Abstract
HIV and AIDS are major public health problems in Cameroon where the HIV prevalence is 5.5%. Candidiasis is the leading opportunistic mycosis in HIV and AIDS patients. The objective of this study was to determine the in vitro antifungal susceptibility pattern of Candida albicans in HIV and AIDS patients to eight antifungal agents in the Nylon Health District of Douala in Cameroon. Three hundred and four HIV and AIDS patients were recruited between March and August 2007 to participate in a cross-sectional study. All subjects who fulfilled the inclusion criteria were enrolled. Informed consent was obtained from all subjects before samples were collected. Three samples comprising oral swabs, vagina/urethra swabs and a mid-stream urine were collected from each subject. Specimens were cultured on sabouraud dextrose agar and C. albicans isolates were identified using the germ tube technique. The disk diffusion method was used for antifungal susceptibility testing using eight antifungal agents. The prevalence of candidiasis in the study population was 67.8% (95% CI: 62.5-73.1%) and that of C. albicans was 42.8% (95% CI: 37.2-48.4%). Oral swabs had the highest prevalence of C. albicans followed by vaginal/urethral samples (52.6% vs. 29.7% respectively). Forty (30.8%) subjects had C. albicans infection at more than one collection site. There was a statistically significant difference in the infectivity of C. albicans with age, sex and site of infection (P<0.05). C. albicans isolates were most sensitive to ketoconazole (80%) followed by econazole (64.6%) while fluconazole and 5-flurocytosin recorded the poorest sensitivities (22.9% vs 24.6%, respectively). There was a statistically significant difference in the sensitivity pattern of antifungal agents with respect to the site of isolation of the organism (P<0.05). Ketoconazole is the drug of choice for the treatment of C. albicans infection in HIV and AIDS patients in the Nylon Health District of Douala, Cameroon.
Key words: in vitro susceptibility, antifungal drugs, Candida albicans, HIV, AIDS, Cameroon
Introduction
Fungal diseases are a major public health problem among HIV and AIDS patients and infections due to Candida and Cryptococcus are the most common. Over the last decade, the incidence of fungal infections has increased dramatically, and HIV and AIDS account largely for this increase.1 Most candida infections are mucocutaneous and do not cause mortality. However, in patients with advanced HIV infection and AIDS, these mucosal infections can become refractory to antifungal therapy and may lead to severe oropharyngeal and oesophageal candidiasis that may initiate a vicious cycle of poor oral intake, malnutrition, wasting, and early death.1 The interaction between the larger number of lactobacilli normal flora and the small number of these fungi creates an equilibrium between them which is probably based on competition for food.2 When this balance is upset, however, as occurs during intensive antibiotic therapy, C. albicans multiplies uncontrolled. Other predisposing factors to candidal infection are late pregnancy, the use of oral contraceptives and uncontrolled diabetes.2
Treatment of candida infection varies substantially and is based on the anatomic location of the infection, the patient’s underlying disease and immune status, the patient’s risk factors for infection, the Candida species responsible for infection, and the susceptibility of the strain to antifungal drugs.
Few drugs are available that treat fungal infections since the drug must kill an eukaryotic organism in an eukaryotic host.3 Currently available antifungals have a small number of targets, including cell membrane (ergosterol) and its biosynthesis, nucleic acid synthesis and cell wall synthesis.3 Of the five classes of systemic antifungal compounds currently in clinical use, the polyenes, the azoles, and the allylamines all target ergosterol, the major sterol in fungal cell membranes that is not present in animals, whereas the fluoropyrimidines and the echinocandins have other targets, including the inhibition of DNA synthesis and RNA miscoding.4 New molecules and targets are under study: echinocandin lipopetides are a novel class of antifungal lipopetides that inhibit the synthesis of 1.3 beta-D glucan, a polypeptide in the cell wall of many pathogenic fungi. They appear to have potent broad spectrum fungicidal in vitro activity against Candida Spp and this antifungal activity has been demonstrated in animal models and patients.5 Synergistic activity has been demonstrated and it has been suggested that the effectiveness of polyene (e.g. Amphotericin B) and imidazole (e.g. clotrimazole) antifungals may depend upon the level of unsaturation and ergesterol in yeast membrane.6 During infection by Candida albicans, arachidonic acid (AA) is released from phospholipids of infected host cells and used by C. albicans as the sole source of carbon for the production of eicosanoids. AA can be incorporated into the phospholipids of yeast influencing the saturation level and fluidity of yeast cell membrane. An improved susceptibility of C. albicans and C. dubliniensis to amphotericin B and clotrimazole has been demonstrated.6
The clinical outcome of candidiasis treatment depends not only on the susceptibility of the pathogen to a given drug but also on factors including pharmacokinetics, drug interactions, immune status, and patient compliance, as well as several specific conditions such as the occurrence of biofilms on surfaces of catheters and prosthetic valves.7 Drug resistance can be measured as the Minimum Inhibitory Concentration (MIC) that curtails the growth of the fungus under standardized in vitro test conditions.8
Recently, several reports have noted the failure of azole drugs, particularly fluconazole, to treat recurrent cases of oropharyngeal candidiasis.9 While factors such as diminishing cellular immunity, drug interactions, or decreased drug absorption may account for some of these treatment failures, increasing evidence suggests that Candida organisms are developing drug resistance.
