1038/ncb1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemke M, Wabnitz GH, Funke F, Funk B, Kirchgessner H, Samstag Y. Oxidation of cofilin mediates T-cell hyporesponsiveness under oxidative stress conditions. Immunity. 2008;29:404–413. doi: 10.1016/j.immuni.2008.06.016. [DOI] [PubMed] [Google Scholar]
- Kostylev MA, Kaufman AC, Nygaard HB, Patel P, Haas LT, Gunther EC, Vortmeyer A, Strittmatter SM. Prion-protein-interacting amyloid-β oligomers of high molecular weight are tightly correlated with memory impairment in multiple Alzheimer mouse models. J Biol Chem. 2015;290:17415–17438. doi: 10.1074/jbc.M115.643577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotiadis VN, Leadsham JE, Bastow EL, Gheeraert A, Whybrew JM, Bard M, Lappalainen P, Gourlay CW. Identification of new surfaces of cofilin that link mitochondrial function to control of multi-drug resistance. J Cell Sci. 2012;125:2288–2299. doi: 10.1242/jcs.099390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueh HY, Charras CT, Mitchison TJ, Brieher WM. Actin disassembly by cofilin, coronin, and Aip1 occurs in bursts and is inhibited by barbed-end cappers. J Cell Biol. 2008;182:341–353. doi: 10.1083/jcb.200801027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurita S, Watanabe Y, Gunji E, Ohashi K, Mizuno K. Molecular dissection of the mechansism of substrate recognition and F-actin-mediated activation of cofilin-phosphatase Slingshot-1. J Biol Chem. 2008;283:32542–32552. doi: 10.1074/jbc.M804627200. [DOI] [PubMed] [Google Scholar]
- Laske C. Alzheimer disease: Blood-based biomarkers in AD-a silver lining on the horizon. Nat Rev Neurol. 2012;8:541–542. doi: 10.1038/nrneurol.2012.173. [DOI] [PubMed] [Google Scholar]
- Lauren J, Gimbel DA, Nygaard HB, Gilbert JW, Strittmatter SM. Cellular prion protein mediates impairment of synaptic plasticity by amyloid-beta oligomers. Nature. 2009;457:1128–1132. doi: 10.1038/nature07761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee TM. Octodon degus: a diurnal, social, and long-lived rodent. ILAR J. 2004;45:14–24. doi: 10.1093/ilar.45.1.14. [DOI] [PubMed] [Google Scholar]
- Lee G, Leugers CJ. Tau and tauopathies. Prog Mol Biol Transl Sci. 2012;107:263–293. doi: 10.1016/B978-0-12-385883-2.00004-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin MC, Galletta BJ, Sept D, Cooper JA. Overlapping and distinct functions for cofilin, coronin and Aip1 in actin dynamics in vivo. J Cell Sci. 2010;123:1329–1342. doi: 10.1242/jcs.065698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzen A, Samosh J, Vandewark K, Anborgh PH, Seah C, Magalhaes AC, Cregan SP, Ferguson SS, Pasternak SH. Rapid and direct transport of cell surface APP to the lysosome defines a novel selective pathway. Mol Brain. 2010;3:11. doi: 10.1186/1756-6606-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loubet D, Dakowski C, Pietri M, Pradines E, Bernard S, Callebert J, Ardila-Osorio H, Mouillet-Richard S, Launay J-M, Kellermann O, et al. Neuritogenesis: the prion protein controls β1 integrin signaling activity. FASEB J. 2012;26:678–690. doi: 10.1096/fj.11-185579. [DOI] [PubMed] [Google Scholar]
- Maciver SK, Weeds AG. Actophorin preferentially binds monomeric ADP-actin over ATP-bound actin: consequences for cell locomotion. FEBS Lett. 1994;347:251–256. doi: 10.1016/0014-5793(94)00552-4. [DOI] [PubMed] [Google Scholar]
