Abstract
The therapeutic impact of inappropriate prescribing of antibiotics is debatable, particularly in situations where infections are treated empirically with multiply prescribed antibiotics. Prescribers may remain under the illusion that such prescriptions are appropriate on the basis of any observed positive treatment outcomes, even though an antibiotic prescribed in such combination therapy may actually be infective against infecting pathogens. This, inevitably, promotes inappropriate antibiotic prescribing. Prescribers may be motivated to make more conscious attempts to prescribe antibiotics appropriately if it is proven that judicious prescribing of antibiotics has positive impacts on treatment outcomes. The objective of this study was to determine the impact of appropriate prescribing of antibiotics on treatment outcomes, days of patient hospitalization and costs related to antibiotic treatment. Observational data on antibiotic treatment were collected for a one-month period from case notes of all inpatients (n=307) and outpatients (n=865) at five government and mission hospitals in Lesotho. Prescriptions were classified into categories of appropriateness based on extents to which antibiotics were prescribed according to principles. Treatment success rates, mean days of hospitalization and costs of antibiotic treatments of inpatients treated with specified prescription categories were determined. Appropriate prescribing of antibiotics for inpatients had positive impacts on treatment outcomes, patients’ days of hospitalization for infections and costs of antibiotic treatments. In outpatient settings, appropriate prescribing of antibiotics failed to show any significant impact on costs of antibiotics. Appropriate prescribing of antibiotics had a positive impact on patients’ recovery and costs of antibiotic treatments in inpatient settings.
Key words: antibiotics, empiric treatment, treatment outcomes, days of hospitalization, hospitalization costs
Introduction
Injudicious prescribing of antibiotics in the management of infectious diseases results in overprescribing of antibiotics and, ultimately, development of microbial resistance to antibiotics.1-3 In situations where antibiotics are selected presumptively, as occurs particularly in the empiric treatment of infections, inappropriate prescribing of antibiotics can pose a major problem in antibiotic usage. Prescribers in such instances may over-prescribe antibiotics in combinations to cover for diagnostic imprecision.4 This is particularly common in developing countries where empiric prescribing of antibiotics is a mainstay of treating infections. Most developing countries lack functional or efficient systems of operating microbiology laboratories,5,6 a situation not conducive to routine identification and antibiotic sensitivity testing of pathogens.
Inappropriate prescribing of antibacterial agents is accepted as a cause of treatment failures and increased costs of treating infections.7 Inappropriate prescribing has been associated with microbial resistance development to antibiotics, and successful intervention studies to improve antibiotic prescribing have actually been found to reduce antimicrobial resistance.8 Timely antibacterial treatment is associated with reduced length of hospital stays and reduced mortality.9,10 Therapeutic deficiencies identified with some antibiotic prescriptions may not be seen as results of inappropriate prescribing, particularly in settings where the drugs are prescribed in combination to treat infections empirically. It is possible for only a subset of such prescribed antibiotics to be effective against the pathogens causing the infection, making the therapeutic infectiveness of others in the prescribed set less obvious. In other circumstances, as seen for example in cases of viral infections closely mimicking bacterial infections in clinical presentations, patients’ recovery may not in any way be attributable to prescribed antibiotics.11 Prescribers may interpret outcomes of treatments they offered in all these instances as results of the effectiveness of the prescribed antibiotics, potentially eliminating their recognition of therapeutic inadequacies of ill prescribed antibiotics. Together, these situations explain the perpetuation of inappropriate prescribing of antibiotics in clinical practice despite the negative effects the practice has on patient management for infections. Studies that investigate the impacts of appropriately and inappropriately prescribed antibiotics on treatment outcome parameters are scarce. We believe that prescribers may become less resistant to prescribing antibiotics appropriately if they are aware of the impacts antibiotic prescribing have on treatment outcomes. This is our motivation for conducting this study.
Objective
The study has the primary objective of observing the impact of appropriate prescribing of antibiotics on patient recovery, days of patient hospitalization and costs of antibiotic treatment in public hospitals in Lesotho.
