Abstract
BACKGROUND
In patients with chronic-phase chronic myeloid leukemia (CP-CML), imatinib resistance is of increasing importance. Imatinib dose escalation was the main treatment option before dasatinib, which has 325-fold more potent inhibition than imatinib against unmutated Bcr-Abl in vitro. Data with a minimum of 2 years of follow-up were available for the current study of dasatinib and high-dose imatinib in CP-CML resistant to imatinib at daily doses from 400 mg to 600 mg.
METHODS
A phase 2, open-label study was initiated of 150 patients with imatinib-resistant CP-CML who were randomized (2:1) to receive either dasatinib 70 mg twice daily (n = 101) or high-dose imatinib 800 mg (400 mg twice daily; n = 49).
RESULTS
At a minimum follow-up of 2 years, dasatinib demonstrated higher rates of complete hematologic response (93% vs 82%; P = .034), major cytogenetic response (MCyR) (53% vs 33%; P = .017), and complete cytogenetic response (44% vs 18%; P = .0025). At 18 months, the MCyR was maintained in 90% of patients on the dasatinib arm and in 74% of patients on the high-dose imatinib arm. Major molecular response rates also were more frequent with dasatinib than with high-dose imatinib (29% vs 12%; P = .028). The estimated progression-free survival also favored dasatinib (unstratified log-rank test; P = .0012).
CONCLUSIONS
After 2 years of follow-up, dasatinib demonstrated durable responses and improved response and progression-free survival rates relative to high-dose imatinib.
Keywords: dasatinib, drug resistance, imatinib, chronic myeloid leukemia
The development of targeted therapies to treat chronic-phase chronic myeloid leukemia (CP-CML) resulted in a positive shift in outcomes in patients with this disease. The first Bcr-Abl tyrosine kinase inhibitor (TKI) available as treatment for CP-CML was imatinib (Gleevec; Novartis, Basel, Switzerland), which became a first-line therapy.1–4 Despite the favorable results obtained with imatinib,5 resistance occurred in a subset of patients with CP-CML.3,5–8 This led to the development of recommendations defining response milestones.1,2 In the International Randomized Interferon Versus STI571 or IRIS study, lack of complete cytogenetic response [CCyR] could be identified in approximately 25% of imatinib-treated patients within 18 months after therapy initiation.3 Currently, the achievement of a CCyR by 18 months after starting imatinib therapy1,2 is considered a minimally acceptable response.
Previous treatment options for imatinib-resistant patients were limited and primarily included imatinib dose escalation up to 800 mg daily.2 Data from small series demonstrated responses in a subset of imatinib-resistant patients after imatinib dose escalation.7,9–12 Dasatinib (Sprycel; Bristol-Myers Squibb, New York, NY) was the first TKI approved to treat patients with imatinib-resistant and imatinib-intolerant CP-CML. Later, nilotinib (Tasigna; Novartis) also became available. In vitro studies demonstrated that dasatinib was 325-fold more potent than imatinib at inhibiting unmutated BCR-ABL.13 The potency of dasatinib, coupled with its activity against imatinib-resistant mutations in vitro,14 provided a compelling preclinical rationale for its investigation in patients with imatinib resistance. Phase 1, 2, and 3 studies have demonstrated the effectiveness of dasatinib in CP-CML after imatinib resistance or intolerance,15–19 with durable responses after 2 years of follow-up.20
In the current study, we evaluated dasatinib and high-dose imatinib in patients with CP-CML and resistance to imatinib at doses from 400 mg to 600 mg daily. Data with a minimum of 2 years of follow-up are presented here.
MATERIALS AND METHODS
The study methods and eligibility criteria have been reported previously17 and are described briefly below. This multicenter, randomized, open-label, phase 2 study (START-R) evaluated patients who had CP-CML with imatinib resistance at standard doses of 400 mg to 600 mg daily. Criteria for CP-CML diagnosis were defined previously.17,19 Primary imatinib resistance was defined as no complete hematologic response (CHR) after 3 months, no cytogenetic response after 6 months, no major cytogenetic response (MCyR) (>35% Philadelphia chromosome-positive cells) after 12 months, or a continuously increasing white blood cell (WBC) count on 2 consecutive evaluations at least 2 weeks apart from nadir to a WBC count ≥20,000/mm3 or an absolute increase in the WBC count >50,000/mm3 above the lowest value. Acquired imatinib resistance was defined as disease recurrence after a previous hematologic response or MCyR. Imatinib resistance could occur only while the patient was receiving at least 400 mg to 600 mg daily of imatinib.
Eligible patients were aged ≥18 years and dasatinib-naive. Patients with imatinib intolerance or who previously received imatinib >600 mg daily were ineligible. In addition, patients previously identified (screening was not required) with one of the following BCR-ABL mutations, which are known to confer a high degree of imatinib resistance in vitro, were excluded: L248V, G250E, Q252H/R, Y253H/F, E255K/V, T315I/D, F317L, and H396P/R.
All patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki and was approved by each center’s Institutional Review Board/Ethics Committee. The study was registered as a National Clinical Trial (NCT) (NCT00103844) (available at: www.clinicaltrials.gov).
Patients were randomized at a 2:1 ratio to receive either oral dasatinib 70 mg twice daily (140 mg daily) or oral high-dose imatinib 800 mg (400 mg twice daily). Randomization was stratified by study site and cytogenetic response on imatinib (any response vs no cytogenetic response). Dasatinib could be dose escalated to 90 mg twice daily for disease progression or no MCyR at 12 weeks. Dose reductions of dasatinib to 50 mg or 40 mg twice daily were permitted for toxicity. No imatinib dose escalation beyond 800 mg daily was allowed, but imatinib could be reduced to 600 mg daily for toxicity in patients who had not been treated previously at this dose level. Patients could cross over to the alternate treatment arm for disease progression or intolerable toxicity17 according to protocol guidelines. For the high-dose imatinib arm only, patients also could cross over if they did not achieve an MCyR or a ≥30% reduction in metaphases by 12 weeks. Therapy continued until disease progression or unacceptable toxicity, patient withdrawal, or discontinuation. Cytogenetic and hematologic evaluations were performed as described previously.17 Quantitative reverse transcriptase-polymerase chain reaction analysis was performed every 4 weeks up to Week 12 and every 3 months thereafter.
