Abstract
A new species (Mayoa portugallica genus novum species novum) of highly characteristic inaperturate, striate fossil pollen is described from the Early Cretaceous (Barremian–Aptian) of Torres Vedras in the Western Portuguese Basin. Based on comparison with extant taxa, Mayoa is assigned to the tribe Spathiphylleae (subfamily Monsteroideae) of the extant monocotyledonous family Araceae. Recognition of Araceae in the Early Cretaceous is consistent with the position of this family and other Alismatales as the sister group to all other monocots except Acorus. The early occurrence is also consistent with the position of Spathiphylleae with respect to the bulk of aroid diversity. Mayoa occurs in the earliest fossil floras (from circa 110 to 120 million years ago) that contain angiosperm flowers, carpels, and stamens. The new fossil provides unequivocal evidence of monocots in early angiosperm assemblages that also include a variety of key “magnoliid” lineages (e.g., Chloranthaceae) but only a limited diversity of eudicots.
Keywords: fossil angiosperms, angiosperm evolution, striate pollen, zona-aperturate pollen, extinctions
Extant monocotyledons (monocots) are a diverse group of flowering plants that include orchids, lilies, palms, sedges, grasses, gingers, and many other groups. Together they comprise about one-fifth of the extant angiosperm diversity and include 50,000 species (1). Despite their diversity, monocots have long been recognized as a natural group, and this is borne out by modern phylogenetic studies of both morphological and molecular sequence data (e.g., refs. 2–4). Based on their evolutionary distinctiveness, it has long been assumed that monocots diverged very early in angiosperm evolution (5, 6); however, direct fossil evidence from the earliest phases of angiosperm evolution has remained sparse (1). According to Gandolfo et al. (7) the first unequivocal monocot fossil is from the Turonian (Late Cretaceous, 93.5–89 million years ago).
Recent phylogenetic analyses based on extensive, combined sequence data from the chloroplast and nuclear genomes resolve monocots as part of a polychotomy with several magnoliid lineages (Piperales, Chloranthaceae, Laurales, Magnoliales, and Winterales) (4). Sometimes included in this polychotomy are Ceratophyllum and eudicots, which comprise the bulk of angiosperm diversity. Only a small number of relatively species-poor magnoliid lineages diverge below this point [Amborellaceae, Nymphaeaceae, and Austrobaileyales (Illiciaceae, Schisandraceae, Trimeniaceae, Austrobaileyaceae)] (4, 8, 9). This pattern of relationship is consistent with the idea that monocots diverged and began to diversify at an early stage in angiosperm evolution.
Estimates of the age of monocot lineages incorporating molecular clock assumptions imply that about six major lineages of monocots had differentiated in the Early Cretaceous 100 million years ago (10). Other studies have dated the divergence of monocots from other angiosperms at ≈140–150 million years ago (11). Although recent studies of well preserved fossil flowers have extended the history of many magnoliid and some eudicot lineages into the Early Cretaceous (12–14), this has not been possible for monocots.
Fossils of diverse monocots are known from the Late Cretaceous and earliest Cenozoic (1, 7, 15–18), but the best evidence for monocots in the Early Cretaceous is provided by pollen assigned to Liliacidites (19, 20), fragmentary leaf remains assigned to Acaciaephyllum (19), and the Pennistemon plant, comprising stamens with in situ pollen and carpels (21). In all these cases, the relationship to monocots is not fully secure, especially the relationship to particular subgroups of monocots. Here we report on a distinctive pollen type, referable to the Araceae, that extends the fossil history of an extant family of monocots into the earliest fossil assemblages containing well preserved angiosperm stamens and carpels.
Materials and Methods
Geological Occurrence. The fossil specimen described here is from a large open-cast clay pit situated in the northeastern outskirts of Torres Vedras ≈1 km northeast of Forte de Forca on the road toward Sarge (39°06′13″N, 9°14′47″W, Carta Geológica de Portugal Torres Vedras 30C) (22) in the Western Portuguese (Lusitanian) Basin. The locality has now been overtaken by town development, but until recently it showed a thick sequence of Lower Cretaceous clastic sediments. According to Rey (23), the sedimentary sequence in this area belongs to the Lugar d'Almen Formation, the Fonte Granda Formation, and the Almargem Formation. The Almargem Formation is divided into an upper and a lower member separated by a ferruginous crust. Rey (23) assigned the lower member to the Late Barremian–Early Aptian (Bedoulian) and the upper member to the Aptian (Gargasian). Subsequent stratigraphic correlations indicate that the upper member may be of Late Aptian–Early Albian age (24) or Early to Late Albian (25). Sample 44, which yielded the fossil specimen described here, was collected from a sandy, lignitic horizon just below the ferruginous crust in sediments corresponding to the basal member of the Almargem Formation, indicating a Late Barremian–Early Aptian age.