Materials and Methods
This was an observational study designed as a case series study.12 Data on prescribed antibiotics were collected from case reports of all patients treated for infections in inpatient and outpatient departments. We also collected information from nursing notes and interviewed nurses where necessary to ascertain adequate data on patients’ recovery status. Data collection was for a one-month period from June 15 to July 15, 2009. Interactions with prescribers for clarifications on observed manners of infection diagnosis and treatment were avoided for purposes of obtaining unbiased baseline information on manners in which antibiotics were prescribed. We selected five study site hospitals (three government and two Christian mission owned hospitals) on the basis of their sizes and ownership. Together, they had a total bed capacity of 2466, nearly half the total hospital bed capacity in Lesotho.13
We assessed all antibiotic prescriptions for their appropriateness. We used a prescription assessment instrument that comprised a set of criteria we developed from literature-documented principles of antibiotic prescribing.4,14-21 We obtained additional information from the literature on the etiologies of infections and the characteristics of antibiotics. We compiled these as an additional research tool and used it as further information to make decisions on the conformities of prescriptions to our assessment procedure.22 Prescriptions were assessed principally to establish whether antibiotics were prescribed on the basis of their need or their clinical effectiveness against infecting pathogens. It was necessary in principle for a prescriber to establish a site of infection and identify hallmark signs and symptoms of infection.14 Prescribed antibiotic(s) must also have the appropriate spectra of activity covering bacterial pathogens commonly associated with infection at an anatomical site in question.4 They must be prescribed in the appropriate doses and must be compatible and not antagonistic with other antibiotics prescribed together with them.4,14 We classified prescriptions conforming to given sets of criteria combinations into seven categories of appropriateness defined as follows:
-
-
Prescription category A1: antibiotics appropriately prescribed for the empiric treatment of infections for which bacteria pathogens were considered absolute etiologies;
-
-
Prescription category A2: antibiotics prescribed appropriately for the treatment of infections according to general principles of antibiotic prescribing but also considered inappropriate on the basis of the agents being prescribed for infections for which bacteria pathogens may only be possible etiologies;
-
-
Prescription category B: antibiotics inappropriately prescribed for the empiric treatment of infections with absolute bacterial etiologies;
-
-
Prescription category C: antibiotics prescribed based on available results of culture sensitivity tests;
-
-
Prescription category D: antibiotics appropriately prescribed for the prevention of infections;
-
-
Prescription category E: antibiotics inappropriately prescribed for the prevention of infections;
-
-
Prescription category F: antibiotics inappropriately prescribed for unjustified indications.
We determined only for inpatients the impacts of appropriateness of antibiotic prescribing on patients’ recovery status and their days of hospitalization. Geographical challenges did not permit us to carry out the impacts of appropriateness of antibiotic prescribing on outpatients’ recovery status. We did, however, determine differences in costs of antibiotic treatment among patient groups treated with respective antibiotic prescriptions between the two patient categories. We calculated both treatment success rates (TSR) of patient groups treated with respective antibiotic prescriptions categorized as appropriate or inappropriate. We did not consider death of patients as necessarily being a cause of non-response to antibiotic treatment since other factors unrelated to antibiotic treatment could cause a patient’s death. We determined TSRs with deaths in the denominator (TSR1) and without deaths in the denominator (TSR2). We did not include prescriptions that were prophylatics in TSR determinations, as we had no means of determining infection prophylaxis success rates. Nearly all prescriptions in this category were in the inpatient surgical ward. We compared the frequency of improvement across groups using Pearson’s χ2 test. We also determined the mean of days of patient hospitalization for infections and the costs of antibiotic treatment (total cost and, for inpatients, cost per day of hospitalization) of patient groups treated with antibiotic prescriptions categorized as appropriate or inappropriate. We also compared individual prescription categories (A1 vs A2 vs B vs F), but we only mention several interesting results because the primary objective is to compare the appropriate vs. inappropriate categories as a whole. We determined statistical significance between prescription categories using the Mann-Whitney test. For days of hospitalization, we also compared the distributions of appropriate (A1, A2) and inappropriate (B, F) prescriptions using a survival analysis23 (survdiff function in the R package survival v.2.36-14). Ethical permissions in accordance with the Helsinki Declaration were received from both the Ministry of Health of Lesotho through its ethics committee for public hospitals and individual CHAL hospitals, as well as the ethics committee of the North-West University (South Africa).