The primary endpoint of the current study was the estimated MCyR rate at 12 weeks. Secondary endpoints were the precrossover major molecular response (MMR) rate; the MCyR and CHR rates at any time; the duration of and time to MCyR and CHR; cytogenetic and hematologic responses after crossover; and safety. Before crossover to the alternate treatment arm for progression or intolerable toxicity, the CCyR rate, the progression-free survival (PFS) rate, the time to treatment failure, and BCR-ABL mutations also were assessed. Additional analyses included cytogenetic response by prior imatinib dose and prior cytogenetic response on imatinib and by primary or acquired imatinib resistance. The MMR was defined as a BCR-ABL level ≤0.1% on the international scale based on standard methodology.21,22 Disease progression was defined as progression to accelerated or blast phase, loss of CHR or MCyR, or increasing WBC count (doubling of the count from nadir to >20,000/mm3 or an increase >50,000/mm3 on 2 occasions at least 2 weeks apart). PFS was calculated as the time from randomization until progression, treatment discontinuation because of progression before crossover, or death. The time to treatment failure was calculated as the time from randomization to progression (as described for PFS), the time off study or crossed over, or death.
Analyses reported were based on data precrossover unless otherwise specified. Adverse events (AEs) were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Statistical Analysis
Sample size determination was based on the primary endpoint. At least 100 dasatinib-treated patients were estimated for a maximum 20% width of the 95% confidence interval (95% CI), and at least 50 imatinib-treated patients were estimated for a maximum width of 29% for the 95%CI.
In the initial study design, no formal statistical comparison between treatment arms was planned. The comparisons performed were unadjusted for multiplicity. Response rates were estimated with an exact 95%CI based on the Clopper and Pearson method.23 The Agresti and Min method24 was used to calculate differences in response rates with corresponding 95%CIs, and for post-hoc response rate comparisons. Kaplan-Meier curves with median and corresponding Brookmeyer and Crowley 95%CIs25 were used to evaluate the duration of responses, time to treatment failure, and PFS. For PFS and time to treatment failure, a 2-sided post-hoc, unstratified log-rank test was used to compare treatments. The time to response was summarized with Kaplan-Meier estimates.
RESULTS
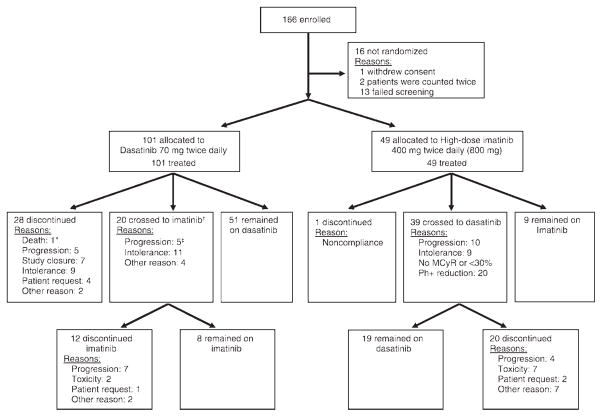
Overall, 150 patients were enrolled and treated from February 2005 to November 2005 (Fig. 1); follow-up to November 2007 provided 2 years of study data. The median follow-up was 26 months (range, 6.9–32.7 months). Most baseline characteristics were well balanced between groups (Table 1), and the median time since CML diagnosis was 59 months for all patients. An exception was a higher incidence of baseline BCR-ABL mutations in patients who were randomized to receive dasatinib.
FIGURE 1.
Consolidated Standards of Reporting Trials (CONSORT) diagram is shown. Randomization was in a 2:1 ratio of dasatinib:imatinib. An asterisk indicates that patient death was within 30 days of dasatinib discontinuation; dagger, 2 patients who were not listed had withdrawn from dasatinib therapy for intolerance (n = 1) or for another reason (n = 1) but were not yet receiving high-dose imatinib; double dagger, 1 patient had developed disease progression and intolerance. MCyR indicates major cytogenetic response; Ph+, Philadelphia chromosome positive.
Table 1.