Preparation. Plant fossils were isolated from the sediment by sieving in water. Adhering mineral matrix was removed by treatment with HF (40%) and HCl (10%) followed by rinsing in water. The material was then air dried and sorted under a binocular microscope (Zeiss Stemi SVII). The fossil was mounted on an aluminum stub with nail polish, coated with gold for 60 sec, and studied by using a Hitachi Field Emission scanning electron microscope (SEM) at 1–2 kV. After SEM documentation, a small piece was removed for ultrastructural studies, dehydrated in an ethanol-propylene oxide series, embedded in Epon, and sectioned at 500–700 Å. Sections were stained with uranyl acetate and lead citrate and studied by using a FEI Morgagni 268D transmission electron microscope (Eindhoven, The Netherlands). The preparations are in the paleobotanical collections of the Swedish Museum of Natural History.
Fossil Assemblage. Most of the organic residue from sample 44 consists of unidentifiable charcoalified or lignitized plant fragments. Nevertheless, the flora is quite diverse and includes megaspores, conifer twigs, and dispersed angiosperm fruits, seeds, and stamens. There are also a few inflorescence fragments with flowers. Approximately 15 different kinds of angiosperm pollen have been identified from stamen fragments and small coprolites. All are monoaperturate or inaperturate, indicating a relationship with magnoliids (sensu lato) and monocotyledons. More precise systematic assignments are problematic, but the sample does contain pistillate flowers and stamens with Asteropollis pollen that closely resemble extant Hedyosmum (Chloranthaceae) (12).
Systematics
Description and Comments on the Fossil Material. The fossil pollen occurs in large masses attached to a small, elongate, cutinized fragment with a length of ≈1.2 mm and a width of 0.3 mm. The structure has faint, irregular ridges, but preservation is too poor to allow definitive interpretation of flower or inflorescence structure. Pollen occurs abundantly over the whole surface of the cutinized fragment and is well preserved (Fig. 1).
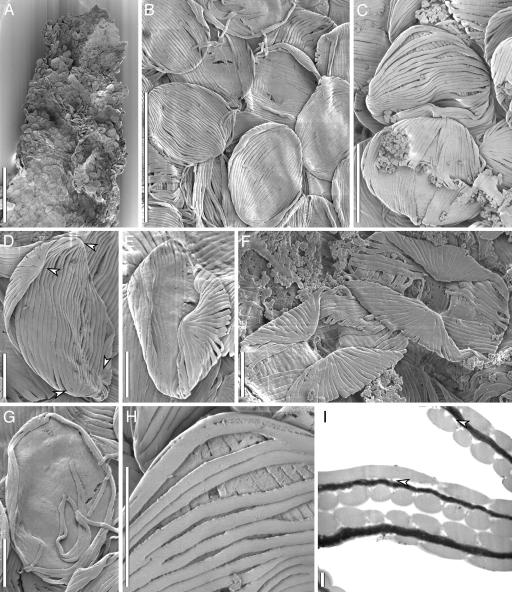
Fig. 1.
Scanning (A–H) and transmission (I) electron micrographs of fossil Mayoa portugallica (S136663, Sample Torres Vedras 44). (A) Part of cutinized structure showing masses of pollen. (Scale bar: 300 μm.) (B) Compressed pollen showing shape and surface ornamentation. (Scale bar: 30 μm.) (C) Two pollen grains with partly separated ribs, upper grain showing the striations on both sides of the grain. (Scale bar: 12 μm.) (D) Pollen grain showing the two pairs of divergence points marked with arrows. (Scale bar: 6 μm.) (E and F) Folded pollen grains showing the crossed arrangement of the ribs. (Scale bar: 6 μm.) (G) Fractured grain exposing the thin inner granular layer of the pollen wall. (Scale bar: 6 μm.) (H) Detail of pollen wall showing outer surface and granular inner surface of ribs. (Scale bar: 6 μm.) (I) Section of pollen grains showing lighter-staining outer layer (exine: ribs and infratectal granules) and darker-staining inner layer (endexine); infratectal granules (arrows) minute, rarely columellae-like; because of the crosswise pattern of the ribs, some ribs are seen in longitudinal sections and others in transverse sections. (Scale bar: 0.3 μm.)
All grains are strongly compressed. They are inaperturate, elliptical in outline, and ≈20–23 × 15–18 μm in size (measured on scanning electron micrographs) with rounded or slightly pointed ends. The lack of an aperture makes orientation of the pollen problematic.