There is a lack of data on the sensitivity pattern of fungal infections in Cameroon. Hence, the in vitro susceptibility pattern of antifungal drugs for Candida albicans infection in HIV and AIDS patients is important because it identifies drug resistance to some antifungal agents and guides clinicians in the management of patients with candidiasis and the prophylactic use of antifungal agents.
Materials and Methods
Study area
The study took place in the Nylon Health District of Douala, Cameroon. Douala is located in the Littoral region (one of 10 regions in the country) and the major urban center. It has the country’s main seaport and airport. The population of Cameroon is about 18 million but that of Douala is about two million. The Nylon Health District has a population of 378,644 inhabitants. The HIV and AIDS pandemic is a serious public health problem in Cameroon which had a national prevalence of 12% and was ranked 25th in the world for prevalence.8 By late 2004, the prevalence of the disease had started decreasing to as low as 5.5%. Two-thirds of those currently infected are youths and 60% of annual infections fall within this category of citizens. The estimated number of people living with HIV and AIDS for 2002 was 920,000, of which 69,000 were children (compared to 22,000 in 1999) between 0-14 years and 860,000 were aged 15-49 years, including 500,000 women.11 HIV and AIDS constitute major health consultations and hospitalization in the country, and most beds are occupied by AIDS patients.
Study design and sample collection
A cross-sectional urban hospital-based study was conducted from March to August 2007 at the Nylon District Hospital in Douala, Cameroon. HIV and AIDS patients were recruited after giving written informed consent and accepting to be tested for oral, vagina/urethra and urinary candidiasis free of cost. Confidentiality was maintained by the use of a double coding system for research purposes. Specimens were cultured on sabouraund dextrose agar and disk diffusion antifungal susceptibility testing was performed following procedures described by the National Committee for Clinical Laboratory Sciences10 on all C. albicans isolates. All patients were given their results and those infected with C. albicans were referred to their consulting medical personnel for appropriate treatment and follow up.
Laboratory processing of samples
A wet preparation of each specimen was made from the oral and urethral/vagina swabs. Urine specimens were centrifuged and the sediment used to prepare a wet preparation and examined microscopically for the presence of small, round to oval, thin-walled, clusters of budding yeast cells and branching pseudohyphae. Gram stained smears were made and all specimens plated on sabouraud agar plates and incubated at 35°C for 24-48 h. Colonies were typically white to cream color with a smooth, glabrous to waxy surface. C. albicans isolates were identified using morphological characteristics and the germ tube test.
The disk diffusion method was used to test the susceptibility of C. albicans to fluconazole, econazole, miconazole, nystatin, 5-flurocytocin, ketoconazole, clotrimazole and amphotericin-B following the protocol developed by the National Committee for Clinical Laboratory Standards (NCCLS)12 for antifungal disk diffusion susceptibility testing of yeasts. The disk diffusion zone diameter correlated inversely with MICs using the Bauer-Kirby curve. The final inoculation was prepared at 5×105 CFU/mL using 0.5 McFarland turbidity standards. Plates were inoculated for 24 h.