- Madineni A, Alhadidi Q, Shah ZA. Cofilin inhibition restores neuronal cell death in oxygen-glucose deprivation model of ischemia. Mol Neurobiol. 2014 Dec 20; doi: 10.1007/s12035-014-9056-3. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maloney MT, Minamide LS, Kinley AW, Boyle JA, Bamburg JR. Beta-secretase-cleaved amyloid precursor protein accumulates at actin inclusions induced in neurons by stress or amyloid beta: a feedforward mechanism for Alzheimer's disease. J Neurosci. 2005;25:11313–11321. doi: 10.1523/JNEUROSCI.3711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsden IT, Minamide LS, Bamburg JR. Amyloid-β-induced amyloid-β secretion: a possible feed-forward mechanism in Alzheimer's disease. J Alzheimers Dis. 2011;24:681–691. doi: 10.3233/JAD-2011-101899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateo I, Sanchez-Juan P, Rodriguez-Rodriguez E, Infante J, Fernande-Viadero C, Pena N, Berciano J, Combarros O. 14-3-3ζ and tau genes interactively decrease Alzheimer’s disease risk. Dement Geriatr Cogn Disord. 2008;25:317–320. doi: 10.1159/000119123. [DOI] [PubMed] [Google Scholar]
- Mattson MP. Pathways towards and away from Alzheimer's disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM. The presence of sodium dodecyl sulphate-stable Aβ dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGough A, Pope B, Chiu W, Weeds A. Cofilin changes the twist of F-actin: implications for actin filament dynamics and cellular function. J Cell Biol. 1997;138:771–781. doi: 10.1083/jcb.138.4.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi J, Shaw AE, Pak CW, Walsh KP, Minamide LS, Bernstein BW, Kuhn TB, Bamburg JR. A genetically encoded reporter for real-time imaging of cofilin-actin rods in neurons. PLoS One. 2013;8:e83609. doi: 10.1371/journal.pone.0083609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikati MA, Breitsprecher D, Jansen S, Reisler E, Goode BL. Coronin enhances actin filament severing by recruiting cofilin to filament sides and altering F-actin conformation. J Mol Biol. 2015;427:3137–3147. doi: 10.1016/j.jmb.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol. 2000;2:628–636. doi: 10.1038/35023579. [DOI] [PubMed] [Google Scholar]
- Minamide LS, Maiti S, Boyle JA, Davis RC, Coppinger JA, Bao Y, Huang TY, Yates J, Bokoch GM, Bamburg JR. Isolation and characterization of cytoplasmic cofilin-actin rods. J Biol Chem. 2010;285:5450–5460. doi: 10.1074/jbc.M109.063768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyasaka T, Sato S, Takebayashi Y, Takashima A. Microtubule destruction induces tau liberation and its subsequent phosphorylation. FEBS Lett. 2010;584:3227–3232. doi: 10.1016/j.febslet.2010.06.014. [DOI] [PubMed] [Google Scholar]
- Mohamed NV, Herrou T, Plouffe V, Piperno N, Leclerc N. Spreading of tau pathology in Alzheimer's disease by cell-to-cell transmission. Eur J Neurosci. 2013;37:1939–1948. doi: 10.1111/ejn.12229. [DOI] [PubMed] [Google Scholar]
- Moore BD, Chakrabarty P, Levites Y, Kukar TL, Baine AM, Moroni T, Ladd TB, Das P, Dickson DW, Golde TE. Overlapping profiles of Aβ peptides in the Alzhemier's disease and pathological aging brains. Alzheimers Res Ther. 2012;4:18. doi: 10.1186/alzrt121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moraga DM, Nunez P, Garrido J, Maccioni RB. A tau fragment containing a repetitive sequence induces bundling of actin filaments. J Neurochem. 1993;61:979–986. doi: 10.1111/j.1471-4159.1993.tb03611.x. [DOI] [PubMed] [Google Scholar]
- Morgan TE, Lockerbie RO, Minamide LS, Browning MD, Bamburg JR. Isolation and characterization of a regulated form of actin depolymerizing factor. J Cell Biol. 1993;122:623–633. doi: 10.1083/jcb.122.3.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Yahara I. Two activities of cofilin, severing and accelerating directional depolymerization of actin filaments, are affected differentially by mutations around the actin-binding helix. EMBO J. 1999;18:6752–6761. doi: 10.1093/emboj/18.23.6752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriyama K, Iide K, Yahara I. Phosphorylation of Ser-3 of cofilin regulates its essential function on actin. Genes Cells. 1996;1:73–86. doi: 10.1046/j.1365-2443.1996.05005.x. [DOI] [PubMed] [Google Scholar]
- Morton WM, Ayscough KR, McLaughlin PJ. Latrunculin alters the actin-monomer subunit interface to prevent polymerization. Nat Cell Biol. 2000;2:376–378. doi: 10.1038/35014075. [DOI] [PubMed] [Google Scholar]