Results
Assessment and categorization of prescriptions
Summary statistics are shown in Table 1. For inpatients, we report results separately for the surgical and medical wards. A total of 307 inpatient and 865 outpatient prescriptions accounting for 584 and 1073 total antibiotics prescribed, respectively, during the study period, were assessed for their appropriateness. Patients were most commonly adults (73% to 88%) and female (55% to 58%).
Table 1.
Summary of study variables.
| No. of patients | Prescriptions per patient | % female patients | % adult patients | |
|---|---|---|---|---|
| Outpatient | 865 | 1.22 | 58% | 79% |
| Inpatient | 307 | 1.89 | 57% | 79% |
| Medical ward | 190 | 1.68 | 58% | 73% |
| Surgical ward | 117 | 2.17 | 55% | 88% |
Table 2 shows the body systems affected in the antibiotic prescriptions we analyzed. When more than one body system was reported, we only report the first body system. The inpatient medical ward and outpatients have roughly the same distribution, with differences noted in the systems skin and soft tissue and non-infections conditions. Both groups had respiratory tract as the body system with the highest percentage of prescriptions. Patients in the inpatient surgical ward were predominantly prescribed antibiotics related to the skin and soft tissue.
Table 2.
Body systems affected when antibiotics were prescribed in hospital wards.
| Body system | Inpatient medical | Inpatient surgical | Outpatient |
|---|---|---|---|
| Respiratory tract | 43.7 | 3.4 | 47.3 |
| Gastrointestinal tract/abdominal cavity | 14.2 | 3.4 | 7.4 |
| Genitourinary tract infections | 11.6 | 6.8 | 15.5 |
| Skin and soft tissue | 4.2 | 63.2 | 16.0 |
| Bones | 0.5 | 4.3 | 0.1 |
| Central nervous system | 2.6 | 2.6 | 0 |
| Blood | 1.1 | 0 | 0 |
| Pyrexia (no specific site) | 1.6 | 0 | 1.2 |
| Non-infectious conditions | 20.5 | 16.2 | 12.6 |
Data are percent in each hospital ward, based on no. of patients from Table 1. Each column adds to 100 within rounding error.
Figure 1 shows the percentage of each prescription category. For outpatients, the most common categories were A1 (34.6%) and A2 (43.8%). We consider both categories appropriate, although where possible we consider A1 and A2 separately to test our hypothesis. The A2 prescriptions were most commonly for the respiratory tract in the inpatient medical ward (46.5% of A2 prescriptions in that ward) and for outpatients (76.9% of A2 prescriptions among outpatients).
Figure 1.
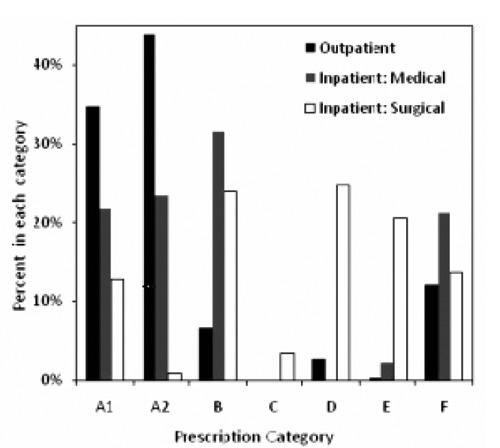
Percentage of prescriptions per category for outpatients and inpatients (medical ward and surgical ward). Sample sizes (no. of patients) in Table 1.
The inpatients received a higher percentage of inappropriate prescriptions (B 31.5% and 23.9%; F 21.2% and 13.7%). The percentage of appropriate prescriptions was significantly different between the two patient types (inpatient vs outpatient) and between the two inpatient wards (P<0.05 by χ2 test). For outpatients, prescriptions could be written by either doctors (N=745) or nurses (N=118). There was no significant difference in the categories (P>0.05 by χ2 test), and we combined the prescriptions of doctors and nurses in all analyses. Few prescriptions were in category C, and categories D and E were found in low percentages except in the inpatient surgical ward. Therefore, we did not consider groups C, D, and E in further analyses.