Procedures for performing the disk diffusion test
All identified isolates were subcultured onto Sabouraud dextrose agar to ensure purity and viability. The incubation temperature throughout was maintained at 35°C. Inoculum was prepared by picking five distinct colonies of approximately 1 mm in diameter from a 24-h culture of Candida albicans. Colonies were suspended in 5 mL of sterile 0.145 mol/L saline (8.5 g/L NaCl; 0.85% saline). The resulting suspension was vortexed for 15 s and its turbidity was adjusted visually by adding sufficient sterile saline or more colonies to adjust the transmittance. This procedure yielded a yeast stock suspension of 1×106 to 5×106 cells per mL and produced semi-confluent growth with most Candida albicans isolates. Within 15 min of adjusting the turbidity of the inoculum suspension, a sterile cotton swab was dipped into the suspension. The swab was rotated several times and pressed firmly against the inside wall of the tube above the fluid level. This helped to remove excess fluid from the swab.12 The dried surface of a sterile Mueller-Hinton + GMB agar plate was inoculated by evenly streaking the swab over the entire agar surface. This procedure was repeated by streaking two more times, rotating the plate approximately 60° each time to ensure an even distribution of inoculum and the rim of the agar was swabbed. The lid was left ajar for 3-15 min to allow for any excess surface moisture to be absorbed before applying the drug-impregnated disks. Variations in inoculum density were avoided by ensuring that the degree of turbidity level was as similar as possible.
Application of disks to inoculated agar plates
Antimicrobial disks were dispensed onto the surface of the inoculated agar plate with a forceps. Each disk was pressed down to ensure complete contact with the agar surface and they were distributed evenly so that they were no closer than 24 mm from center to center. Because the drug diffuses almost instantaneously, a disk was not removed once it had come into contact with the agar surface. Instead, a new disk was placed in another location on the agar. The plates were inverted and incubated at 35°C for 15 min after which the disks were applied.
Reading the plates and interpreting the results
Plates were examined after 20-24 h of incubation. For satisfactorily streaked plates with the correct inoculums, the resulting zones of inhibition were uniformly circular and there was a semiconfluent lawn of growth. The plate was held a few inches above a black, non-reflecting background illuminated with reflected light. The zone diameters were measured to the nearest whole millimeter at the point at which there was a prominent reduction in growth. Pinpoint microcolonies at the zone edge or large colonies within a zone were encountered frequently and these were ignored.12 Whenever these colonies were sub-cultured and retested, identical results were usually obtained, i.e. a clear zone with micro colonies at the zone edge or large colonies within the zone. Plates were read at 48 h only when insufficient growth was observed after 24 h incubation.
Interpretation of the disk diffusion test results
A susceptible category implied that an infection due to the strain may be appropriately treated with the dose of antimicrobial agent recommended for that type of infection, unless otherwise contraindicated. This was obtained after measurement of the zone diameter to the nearest whole mm and compared with reference values.12 The susceptible-dose dependent (intermediate) category included isolates with antimicrobial agent MICs that approach usually attainable blood and tissue levels and for which response rates may be lower than for susceptible isolates. Susceptibility was dependent on achieving the maximal possible blood level. This was obtained after measurement of the zone diameter to the nearest whole mm and compared with reference values.12 Resistant strains were those that were not inhibited by the usually achievable concentrations of the agent with normal dosage schedules or when zone diameters have been in a range at which clinical efficacy has not been reliable in treatment studies. This was obtained after measurement of the zone diameter to the nearest whole mm and compared with reference values.12
Data management and analysis
Data was entered into a log book during working days by a laboratory technician and cross-checked weekly by one of the authors for completeness and use of correct codes. The data were entered into Microsoft Excel and transferred to SPSS version 11 for statistical analysis. The hypothesis was that HIV and AIDS patients have a decreased CD4+ T-cell count which predisposes them to C. albicans infection and subsequent resistance to available antifungal drugs.
At the initial step of analyses, frequency distributions of each variable were produced and the information grouped according to site of sample collection and sensitivity pattern. Associations were established between variables of different measures through cross-tabulations. Further analysis included data summary such as proportions, percentages, and standard deviation. In these analyses, such methods as the c2 test, Fisher’s exact test for the test of significance of associations between categorical variables, and Student’s t-test and ANOVA to test statistical significance of hypotheses for continuous variables were used.