- Munsie LN, Truant R. The role of cofilin-actin rod stress response in neurodegenerative diseases uncovers potential new drug targets. Bioarchitecture. 2012;2:203–208. doi: 10.4161/bioa.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munsie LN, Desmond CR, Truant R. Cofilin nuclear-cytoplasmic shuttling affects cofilin-actin rod formation during stress. J Cell Sci. 2012;125:3977–3988. doi: 10.1242/jcs.097667. [DOI] [PubMed] [Google Scholar]
- Murrell J, Farlow M, Ghetti B, Benson MD. A mutation in the amyloid precursor protein associated with hereditary Alzheimer's disease. Science. 1991;254:97–99. doi: 10.1126/science.1925564. [DOI] [PubMed] [Google Scholar]
- Murrin LC, Talbot JN. RanBPM, a scaffolding protein in the immune and nervous systems. J Neuroimmune Pharmacol. 2007;2:290–295. doi: 10.1007/s11481-007-9079-x. [DOI] [PubMed] [Google Scholar]
- Nadkarni AV, Brieher WM. Aip1 destabilizes cofilin-saturated actin filaments by severing and accelerating monomer dissociation from ends. Curr Biol. 2014;24:2749–2757. doi: 10.1016/j.cub.2014.09.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata-Ohashi K, Ohta Y, Goto K, Chiba S, Mori R, Nishita M, Ohashi K, Kousaka K, Iwamatsu A, Niwa R, et al. A pathway of neuregulin-induced activation of cofilin-phosphatase Slingshot and cofilin in lamellipodia. J Cell Biol. 2004;165:465–471. doi: 10.1083/jcb.200401136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson P, Wang W-X. miR-107 is reduced in Alzheimer's disease brain neocortex: validation study. J Alzheimer’s Dis. 2010;21:75–79. doi: 10.3233/JAD-2010-091603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngo KX, Kodera N, Katayama E, Ando T, Uyeda TQP. Cofilin-induced unidirectional cooperative conformational changes in actin filaments revealed by high-speed atomic force microscopy. eLIFE. 2015;2:4. doi: 10.7554/eLife.04806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida E. Opposite effects of cofilin and profilin from porcine brain on rate of exchange of actin-bound adenosine 5'-triphosphate. Biochemistry. 1985;26:1160–1164. doi: 10.1021/bi00326a015. [DOI] [PubMed] [Google Scholar]
- Nishida E, iida K, Yonezawa N, Kayasu S, Yahara I, Sakai H. Cofilin is a component of intranuclear and cytoplasmic rods induced in cultured cells. Proc Nat Acad Sci USA. 1987;84:5262–5266. doi: 10.1073/pnas.84.15.5262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niwa R, Nagata-Ohashi K, Takeichi M, Mizuno K, Uemura T. Control of actin reorganization by Slingshot, a family of phosphatases that dephosphorylate ADF/cofilin. Cell. 2002;108:233–246. doi: 10.1016/s0092-8674(01)00638-9. [DOI] [PubMed] [Google Scholar]
- Normoyle KP, Brieher WM. Cyclase associated protein (CAP) acts directly on F-actin to accelerate cofilin-mediated actin severing across the range of physiological pH. J Biol Chem. 2012;287:35722–35732. doi: 10.1074/jbc.M112.396051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunomura A, Chiba S, Eto M, Saito M, Makino I, Miyagishi T. Apolipoprotein E polymorphism and susceptibility to early- and late-onset sporadic Alzheimer's disease in Hokkaido, the northern part of Japan. Neurosci Lett. 1996;206:17–20. doi: 10.1016/0304-3940(96)12415-0. [DOI] [PubMed] [Google Scholar]
- Ochs K, Malaga-Trillo E. Common themes in PrP signaling: the Src remains the same. Front Cell Dev Biol. 2014;2:63. doi: 10.3389/fcell.2014.00063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okreglak V, Drubin DG. Loss of Aip1 reveals a role in maintaining the actin monomer pool and an in vivo oligomer assembly pathway. J Cell Biol. 2010;188:769–777. doi: 10.1083/jcb.200909176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton A, Pope B, Weeds A, Koffer A. Latrunculin B or ATP depletion induces cofilin-dependent translocation of actin into nuclei of mast cells. J Biol Chem. 2003;278:14394–14400. doi: 10.1074/jbc.M206393200. [DOI] [PubMed] [Google Scholar]
