Abstract
Stimulation of cells with various peptide growth factors induces the production of phosphatidylinositol 3,4,5-trisphosphate (PIP3) through activation of phosphatidylinositol 3-kinase. The action of this enzyme is reversed by that of the tumor suppressor PTEN. With the use of cells overexpressing NADPH oxidase 1 or peroxiredoxin II, we have now shown that H2O2 produced in response to stimulation of cells with epidermal growth factor or platelet-derived growth factor potentiates PIP3 generation and activation of the protein kinase Akt induced by these growth factors. We also show that a small fraction of PTEN molecules is transiently inactivated as a result of oxidation of the essential cysteine residue of this phosphatase in various cell types stimulated with epidermal growth factor, platelet-derived growth factor, or insulin. These results suggest that the activation of phosphatidylinositol 3-kinase by growth factors might not be sufficient to induce the accumulation of PIP3 because of the opposing activity of PTEN and that the concomitant local inactivation of PTEN by H2O2 might be needed to increase the concentration of PIP3 sufficiently to trigger downstream signaling events. Furthermore, together with previous observations, our data indicate that peroxiredoxin likely participates in PIP3 signaling by modulating the local concentration of H2O2.
Keywords: hydrogen peroxide, peptide growth factor receptor, peroxiredoxin
The intracellular generation of H2O2 in response to the activation of cell surface receptors is not limited to phagocytic cells, because peptide growth factors such as insulin, epidermal growth factor (EGF), and platelet-derived growth factor (PDGF) have been shown to induce the production of H2O2 in various nonphagocytic cells (1). Although the amount of H2O2 produced in growth factor-stimulated nonphagocytic cells is minuscule compared with that generated in activated phagocytic cells (2), scavenging or inhibition of H2O2 production results in attenuation of mitogenic signaling triggered by growth factors (3, 4). These observations complement the results of many previous studies showing that exposure of normal cells to low levels of H2O2 is sufficient to increase their proliferation (5) and that many types of cancer cells manifest increased production of H2O2 (6).
Protein tyrosine phosphatases (PTPs) have been suggested to be direct targets of H2O2 in the propagation of its signal in cells stimulated with various growth factors (7–9). Growth factor signaling is initiated by activation of the corresponding receptor tyrosine kinase and consequent phosphorylation both of the receptor itself and of various other substrates. In general, PTPs exert an inhibitory effect on growth factor signaling by opposing the tyrosine phosphorylation initiated by the activated receptor kinases. PTPs contain an essential cysteine residue that must be in the reduced state to form a thiol-phosphate intermediate in the catalytic mechanism (10) and that is readily oxidized by H2O2 as a result of its molecular environment (7).
Stimulation of cells with growth factors also induces the transient activation of class I phosphatidylinositol (PI) 3-kinase, and the consequent production of PI 3,4,5-trisphosphate (PIP3) is important for the activation of a variety of downstream signaling molecules, including the protein kinase Akt, that mediate promotion of cell proliferation and cell survival (10). The reaction catalyzed by PI 3-kinase is reversed by PTEN (phosphatase with sequence homology to tensin), which functions as a PIP3 3-phosphatase (10). Indeed, by negatively modulating the PI 3-kinase signaling pathway, PTEN acts as a tumor suppressor (10). PTEN is also a member of the PTP family, and we previously demonstrated that Cys-124 in the catalytic site of human PTEN is readily oxidized by exogenous H2O2 to form a disulfide with Cys-71 (11). In contrast, the catalytic cysteine of other PTPs is glutathionylated (12) or forms a cyclic sulfenylamide with the nitrogen of a neighboring serine residue (13). The active site pockets of PTEN and of other PTPs differ markedly in terms of size and charge distribution (14).
A substantial proportion (≈10%) of PTEN molecules was oxidatively inactivated by H2O2 produced in RAW macrophages that were maximally stimulated by the combination of lipopolysaccharide and phorbol 12-myristate 13-acetate (15), and this inactivation of PTEN appeared to contribute to the activation of Akt induced by these agents. However, the insulin-induced activation of Akt in these phagocytic cells, although similar in magnitude to that induced by lipopolysaccharide plus phorbol 12-myristate 13-acetate, was independent of oxidant generation, suggesting that H2O2 production might not play a role in PIP3 generation and Akt activation in growth factor-stimulated cells (15).