Results
Of the 304 patients who were positive for HIV/AIDS, 206 (67.8%, 95% CI: 62.5-73.1%) had candidiasis and 130 (42.8¨%, 95% CI: 37.2-48.4%) had C. albicans. The prevalence of C. albicans decreased from oral (n=92; 52.6%) and vagina/urethra (n=52; 29.7%) swabs to mid-stream urine (n=31; 17.7%). Of the 897 specimens collected from the three sites, 335 (37.3%) were positive for candidiasis and 175 (19.5%) for C. albicans. Forty (22.9%) patients had C. albicans in more than one site and 8 (4.6%) in all three sites. The distribution of C. albicans according to the various sample collection sites is shown in Figure 1. Most cases of C. albicans were recorded in the 21-40 year age group (Table 1). There was a statistically significant difference between the prevalence of C. albicans isolated among the age groups and the site of specimen collected (P<0.05) but there was no statistically significant difference between the prevalence of C. albicans and the sex of the patient (P>0.05).
Figure 1.
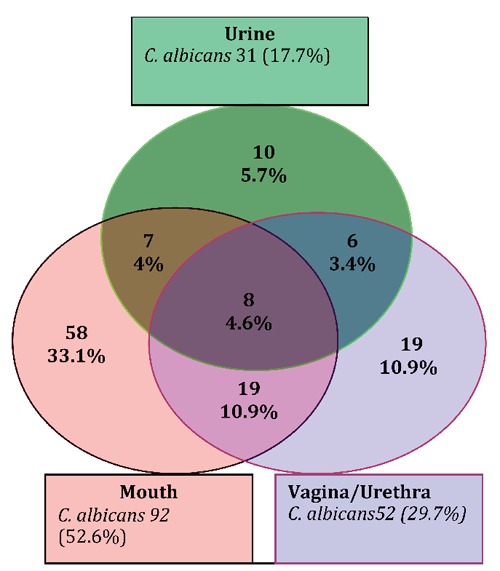
Prevalence of Candida albicans in different body sites of HIV and AIDS patients in Nylon-Douala (n=175).
Table 1.
Distribution of C. albicans in the mouth, vagina/urethral and urine according to age group and gender.
| Age group (years) |
Parameter | Mouth n=92 n. (%) |
Vaginal/urethral n=52 n. (%) |
Urine n=31 n. (%) |
|---|---|---|---|---|
| 1-20 | 3 (3.3) | 1(1.9) | 2(6.5) | |
| 21-40 | 57(61.9) | 34(65.4) | 16(51.6) | |
| 41-60 | 32(34.8) | 17(32.7) | 13(41.9) | |
| Gender | Female | 70(76.1) | 42(80.8) | 24(77.4) |
| Male | 22(23.9) | 10(19.2) | 7(22.6) |
Overall, C. albicans recorded the highest susceptibility to ketoconazole at all three sites: n=140 (80%) followed by econazole (n=113, 64.6%), nystatin (n=104, 59.4%), miconazole (n=87, 49.7%), clotrimazole (n=81, 46.3%), amphotericin-B (n=50, 28.6%), 5-fluorocytosin (n=43, 24.6%) and fluconazole (n=40, 22.9 %). There was no statistically significant difference between drug sensitivity patterns and the site of specimen collected (P>0.05) (Table 2).
Table 2.
Susceptibility pattern of antifungals to C. albicans using the disk diffusion method (n=175).