The surface of the grains is distinctly striate (polyplicate) with uniform, straight, closely spaced ribs (muri) separated by narrow grooves (striae) (Fig. 1 B–I). The striations form a distinct pattern: The two sets of ribs on the different sides of each pollen grain run perpendicular or somewhat oblique to one another. Each set of ribs covers approximately half of the grain, and the grains are typically compressed in such a way that the two sets face in opposite directions (Fig. 1 B and C). The two sets of ribs cross at angles ranging between 45° and 90°, and in many grains both sets are visible (Fig. 1 C–F and H). Each set of ribs is slightly longer than the long axis of the grains, and the two points from which the ribs diverge usually extend onto the opposite surface (Fig. 1 D–F). There are ≥30 ribs on each side of the grain. Bifurcations are sparse but occur most frequently near the divergence points where the number of ribs is ≈10. The three-dimensional form of the grains is reconstructed in Fig. 2.
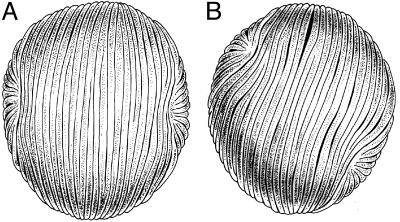
Fig. 2.
Three-dimensional reconstructions of Mayoa portugallica pollen grains showing the two set of ribs running perpendicular (A) and oblique (B) to each other (drawing by P. von Knorring).
Each rib is ≈0.5–1 μm wide with a nearly flat, smooth outer surface. The ribs are solid in cross section and ≈1 μm high (Fig. 1I). Laterally, and also on the inner surface, there is a very thin granular or, rarely, weakly columellate layer (Fig. 1 H and I). Most specimens show a thin, (≈0.025 μm thick), continuous granular layer extending below the ribs (Fig. 1 G and H). Transmission electron micrographs show that this granular layer is a typical, darker-staining endexine (Fig. 1I). The pollen wall including the endexine often splits between the ribs, and all ribs may be detached from one another except at the divergence points.
Relationships. In the absence of other features, the phylogenetic relationships of the fossil material must be determined solely on the basis of the distinctive structure of the pollen. Compared with pollen of angiosperms and other seed plants, the most distinctive features of this pollen are its inaperturate condition, the crossed pattern of narrow, straight ribs, and the easily detached ektexine that rests on a thin, inconspicuous granular endexine.
Pollen with distinctly ribbed ornamentation is widespread in angiosperms and also in the Gnetales (26). The two genera of Gnetales (Ephedra and Welwitschia) that have ribbed pollen are both quite different from the fossil. Both genera have a much more distinct granular infratectal layer that also fills out the central part of the ribs and a thick, distinctly laminar endexine that is well preserved in gnetalean fossils (27). Based on these features a relationship to Ephedra and Welwitschia can be excluded.
Among angiosperms, eudicot pollen is fundamentally different from the fossil material, and a relationship to this large group of angiosperms can be excluded. Among magnoliids, inaperturate and striate pollen is known in Hortonia (Hortoniaceae) and Dahlgrenodendron (Lauraceae). Both differ from the fossil in that they have striations that extend between two divergence points in a single direction, and they lack an inner layer (foot layer and endexine) below the ribs (28–30). Pollen of Dahlgrenodendron also has much coarser, slightly undulating anastomosing ribs characterized by abundant, minute perforations. The tectum in Dahlgrenodendron also forms an irregular, reticulate pattern at the points of divergence (31). In Hortonia the striations are spiral. The ribs are hollow, formed from rope-like cylindrical strands, and has covered by very fine spines (28, 30).
Among monocots, inaperturate pollen with prominent striae occurs in both Araceae and Zingiberaceae (32). Striate pollen of Zingiber (section Cryptanthium) is distinguished from the fossil in that Zingiber pollen has a distinctly or weakly spiral arrangement of the ribs and has exceptionally large (110–135 μm long) grains (33). The exine is also only weakly developed and susceptible to acetolysis, which also removes the thick intine (32). Low resistance of exine to acetolysis is thought to correlate with low fossilization potential (29).
Among Araceae, inaperturate, striate pollen occurs in two subfamilies: Monsteroideae and Aroideae sensu Mayo et al. (34). In Monsteroideae these features occur together in both genera of Spathiphylleae (Holochlamys, Spathiphyllum), whereas in Aroideae they occur in Thomsonieae (Amorphophallus, Pseudodracontium), Arisareae (Arisarum), Ambrosineae (Ambrosina), Colocasieae (Colocasia pro parte, Protarum, Steudnera), and Pistieae (Pistia) (35).
Pollen of Amorphophallus and Pseudodracontium (Thomsonieae) has been surveyed in detail based on 269 collections representing 145 species of Amorphophallus and seven species of Pseudodracontium (36). Grains are striate in all species of Pseudodracontium and in 67 species of Amorphophallus. In Pseudodracontium the ribs terminate at the ends of the grains in distinct, psilate caps, a feature not seen in the fossil material. Wall stratification in these two genera is also very different from that in the fossil material. In both genera, the pollen wall has a thick acetolysis-resistant inner layer, interpreted as endexine (32, 36), and an acetolysis-susceptible outer layer that forms the ribs. van der Ham et al. (36) interpreted the ribs as formed from ektexine, whereas Hesse et al. (32) noted that ektexine is lacking in both Amorphophallus and Pseudodracontium. In the fossil it is the ribs that are well developed and robust after fossilization, whereas an inner layer is either thin or absent. In addition, neither Amorphophallus nor Pseudodracontium shows the characteristic crossed arrangement of the ribs seen in the fossil material.