We now show that H2O2 produced in growth factor-stimulated cells governs both the accumulation of PIP3 and the activation of Akt and that peroxiredoxin (Prx) II, a recently identified cytosolic peroxidase, likely participates in growth factor signaling by removing H2O2 in a time-dependent manner. We also show that a small fraction of PTEN undergoes transient oxidation in growth factor-stimulated cells, suggesting that H2O2 might propagate growth factor signaling by inactivating PTEN as well as PTPs.
Materials and Methods
Materials. DMEM, FBS, penicillin, and streptomycin were obtained from Life Technologies (Grand Island, NY). EGF was from Calbiochem. PDGF isoform BB, the 4G10 mAb to phosphotyrosine, and rabbit polyclonal Abs to PTEN were from Upstate Biotechnology (Lake Placid, NY). Rabbit polyclonal Abs to Akt and mAbs to PTEN (A2B1) and to the hemagglutinin epitope (HA) were from Santa Cruz Biotechnology. Rabbit polyclonal Abs to phospho-Akt (Ser-473) were from Cell Signaling Technology (Beverly, MA), and horseradish peroxidase-conjugated goat Abs to mouse or rabbit IgG were from Amersham Pharmacia Biotech. DTT and N-ethylmaleimide (NEM) were from Sigma, and [32P]Pi was from ICN.
Cell Lines. NIH 3T3 cells overexpressing human NADPH oxidase 1 (Nox1) were provided by J. D. Lambeth (Emory University School of Medicine, Atlanta) (16). HeLa cells overexpressing wild-type or a dominant negative mutant (DN) of human Prx II were described previously (17). HeLa and NIH 3T3 cells were grown under an atmosphere of 5% CO2 at 37°C in DMEM supplemented with 10% FBS, 100 units/ml penicillin, and 100 units/ml streptomycin. HEK293 cells were grown under the same conditions, with the exception that FBS was replaced with 10% horse serum. The cells were deprived of serum by incubation for 20 h either in DMEM alone (HeLa and HEK293 cells) or in DMEM supplemented with 0.5% calf serum (NIH 3T3 cells) before stimulation.
Measurement of PIP3. Serum-deprived HeLa or NIH 3T3 cells in 24-well plates were washed, incubated for 1 h in phosphate-free DMEM, labeled for 90 min with 0.5 ml of the same medium containing [32P]Pi (1 mCi/ml; 1 Ci = 37 GBq), and then stimulated with growth factor. The cells were then lysed by the addition of 0.5 ml of ice-cold 20% trichloroacetic acid. Phospholipids extracted from the cell pellet were deacylated, and the resulting glycerophosphoinositol phosphates were analyzed by HPLC as described (18).
Assay of PI 3-kinase Activity. NIH 3T3 cells that had been stimulated with 30 ng/ml PDGF for the indicated times in 24-well plates were lysed in 1 ml of ice-cold lysis buffer [20 mM Hepes-NaOH, pH 7.4/2 mM EGTA/25 mM β-glycerophosphate/1% Triton X-100/10% glycerol/1 mM DTT/1 mM Na3VO4/5 mM NaF/50 μg/ml leupeptin/50 μg/ml aprotinin/1 mM 4-(2-aminoethyl)benzene-sulfonyl fluoride]. The lysates were centrifuged, and the resulting supernatants (500 μg of protein) were subjected to immunoprecipitation with the 4G10 mAb to phosphotyrosine (2 μg). The immunoprecipitates were then assayed for PI 3-kinase activity as described (33).
Transient Transfection of HeLa Cells. HeLa cells (0.5 × 106) were detached from culture plates with trypsin, suspended in 0.1 ml of Nucleofector solution (Amaxa, Cologne, Germany), and transfected with 10 μg of DNA by electroporation with an Amaxa Nucleofector apparatus. DMEM (0.5 ml) containing 10% FBS was added to the cuvette, and the transfected cells were then transferred to six-well plates at a density of 1 × 105 per well. Cells were allowed to express the exogenous gene for 24 h before serum deprivation for 20 h and subsequent stimulation.