| Collection site | Susceptibility pattern | Antifungal drugs tested | |||||||
|---|---|---|---|---|---|---|---|---|---|
| KCA N. (%) |
ECO N. (%) |
NY N. (%) |
MIC N. (%) |
CLO N. (%) |
AMB N. (%) |
AFU N. (%) |
FLU N. (%) |
||
| Mouth (n=92) | Sensitive | 69 (73.6) | 61 (66.7) | 54 (60.0) | 50 (57.0) | 43 (47.8) | 28 (31.1) | 26 (28.9) | 17 (19.1) |
| Intermediate | 8 (9.2) | 20 (21.1) | 29 (32.2) | 14 (10.5) | 17 (16.7) | 15 (14.4) | 5 (3.3) | 9 (6.7) | |
| Resistant | 15 (17.2) | 11 (12.2) | 9 (7.8) | 28 (32.6) | 32 (35.6) | 49 (54.4) | 61 (67.8) | 66 (74.2) | |
| Vagina/urethral (n=52) | Sensitive | 46 (85.2) | 36 (68.6) | 32 (60.4) | 20 (55.6) | 20 (38.9) | 16 (29.6) | 11 (22.6) | 12 (22.2) |
| Intermediate | 4 (7.4) | 7 (13.7) | 11 (22.6) | 16 (14.8) | 10 (20.4) | 14 (27.8) | 1 (1.9) | 2 (3.7) | |
| Resistant | 2 (7.4) | 9 (17.6) | 9 (17.0) | 16 (29.6) | 22 (4.7) | 20 (42.6) | 40 (75.5) | 38 (74.1) | |
| Urine (n=31) | Sensitive | 25 (80.6) | 16 (51.6) | 18 (58.1) | 17 (54.8) | 18 (58.1) | 6 (19.4) | 6 (19.4) | 11 (35.5) |
| Intermediate | 1 (3.2) | 11 (35.5) | 8 (25.8) | 5 (16.1) | 0 (0) | 2 (6.5) | 1 (6.5) | 0 (0) | |
| Resistant | 5 (16.1) | 4 (12.9) | 5 (16.1) | 9 (29.0) | 13 (41.9) | 23 (74.2) | 23 (74.2) | 20 (64.5) | |
KCA, ketoconazole; ECO, econazole; NY, nystatin; MIC, miconazole; CLO, clotrimazole; AMB, amphotericin B; AFU, 5-fluorocytosin; FLU, fluconazole.
Discussion
In humans, C. albicans live as normal commensals in the gut, mouth and vaginal mucosa. Their number is maintained at a low and non-infective level because they are kept under control by both specific and non-specific immune barriers.
Although HIV and AIDS are chronic and debilitating health conditions that predispose patients to opportunistic infection, of which candidiasis is the most prevalent in West and Central Africa, there are few data on the susceptibility pattern of antifungal agents to C. albicans in HIV and AIDS patients. The in vitro antifungal susceptibility profile of C. albicans isolated from HIV and AIDS patients in the Nylon Health District of Douala in Cameroon, therefore, provides important data which can guide clinicians in the management of HIV and AIDS patients.
In this study, the prevalence of C. albicans recorded (42.8%) is lower compared to studies from Thailand (66.6% in adults and 70% in children), Hong Kong (54.8%), Italy (61.9 %), Mexico (92%) and India (65.3%).13-17 The low prevalence could be explained by the habit of using bicarbonates for mouthwash and automedication. In Cameroon, all HIV and AIDS patients with CD4+ T-cell counts less than 200 cells/uL were systematically placed on antiretroviral therapy and those presenting with clinical symptoms of candidiasis were offered fluconazole free of charge.18
Most of these C. albicans infections were in the mouth, followed by the vagina/urethra and urine, respectively (52.6%, 29.7% and 17.7%). C. albicans was recovered in some patients (40%) from more than one site. This could also be explained by the fact that the mouth and vagina are sites where C. albicans exist as a normal flora.2 The low carriage rate could also be related to the method of sample collection whereby the use of oral swabs and unstimulated saliva may not detect as many Candida as using an oral rinse. Patients in the 21-40 year old age group and more females than males were infected. This study confirms the findings by the National AIDS Control Committee Central Technical Group of the Ministry of Public Health of Cameroon.19
In this study, C. Candida albicans isolates were susceptible to ketoconazole with the least susceptibility to fluconazole at all three sites. The development of fluconazole resistance is an emerging trend. In 1995, 40 % of patients on long-term therapy carried resistant strains: 43% in 1997 and 45% in 2000.1 In this study, we recorded a much higher rate of fluconazole resistance (70.9%). We attributed this to the fact that HIV and AIDS patients with clinical symptoms of candidiasis were systematically placed on fluconazole therapy free of charge without prior antifungal susceptibility testing.
While C. albicans recorded remarkable susceptibility to ketoconazole, fluconazole and 5-fluorocytosin recorded the poorest susceptibility at the Nylon Health District compared to most regions in the world. We recommend that ketoconazole should be the drug of choice whilst C. albicans strains resistant to fluconazole are characterized and their foci in Cameroon determined.
Since in the long term, monotherapy is likely to bring about resistance, it is important to assess the synergistic pattern of ketoconazole with other antifungals. Although 5-fluorocytosin recorded the poorest susceptibility, its use in combination with ketoconazole will probably induce an increased susceptibility to C. albicans and counteract resistance because of their different targets on the pathogenic cells.