Striate pollen of Ambrosina (Ambrosineae) and Arisarum (Arisareae) is similar to pollen of Amorphophallus and Pseudodracontium. In both genera the pollen has a thick, spongy endexine and ribs that are acetolysis-susceptible (32). In addition, in Ambrosina the relatively broad ribs are covered with numerous, fine perforations, whereas in Arisarum the ribs are linked to each other laterally by a perforate layer as in some species of Amorphophallus (32).
Striate pollen of Pistia (Pistieae) has narrow ribs, each with a sharp, angular profile. The ribs extend in one direction between two poles. The pollen wall consists mainly of a thick, spongy endexine, and ektexine is lacking. The ribs are acetolysis-susceptible (37), have low fossilization potential (32), and are not comparable to the resistant ribs of the fossil pollen.
Protarum and Steudnera (Colocasieae) both show crossed sets of ribs on the opposite faces of the pollen, as seen in the fossil material; however, the pollen wall is quite different. Pollen of Steudnera lacks ektexine, and the endexine is thick and spongy (32). Given that Protarum is systematically close to Pistia and Steudnera (34, 38) it is likely that its pollen wall is also similar.
The strongest resemblance between the fossil pollen and pollen of extant Araceae is with the two very closely related genera of Spathiphylleae: Spathiphyllum and Holochlamys. Both have inaperturate, striate pollen of medium size (mean size, 32 and 33 μm, respectively). The pollen wall consists mainly of acetolysis-resistant ribs (formed from ektexine), a very thin, granular (Holochlamys) to weakly columellate (Spathiphyllum) infratectal layer, and a very thin granular endexine (32). Pollen of some species of Spathiphyllum has very fine ribs similar to the fossil, but in those species surveyed (35), the ribs run in only one direction extending between two distinct divergence points. In Holochlamys the same crossed pattern of ribs is present as in the fossil material. The ribs radiate from two pairs of more or less asymmetrically placed divergence points.
Holochlamys comprises a single species (Holochlamys beccarii) mainly restricted to the island of New Guinea, but extending into New Britain (34) and west to Sumatra (J. Bogner, personal communication). Spathiphyllum is more diverse (≈41 species) and is disjunct between the Neotropics and Southeast Asia. Holochlamys and Spathiphyllum are very closely related, and the former may be nested phylogenetically in the latter (S. J. Mayo, personal communication). Holochlamys is distinguished from Spathiphyllum mainly by its deliquescent and clasping spathe, as opposed to a persistent and fully expanded (rarely arched) spathe. Holochlamys also has a unilocular ovary with a basal placenta, rather than an ovary with three (or more rarely two or four) locules and axile placentation. Ovules are also more numerous in Holochlamys.
The similarity between the fossil material and Holochlamys is striking based on distinctive palynological features that are not widespread among seed plants; however, in the absence of information on other parts of the plant, we refrain from assigning this material to the extant genus. Nevertheless, we conclude that the fossil pollen provides the first unequivocal evidence of araceous monocots in the Early Cretaceous and indicates the presence of subfamily Monsteroideae, specifically the tribe Spathiphylleae. This proposed relationship will be tested as other parts of this plant, and similar fossils, are discovered by future paleobotanical work.
Mayoa. Friis, Pedersen et Crane genus novum.
Derivation of generic name. In honor of Simon J. Mayo in recognition of his contributions to understanding the diversity of Araceae.
Generic diagnosis. Pollen inaperturate. Surface of pollen wall is striate with densely spaced ribs (muri) separated by narrow grooves (striae). Ribs are arranged in a distinctive pattern in which two sets of ribs each radiate from a pair of divergence points. Main axes of the two sets of ribs are at an angle of 45–90° to each other. Pollen wall is thin and consists of an outer exine of solid ribs supported by a very thin granular infratectal layer and an inner thin granular endexine that forms a continuous layer under the ribs.
Type Species. Mayoa portugallica Friis, Pedersen et Crane genus novum, species novum.
Mayoa portugallica. Friis, Pedersen et Crane genus novum, species novum.
Derivation of specific epithet. From Portugal where the fossil was collected.
Specific diagnosis. As for the genus.
Holotype. S136663 (only specimen).
Type locality and stratum. Open-cast clay pit northeast of Torres Vedras (39°06′13″N, 9°14′47″W), Portugal; basal member of the Almargem Formation (Late Barremian or Early Aptian).