- Penzes P, Rafalovich I. Regulation of the actin cytoskeleton in dendritic spines. Adv Exp Med Biol. 2012;970:81–95. doi: 10.1007/978-3-7091-0932-8_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez VI, Buffenstein R, Masamsetti V, Leonard S, Salmon AB, Mele J, Andziak B, Yang T, Edrey Y, Friguet B, et al. Protein stability and resistance to oxidative stress are determinants of longevity in the longest-living rodent, the naked mole-rat. Proc. Natl Acad Sci USA. 2009;106:3059–3064. doi: 10.1073/pnas.0809620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterburs P, Heering J, Link G, Pifzenmaier K, Olayioye MA, Hausser A. Protein kinase D regulates cell migration by direct phosphorylation of the cofilin phosphatase slingshot 1 like. Cancer Res. 2009;69:5634–5638. doi: 10.1158/0008-5472.CAN-09-0718. [DOI] [PubMed] [Google Scholar]
- Pfannstiel J, Cyrklaff M, Habermann A, Stoeva S, Griffiths G, Shoeman R, Faulstich H. Human cofilin forms oligomers exhibiting actin binding activity. J Biol Chem. 2001;276:49476–49484. doi: 10.1074/jbc.M104760200. [DOI] [PubMed] [Google Scholar]
- Pollard TD, Cooper JA. Actin, a central player in cell shape and movement. Science. 2009;326:1208–1212. doi: 10.1126/science.1175862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope BJ, Zierler-Gould KM, Kuhne R, Weeds AG, Ball LJ. Solutions structure of human cofilin: actin binding, pH sensitivity, and relationship to actin depolymerizing factor. J Biol Chem. 2004;279:4840–4848. doi: 10.1074/jbc.M310148200. [DOI] [PubMed] [Google Scholar]
- Pozueta J, Lefort R, Shelanski ML. Synaptic changes in Alzheimer's disease and its models. Neuroscience. 2013;251:51–65. doi: 10.1016/j.neuroscience.2012.05.050. [DOI] [PubMed] [Google Scholar]
- Price DL, Sisodia SS, Gandy SE. Amyloid beta amyloidosis in Alzheimer's disease. Curr Opin Neurol. 1995;8:268–274. doi: 10.1097/00019052-199508000-00004. [DOI] [PubMed] [Google Scholar]
- Qureshi HY, Han D, MacDonald R, Paudel HK. Overexpression of 14-3-3ζ promotes tau phosphorylation at ser262 and accelerates proteosomal degredation of synaptophysin in rat primary hippocampal neurons. PLoS One. 2013 Dec 19;8(12):e84615. doi: 10.1371/journal.pone.0084615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman T, Davies DS, Tannenberg RK, Fok S, Shepherd C, Dodd PR, Cullen KM, Goldsbury C. Cofilin rods and aggregates concur with tau pathology and the development of Alzheimer's disease. J Alzheimers Dis. 2014;42:1443–1460. doi: 10.3233/JAD-140393. [DOI] [PubMed] [Google Scholar]
- Ren S, Ouyang DY, Saltis M, Xu LH, Zha QB, Cai JY, He XH. Anti-proliferative effect of 23,24-dihydrocucurbitacin F on human prostate cancer cells through induction of actin aggregation and cofilin-actin rod formation. Cancer Chemother Pharmacol. 2012;70:415–424. doi: 10.1007/s00280-012-1921-z. [DOI] [PubMed] [Google Scholar]
- Roucou X. Regulation of PrPC signaling and processing by dimerization. Front Cell Devel Biol. 2014;2:57. doi: 10.3389/fcell.2014.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez KA, Wywial E, Perez VI, Lambert AJ, Edrey YH, Lewis KN, Grimes K, Lindsey ML, Brand MD, Buffenstein R. Walking the oxidative stress tightrope: A perspective from the naked mole-rat, the longest-living rodent. Curr Pharm Des. 2014;17:2290–2307. doi: 10.2174/138161211797052457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh SE, Woo JA, Lakshmana MK, Ankala V, Boggess T, Liu T, Hong YH, Mook-Jung I, Kim SJ, Kang DE. Mitochondrial dysfunction and calcium deregulation by the RanBP9-cofilin pathway. FASEB J. 2013;27:4776–4789. doi: 10.1096/fj.13-234765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvany L, Muller J, Guccione E, Rorth P. The core and conserved role of MAL is homeostatic regulation of actin levels. Genes Dev. 2014;28:1048–1053. doi: 10.1101/gad.237743.