Impact of appropriateness of antibiotic prescribing on treatment outcomes, days of hospitalization for infection treatment and costs of antibiotic treatment
Table 3 shows the treatment outcomes for the inpatient medical and surgical wards for the prescription categories A1 and A2 (appropriate) vs B and F (inappropriate). In both wards, A1 prescriptions have a higher treatment success rate than B and F by both TSR metrics. In the medical ward, A2 has a lower TSR1 but a higher TSR2 than B and F. There were not enough F prescriptions in either ward nor A2 prescriptions in the surgical ward to make meaningful comparisons among these groups alone.
Table 3.
Treatment success rate for medical and surgical wards (inpatients) by prescription category.
| Prescription category | Improved | Not improved | Died | % Died | TSR1* | TSR2° | |
|---|---|---|---|---|---|---|---|
| Medical ward | |||||||
| A1 | 29 | 5 | 6 | 15.0 | 72.5 | 85.3 | |
| A2 | 26 | 7 | 10 | 23.3 | 60.5 | 78.8 | |
| B | 35 | 12 | 10 | 17.5 | 61.4 | 74.5 | |
| F | 1 | 0 | 0 | 0 | 100 | 100 | |
| A1, A2 | 55 | 12 | 16 | 19.3 | 66.3 | 82.1 | |
| B, F | 36 | 12 | 10 | 17.2 | 62.1 | 75.0 | |
| Total | 91 | 24 | 26 | 18.4 | 64.5 | 79.1 | |
| Surgical ward | |||||||
| A1 | 11 | 2 | 1 | 7.1 | 78.6 | 84.6 | |
| A2 | 1 | 0 | 0 | 0 | 100 | 100 | |
| B | 20 | 7 | 0 | 0 | 74.1 | 74.1 | |
| F | 0 | 0 | 1 | 100 | 0 | 0 | |
| A1, A2 | 12 | 2 | 1 | 6.7 | 80.0 | 85.7 | |
| B, F | 20 | 7 | 1 | 3.6 | 71.4 | 74.1 | |
| Total | 37 | 9 | 2 | 4.2 | 77.1 | 80.4 | |
TSR, treatment success rate.
*TSR1=Improved/(Improved+NotImproved+Died)
°TSR2=Improved/(Improved+NotImproved). No differences between individual groups (A1, A2, B) nor between aggregate groups (A1, A2 vs B, F) were statistically significant.
Table 4 shows the average number of days of hospitalization for inpatients by prescription category and hospital ward. The differences across wards (medical vs surgical) were greater than the differences within wards (A1-A2 vs B-F). The patients treated with appropriately prescribed antibiotics (A1-A2) had significantly shorter hospital stays than patients treated with inappropriate antibiotics (B-F) (P<0.05 by Mann Whitney Test) in the medical ward but not in the surgical ward (P>0.05). The conclusions were the same when deaths were excluded from the calculations. In the medical ward, the categories A1 and A2 were each significantly different from category B, and A1 and A2 were not significantly different from each other. A survival analysis modeling time of hospital stay with deaths treated as censored confirmed that the length of stay was less for appropriate prescriptions in the medical ward but that there was no difference in the surgical ward (P<0.05 corresponding to a significant difference by χ2).
Table 4.
Days in hospital by prescription category and ward.
| Ward | Category | Including deaths | Excluding deaths | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | ||
| Medical | Appropriate | ||||||
| A1+A2 | 6.6 | 4.3 | 6.0 | 6.9 | 3.9 | 6.0 | |
| Inappropriate | |||||||
| B+F | 9.2 | 5.8 | 8.0 | 9.4 | 6.0 | 8.0 | |
| Surgical | Appropriate | ||||||
| A1+A2 | 16.3 | 13.6 | 12.0 | 17.2 | 13.5 | 14.0 | |
| Inappropriate | |||||||
| B+F | 20.4 | 19.1 | 14.5 | 20.9 | 19.1 | 15.0 | |
SD, standard deviation.
Table 5 shows the total cost of antibiotics during the inpatient hospital stays and for outpatient prescriptions. The inpatients with appropriate prescriptions had significantly lower total costs than patients given inappropriate prescriptions (P<0.05 in both wards). For the individual categories in the medical ward, A1, A2, and F were all significantly less than B but were not significantly different from each other. In the surgical ward, only A1 and F were significantly less than B.