Baseline Demographics and Disease Characteristics
| No. of Patients (%) | ||
|---|---|---|
| Demographics/Characteristics | Dasatinib, n=101 | High-Dose Imatinib, n=49 |
| Median age (range), y | 51 (24–85) | 51 (24–80) |
| Men | 53 (52) | 22 (45) |
| Median CML duration from diagnosis to first dasatinib dose (range), mo | 64 (6–166) | 52 (14–133) |
| Maximum previous imatinib dose, mg | ||
| 400 | 36 (36) | 14 (29) |
| 500 | 2 (2) | 1 (2) |
| 600 | 63 (62) | 34 (69) |
| Previous imatinib treatment duration, y | ||
| <1 | 12 (12) | 5 (10) |
| 1–3 | 44 (44) | 29 (59) |
| >3 | 45 (45) | 15 (31) |
| Reason for imatinib resistance* | ||
| Lost MCyR | 21 (21) | 14 (29) |
| Lost CHR | 24 (24) | 15 (31) |
| Increasing WBC count | 4 (4) | 2 (4) |
| No CHR after 3 mo | 3 (3) | 2 (4) |
| No CyR after 6 mo | 39 (39) | 16 (33) |
| No MCyR after 12 mo | 39 (39) | 24 (49) |
| Response status at entry | ||
| In CHR | 51 (50) | 27 (55) |
| In MCyR | 6 (6) | 0 (0) |
| Baseline BCR-ABL mutation, n/N (%)/† | 41/93 (44) | 11/46 (24) |
| P-loop mutation | 10/93 (11) | 2/46 (4) |
| T315I | 3/93 (3) | 0 (0) |
| Protocol-specified mutations‡ | 17/93 (18) | 2/46 (4) |
| Best previous cytogenetic response to imatinib | ||
| CCyR | 15 (15) | 4 (8) |
| PCyR | 13 (13) | 10 (20) |
| No cytogenetic response | 39 (39) | 15 (31) |
| CHR | 93 (92) | 47 (96) |
| Treatment history | ||
| Hydroxyurea/anagrelide | 97 (96) | 46 (94) |
| Interferon | 74 (73) | 33 (67) |
| Chemotherapy | 39 (39) | 18 (37) |
| Bone marrow transplantation | 7 (7) | 2 (4) |
| Hematologic analysis | n=100 | n=48 |
| Median WBC count (range), ×109/L | 7.6 (1.8–153.2) | 7.4 (1.8–133) |
| WBC count ≥20,000×109/L | 11 (11) | 7 (14) |
| Median platelet count (range), ×109/L | 261 (55–1903) | 248 (80–2318) |
CML indicates chronic myeloid leukemia; MCyR, major cytogenetic response; CHR, complete hematologic response; WBC, white blood cell; CyR, any cytogenetic response; CCyR, complete cytogenetic response; PCyR, partial cytogenetic response.
May have had more than 1 reason for resistance.
Data were available for 139 patients (93 patients in the dasatinib group and 46 patients in the imatinib).
Protocol-specified mutations included L248V, G250E, Q252H/R, Y253H/F, E255K/V, T315I/D, F317L, and H369P/R.
Adapted with permission from Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5140.
Response Rates
The CHR rate was higher with dasatinib (93%; 94 of 101 patients) than with high-dose imatinib (82%; 40 of 49 patients; P = .034). At 24 months, a greater proportion of patients receiving dasatinib (84%; 95% CI, 76%–93%) than patients receiving high-dose imatinib (73%; 95% CI, 49%–96%) were without loss of CHR. In patients without a CHR at baseline, the CHR rate was 86% (43 of 50 patients) on dasatinib and 72% (16 of 22 patients) on high-dose imatinib.
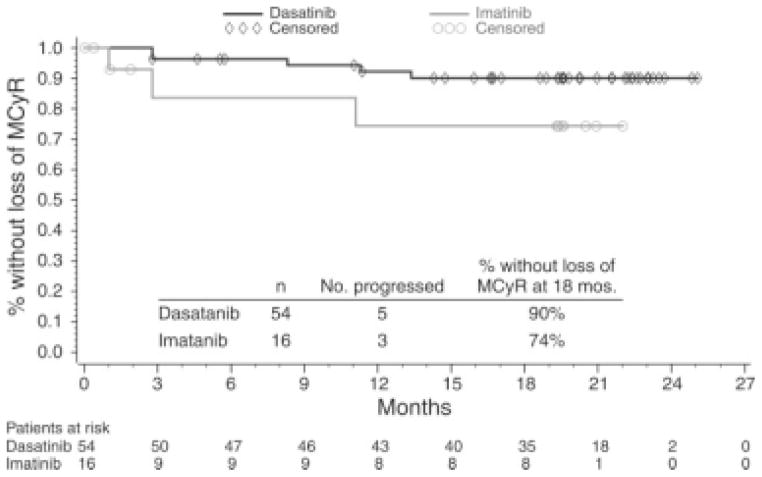
The MCyR rate was higher with dasatinib at 53% (54 of 101 patients) than with high-dose imatinib at 33% (16 of 49 patients; P = .017), and the CCyR rates were 44% (44 of 101 patients) and 18% (9 of 49 patients) with dasatinib and high-dose imatinib, respectively (P = .0025) (Table 2). Ninety percent (95% CI, 82%–98%) of patients receiving dasatinib and 74% (95% CI, 49%–100%) of patients receiving high-dose imatinib maintained their MCyR at 18 months (Fig. 2). For patients who achieved an MCyR, 45% (45 of 101 patients) and 18% (9 of 49 patients) of those receiving dasatinib and high-dose imatinib, respectively, still were on study with an MCyR. Among the patients who achieved MCyR, the reasons for discontinuing dasatinib or high-dose imatinib included loss of MCyR (5% and 6% of patients, respectively), intolerance (3% and 4% of patients, respectively), or other reasons (1% and 4%, of patients, respectively). In patients without a baseline MCyR, the MCyR rate was 52% (49 of 95 patients) with dasatinib and 33% (16 of 49 patients) with high-dose imatinib. The CCyR rate was 42% (41 of 97 patients) with dasatinib and 18% (9 of 49 patients) with high-dose imatinib among those who were not in CCyR at baseline.
Table 2.