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sari-Hassoun M, Clement MJ, Hamdi I, Bollot G, Bauvais C, Joshi V, Toma F, Burgo A, Cailleret M, Rosales-Hernandez MC, et al. Cucurbitacin I elicits the formation of actin/phospho-myosin II co-aggregates by stimulation of the RhoA/ROCK paythway and inhibition of LIM-kinase. Biochem Pharmacol. 2015 Dec 18; doi: 10.1016/j.bcp.2015.12.013. 2015 pii: S0006-2952(15)00768-6. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA. Alzheimer's disease-related alterations in synaptic density: neocortex and hippocampus. J Alzheimer's Dis. 2006;9(suppl. 3):101–115. doi: 10.3233/jad-2006-9s312. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Schilling S, Lauber T, Schaupp M, Manhart S, Scheel E, Bohm G, Demuth HU. On the seeding and oligomerization of pGlu-amyloid peptides (in vitro) Biochemistry. 2006;45:12393–12399. doi: 10.1021/bi0612667. [DOI] [PubMed] [Google Scholar]
- Schliebs R, Arendt T. The cholinergic system in aging and neuronal degeneration. Behav Brain Res. 2011;221:555–563. doi: 10.1016/j.bbr.2010.11.058. [DOI] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, Pericak-Vance MA, Goldgaber D, Roses AD. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scoville D, Stamm JD, Altenbach C, Shvetsov A, Kokabi K, Rubenstein PA, Hubbell WL, Reisler E. Effects of binding factors on structural elements of F-actin. Biochemistry. 2009;48:370–378. doi: 10.1021/bi801649j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano-Pozo A, Mielke ML, Gomez-Isla T, Betensky RA, Growdon JH, Frosch MP, Hyman BT. Reactive glia not only associates with plaques but also parallels tangles in Alzheimer’s disease. Am J Pathol. 2011;179:1373–1384. doi: 10.1016/j.ajpath.2011.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, et al. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw AE, Minamide LS, Bill CL, Funk JD, Maiti S, Bamburg JR. Cross-reactivity of antibodies to actin-depolymerizing factor family proteins and identification of the major epitope recognized by a mammalian actin-depolymerizing factor/cofilin antibody. Electrophoresis. 2004;25:2611–2620. doi: 10.1002/elps.200406017. [DOI] [PubMed] [Google Scholar]
- Shi Q, Jing YY, Wang SB, Chen C, Sun H, Xu Y, Gao C, Zhang J, Tian C, Guo Y, et al. PrP octarepeats region determined the interaction with caveolin-1 and phosphorylation of caveolin-1 and Fyn. Med Microbiol Immunol. 2013;202:215–227. doi: 10.1007/s00430-012-0284-8. [DOI] [PubMed] [Google Scholar]
- Sisodia SS, Price DL. Role of the beta-amyloid protein in Alzheimer's disease. FASEB J. 1995;9:366–370. doi: 10.1096/fasebj.9.5.7896005. [DOI] [PubMed] [Google Scholar]
- Sluchanko NN, Gusev NB. Probable participation of 14-3-3 in tau protein oligomerization and aggregation. J Alzheimers Dis. 2011;27:467–476. doi: 10.3233/JAD-2011-110692. [DOI] [PubMed] [Google Scholar]
- Soosairajah J, Maiti S, Wiggan O, Sarmiere P, Moussi N, Sarcevic B, Sampath R, Bamburg JR, Bernard O. Interplay between components of a novel LIM kinase-slingshot phosphatase complex regulates cofilin. EMBO J. 2005;24:473–486. doi: 10.1038/sj.emboj.7600543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strittmatter WJ, Saunders AM, Schmechel D, Pericak-Vance M, Enghild J, Salvesen GS, Roses AD. Apolipoprotein E: high-avidity binding to beta-amyloid and increased frequency of type 4 allele in late-onset familial Alzheimer disease. Proc Natl Acad Sci U S A. 1993;90:1977–1981. doi: 10.1073/pnas.90.5.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Tong Y, Qing H, Chen CH, Song W. Increased BACE1 maturation contributes to the pathogenesis of Alzheimer's disease in Down syndrome. FASEB J. 2006a;20:1361–1368. doi: 10.1096/fj.05-5628com. [DOI] [PubMed] [Google Scholar]