Discussion
Monocot Diversification. It has long been recognized that monocots diverged from other angiosperms very early in flowering plant evolution, but decisive paleobotanical evidence of monocots from the earliest phases of angiosperm evolution has been sparse (1, 21, 39). The recognition of pollen closely resembling that of Holochlamys from the Torres Vedras locality indicates that some differentiation of Araceae was already under way by the late Early Cretaceous. Together with the position of aroids in monocot phylogeny, the discovery provides the clearest evidence yet that several lineages of monocots had already diverged and were undergoing diversification. Nevertheless, it is significant that the fossil material is not nested well inside the monocots. Instead, its relationships are within Araceae, which is the sister group to all other Alismatales. Alismatales are, in turn, the sister group to all other monocots except Acorus.
Within Araceae, Mayoa is most similar to Holochlamys and Spathiphyllum. These genera diverge near the base of a clade that contains the bulk of aroid diversity. Only Gymnostachyoideae (1 spp.), Orontioideae (7 spp.), and Lemnoideae (35 spp.) diverge below this point. Therefore, the phylogenetic position of Araceae and of Mayoa within the Araceae implies some, but not necessarily extensive, differentiation of extant lineages of Araceae and other monocots in the late Early Cretaceous.
Several other plant fossils from the Early Cretaceous of Portugal may be of alismatalean, or even araceous, affinity. At the Torres Vedras locality, another sample (Torres Vedras 149) has yielded pollen masses (probable coprolites) composed of distinctive zona-aperturate grains (Figs. 3 A–C). The pollen is ≈30–35 μm in diameter and circular to elliptical in the plane of the aperture, which extends for almost the full circle of the grains and separates the pollen into two shallow, almost equal, dome-shaped halves.
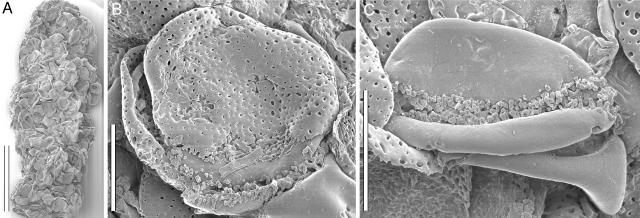
Fig. 3.
Scanning electron micrographs of fossil coprolite with zona-aperturate pollen from Torres Vedras (S136702, Sample Torres Vedras 149). (A) Coprolite with pollen masses. (Scale bar: 150 μm.) (B) Pollen grain with both halves preserved showing punctate tectum and irregular exine elements of aperture membrane. (Scale bar: 15 μm.) (C) Naked pollen grain showing smooth surface of foot layer and exine of aperture membrane. (Scale bar: 15 μm.)
The pollen wall is ≈2.5 μm thick. The tectum is psilatepunctate with perforations of varying size, shape, and density. The diameter of the individual perforations increases from the outside toward the inner surface of the tectum, giving this a more coarsely foveolate appearance. The inner surface of the tectum sometimes shows very faintly developed granulae, but there are no columellae, and the tectum is only loosely attached to the thick, smooth, homogenous foot layer. The pollen wall is thinner over the aperture and characterized by irregular exine elements. The endexine is granular, thin in nonapertural regions, and thickened under the aperture.
Pollen of this general form has been reported in several families of magnoliid angiosperms (Annonaceae, Atherospermataceae, Eupomatiaceae, and Nymphaeaceae) and monocots (Arecaceae, Araceae, Dasypogonaceae, Laxmanniaceae, and Rapateaceae) (40, 41). Pollen with a punctate-foveolate tectum and an aperture that is equally wide for its full length is known only for members of the two araceous subfamilies: Monstereae (e.g., Monstera) and Zamioculcadeae (e.g., Gonatopus) (35, 41, 42).
The Pennistemon plant described from the Early Cretaceous Vale de Agua and Buarcos localities in the Western Portuguese Basin also shows features seen among Alismatales, including Araceae. The plant is known from monocolpate and acolumellate, coarsely reticulate pollen (Pennipollis-type) found in situ in stamens of Pennistemon as well as on the surface of Pennicarpus fruits and in many coprolites (21). Dispersed pollen of the Pennipollis type is widespread in Lower and mid-Cretaceous (Barremian? to Cenomanian or perhaps Turonian) sediments from low to midpaleolatitudes (21). The reticulum is loosely attached to the foot layer by a very thin granular infratectal layer and the muri are ornamented with tiny spines. A similar kind of coarse reticulum, composed of spiny muri only weakly attached to the foot layer, occurs in Alismatales among certain Araceae (e.g., Anthurium) and Potamogetonaceae. However, Pennipollis is distinguished from these species of Alismatales in aperture configuration.