Hematologic and Cytogenetic Response Rates Before and After Crossover
| No. of Patients (%) | ||||
|---|---|---|---|---|
| Initial Treatment | Postcrossover | |||
| Response | Dasatinib, n=101 | High-Dose Imatinib, n=49 | From High-Dose Imatinib to Dasatinib, n=39 | From Dasatinib to High-Dose Imatinib, n=20 |
| Complete hematologic response | 94 (93)* | 40 (82) | 37 (95) | 13 (65) |
| 95% CI | 86.2–97.2 | 68–91.2 | 82.7–99.4 | 40.8–84.6 |
| Cytogenetic responses | ||||
| MCyR at 12 wk | 36 (36)† | 14 (29) | ||
| 95% CI | 26.4–45.8 | 16.6–43.3 | — | — |
| CCyR at 12 wk | 22 (22)‡ | 4 (8) | — | — |
| MCyR | 54 (53)§ | 16 (33) | 19 (49) | 3 (15) |
| 95% CI | 43.3–63.5 | 19.9–47.5 | 32.4–65.2 | 3.2–37.9 |
| Previous imatinib, 600 mg/d | 32/63 (51) | 8/34 (24) | — | — |
| Previous imatinib, 400 mg/d | 22/36 (61) | 7/14 (50) | — | — |
| CCyR | 44 (44)|| | 9 (18) | 15 (38) | 0 (0) |
| PCyR | 10 (10) | 7 (14) | 4 (10) | 3 (15) |
| MCyR in patients with previous | ||||
| CyR on imatinib | ||||
| All | 34/62 (55) | 15/34 (44) | — | — |
| Previous imatinib, 600 mg/d | 20/40 (50) | 8/23 (35) | ||
| Previous imatinib, 400 mg/d | 14/20 (70) | 6/10 (60) | ||
| MCyR in patients without a previous | ||||
| CyR on imatinib | ||||
| All | 20/39 (51) | 1/15 (7) | ||
| Previous imatinib, 600 mg/d | 12/23 (52) | 0/11 (0) | ||
| Previous imatinib, 400 mg/d | 8/16 (50) | 1/4 (25) | ||
| Cytogenetic response by imatinib resistance¶ | — | — | ||
| Primary resistance | n=53 | n=24 | — | — |
| MCyR | 30 (57) | 7 (29) | ||
| CCyR | 22 (42) | 3 (13) | ||
| Acquired resistance | n=43 | n=24 | — | — |
| MCyR | 21 (49) | 8 (33) | ||
| CCyR | 19 (44) | 5 (21) | ||
| Cytogenetic response with protocol-specified mutations# | n=17 | n=2 | — | — |
| MCyR | 7 (41) | 0 (0) | ||
| CCyR | 4 (24) | 0 (0) | ||
95% CI indicates 95% confidence interval; MCyR, major cytogenetic response; CCyR, complete cytogenetic response; PCyR, partial cytogenetic response; CyR, any cytogenetic response.
P =.0341.
P =.402.
P =.041.
P =.017.
P =.0025.
Six patients had no reason for previous imatinib resistance available.
Patients with protocol-specified mutations, including L248V, G250E, Q252H/R, Y253H/F, E255K/V, T315I/D, F317L, and H396P/R.
FIGURE 2.
The duration of major cytogenetic response (MCyR) before patients crossed over to the alternate treatment arm is shown. Patients who achieved an MCyR and crossed over for intolerance before disease progression were censored at the date of the first treatment postcrossover. Patients without disease progression and those who remained alive at the time of last follow-up were censored on the date of their last cytogenetic assessment.
In patients who previously received imatinib at doses of 400 or 600 mg daily, the MCyR rate was higher with dasatinib than with high-dose imatinib both overall and among those with and without a prior cytogenetic response on imatinib (Table 2). Among patients with primary and acquired imatinib resistance, the MCyR and CCyR rates were higher for dasatinib relative to high-dose imatinib (Table 2).
The MMR rates were 29% (29 of 101 patients) with dasatinib and 12% (6 of 49 patients) with high-dose imatinib (P = .028). In addition, the MMR rate was 64% (28 of 44 patients) with dasatinib and 56% (5 of 9 patients) with high-dose imatinib in patients who had a CCyR and molecular response assessment. The Kaplan-Meier estimated proportion of patients without treatment failure at 24 months was 59% with dasatinib and 18% with high-dose imatinib (Fig. 3).
FIGURE 3.
The time to treatment failure is shown. This was defined as the time from randomization to disease progression, off-study or crossover, or death. Patients who had not crossed over to the alternate treatment arm or who were off study because of study closure were censored at the date of their last hematologic or cytogenetic assessment.
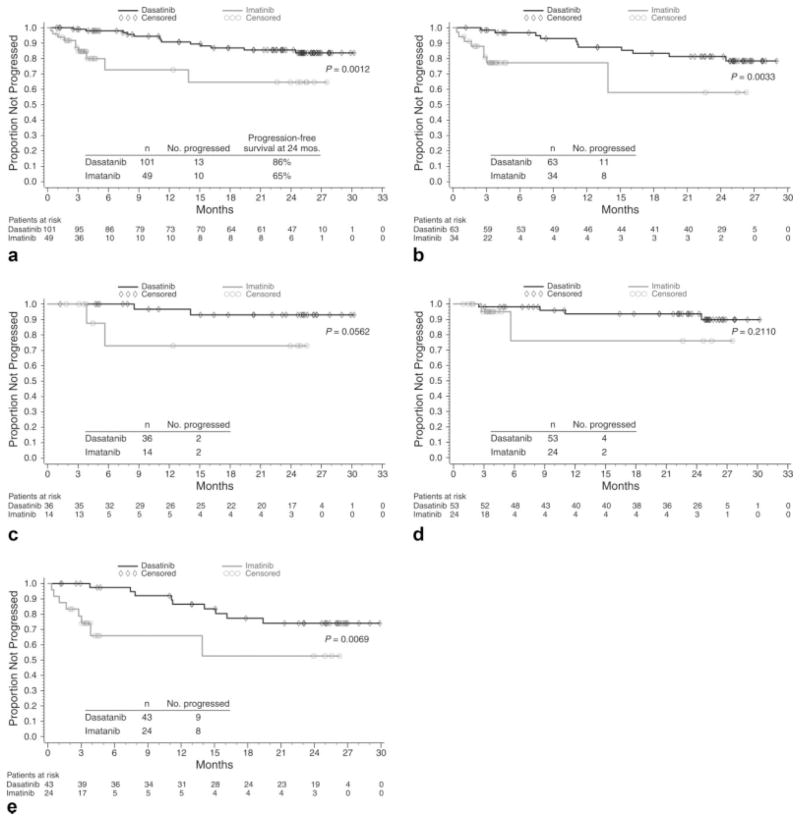
Progression-Free Survival
The estimated PFS favored dasatinib relative to high-dose imatinib (P = .0012) (Fig. 4a). In patients who previously received imatinib at doses of 400 mg or 600 mg daily, the PFS also was improved for dasatinib (Fig. 4b and 4c). The estimated PFS was prolonged with dasatinib in patients who had primary and acquired imatinib resistance (Fig. 4d,e).