Table 5.
Total antibiotic cost (ZAR) by prescription category and ward [1 ZAR (South African Rand)=0.14486 USD at the time of study].
| Ward | Category | Including deaths | Excluding deaths | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | ||
| Medical | Appropriate | ||||||
| A1+A2 | 96.6 | 106.3 | 67.8 | 102.6 | 112.7 | 71.5 | |
| Inappropriate | |||||||
| B+F | 139.0 | 110.4 | 107.0 | 133.1 | 96.6 | 103.4 | |
| Surgical | Appropriate | ||||||
| A1+A2 | 196.8 | 175.2 | 138.1 | 208.0 | 175.5 | 161.3 | |
| Inappropriate | |||||||
| B+F | 347.8 | 405.3 | 236.3 | 355.6 | 406.8 | 248.1 | |
| All patients | |||||||
| Outpatient | Appropriate | ||||||
| A1+A2 | 9.8 | 13.6 | 6.5 | - | - | - | |
| Inappropriate | |||||||
| B+F | 8.8 | 8.9 | 5.6 | - | - | - | |
SD, standard deviation.
Table 6 shows the antibiotic costs per day of hospitalization. The differences between the prescription categories and between the wards are less pronounced than in terms of total cost.
Table 6.
Antibiotic cost per day of hospitalization (ZAR per day) by prescription category and ward [1 ZAR (South African Rand)=0.14486 USD at the time of study].
| Ward | Category | Including deaths | Excluding deaths | ||||
|---|---|---|---|---|---|---|---|
| Mean | SD | Median | Mean | SD | Median | ||
| Medical | Appropriate | ||||||
| A1+A2 | 14.0 | 7.8 | 11.4 | 14.1 | 8.0 | 11.4 | |
| Inappropriate | |||||||
| B+F | 15.4 | 8.1 | 11.7 | 14.4 | 6.8 | 11.7 | |
| Surgical | Appropriate | ||||||
| A1+A2 | 11.9 | 1.4 | 11.6 | 11.7 | 1.2 | 11.5 | |
| Inappropriate | |||||||
| B+F | 17.0 | 10.9 | 12.6 | 17.1 | 11.0 | 12.5 | |
SD, standard deviation.
The difference between appropriate and inappropriate is significant in the medical ward (P<0.05) and marginally significant in the surgical ward (P<0.10). For the individual categories, A2 was significantly less than A1, and all categories were significantly less than B.
Considering Table 4, 5, and 6 together, we conclude that the length of hospital stay is the primary cause of higher antibiotic costs for inpatients in this survey. For outpatients (Table 5), there is no significant difference in cost between the appropriate and inappropriate categories, and the total costs are most similar to the inpatient costs per day.
Discussion
Assessment of antibiotic prescriptions
A fundamental tenet in the appropriate prescribing of antibiotics to treat infections is the establishment of bacterial pathogens as etiological agents before antibiotics are prescribed. Classical definitions of a judiciously prescribed antibiotic tie down the appropriateness of a prescribed antibiotic to this principle.2,3 In empiric or non-definitive antibiotic treatment of infections, the presence of bacterial pathogens is often assumed. Such prescriptions can be justified on the basis of presenting clinical signs and symptoms and an identified site of infection that accurately suggest bacterial pathogens as a cause of the treated condition. Antibiotics can be considered prescribed appropriately in such circumstances provided the selection of the agents are based on their costs and therapeutic effectiveness against bacterial pathogens known to be commonly associated with infections at the site as principles require.14 These considerations form the basis of the assessment of antibiotic prescriptions in this study.