Other Fossil Araceae. Dispersed striate pollen assigned to the extinct genus Jugella from the Early Cretaceous of the Prikaspian Basin (Berriasian–Barremian) and Western Siberia (Barremian) were referred to the Araceae based on similarities with pollen of Spathiphyllum (43). Jugella, however, differs from Spathiphyllum, Holochlamys, and Mayoa in being monocolpate. Similar monocolpate and striate pollen is also characteristic for Welwitschia (Gnetales), but the lack of ultrastructural details in Jugella precludes a closer assessment of its systematic position.
The Late Cretaceous record of Araceae is sparse. Monstereae are represented by an aerial stem with root scars and remains of leaf sheaths from the Maastrichtian of India (16). In Europe, a suite of small Araceae seeds (similar to Epipremnum and Spathiphyllum) has been recovered from the Campanian–Maastrichtian Mira locality of western Portugal (E.M.F. and K.R.P., unpublished observation), and leaves (similar to Lysichiton) have been recorded from the Late Cretaceous (Early Campanian) Grünbach locality of Lower Austria (18). From North America an orontioid inflorescence with attached fruits and seed is known from the Late Cretaceous (Campanian) of Canada (J. Bogner, personal communication). Hickey¶ also reported leaves of Pistia in Maastrichtian strata of Wyoming and Montana, but the material remains to be documented in detail.
By the Early Cenozoic there is clear paleobotanical evidence that Araceae were already diverse and widespread (1, 17, 34, 44–47). The European record is particularly rich and includes distinct zona-aperturate pollen similar to that of extant Monsteroideae (41), a variety of curved seeds related to Monsteroideae and Lasioideae (44, 45, 48), numerous seeds of Pistia (49), and many araceous leaves (50), including some similar to those of Lemnaceae (51).
Ecology of Early Cretaceous Araceae. The recognition of a coprolite composed more or less exclusively of Monstera-like pollen provides evidence of insect feeding on putative Early Cretaceous araceous floral material. Coprolites containing only Pennipollis pollen are also known, implying pollen feeding in other putative early monocots. Other extrapolations about the ecology of Early Cretaceous Araceae must be based on inferences from the biology of extant taxa.
All Araceae are insect-pollinated and protogynous (34), but pollination systems are diverse. The trap mechanisms of inflorescences with unisexual flowers have been investigated in detail (e.g., Arum), but pollination in genera with bisexual flowers and simpler, spreading spathes is not nearly as well studied (34). According to Grayum (35, 52), striate and foveolate pollen is generally associated with unspecialized pollination biology. Inflorescences of Spathiphyllum in Central and South America are visited by euglossine and trigonid bees (35, 53). A recent compilation of pollinators and visitors to Spathiphyllum also lists chrysomelid beetles, drosophilid flies, and tephritid flies (54).
Almost all extant Araceae require abundant water and generally high humidity. Today they are especially diverse and abundant in the humid tropics. Spathiphyllum and Holochlamys are mainly plants of shaded, wet sites in tropical, humid forest. They occur particularly along small streams, on riverbanks, and on rocks. This set of ecological preferences is consistent with the dimly lit, disturbed, forest understory habitats and/or shady, streamside settings inferred for the ecology of early angiosperms. This inference is based mainly on studies of the morphology and ecophysiology of extant groups (Amborella, Nymphaeales, Austrobaileya, and Chloranthaceae) thought to have differentiated early in angiosperm evolution (55, 56).
Biogeography. The recognition of fossil pollen that can be assigned to the Spathiphylleae in the Early Cretaceous of Southwest Europe has interesting implications for understanding the biogeography of Araceae. Several groups of Araceae show trans-Pacific biogeographic patterns and this is also the case for Spathiphylleae, which are present in the tropics of South America and Southeast Asia but not in Africa (57). About three species of Spathiphyllum and the single species of Holochlamys occur in the eastern Indo–Malesian region, whereas ≈38 species of Spathiphyllum occur in the Neotropics, from Central America to ≈15°S (57, 58). Spathiphyllum sect Massowia ranges from central and northern South America and Cocos Island across the Pacific to east Malesia (S. J. Mayo, personal communication). Such distributions have often been difficult to explain (57). Recognition that the Spathiphylleae were once present in Europe close to the margin of the former Tethys Ocean implies that the trans-Pacific distribution is relictual in part, reflecting extinction in Europe, Africa, and other parts of the world.
Acknowledgments
We thank J. Bogner, J. A. Doyle, P. K. Endress, M. Hesse, and S. J. Mayo for valuable comments on this work; B. Krunderup for help with TEM sections; A. B. Maunsbach for access to TEM facilities in Aarhus; and P. von Knorring for line drawings. This study was supported by grants from the Swedish Research Council (to E.M.F.) and the Carlsberg Foundation (to K.R.P.).
Author contributions: E.M.F., K.R.P., and P.R.C. performed research, analyzed data, and wrote the paper.
Footnotes
Hickey, L. J. (1991) Am. J. Bot. 78, Suppl. 6, 115 (abstr.).