FIGURE 4.
Progression-free survival is shown (a) overall, for patients who received prior imatinib at doses of (b) 600 mg or (c) 400 mg, and for patients with (d) primary resistance and (e) acquired resistance. Patients without disease progression and those who remained alive at the time of last follow-up were censored at the last dosing date if they were off initial treatment or at the last assessment date if they were receiving initial treatment.
Postcrossover Response Rates
Thirty-nine patients (80%) crossed from the high-dose imatinib arm to the dasatinib arm, whereas 20 patients (20%) receiving dasatinib crossed over to high-dose imatinib (Fig. 1). The MCyR rate was 49% (19 of 39 patients) and 15% (3 of 20 patients) with dasatinib and high-dose imatinib, respectively (Table 2).
Safety
The median treatment duration was longer for dasatinib at 23 months (range, 0.16 months–29.4 months), whereas high-dose imatinib was received for a median of 3 months (range, 0.16 months–26.3 months). The median average dose was 105 mg daily (range, 42–177 mg daily) for dasatinib and 796 mg daily (range, 358–800 mg daily) for high-dose imatinib.
Treatment-related AEs were reported in 93% (94 of 101 patients; (grade 3/4, 61%; 62 of 101 patients) of patients on the dasatinib arm and in 90% (44 of 49 patients; grade 3/4, 39%; 19 of 49 patients) of patients on the high-dose imatinib arm, and patients may have had >1 AE. Treatment-related nonhematologic AEs generally were grade 1/2 in both arms (Table 3). The more frequent grade 3/4 nonhematologic events (≥2%) were fluid retention (including pleural effusion), dyspnea, infection, fatigue, headache, diarrhea, and abdominal and musculo-skeletal pain (Table 3). Five of the 25 instances of pleural effusion reported with dasatinib were grade 3, and no grade 4 events were reported. Occurrences of grade 3/4 neutropenia, thrombocytopenia, and leukopenia were more frequent with dasatinib (Table 3). There were 2 deaths reported on the dasatinib arm. One death occurred within 30 days of the last dose and was caused by multiorgan failure, which was considered unrelated to dasatinib; the other death occurred 44 days after study discontinuation because of progression to blast crisis.
Table 3.
Adverse Events Associated With Treatment
| Initial Treatment: No. of Patients (%) | ||||
|---|---|---|---|---|
| Dasatinib, n5101 | High-Dose Imatinib, n=49 | |||
| Adverse Event | All Grades* | Grade 3–4 | All Grades | Grade 3–4 |
| Nonhematologic treatment-related adverse events in ≥10% of patients | ||||
| Diarrhea | 37 (37) | 3 (3) | 14 (29) | 1 (2) |
| Fatigue | 33 (33) | 3 (3) | 11 (22) | 2 (4) |
| Headache | 26 (26) | 2 (2) | 5 (10) | 1 (2) |
| Nausea | 24 (24) | 0 | 16 (33) | 0 |
| Dyspnea | 23 (23) | 5 (5) | 2 (4) | 0 |
| Musculoskeletal pain | 21 (21) | 1 (1) | 6 (12) | 1 (2) |
| Rash | 18 (18) | 0 | 10 (20) | 0 |
| Bleeding | 18 (18) | 1 (1) | 4 (8) | 0 |
| Anorexia | 17 (17) | 0 | 4 (8) | 0 |
| Asthenia | 15 (15) | 0 | 2 (4) | 0 |
| Abdominal pain | 15 (15) | 0 | 4 (8) | 1 (2) |
| Pyrexia | 14 (14) | 0 | 5 (10) | 0 |
| Infection | 14 (14) | 4 (4) | 3 (6) | 0 |
| Vomiting | 10 (10) | 0 | 12 (24) | 0 |
| Upper respiratory tract infection/inflammation | 11 (11) | 1 (1) | 3 (6) | 0 |
| Fluid retention | 39 (39) | 7 (7) | 21 (43) | 0 |
| Superficial edema | 20 (20) | 1 (1) | 21 (43) | 0 |
| Pleural effusion | 25 (25) | 5 (5) | 0 | 0 |
| Grade 3–4 cytopenias† | ||||
| Leukopenia | – | 24 (24) | – | 8 (16) |
| Neutropenia | – | 64 (63) | – | 19 (39) |
| Thrombocytopenia | – | 58 (57) | – | 7 (14) |
| Anemia | – | 20 (20) | – | 4 (8) |
Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 3.0).
Hematologic adverse events were reported based on laboratory values.
Dose Modifications and Discontinuations
Dose interruptions occurred in 86 patients (85%) and dose reductions occurred in 71 patients (70%) in the dasatinib arm. Initial dose interruptions and reductions were for hematologic toxicities in 62 patients (61%) and 47 patients (47%), respectively, and for nonhematologic toxicities in 18 patients (18%) and 14 patients (14%), respectively. On the high-dose imatinib arm, dose interruptions were recorded for 17 patients (35%), and dose reductions were recorded for 6 patients (12%). Initial high-dose imatinib treatment interruptions and reductions were for hematologic toxicities in 8 patients (16%) and 2 patients (4%), respectively, and for nonhematologic toxicities in 4 patients (8%) and 2 patients (4%), respectively. Initial dasatinib dose escalations occurred in 38 patients (38%), primarily because there were no MCyRs at 12 weeks in 13% of patients, and 10% of patients developed disease progression. A lower proportion of patients who received dasatinib (50%; 50 of 101 patients) than patients who received high-dose imatinib (82%; 40 of 49 patients) withdrew from initial therapy (Fig. 1).