Detection of Oxidized PTEN by Alkylation with Biotin-Conjugated Maleimide. Serum-deprived cells (1 × 106 per 100-mm dish) were washed with PBS and stimulated with H2O2 or growth factor for the indicated times. The medium was then removed, and the cells were rapidly frozen by placing the culture dish on dry ice. The frozen cells were transferred to an anaerobic chamber and incubated for 1 h at room temperature with 1 ml of oxygen-free extraction buffer [50 mM sodium phosphate, pH 7.0/1 mM EDTA/10 mM NEM/10 mM iodoacetic acid/1% Triton X-100/5 mM NaF/50 μg/ml leupeptin/50 μg/ml aprotinin/1 mM 4-(2-aminoethyl)benzene-sulfonyl fluoride]. The cells were then removed from the dish and transferred to a 15-ml conical tube. The dish was washed with 1 ml of oxygen-free extraction buffer, and the solution was combined with the cells in the conical tube. The combined sample was centrifuged, and the protein concentration of the supernatant was determined. After the addition to the supernatant of SDS to a final concentration of 1%, the mixture was maintained for 2 h at room temperature in the dark, and the denatured proteins (100 μg) were then precipitated by the addition of trichloroacetic acid to a final concentration of 10% and further incubation for 1 h at room temperature. The protein precipitate was washed twice with acetone (cooled on dry ice) to remove traces of trichloroacetic acid, NEM, and iodoacetic acid and was then both solubilized and reduced by incubation for 30 min at 50°C in 0.1 ml of oxygen-free reducing buffer (50 mM Hepes-NaOH, pH 7.7/1 mM EDTA/2% SDS/4 mM DTT) in an anaerobic chamber. The reduced proteins were biotinylated by incubation for 30 min at 50°C with 0.9 ml of a solution containing 50 mM sodium phosphate (pH 7.0), 1 mM EDTA, and 1 mM biotin that had been conjugated to polyethylene oxide-maleimide (Pierce). The reaction was stopped by the addition of DTT to a final concentration of 1 mM, and proteins were precipitated by the addition of trichloroacetic acid (final concentration, 10%) and incubation for 1 h. The precipitate was collected by centrifugation, washed with dry ice-chilled acetone, and solubilized in 0.2 ml of a solution containing 50 mM Hepes-NaOH (pH 7.7), 1 mM EDTA, and 2% SDS. The sample was then diluted with 0.2 ml of the same solution without SDS, and a 40-μl portion of the resulting mixture was subjected to immunoblot analysis with rabbit Abs to PTEN. The remaining 360 μl of the mixture were further diluted by the addition of an equal volume of a solution containing 20 mM Hepes-NaOH (pH 7.7), 100 mM NaCl, and 1 mM EDTA, and biotinylated proteins were then precipitated by incubation for 1 h at room temperature with 3 μl of packed UltraLink Immobilized NeutrAvidin (Pierce). The beads were washed five times with a solution containing 20 mM Hepes-NaOH (pH 7.7), 200 mM NaCl, 1 mM EDTA, and 0.5% SDS, after which biotinylated proteins were released from the beads by boiling in SDS/PAGE sample buffer and subjected to immunoblot analysis with rabbits Abs to PTEN.
Results
Effect of Nox1 Overexpression on PDGF-Induced PIP3 Generation. The source of H2O2 generated in nonphagocytic cells in response to growth factor stimulation has been identified as Nox1 or Nox4, both of which are homologs of gp91phox (now renamed Nox2), the catalytic subunit of NAD(P)H oxidase in phagocytic cells (19–21). NIH 3T3 cells overexpressing Nox1 produce greater amounts of H2O2 and proliferate to a greater extent compared with control cells (16). We first investigated whether the increased intracellular level of H2O2 resulting from Nox1 overexpression affects the intracellular abundance of PIP3.
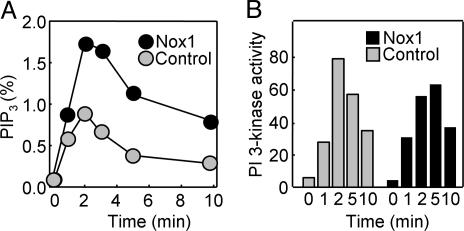
NIH 3T3 cells stably overexpressing Nox1 produced a greater amount of PIP3 in response to stimulation with PDGF than did NIH 3T3 cells transfected with the corresponding empty vector (Fig. 1A). PIP3 is metabolized either by 5-phosphatases or by the 3-phosphatase PTEN. The amount of PI(3,4)P2 was also transiently increased in both Nox1-overexpressing and control NIH 3T3 cells in response to PDGF stimulation, and this phospholipid accumulated to a greater extent in the Nox1-overexpressing cells (data not shown), suggesting that the lower level of PIP3 seen in control cells was not the result of faster dephosphorylation by a 5-phosphatase. PDGF-dependent activation of PI 3-kinase was examined with the use of an in vitro assay of kinase activity with PI as the substrate. Total PI 3-kinase activity in immunoprecipitates prepared with a mAb to phophotyrosine showed a transient increase that was similar in extent in Nox1-overexpressing and control NIH 3T3 cells stimulated with PDGF (Fig. 1B).