- Sun X, He G, Song W. BACE2 as a novel APP theta-secretase, is not responsible for the pathogenesis of Alzheimer's disease in Down syndrome. FASEB J. 2006b;20:1369–1376. doi: 10.1096/fj.05-5632com. [DOI] [PubMed] [Google Scholar]
- Tahtamouni LH, Shaw AE, Hasan MH, Yasin SR, Bamburg JR. Non-overlapping activities of ADF and cofilin-1 during the migration of metastatic breast tumor cells. BMC Cell Biology. 2013;14:45. doi: 10.1186/1471-2121-14-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tai LM, Ghura S, Koster KP, Liakaite V, Maienschein-Cline M, Kanabar P, Collins N, Ben-Aissa M, Lei AZ, Bahroos N, et al. APOE-modulated Aβ-induced neuroinflammation in Alzheimer's disease: current landscape, novel data, and future perspective. J Neurochem. 2015;133:465–488. doi: 10.1111/jnc.13072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Z, Ioja E, Bereczki E, Hultenby K, Li C, Guan Z, Winblad B, Pei J-J. mTor mediates tau localization and secretion: implication for Alzheimer's disease. Biochim Biophys Acta. 2015;1853:1646–1657. doi: 10.1016/j.bbamcr.2015.03.003. [DOI] [PubMed] [Google Scholar]
- Tanzi RE, Bertram L. Twenty years of the Alzheimer's disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- Taulet N, Delorme-Walker VD, DerMardirossian C. Reactive oxygen species regulate protrusion efficiency by controlling actin dynamics. PLoS One. 2012;7:e42342. doi: 10.1371/journal.pone.0041342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasella A, Blangy A, Brancolini C. A receptor-interacting protein (Rip1) independent necrotic death under the control of protein phosphatase Pp2a that involves the reorganization of actin cytoskeleton and the action of cofilin-1. J Biol Chem. 2014;289:25699–25710. doi: 10.1074/jbc.M114.575134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama T, Nagata T, Shimada H, Teroka R, Fukushima A, Kanemitsu H, Takuma H, Kuwano R, Imagawa M, Ataka S, et al. A new amyloid β variant favoring oligomerization in Alzheimer’s type dementia. Ann Neurol. 2008;63:377–387. doi: 10.1002/ana.21321. [DOI] [PubMed] [Google Scholar]
- Toshima J, Toshima JY, Takeuchi K, Mori R, Mizuno K. Cofilin phosphorylation and actin reorganization activities of testicular protein kinase 2 and its predominant expression in testicular Sertoli cells. J Biol Chem. 2001;276:31449–31458. doi: 10.1074/jbc.M102988200. [DOI] [PubMed] [Google Scholar]
- Treisman R. Shedding light on nuclear actin dynamics and function. Trends Biochem Sci. 2013;38:376–377. doi: 10.1016/j.tibs.2013.06.004. [DOI] [PubMed] [Google Scholar]
- Tu S, Okamoto S-I, Lipton SA, Xu H. Oligomeric Aβ-induced synaptic dysfunction in Alzheimer’s disease. Mol Neurodegen. 2014;9:48. doi: 10.1186/1750-1326-9-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubhi K, Masliah E. Alzhemier’s disease: recent advances and future prospectives. J Alzheimers Dis. 2012;33(Suppl 1):S185–S189. doi: 10.3233/JAD-2012-129028. [DOI] [PubMed] [Google Scholar]
- Um JW, Kaufman AC, Kostylev M, Heiss JK, Stagi M, Takahashi H, Kerrisk ME, Vortmeyer A, Wisniewski T, Koleske AJ, et al. Metabotropic glutamate receptor 5 is a coreceptor for Alzheimer Aβ oligomer bound to cellular prion protein. Neuron. 2013;79:887–902. doi: 10.1016/j.neuron.2013.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umahara T, Uchihara T, Tsuchiya K, Nakamura A, Iwamoto T, Ikeda K, Takasaki M. 14-3-3 proteins and zeta isoform containing neurofibrillary tangles in patients with Alzheimer’s disease. Acta Neuropathol. 2004;108:279–286. doi: 10.1007/s00401-004-0885-4. [DOI] [PubMed] [Google Scholar]
- Van Cauwenberghe C, Van Broeckhoven C, Sleegers K. The genetic landscape of Alzheimer disease: clinical implications and perspectives. Genet Med. 2015 doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rheenen J, Song X, van Roosmalen W, Cammer M, Chen X, Desmarais V, Yip SC, Backer JM, Eddy RJ, Condeelis JS. EGF-induced PIP2 hydrolysis releases and activates cofilin locally in carcinoma cells. J Cell Biol. 2007;179:1247–1259. doi: 10.1083/jcb.200706206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasco ME, Smith MA, Siedlak SL, Nunomura A, Perry G. Striation is the characteristic neuritic abnormality in Alzheimer disease. Brain Res. 1998;813:329–333. doi: 10.1016/s0006-8993(98)01034-8. [DOI] [PubMed] [Google Scholar]