Discontinuations because of AEs occurred in 23 patients (23%) receiving dasatinib and in 10 patients (20%) receiving high-dose imatinib. Patient discontinuations because of hematologic AEs occurred in 10% (10 of 101 patients) and 8% (4 of 49 patients) of patients receiving dasatinib and high-dose imatinib, respectively, and nonhematologic AEs occurred in 13% (13 of 101 patients) and 12% (6 of 49 patients), respectively. Nonhematologic AEs that resulted in treatment discontinuation included 5 grade 1/2 pleural effusions (5%, 5 of 101 patients) in the dasatinib arm and 1 grade 4 joint effusion (2%, 1 of 49 patients) in the high-dose imatinib arm.
Postcrossover Safety
After crossover, the median treatment duration was 89 weeks (range, 3 weeks–113 weeks) on the dasatinib arm (n = 39) and 28 weeks (range, 4–100 weeks) on the high-dose imatinib arm (n = 20). Before crossover in patients who withdrew from initial treatment (Fig. 1), the median treatment duration had been 34 weeks (range, 1 weeks–108 weeks) on dasatinib (n = 22) and 13 weeks (range, 1 weeks–68 weeks) on high-dose imatinib (n = 39).
After crossover, the treatment-related AE incidence was 95% (37 of 39 patients) with dasatinib and 85% (17 of 20 patients) with high-dose imatinib; grade 3/4 AEs occurred in 54% (21 of 39 patients) and 25% (5 of 20 patients), respectively. The most frequent treatment-related AEs were consistent with those reported before crossover. Three deaths were reported on the dasatinib arm, including 2 deaths that occurred within 30 days of the last dose and were considered to be unrelated to dasatinib (1 patient had lung infection and sepsis and 1 patient had acute heart failure) and 1 death caused by disease progression.
DISCUSSION
The 2-year follow-up data for the current study demonstrate the durability of efficacy and provide mature safety information. Consistent with the initial study report,17 dasatinib demonstrated higher MCyR, CCyR, and MMR rates (53%, 44%, and 29%, respectively) relative to high-dose imatinib (33%, 18%, and 12%, respectively). Efficacy results were achieved in pretreated patients who had a median prestudy CML duration of 5 years who then received dasatinib for an additional median 2 years. The MCyR was durable for dasatinib, because 90% of dasatinib-treated patients maintained an MCyR at 18 months compared with 74% of high-dose imatinib-treated patients. The estimated PFS at 2 years was greater with dasatinib (86%) relative to high-dose imatinib (65%), and the estimated time to treatment failure was more prolonged with dasatinib. The time to treatment failure provided a composite endpoint of efficacy and safety and produced results that were consistent with other study endpoints. In addition, among patients who crossed over to the other treatment arm, an MCyR occurred more frequently with dasatinib (49%) than with high-dose imatinib (15%). The MCyR and CCyR rates in this study are promising given the association between cytogenetic response and survival and disease progression in patients with CP-CML.5,26,27 Overall, dasatinib was found to have durable response rates and prolonged PFS, suggesting improved efficacy over high-dose imatinib in this CP-CML population.
Because a previous response on lower doses of imatinib may suggest the presence of disease that is inherently more sensitive to TKI therapy, the achievement of a response in patients without a prior cytogenetic response to imatinib may be expected to be difficult. In the current study, however, patients who were receiving dasatinib were able to achieve an MCyR regardless of their previous imatinib dose (400 mg or 600 mg daily) and lack of a prior cytogenetic response. Patients who were receiving imatinib at doses ≥ 400 mg daily who did not achieve any cytogenetic response appeared more likely to achieve an MCyR with a change to dasatinib than with an imatinib dose escalation to 800 mg daily.
The most common mechanisms for secondary or acquired imatinib resistance are mutations in the BCR-ABL kinase domain.6,13,28 Despite an imbalance of BCR-ABL mutations at baseline, a higher CCyR rate was reported with dasatinib than with high-dose imatinib (43% vs 18%). These responses with dasatinib are supported by in vitro data demonstrating that imatinib-resistant cell lines, except for T315I, may have varying levels of dasatinib sensitivity.13,14 It is noteworthy that the dasatinib treatment arm had a higher proportion of protocol-specific mutations (18% vs 4%), which confer a high degree of imatinib resistance in vitro.
The cytopenias and nonhematologic treatment-related AEs that were reported after 2 years of follow-up generally were consistent with those in the earlier study report.17 Interpretation of safety data and differences between treatment arms may be limited, because the study was predisposed to differences in treatment duration. The median treatment duration of 23 months for dasatinib and 3 months for high-dose imatinib allowed more opportunity for dasatinib-treated patients to experience an AE than imatinib-treated patients. In addition, the eligibility requirement of adequate tolerability of 400 mg to 600 mg daily of imatinib effectively preselected patients with few AEs while receiving imatinib. It is interesting to note that the safety results in this study apply to the dasatinib dosing regimen of 70 mg twice daily. A subsequent large study demonstrated improved safety with a lower, single daily dose.18
The results of this randomized study demonstrated that the dasatinib dosing regimen of 70 mg twice daily had improved response rates with prolonged PFS compared with high-dose imatinib. Dasatinib also demonstrated a positive effect regardless of the prior imatinib dose received by patients. Particularly noteworthy is the frequency of CCyR in dasatinib-treated patients in this study. The achievement of CCyR has been associated with a low risk of disease progression and improved survival in patients with CP-CML who are treated with imatinib.5,27 Thus, dasatinib rather than high-dose imatinib should be considered the preferred treatment option for patients with CP-CML who have experienced resistance to imatinib doses of 400 mg to 600 mg.