Fig. 1.
Effects of Nox1 overexpression on the production of PIP3 and on PI 3-kinase activity in PDGF-stimulated cells. (A) NIH 3T3 cells either stably expressing Nox1 or stably transfected with the empty pEF-PAC vector (control) were labeled with [32P]Pi and stimulated for the indicated times with 30 ng/ml PDGF. The amount of [32P]PIP3 in the cells was then determined and expressed as a percentage of total radioactivity associated with the phospholipid fraction. Data are means of values obtained from two separate experiments. (B) Control and Nox1-overexpressing NIH 3T3 cells were stimulated with PDGF as in A, and cell lysates were processed for assay of PI 3-kinase activity. Data are expressed in arbitrary units and are from an experiment that was performed twice with similar results.
Effect of Prx II Overexpression on EGF-Induced PIP3 Generation. Members of the Prx family of peroxidases catalyze the reduction of H2O2 with the use of reducing equivalents provided by thioredoxin (22). Prx II is a cytosolic Prx isoform and eliminates intracellular H2O2 generated in response to cell stimulation with PDGF or EGF (23). We previously established and characterized HeLa cell lines that stably express wild-type (WT) or a DN of Prx II (17). The level of Prx II expression in these cell lines was about twice that in control cells transfected with the corresponding empty vector. The transient increase in the intracellular concentration of H2O2 induced by EGF or tumor necrosis factor α was inhibited by expression of Prx II-WT and potentiated by expression of Prx II-DN.
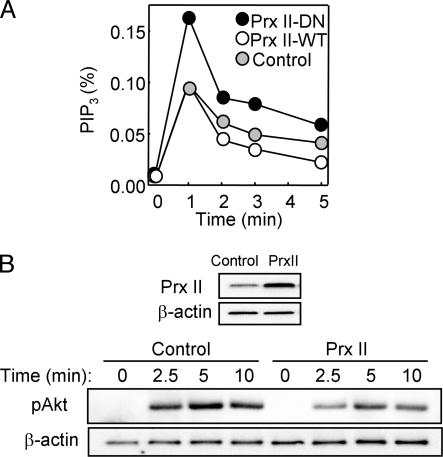
We measured the level of PIP3 in these stably transfected HeLa cell lines after their stimulation with EGF (Fig. 2A). PIP3 increased rapidly to peak at ≈1 min after exposure of the cells to EGF and then decreased in a biphasic manner. Compared with control cells, the amount of PIP3 in cells expressing Prx II-DN was greater at all time points, whereas that in cells expressing Prx II-WT was smaller at time points after 1 min.
Fig. 2.
Effects of Prx II on PIP3 accumulation and Akt phosphorylation in EGF-stimulated HeLa cells. (A) HeLa cells stably expressing Prx II-WT or Prx II-DN or those transfected with the empty pCR3.1 vector (control) were labeled with [32P]Pi and stimulated for the indicated times with 25 ng/ml EGF. The amount of [32P]PIP3 in the cells was then determined and is expressed as a percentage of the total radioactivity associated with the phospholipid fraction. Data are representative of three independent experiments. (B) HeLa cells were transfected for 48 h with pCR3.1 encoding Prx II-WT (PrxII) or with the empty vector, and cell lysates were subsequently subjected to immunoblot analysis with Abs to Prx II and to β-actin (loading control). The transfected cells were also stimulated with 2 ng/ml EGF for the indicated times, after which cell lysates were subjected to immunoblot analysis with Abs specific for Akt phosphorylated on Ser-473 (pAkt); the filter was reprobed with Abs to β-actin. Data are representative of three independent experiments.