References
- 1.Herendeen, P. S. & Crane, P. R. (1995) in Monocotyledons: Systematics and Evolution, eds. Rudall, P. J., Cribb, P. J., Cutler, D. F. & Humphries, C. J. (Royal Botanic Gardens, Kew, United Kingdom), pp. 1–21.
- 2.Loconte, H. & Stevenson, D. W. (1991) Cladistics 7, 267–296. [DOI] [PubMed] [Google Scholar]
- 3.Chase, M. W., Soltis, D. E., Soltis, P. S., Rudall, P. J., Fay, M. F., Hahn, W. H., Sullivan, S., Joseph, J., Molvray, M., Kores, P. J., et al. (2000) in Monocots: Systematics and Evolution, eds. Wilson, K. L. & Morrison, D. A. (Commonwealth Scientific and Industrial Research Organization, Melbourne), pp. 3–16.
- 4.Soltis, D. E., Soltis, P. S., Chase, M. W., Mort, M. E., Albach, D. C., Zanis, M., Savolainen, V., Hahn, W. H., Hoot, S. B., Fay, M. F., et al. (2000) Bot. J. Linn. Soc. 133, 381–461. [Google Scholar]
- 5.Bessey, C. E. (1896) Bot. Gaz. (Crawfordsville) 22, 229–232. [Google Scholar]
- 6.Arber, E. A. N. & Parkin, J. (1908) Ann. Bot. New Ser. 12, 489–515. [Google Scholar]
- 7.Gandolfo, M. A., Nixon, K. C. & Crepet, W. L. (2000) in Monocots: Systematics and Evolution, eds. Wilson, K. I. & Morrison, D. A. (Commonwealth Scientific and Industrial Research Organization, Melbourne), pp. 44–51.
- 8.Soltis, P. S., Soltis, D. E. & Chase, M. W. (1999) Nature 402, 402–404. [DOI] [PubMed] [Google Scholar]
- 9.Qiu, Y.-L., Lee, J., Bernasconi-Quadroni, F., Soltis, D. E., Soltis, P. S., Zanis, M., Zimmer, E. A., Chen, Z., Savolainen, V. & Chase, M. W. (1999) Nature 402, 404–407. [DOI] [PubMed] [Google Scholar]
- 10.Bremer, K. (2000) Proc. Natl. Acad. Sci. USA 97, 4707–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chaw, S. M., Chang, C. C., Chen, H. L. & Li, W. H. (2004) J. Mol. Evol. 58, 424–441. [DOI] [PubMed] [Google Scholar]
- 12.Friis, E. M., Crane, P. R. & Pedersen, K. R. (1997) in Evolution and Diversification of Land Plants, eds. Iwatsuki, K. & Raven, P. H. (Springer, Tokyo), pp. 121–156.
- 13.Crane, P. R., Pedersen, K. R., Friis, E. M. & Drinnan, A. N. (1993) Syst. Bot. 18, 328–344. [Google Scholar]
- 14.Pedersen, K. R., Friis, E. M., Crane, P. R. & Drinnan, A. N. (1994) Rev. Palaeobot. Palynol. 80, 291–303. [Google Scholar]
- 15.Daghlian, C. P. (1981) Bot. Rev. 47, 517–555. [Google Scholar]
- 16.Bonde, S. D. (2000) Palaeobotanist 49, 85–92. [Google Scholar]
- 17.Greenwood, D. R. & Conran, J. G. (2000) in Monocots: Systematics and Evolution, eds. Wilson, K. I. & Morrison, D. A. (Commonwealth Scientific and Industrial Research Organization, Melbourne), pp. 52–59.
- 18.Kvaček, J. & Herman, A. B. (2004) Rev. Palaeobot. Palynol. 128, 323–353. [Google Scholar]
- 19.Doyle, J. A. (1973) Q. Rev. Biol. 48, 399–413. [Google Scholar]
- 20.Walker, J. W. & Walker, A. G. (1984) Ann. Mo. Bot. Gard. 71, 464–521. [Google Scholar]
- 21.Friis, E. M., Pedersen, K. R. & Crane, P. R. (2000) Grana 39, 226–245. [Google Scholar]
- 22.Zbyszewski, G., Moitinho d'Almeida, F. & Torre de Assunção, C. (1955) Carta geológica de Portugal na escala de 1/50 000. Notícia explicativa da folha 30-C Torres Vedras (Serviços Geológicos de Portugal, Lisbon).
- 23.Rey, J. (1993) Comun. Inst. Geol. Mineiro 79, 75–85. [Google Scholar]
- 24.Dinis, J. L. (2001) Comun. Inst. Geol. Mineiro 88, 127–160. [Google Scholar]
- 25.Heimhofer, U. (2004) Ph.D. thesis (Eidgenössische Technische Hochschule, Zurich), pp. 1–166.
- 26.Rydin, C., Pedersen, K. R. & Friis, E. M., Proc. Natl. Acad. Sci. USA, in press.