The recommended dose of dasatinib recently was modified to 100 mg once daily for the treatment of imatinib-resistant or imatinib-intolerant CP-CML based on results of a phase 3, dose-optimization study with a median treatment duration of 8 months in patients who had CP-CML with resistance, suboptimal response, or intolerance to imatinib.18 Study results demonstrated similar efficacy between doses of 100 mg once daily and 70 mg twice daily, with a lower incidence of cytopenia and a significantly lower incidence of grade 3/4 thrombocytopenia (P = .004) and any grade of pleural effusion (P = .024) noted for the dosing regimen of 100 mg once daily.18 Overall, dasatinib at a dose of 100 mg once daily demonstrated a more favorable risk-benefit ratio than dasatinib at a dose of 70 mg twice daily. In that study and in the current report, the average median dasatinib dose administered to patients who intended to take 70 mg twice daily was approximately 100 mg daily. The equivalent efficacy and improved tolerability of dasatinib at a dose of 100 mg once daily relative to 70 mg twice daily further support dasatinib as a favorable therapeutic option in patients who have CP-CML with resistance to imatinib doses of 400 mg to 600 mg.
Footnotes
Presented previously in part as an oral presentation at the 49th Annual Meeting and Exposition of the American Society of Hematology, Atlanta, Georgia, December 8–11, 2007; and as a poster presentation at the 44th Annual Meeting of the American Society of Clinical Oncology, Chicago, Illinois, May 30 to June 3, 2008; and at the 13th Congress of the European Hematology Association, Copenhagen, Denmark, June 12–15, 2008.
The following principal investigators, in addition to the authors, also participated in the CA180-017 study: J. J. Garcia, I. Otero, and D. Riveros (Argentina); C. Arthur (Australia); A. Bosly and W. Schroyens (Belgium); P. E. Dorlhiac Llacer and A. Moellman Coelho (Brazil); S. Assouline, D. Forrest, C. Gambacorti-Passerini, P. Laneuville, and A. Turner (Canada); M. Varik (Estonia); K. Porkka (Finland); T. Facon, F. Guilhot, M. Michalet, J. Reiffers, and P. Rousselot (France); C. Bokemeyer, A. Hochhaus, and D. Niederwieser (Germany); T. Masszi (Hungary); A. Nagler (Israel); D. W. Kim (Korea); H. Hjorth-Hansen (Norway); L. Casanova and J. Navarro (Peru); P. Caguioa (Philippines); A. Dmoszynska, W. Jedrzejczak, A. Hellmann, T. Robak, and A. Skotnicki (Poland); F. Cabanillas (Puerto Rico); N. Khoroshko, A. Zaritsky, and A. Golenkov (Russia); J. Lombard, P. Ruff, and G. Cohen (South Africa); L. Stenke and H. Wadenvik (Sweden); L. Y. Shih (Taiwan); J. Apperley, T. Holyoake, and S. O’Brien (United Kingdom); and J. Dipersio, J. K. Giguere, R. Lloyd, J. McGuirk, M. S. Murali, R. M. Stone, and R. Paquette (United States). In addition, K. Gialelis served as protocol manager, and A. Zhao and E. Cormier served as protocol data managers.
Conflict of Interest Disclosures
Funding for this research was provided by Bristol-Myers Squibb. Professional medical writing and editorial assistance was funded by Bristol-Myers Squibb and was provided by E. Dolgos.
Dr. Kantarjian has received research grants from Bristol-Myers Squibb and Novartis.
Dr. Holowiecki has received research grants from Bristol-Myers Squibb and Novartis.
Dr. Hughes has received honoraria and research funding from Bristol-Myers Squibb and Novartis and has acted in an advisory role for both companies.
Dr. Bleickardt is an employee of Bristol-Myers Squibb and owns stock in the company.
Dr. Dejardin is an employee of Bristol-Myers Squibb and owns stock and stock options.
Dr. Cortes has received research support from Bristol-Myers Squibb, Novartis, and Wyeth.
Dr. Shah has acted as a consultant for Bristol-Myers Squibb and Novartis.
References
- 1.National Comprehensive Cancer Network, Inc. [Accessed August 29, 2008];The Chronic Myelogenous Leukemia Clinical Practice Guidelines in Oncology. Version 1.2009. Available at http://www.nccn.org.