In certain cases, some infections or even non-infectious clinical conditions with non-bacterial causes may present with signs and symptoms similar to infections with bacterial causes, which makes their differentiation difficult. Viral infections of the respiratory tract, chronic obstructive airway disease with symptoms of chronic cough and excessive production of sputum and vulvovaginal symptoms caused by retained foreign bodies, for example, typically exemplify this.16,24,25 We found that respiratory tract infections were the most common body system affected in outpatients and the inpatient medical ward (Table 2). A prescriber may adhere to principles of antibiotic prescribing in such instances and still prescribe the drugs inappropriately if the clinical condition has non-bacterial etiologies. Prescription category A2 categorized these prescriptions as appropriately prescribed according to principles on one hand but which on the other hand were inappropriate on the grounds of their being prescribed for suspected bacterial infections only. We found that 44% of outpatient prescriptions and 14% of inpatient prescriptions were in this A2 category (Figure 1). Category A2 was most often found in cases of suspected respiratory tract infections that, among outpatients commonly have viral aetiologies.26
Impact of appropriateness of antibiotic prescribing on treatment evaluation parameters
Treatment outcomes
Calculated TSRs of patient groups treated with various antibiotic prescription categories are summarized in Table 2. Inpatients who received treatments with appropriate prescriptions had a higher rate of improvement compared to patients who received inappropriate prescriptions. However, due to the relatively small differences between groups and the small sample size, we cannot conclude that the results were statistically significant. Leibovici et al. in similar studies also found that appropriate empirical antibiotic treatment was associated with a significant reduction in fatality in patients with bloodstream infection.27
In the medical ward, we found that A1 had the highest improvement rate and that A2 and B were essentially identical. This finding is important as it shows positive impacts of prescribing antibiotics appropriately in the treatment of infections with absolute, rather than suspected bacterial etiologies. It emphasized the lack of therapeutic benefits from prescribing antibiotics in situations where bacterial pathogens are not etiologies.
Patients’ recovery in response to drug treatment can be attributed to factors other than the quality of drug treatment they receive. In a meta-analysis where treatment outcomes were evaluated against adherence, DiMatteo et al. acknowledge that many factors including treatment efficacy, genetic variations in response rates and even limitations in current understanding of disease can affect outcomes.28 In the empiric treatment of infections, factors like the severity of infection, the sensitivities of infecting pathogens to administered antibiotics and the regularity, likewise routes by which prescribed antibiotics are administered, may all determine the nature of patients’ clinical response to antibiotic treatment. In an observational study such as ours, it is not possible to control so many factors. Therefore, these unobserved variables couldn’t be controlled for in statistical modeling and make determination of statistical significance more difficult.
Days of hospitalization
Patient categories treated with prescriptions with appropriately prescribed antibiotics stayed in hospital for fewer days than patients treated with prescriptions with inappropriately prescribed antibiotics (Table 4). The result was statistically significant in the Medical ward but not in the Surgical ward. The difference between categories A1 and A2 was not statistically significant, but both were significantly less than category B. Leibiovici et al. similarly showed that hospital stay of survivors of bacteremic infections was shorter in patients treated with appropriately than inappropriately prescribed antibiotics.27
Antibiotic treatment costs
Average total costs of antibiotic treatments for inpatient groups treated with appropriately prescribed antibiotics were significantly less than those for patient groups treated with inappropriately prescribed antibiotics (Table 5). Patients with appropriately prescriptions also had lower average costs per day (Table 6). However, the difference in average days of hospitalization is much larger than the difference in average cost per day. Therefore, we conclude that the differences in average length of stay are the more important variable. Unlike inpatients, antibiotic treatments of outpatient groups treated with appropriately and inappropriately prescribed antibiotics showed no significant differences in costs (Table 5). To our knowledge, no previous study has compared costs of antibiotic treatments in instances where the agents were prescribed appropriately and inappropriately in a clinical environment. Results of some studies that evaluated intervention programs initiated to promote rational use of antibiotics did show, however, that diligent antibiotic usage has significant cost reduction effects. Such cost reductions, though, were attributable to less prescribing rather than an appropriate prescribing of the agents.29 Among inpatients, our findings indicate a positive impact of appropriate antibiotic prescribing on costs of antibiotic treatment or hospitalization. The same, however, cannot be said about antibiotic prescribing in outpatient departments.