We also studied the effect of transient overexpression of Prx II-WT in HeLa cells, which resulted in an ≈3-fold increase in the abundance of Prx II compared with that in control cells (Fig. 2B). Transfection efficiency in this system was determined to be ≈80% with an expression vector for green fluorescent protein (data not shown). The transient overexpression of Prx II-WT resulted in inhibition of the EGF-induced phosphorylation of Akt (Fig. 2B), which is often measured as a surrogate for changes in PIP3 abundance in cells. It was not possible to examine the effect of Prx II-DN by transient transfection because, in contrast to the situation in stably transfected cells, only a small fraction of the mutant protein molecules was able to form heterodimers with endogenous Prx II. Heterodimerization with endogenous enzyme molecules is the basis of the dominant negative action of the mutant protein (17). Together, these results suggested that PIP3 accumulates to a greater extent and persists for a longer time in EGF-stimulated cells in which the intracellular concentration of H2O2 is increased.
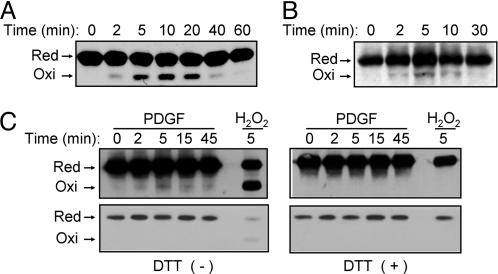
Growth Factor-Induced Oxidation of PTEN Detected by a Mobility Shift on Nonreducing SDS/PAGE. Hydrogen peroxide produced in response to cell stimulation with PDGF or EGF appeared to increase the amount of PIP3 measured either directly or indirectly on the basis of Akt phosphorylation. Therefore, we investigated whether growth factor-induced H2O2 production results in the oxidation of PTEN. We have previously shown that the essential Cys-124 of PTEN forms a disulfide with Cys-71 during oxidation with H2O2 and that the oxidized PTEN migrates faster on SDS/PAGE under nonreducing conditions than does the reduced protein (11). Treatment of HeLa cells with H2O2 induced the oxidation of PTEN, with the amount of the oxidized (faster-moving) form of the protein reaching a maximum at 10 min and decreasing thereafter (Fig. 3A). In contrast, similar immunoblot analysis of HeLa cells incubated for various times with EGF failed to detect the higher-mobility form of PTEN (data not shown). Therefore, we subjected lysates of EGF-treated cells to immunoprecipitation with a mAb to PTEN before immunoblot analysis with rabbit Abs to this protein. Under these conditions, a faint band corresponding to oxidized PTEN was detected in cells stimulated with EGF for 2 min; the intensity of this band peaked at 5 min and decreased gradually thereafter (Fig. 3B). Immunoprecipitates treated with DTT before electrophoresis failed to manifest the faint band (data not shown), suggesting that it indeed resulted from disulfide formation.
Fig. 3.
Detection of oxidized PTEN on the basis of an electrophoretic mobility shift in cells treated with exogenous H2O2 or with growth factors. (A) HeLa cells were incubated for the indicated times with 0.2 mM H2O2, after which cell lysates were subjected to alkylation with NEM and fractionated by nonreducing SDS/PAGE as described (11). Reduced (Red) and oxidized (Oxi) forms of PTEN were detected by immunoblot analysis with rabbit Abs to PTEN. (B) HeLa cells were incubated for the indicated times with 100 ng/ml EGF, after which cell lysates were subjected to alkylation with NEM followed by immunoprecipitation with a mAb (A2B1) to PTEN. The resulting precipitates were subjected to nonreducing SDS/PAGE and immunoblot analysis with rabbit Abs to PTEN as in A. (C) NIH 3T3 cells were transfected for 48 h with a mixture of Effectene (Qiagen) and a pCGN-derived vector encoding human PTEN tagged with HA at its NH2 terminus. The cells were subsequently stimulated for the indicated times with 25 ng/ml PDGF or for 5 min with 5 mM H2O2, after which cell lysates were analyzed as in B with the exception that the PTEN immunoprecipitates were either left untreated (Left) or treated with 1 mM DTT (Right) before immunoblot analysis with Abs to HA. The blots were exposed to x-ray film for 5 min (Upper) or 10 s (Lower).
We next studied NIH 3T3 cells transiently transfected with an expression vector for an HA-tagged form of PTEN. The cells were stimulated with PDGF for various times, and PTEN was then immunoprecipitated with the mAb to PTEN. Immunoblot analysis of the immunoprecipitates with Abs to HA revealed the transient appearance of a faint band corresponding to oxidized HA-tagged PTEN (Fig. 3C). Again, the band was not observed when the immunoprecipitates were exposed to DTT before electrophoresis (Fig. 3C). Given that the oxidized protein was detectable only after overexposure of blots, equal loading of the immunoprecipitated samples onto the gels was demonstrated by exposure of the immunoblots for a shorter time (Fig. 3C).