Acknowledgments
The authors are grateful to the management of the Nylon District Hospital for their collaboration and assistance in sample collection.
References
- 1.Hidalgo DL, Roberts GD, Galgiani JN, et al. Results of a survey of antifungal susceptibility tests in the United States and inter-laboratory comparison of broth dilution testing of flucytosine and amphotericin. B J Clin Microbiol 2006;23:298-301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cheesbrough M. District Laboratory Practice in Tropical Countries. Part 2. London, Cambridge University Press, 2000. [Google Scholar]
- 3.Leah JH, Walsh TJ, Sobel JD, et al. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis 2000;30:662-78. [DOI] [PubMed] [Google Scholar]
- 4.Georgopapadakou NH. Antifungals: mechanism of action and resistance, established and novel drugs. Curr Opin Microbiol 1998;1:547-57. [DOI] [PubMed] [Google Scholar]
- 5.Groll AH, Kolve H. Antifungal agents: In vitro susceptibility testing, pharmacodynamics and prospects for combination therapy. Eur J Clin Microbiol Infect Dis 2004;23:257-70. [DOI] [PubMed] [Google Scholar]
- 6.Ells K, Kock JLF, Van Wyk PWJ, et al. Arachidonic acid increase susceptibility of Candida albicans and candida dubliniensis. J Antimicrobiol Therapy 2008;63:124-8. [DOI] [PubMed] [Google Scholar]
- 7.White TC, Marr KA, Bowden RA. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin Microbiol Rev 1998;11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Talibi D, Raymond M. Isolation of a putative C. albicans transcriptional regulator involved in pleiotropic drug resistance by functional complementation of a pdr1 pdr3 mutation in Saccharomyces cerevisiae. J Bacteriol 1999;181:231-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sangeorzan PL, Jr, Wolf NA, Kukuruga MA. T lymphocytes in the murine vaginal mucosa are phenotypically distinct from those in the periphery. Infect Immun 1994;64:3793-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.UNAIDS/WHO. AIDS epidemic update. Accessed 19 December 2005. Available at: www.unaids.org. 2003 [Google Scholar]
- 11.Ministry of Public Health. Strategic Plan for the fight against HIV and AIDS, pp 2006-10. National AIDS Control Committee, Cameroon. [Google Scholar]
- 12.National Committee for Clinical Laboratory Standards. Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline, pp 19087-1898. NCCLS document M44-A. NCCLS, Wayne, PA, USA, 2004. [Google Scholar]
- 13.Teanpaisan R., Nittayananta W. Prevalence of Candida species in HIV and AIDS patients and HIV and AIDS-free subjects in Thailand. J Oral Pathol Med 1998;27:4-7. [DOI] [PubMed] [Google Scholar]
- 14.Tsang CS, Samaranayake LP. Oral yeasts and coliforms in HIV and AIDS-infected individuals in Hong Kong. Mycoses 2000; 43:303-8. [DOI] [PubMed] [Google Scholar]
- 15.Campisi G, Pizzo G, Milici ME, et al. Candida carriage in the oral cavity of human immunodeficiency virus-infected subjects. Oral Surg Oral Med Oral Pathol 2002;93:281-6. [DOI] [PubMed] [Google Scholar]
- 16.Prieto SA, Luz M, Illnait Z, et al. Oral candidiasis in HIV- seropositive patients and AIDS cases: Clinical, Mycological and therapeutical aspects. Rev Cubana Med Trop 2006;58:375-760. [PubMed] [Google Scholar]
- 17.Gugnani HC, Becker K, Fegeler W, et al. Oropharyngeal carriage of Candida species in HIV/AIDS-infected patients in India. Mycoses 2003;46:299-306. [DOI] [PubMed] [Google Scholar]
- 18.Cameroon Ministry of Public Health. Decision n. 176-B/MSP/CAB, 19/04/2007. Fixant la nouvelle tarification des protocols de prise en charge des personnes vivant avec le VIH et SIDA par les medicamments antiretroviraux (ARV) et les medicaments pour les infections opportunistes au Cameroun, 2007. [Google Scholar]
- 19.National Aids Control Committee Central Technical Group (NACC), Cameroon Ministry of Public Health. Towards universal access to treatment and care for adults and children living with HIV/AIDS in Cameroon. Progress report n. 7. pp 2-5, 2007. [Google Scholar]