Limitations of study
The correctness of interpretations given to information collected from patients’ case notes in prescription assessment depended largely on how much information had been provided. In some cases prescribers omitted vital information that made it difficult to assess prescriptions against the assessment criteria of this study. We therefore had to interpret the available clinical information to determine a prescription’s conformity or non-conformity. Some such interpretations may not necessarily be what prescribers actually did in conscious attempts to demonstrate their adherence to principles of antibiotic prescribing. These limitations somewhat compromise the versatility of prescription assessment methods as well as interpretations of prescription appropriateness analysis results. Patient case notes also lacked information on severity of infections for which antibiotics were prescribed. This introduced uncertainty in establishing the appropriateness of prescriptions. Although we collected a large total number of prescriptions, the survey period of one month did not yield enough data to find statistically significant results when the data was partitioned at the finest levels. We suggest that further studies of the impact of appropriateness of antibiotic prescribing be carried out within a methodological design in which prescribers, prior to data collection, would be informed of the study and also be persuaded to provide all information necessary for adequate analysis of results. Such a study design would also serve as an educational tool for healthcare providers, although in policy terms it would require routine verification that the participants did not merely follow the recommendations during the study period.
Conclusions
This article describes an observational study of antibiotic prescribing in Lesotho. The findings serve to inform clinical practice and could be used to design controlled trials to formally evaluate the safety, efficacy, and effectiveness of the antibiotics and practices observed herein. Appropriate prescribing of antibiotics was associated with higher rates of patients’ recovery, fewer days of patients’ stay in hospital for the treatment of infections as well as lower costs of antibiotic treatments. Factors like severity of infection, pathogens’ antibiotic sensitivity patterns and modes of administration of prescribed antibiotics may influence patients’ response to the empiric treatment of infections other than the appropriateness of prescribed antibiotics. Such factors may complicate correlations between appropriateness of antibiotic prescribing and treatment outcome indicators. Appropriate prescribing of antibiotics did not have a significant impact on costs of antibiotic treatments in outpatient settings. This may be due to relatively cheap traditional antibiotics still being the mainstay of treating infections in Lesotho. These have little differences in their costs.
References
- 1.Malaysian Health Technology Assessment Unit. Rational antibiotic utilization in selected pediatric conditions; 2002. Available from: http://moh.arahe.co/attachments/733 Accessed: 1 March 2006.
- 2.Gaur AH, English BK. The judicious use of antibiotics - An investment towards optimised health care. Indian Paediatr 2006;73:343-50. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. WHO global strategy for containment of antimicrobial resistance; 2001. Available from: http://www.who.int/drugresistance/WHO_Global_Strategy.htm/en/ Accessed: 1 April 2010.
- 4.Chambers H. Antimicrobial agents: General considerations. Hardman JG, Limbird LE. The pharmacological basis of therapeutics. New York: MacGraw-Hill; 2001. 1143-69. [Google Scholar]
- 5.Okeke IN, Aboderin OA, Byarugaba DK, et al. Growing problem of multidrug-resistance enteric pathogens in Africa. Emerg Infect Dis 2007;13:1640-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Archibald LK, Reiller LB. Clinical microbiology in developing countries. Emerg Infect Dis 2001;7:302-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.French GL. Clinical impact and relevance of antibiotic resistance. Adv Drug Deliv Rev 2005;57:1514-27. [DOI] [PubMed] [Google Scholar]
- 8.Davey P, Brown E, Fenelon L, et al. Interventions to improve antibiotic prescribing practices for hospital inpatients. Cochrane Database Syst Rev 2005;(4): CD003543. [DOI] [PubMed] [Google Scholar]