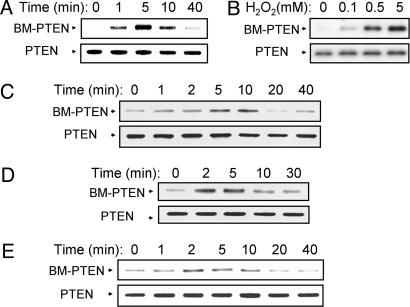
Growth Factor-Induced Oxidation of PTEN Detected by Biotinylation of the Oxidized Cys Residues. We also devised an alternative approach to detect oxidized PTEN. In the procedure, all free sulfhydryl moieties of proteins were first masked by alkylation with a mixture of NEM and iodoacetic acid. Proteins were then incubated with DTT to reduce disulfide linkages, and the newly revealed sulfhydryl groups were labeled with biotin-conjugated maleimide. The biotinylated proteins were precipitated with avidin-conjugated agarose and subjected to immunoblot analysis with Abs to PTEN. We applied this approach, which requires handling of samples under anaerobic conditions, to monitor PTEN oxidation in NIH 3T3 cells treated with H2O2. No substantial amount of biotinylated PTEN was recovered from untreated cells (Fig. 4), indicating that PTEN does not form a disulfide linkage under resting conditions even though it possesses 10 cysteine residues. However, exposure of cells to H2O2 resulted in the transient appearance of a band corresponding to biotinylated PTEN (Fig. 4A), and the intensity of this band depended on the concentration of H2O2 (Fig. 4B), consistent with our previous results obtained with the mobility-shift method (11). The biotinylation method was then applied to three different cell lines stimulated with growth factors. Growth factor-dependent transient increases in the amount of biotinylated PTEN were apparent in EGF-stimulated HeLa cells (Fig. 4C), PDGF-stimulated NIH 3T3 cells (Fig. 4D), and insulin-stimulated HEK293 cells (Fig. 4E).
Fig. 4.
Detection of oxidized PTEN by biotinylation in cells treated with exogenous H2O2 or with growth factors. NIH 3T3 cells were incubated either for the indicated times with 1 mM H2O2 (A) or for 5 min with the indicated concentrations of H2O2 (B); HeLa cells were incubated for the indicated times with 100 ng/ml EGF (C); NIH 3T3 cells were incubated for the indicated times with 25 ng/ml PDGF (D); and HEK293 cells were incubated for the indicated times with 0.5 μg/ml insulin (E). Cell lysates were then prepared and subjected to labeling with biotin-conjugated maleimide as described in Materials and Methods. A portion (10%) of each biotinylated sample was subjected to immunoblot analysis with rabbit Abs to PTEN as a control (Lower), whereas biotinylated proteins in the remaining fraction were precipitated with avidin-conjugated agarose and then subjected to immunoblot analysis with Abs to PTEN to detect biotinylated PTEN (BM-PTEN) (Upper).
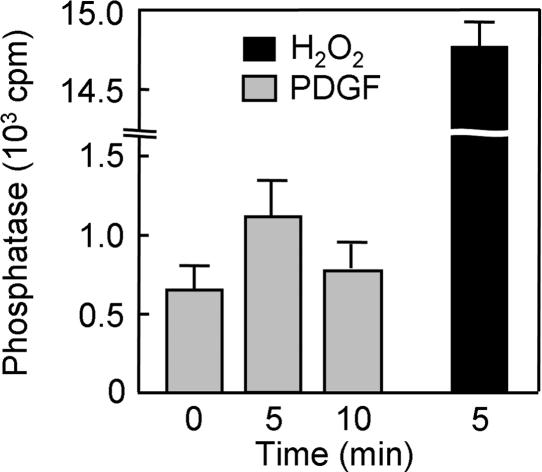
PDGF-Induced Inactivation of PTEN Detected by Assay of Phosphatase Activity. A method has been developed to measure selectively the phosphatase activity of PTEN that has been oxidized in cells (15). In this approach, cell lysates are treated first with NEM to alkylate all available thiols and then with DTT to reduce oxidized cysteine residues. PTEN molecules with a reduced essential cysteine thus become irreversibly inactivated by alkylation, whereas those with oxidized cysteines are protected from alkylation and become reactivated by DTT. PTEN is then immunoprecipitated and assayed for PIP3 3-phosphatase activity. Application of this method to NIH 3T3 cells revealed the formation of oxidized PTEN within 5 min of stimulation of the cells with PDGF (Fig. 5). Total PTEN activity was estimated by exposure of the cells to H2O2 for 5 min; however, quantitative comparison of the activity values was problematic because of the high background PTEN activity measured in untreated cells and an insufficient number of data points.