- 27.Osborn, J. M., Taylor, T. N. & Lima, M. R. d. (1993) Rev. Palaeobot. Palynol. 77, 171–184. [Google Scholar]
- 28.Sampson, F. B. (1993) Grana 32, 154–162. [Google Scholar]
- 29.Hesse, M., Weber, M. & Halbritter, H.-M. (1999) Grana 38, 203–209. [Google Scholar]
- 30.Sampson, F. B. (2000) Int. J. Plant Sci. 161, Suppl. 6, S193–S210. [Google Scholar]
- 31.van der Merwe, J. J. M., van Wyk, A. E. & Kok, P. D. F. (1988) S. Afr. J. Bot. 54, 80–88. [Google Scholar]
- 32.Hesse, M., Weber, M. & Halbritter, H. (2000) in Pollen and Spores: Morphology and Biology, eds. Harley, M. M., Morton, C. M. & Blackmore, S. (Royal Botanic Gardens, Kew, United Kingdom), pp. 227–239.
- 33.Theilade, I., Mærsk-Møller, M.-L., Theilade, J. & Larsen, L. (1993) Grana 32, 338–342. [Google Scholar]
- 34.Mayo, S. J., Bogner, J. & Boyce, P. C. (1997) The Genera of the Araceae (Royal Botanic Gardens, Kew, United Kingdom).
- 35.Grayum, M. H. (1992) Monogr. Syst. Bot. Mo. Bot. Gard. 43, 1–167. [Google Scholar]
- 36.van der Ham, R. W. J. M., Hetterscheid, W. L. A. & van Heuven, B. J. (1998) Rev. Palaeobot. Palynol. 103, 95–142. [Google Scholar]
- 37.Weber, M., Halbritter, H. & Hesse, M. (1999) Int. J. Plant Sci. 160, 415–423. [Google Scholar]
- 38.Renner, S. S. & Zhang, L.-B. (2004) Syst. Biol. 53, 422–432. [DOI] [PubMed] [Google Scholar]
- 39.Gandolfo, M. A., Nixon, K. C., Crepet, W. L. & Friis, E. M. (1998) Nature 394, 532–533. [Google Scholar]
- 40.Sampson, F. B. (1975) Grana 15, 153–157. [Google Scholar]
- 41.Zetter, R., Hesse, M. & Frosch-Radivo, A. (2001) Rev. Palaeobot. Palynol. 117, 267–279. [Google Scholar]
- 42.Hesse, M., Bogner, J., Halbritter, H. & Weber, M. (2001) Grana 40, 26–34. [Google Scholar]
- 43.Mtchedlishvili, N. D. & Shakhmoundes, V. A. (1973) in Palynology of Mesophyte, ed. Chlonova, A. F. (Nauka, Moscow), pp. 137–142 (in Russian with English summary).
- 44.Madison, M. & Tiffney, B. H. (1976) J. Arnold Arbor. 57, 185–201. [Google Scholar]
- 45.Gregor, H. J. & Bogner, J. (1984) Doc. Naturae 19, 1–12. [Google Scholar]
- 46.Mai, D. H. (1985) Flora 176, 449–511. [Google Scholar]
- 47.Mai, D. H. (1995) Tertiäre Vegetationsgeschichte Europas (Fischer, New York).
- 48.Smith, S. Y. & Stockey, R. A. (2003) Int. J. Plant Sci. 164, 239–250. [Google Scholar]
- 49.Friis, E. M. (1985) Biol. Skr. K. Dan. Vidensk. Selsk. 24, 1–165. [Google Scholar]
- 50.Wilde, V. (1989) Cour. Forschungsinst. Senckenberg 115, 1–213. [Google Scholar]
- 51.Stockey, R. A., Hoffman, G. L. & Rothwell, G. W. (1997) Am. J. Bot. 84, 355–368. [PubMed] [Google Scholar]
- 52.Grayum, M. H. (1990) Ann. Mo. Bot. Gard. 77, 628–697. [Google Scholar]
- 53.Williams, N. H. & Dressler, R. L. (1976) Selbyana 1, 349–356. [Google Scholar]
- 54.Gibernall, M. (2003) Aroideana 26, 66–83. [Google Scholar]
- 55.Feild, T. S., Franks, P. J. & Sage, T. L. (2003) Int. J. Plant Sci. 164, 313–324. [Google Scholar]
- 56.Feild, T. S., Arens, A. C., Doyle, J. A., Dawson, T. E. & Donoghue, M. J. (2004) Paleobiology 30, 82–107. [Google Scholar]
- 57.Mayo, S. J. (1993) in The Africa–South America Connection, eds. George, W. & Lavocat, R. (Clarendon, Oxford), pp. 44–58.
- 58.Bunting, G. S. (1960) Mem. N.Y. Bot. Gard. 10, 1–53. [Google Scholar]