- 2.Baccarani M, Saglio G, Goldman J, et al. Evolving concepts in the management of chronic myeloid leukemia: recommendations from an expert panel on behalf of the European LeukemiaNet. Blood. 2006;108:1809–1820. doi: 10.1182/blood-2006-02-005686. [DOI] [PubMed] [Google Scholar]
- 3.O’Brien SG, Guilhot F, Larson RA, et al. Imatinib compared with interferon and low-dose cytarabine for newly diagnosed chronic-phase chronic myeloid leukemia. N Engl J Med. 2003;348:994–1004. doi: 10.1056/NEJMoa022457. [DOI] [PubMed] [Google Scholar]
- 4.Schiffer CA. BCR-ABL tyrosine kinase inhibitors for chronic myelogenous leukemia. N Engl J Med. 2007;357:258–265. doi: 10.1056/NEJMct071828. [DOI] [PubMed] [Google Scholar]
- 5.Druker BJ, Guilhot F, O’Brien SG, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. N Engl J Med. 2006;355:2408–2417. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 6.Hochhaus A, Hughes T. Clinical resistance to imatinib: mechanisms and implications. Hematol Oncol Clin North Am. 2004;18:641–656. doi: 10.1016/j.hoc.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian HM, Talpaz M, O’Brien S, et al. Dose escalation of imatinib mesylate can overcome resistance to standard-dose therapy in patients with chronic myelogenous leukemia. Blood. 2003;101:473–475. doi: 10.1182/blood-2002-05-1451. [DOI] [PubMed] [Google Scholar]
- 8.Hochhaus A, Druker B, Sawyers C, et al. Favorable long-term follow-up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-alpha treatment. Blood. 2008;111:1039–1043. doi: 10.1182/blood-2007-07-103523. [DOI] [PubMed] [Google Scholar]
- 9.Jabbour E, Kantarjian H, Atallah E, et al. Impact of imatinib mesylate dose escalation on resistance and suboptimal responses to standard-dose therapy in patients (pts) with chronic myeloid leukemia (CML) [abstract] Blood. 2007;110:313a–314a. Abstract 1035. [Google Scholar]
- 10.Kantarjian HM, Druker BJ, Guilhot F, et al. Imatinib dose escalation is effective in patients with chronic myeloid leukemia in chronic phase (CML-CP) [abstract] Blood. 2007;110:317a. Abstract 1047. [Google Scholar]
- 11.Marin D, Goldman JM, Olavarria E, et al. Transient benefit only from increasing the imatinib dose in CML patients who do not achieve complete cytogenetic remissions on conventional doses. Blood. 2003;102:2702–2703. doi: 10.1182/blood-2003-06-2042. [DOI] [PubMed] [Google Scholar]
- 12.Zonder JA, Pemberton P, Brandt H, et al. The effect of dose increase of imatinib mesylate in patients with chronic or accelerated phase chronic myelogenous leukemia with inadequate hematologic or cytogenetic response to initial treatment. Clin Cancer Res. 2003;9:2092–2097. [PubMed] [Google Scholar]
- 13.O’Hare T, Walters DK, Stoffregen EP, et al. In vitro activity of Bcr-Abl inhibitors AMN107 and BMS-354825 against clinically relevant imatinib-resistant Abl kinase domain mutants. Cancer Res. 2005;65:4500–4505. doi: 10.1158/0008-5472.CAN-05-0259. [DOI] [PubMed] [Google Scholar]
- 14.Shah NP, Tran C, Lee FY, et al. Overriding imatinib resistance with a novel ABL kinase inhibitor. Science. 2004;305:399–401. doi: 10.1126/science.1099480. [DOI] [PubMed] [Google Scholar]
- 15.Hochhaus A, Kantarjian HM, Baccarani M, et al. Dasatinib induces notable hematologic and cytogenetic responses in chronic-phase chronic myeloid leukemia after failure of imatinib therapy. Blood. 2007;109:2303–2309. doi: 10.1182/blood-2006-09-047266. [DOI] [PubMed] [Google Scholar]
- 16.Hochhaus A, Baccarani M, Deininger M, et al. Dasatinib induces durable cytogenetic responses in patients with chronic myelogenous leukemia in chronic phase with resistance or intolerance to imatinib. Leukemia. 2008;22:1200–1206. doi: 10.1038/leu.2008.84. [DOI] [PubMed] [Google Scholar]
- 17.Kantarjian H, Pasquini R, Hamerschlak N, et al. Dasatinib or high-dose imatinib for chronic-phase chronic myeloid leukemia after failure of first-line imatinib: a randomized phase 2 trial. Blood. 2007;109:5143–5150. doi: 10.1182/blood-2006-11-056028. [DOI] [PubMed] [Google Scholar]
- 18.Shah NP, Kantarjian HM, Kim DW, et al. Intermittent target inhibition with dasatinib 100 mg once daily preserves efficacy and improves tolerability in imatinib-resistant and -intolerant chronic-phase chronic myeloid leukemia. J Clin Oncol. 2008;26:3204–3212. doi: 10.1200/JCO.2007.14.9260. [DOI] [PubMed] [Google Scholar]
- 19.Talpaz M, Shah NP, Kantarjian H, et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias. N Engl J Med. 2006;354:2531–2541. doi: 10.1056/NEJMoa055229. [DOI] [PubMed] [Google Scholar]
- 20.Mauro MJ, Baccarani M, Cervantes F, et al. Dasatinib 2-year efficacy in patients with chronic-phase chronic myelogenous leukemia (CML-CP) with resistance or intolerance to imatinib (START-C) [abstract] J Clin Oncol. 2008;26:374s. Abstract 7009. [Google Scholar]
- 21.Hughes TP, Kaeda J, Branford S, et al. Frequency of major molecular responses to imatinib or interferon alfa plus cytarabine in newly diagnosed chronic myeloid leukemia. N Engl J Med. 2003;349:1423–1432. doi: 10.1056/NEJMoa030513. [DOI] [PubMed] [Google Scholar]
- 22.Hughes T, Deininger M, Hochhaus A, et al. Monitoring CML patients responding to treatment with tyrosine kinase inhibitors: review and recommendations for harmonizing current methodology for detecting BCR-ABL transcripts and kinase domain mutations and for expressing results. Blood. 2006;108:28–37. doi: 10.1182/blood-2006-01-0092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404–413. [Google Scholar]
- 24.Agresti A, Min Y. On small-sample confidence intervals for parameters in discrete distributions. Biometrics. 2001;57:963–971. doi: 10.1111/j.0006-341x.2001.00963.x. [DOI] [PubMed] [Google Scholar]
- 25.Brookmyer R, Crowley JJ. A confidence interval for the median survival time. Biometrics. 1982;38:29–41. [Google Scholar]
- 26.Guilhot F, Chastang C, Michallet M, et al. Interferon alfa-2b combined with cytarabine versus interferon alone in chronic myelogenous leukemia. N Engl J Med. 1997;337:223–229. doi: 10.1056/NEJM199707243370402. [DOI] [PubMed] [Google Scholar]
- 27.de Lavallade H, Apperley JF, Khorashad JS, et al. Imatinib for newly diagnosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. J Clin Oncol. 2008;26:3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 28.Deininger M, Buchdunger E, Druker BJ. The development of imatinib as a therapeutic agent for chronic myeloid leukemia. Blood. 2005;105:2640–2653. doi: 10.1182/blood-2004-08-3097. [DOI] [PubMed] [Google Scholar]