- Verghese PB, Castellano JM, Holtzman DM. Apolipoprotein E in Alzheimer’s disease and other neurological disorders. Lancet Neuro. 2011;10:241–252. doi: 10.1016/S1474-4422(10)70325-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verri M, Pastoris O, Dossena M, Aguilani R, Guerriero F, Cuzzoni G, Venturini L, Ricevuti G, Bongiorno A. Mitochondria alterations, oxidative stress and neuroinflammation in Alzheimer’s disease. Int J Immunopathol Pharmacol. 2012;25:345–353. doi: 10.1177/039463201202500204. [DOI] [PubMed] [Google Scholar]
- Vitriol WA, McMillen LM, Kapustina M, Gomez SM, Vavylonis D, Zheng JQ. Two functionally distinct sources of actin monomers supply the leading edge of lamellipodia. Cell Reports. 2015;11:433–445. doi: 10.1016/j.celrep.2015.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh KP, Minamide LS, Kane SJ, Shaw AE, Brown DR, Pulford B, Zabel MD, Lambeth JD, Kuhn TB, Bamburg JR. Amyloid-β and proinflammatory cytokines utilize a prion protein-dependent pathway to activate NADPH oxidase and induce cofilin-actin rods in hippocampal neurons. PLoS One. 2014;9:e95995. doi: 10.1371/journal.pone.0095995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C, Zhou GL, Vedantam S, Li P, Field J. Mitochondrial shuttling of CAP1 promotes actin- and cofilin-dependent apoptosis. J Cell Sci. 2008;121:2913–2920. doi: 10.1242/jcs.023911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang JZ, Xia YY, Grundke-Iqbal I, Iqbal K. Abnormal hyperphosphorylation of tau: sites, regulation, and molecular mechanism of neurofibrillary degeneration. J Alzheimers Dis. 2013;33(Suppl.1):S123–S139. doi: 10.3233/JAD-2012-129031. [DOI] [PubMed] [Google Scholar]
- Wang W-X, Rajeev BW, Stromberg AJ, Ren N, Tang G, Huang Q, Rigoutsos I, Nelson PT. The expression of microRNA miR-107 decreases early in Alzheimer's disease and may accelerate disease progression through regulation of {beta}-site Amyloid Precursor Protein-Cleaving Enzyme 1. J Neurosci. 2008;28:1213–1223. doi: 10.1523/JNEUROSCI.5065-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Shibasaki F, Mizuno K. Calcium signal-induced cofilin dephosphorylation is mediated by Slingshot via calcineurin. J Biol Chem. 2005;280:12683–12689. doi: 10.1074/jbc.M411494200. [DOI] [PubMed] [Google Scholar]
- Weber M, Wu T, Hanson JE, Alam NM, Solanoy H, Ngu H, Lauffer BE, Lin HH, Dominguez SL, Reeder J, et al. Cognitive deficits, changes in synaptic function, and brain pathology in a mouse model of normal aging. eNeuro. 2015 doi: 10.1523/ENEURO.0047-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman IT, Gervasio OL, Cullen KM, Guillemin GJ, Jeong EV, Witting PK, Antao ST, Minamide LS, Bamburg JR, Goldsbury C. Activated actin-depolymerizing factor/cofilin sequesters phosphorylated microtubule-associated protein during the assembly of Alzheimer-like neuritic cytoskeletal striations. J Neurosci. 2009;29:12994–13005. doi: 10.1523/JNEUROSCI.3531-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman IT, Minamide LS, Goh de L, Bamburg JR, Goldsbury C. Rapid changes in phospho-MAP/tau epitopes during neuronal stress: cofilin-actin rods primarily recruit microtubule binding domain epitopes. PloS One. 2011;6:e20878. doi: 10.1371/journal.pone.0020878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiggan O, Shaw AE, DeLuca JG, Bamburg JR. ADF/cofilin regulates actomyosin assembly through competative inhibition of myosin II binding to F-actin. Dev Cell. 2012;22:530–543. doi: 10.1016/j.devcel.2011.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wildsmith KR, Holley M, Savage JC, Skerrett R, Landreth GE. Evidence for impaired amyloid beta clearance in Alzheimer disease. Alzheimer’s Res Ther. 2013;5:33. doi: 10.1186/alzrt187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winterhoff M, Faix J. Actin-filament disassembly: It takes two to shrink them fast. Curr Biol. 2015;25:R450–R452. doi: 10.1016/j.cub.2015.04.050. [DOI] [PubMed] [Google Scholar]