- 9.Lodise TP, McKinnon PS, Swiderski L, Rybak M. Outcomes analysis of delayed antibiotic treatment for hospital-acquired Staphylococcus aureus bacteremia. Clin Infect Dis 2003;36:1418-23. [DOI] [PubMed] [Google Scholar]
- 10.Harbarth S, Garbino J, Pugin J, et al. Inappropriate initial antimicrobial therapy and its effect on survival in a clinical trial of immunomodulating therapy for severe sepsis. Am J Med 2003;115:529-35. [DOI] [PubMed] [Google Scholar]
- 11.Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of non-specific upper respiratory tract infections in adults: background. Ann Intern Med 2001;134:490-4. [DOI] [PubMed] [Google Scholar]
- 12.Waning B, Montagne M. Pharmacoepidemiological Principles and Practice. New York: McGraw-Hill; 2001. p 209. [Google Scholar]
- 13.Lesotho Ministry of Health & Social Welfare. Health statistical tables; 2002. p 161 Available from: http://www.health.gov.ls/
- 14.Gugliemo BJ. Principles of infectious diseases. Koda-Kimble MA, Young LY, Alldredge BK, Corelli RL, Guglielmo BJ, Kradjan WA, Williams BR, Applied therapeutics: the clinical use of drugs. Philadelphia: Wolters Kluwer Health/Lippincott & Wilkins; 2008. 1, 24, 56. [Google Scholar]
- 15.Drew RH. Prevention and treatment of infections in Neutropenic cancer patients Koda-Kimble MA, Young LY, Alldredge BK, Corelli RL, Guglielmo BJ, Kradjan WA, Williams BR, Applied therapeutics: the clinical use of drugs. Philadelphia: Wolters Kluwer Health/Lippincott & Wilkins; 2008. 1-18, 68. [Google Scholar]
- 16.Gelone PS, O’Donnell J. Respiratory tract infections. Koda-Kimble MA, Young LY, Alldredge BK, Corelli RL, Guglielmo BJ, Kradjan WA, Williams BR, Applied therapeutics: the clinical use of drugs. Philadelphia: Wolters Kluwer Health/Lippincott & Wilkins; 2008. 1-29, 60. [Google Scholar]
- 17.Archer GL, Polk RE. Treatment and prophylaxis of bacterial infections. Kasper DL Brunwald E, Fausi AS, Hauser SL, Longo DL, Jameson JL. Harrison’s principles of internal medicine. New York: McGraw-Hill; 2005. 789-806. [Google Scholar]
- 18.Elliot T, Hastings M, Desselberger U. Lecture notes on medical microbiology. Oxford: Blackwell Science; 2004. [Google Scholar]
- 19.Scottish Intercollegiate Guidelines Network (SIGN),. Antibiotic prophylaxis in surgery: a national clinical guideline. No. 104. Edinburgh: SIGN Publication; 2008. 1-70. [Google Scholar]
- 20.Bronska E, Kalmusova J, Dzupova O, et al. Dynamics of PCR based diagnosis in patients with invasive meningococcal disease. Clin Microbiol Infect 2006;12:137-41. [DOI] [PubMed] [Google Scholar]
- 21.Popa RI, Gray LA, Kallimes DF. Urinary tract infections in the potential vertebroplasty patient: incidence, significance and management. Am J Neuroradiol 2009; 30:227-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kasper DL, Bunwald E, Fausi AS. Harrison’s principles of internal medicine. New York: McGraw-Hill; 2005. 78-2477. [Google Scholar]
- 23.Harrington DP, Fleming TR. A class of rank test procedures for censored survival data. Biometrika 1982;69:553-66. [Google Scholar]
- 24.Holmes K. Sexually transmitted diseases: overview and clinical approach. Kasper DL, Brunwald E, Fausi AS, Longo DL, Jameson JL. Harrison’s principles of internal medicine. New York: McGraw-Hill; 2005. 762-75. [Google Scholar]
- 25.Gonzales R, Bartlett JG, Besser RE, et al. Principles of appropriate antibiotic use for treatment of acute respiratory tract infections in adults: background, specific aims and methods. Ann Intern Med 2001;134:479-86. [DOI] [PubMed] [Google Scholar]
- 26.Hart AM. An evidenced based approach to the diagnosis and management of acute respiratory infections. J Nurse Pract 2007;3:607-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leibovici L, Shraga I, Drucker M, Konigsberger H, Samra Z, Pitlik SD. The benefit of appropriate empirical antibiotic treatment in patients with bloodstream infection. J Intern Med 1998;244:379-86. [DOI] [PubMed] [Google Scholar]
- 28.DiMatteo MR, Giordani PJ, Lepper HS, Croghan TW. Patient adherence and medical treatment outcomes: a meta-analysis. Med Care 2002;40:794-811. [DOI] [PubMed] [Google Scholar]
- 29.Rüttimann S, Keck B, Hartmeier C, et al. Long term antibiotic cost savings from a comprehensive intervention program in a medical department of a university–affiliated teaching hospital. Clin Infect Dis 2004;38:348-56. [DOI] [PubMed] [Google Scholar]