- Wirths O, Breyhan H, Cynis H, Schilling S, Demuth HU, Bayer TA. Intraneuronal pyroglutamate-Aβ3-42 triggers neurodegeneration and lethal neurological deficits in a transgenic mouse model. Acta Neuropathol. 2009;118:487–496. doi: 10.1007/s00401-009-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf M, Zimmermann A-M, Görlich A, Gurniak CB, Sassoe-Pognetto M, Friauf E, Witke W, Rust MB. ADF/cofilin controls synaptic dynamics and regulates synaptic vesicle mobilization and exocytosis. Cerbral Cortex. 2015;25:2863–2875. doi: 10.1093/cercor/bhu081. [DOI] [PubMed] [Google Scholar]
- Woo JA, Jung AR, Lakshmana MK, Bedrossian A, Lim Y, Bu JH, Park SA, Koo EH, Mook-Jung I, Kang DE. Pivotal role of the RanBP9-cofilin pathway in Aβ-induced apoptosis and neurodegeneration. Cell Death Differ. 2012;19:1413–1423. doi: 10.1038/cdd.2012.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JA, Zhao X, Khan H, Penn C, Wang X, Joly-Amado A, Weeber E, Morgan D, Kang DE. Slingshot-cofilin activation mediates mitochondrial and synaptic dysfunction via Aβ ligation to β1-integrin conformers. Cell Death Differ. 2015a;22:921–934. doi: 10.1038/cdd.2015.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo JA, Boggess T, Uhlar C, Wang X, Khan H, Cappos G, Joly-Amado A, De Narvaez E, Majid S, Minamide LS, et al. RanBP9 at the intersection between cofilin and Aβ pathologies: rescue of neurodegenerative changes by RanBP9 reduction. Cell Death Dis. 2015b;6:1676. doi: 10.1038/cddis.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Finkelstein DI, Adlard PA. Interactions of metals and apolipoprotein E in Alzheimer’s disease. Front Aging Neurosci. 2014;6:121. doi: 10.3389/fnagi.2014.00121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang N, Higuchi O, Ohashi K, Nagata K, Wada A, Kangawa K, Nishida E, Mizuno K. Cofilin phosphorylation by LIM-kinase 1 and its role in Rac-mediated actin reorganization. Nature. 1998;393:809–812. doi: 10.1038/31735. [DOI] [PubMed] [Google Scholar]
- Yao J, Hennessey T, Flynt A, Lai E, Beal MF, Lin MT. MicroRNA-related cofilin abnormality in Alzheimer's disease. PLoS ONE. 2011;5:e15546. doi: 10.1371/journal.pone.0015546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yirmiya R, Goshen I. Immune modulation of learning, memory, neural plasticity and neurogenesis. Brain Behav Immun. 2011;25:181–213. doi: 10.1016/j.bbi.2010.10.015. [DOI] [PubMed] [Google Scholar]
- Yonezawa N, Nishida E, Iida K, Yahara I, Sakai H. Inhibition of the interactions of cofilin, destrin and deoxyribonuclease I with actin by phosphoinositides. J Biol Chem. 1990;265:8382–8386. [PubMed] [Google Scholar]
- Zhang YT, Ouyang DY, Xu LH, Zha QB, He XH. Formation of cofilin-actin rods following cucurbitacin-B-induced actin aggregation depends on Slingshot homolog 1-mediated cofilin hyperactivation. J Cell Biochem. 2013;114:2415–2429. doi: 10.1002/jcb.24587. [DOI] [PubMed] [Google Scholar]
- Zhao L, Ma QL, Calon F, Harris-White ME, Yang F, Lim GP, Morihara T, Ubeda OJ, Ambegaokar S, Hansen JE, et al. Role of p21-activated kinase pathway defects in the cognitive deficits of Alzheimer disease. Nat Neurosci. 2006;9:234–242. doi: 10.1038/nn1630. [DOI] [PubMed] [Google Scholar]
- Zhou G-L, Zhang H, Wu H, Ghai P, Field J. Phosphorylation of the cytoskeletal protein CAP1 controls its association with cofilin and actin. J Cell Sci. 2014;127:5052–5065. doi: 10.1242/jcs.156059. [DOI] [PMC free article] [PubMed] [Google Scholar]