Fig. 5.
Detection by phosphatase assay of PTEN oxidized in NIH 3T3 cells stimulated with PDGF. Cells were incubated for 0, 5, or 10 min with 30 ng/ml PDGF or for 5 min with 5 mM H2O2, lysed in the presence of iodoacetic acid, and divided into three equal portions. PTEN was immunoprecipitated from each portion with a mAb to PTEN and then assayed for phosphatase activity under reducing conditions as described (15). Data are expressed as cpm of radioactivity released as Pi from the [32P]PIP3 substrate and are means ± SEM of the triplicate samples.
Discussion
Both elevation of the intracellular H2O2 level by stable expression of Nox1 and modulation of H2O2 accumulation by overexpression of wild-type or a DN of Prx II have indicated in the present study that H2O2 produced in response to stimulation of cells with EGF or PDGF potentiates the accumulation of PIP3 and subsequent activation of Akt. The cellular abundance of PIP3 is thought to be determined both by its synthesis, which is catalyzed by PI 3-kinase, and by its removal catalyzed by the 3-phosphatase PTEN and 5-phosphatases. We have now shown that overexpression of Nox1 did not affect the activity of PI 3-kinase measured in vitro. Furthermore, SHIP-2, a member of the PIP3 5-phosphatase family, is insensitive to oxidation by H2O2 (15). Therefore, the increased accumulation of PIP3 induced by Nox1 overexpression is probably not caused by enhanced activation of PI 3-kinase or inhibition of 5-phosphatase activity; rather, it likely results from oxidative inactivation of PTEN by H2O2. Consistent with this notion, the H2O2-dependent increase in the abundance of PIP3 and activation of Akt were detected in PTEN-expressing glioma cells but not in PTEN-null glioma cells (15, 24).
Endogenously produced H2O2 has also previously been shown to inactivate PTEN in a macrophage cell line, with ≈10% of PTEN molecules undergoing reversible oxidation at the active site cysteine in cells maximally stimulated with the combination of lipopolysaccharide and phorbol 12-myristate 13-acetate (15). However, the amount of reactive oxygen species produced by Nox proteins in nonphagocytic cells is much less than that generated in phagocytic cells, which gave rise to the long-held notion that such production of reactive oxygen species is biologically significant only in phagocytic cells.
We have now shown that PTEN is oxidized and inactivated by H2O2 likely produced by Nox enzymes in various cell types stimulated with EGF, PDGF, or insulin. Although quantitation of the extent of PTEN oxidation in these cells was not possible, given that its measurement was affected by several factors such as cell density and the nature of the culture medium (data not shown), it was substantially less than the 10% level detected previously in the macrophage cell line RAW (15). However, the transient oxidation of PTEN induced by growth factors was demonstrated by two different methods based on the electrophoretic mobility shift of oxidized PTEN or on the biotinylation of oxidized PTEN. Assay of phosphatase activity also provided further support for the transient oxidation of PTEN in PDGF-stimulated cells.
Assembly of the functional Nox1 complex requires GTP-bound Rac, as is also the case for the Nox2 complex in phagocytic cells. Exchange of Rac-bound GDP for GTP is catalyzed by guanine nucleotide exchange factors, and all guanine nucleotide exchange factors for Rac contain a pleckstrin homology domain and are activated by PIP3 or PI(3,4)P2. We have previously shown that H2O2 generation in cells stimulated with EGF or PDGF requires the activation of PI 3-kinase and that Nox1, Rac1, and βPix (a Rac guanine nucleotide exchange factor) form a ternary complex in response to growth factor stimulation (20, 25). PIP3 thus activates Nox1, and the resulting production of H2O2 mediates the inactivation of PTEN, leading to further accumulation of PIP3 and completing a positive feedback loop. Such feedback would be expected to cause a rapid increase in the abundance of H2O2 near the site of colocalization of PIP3 and the Nox complex. This localized H2O2 accumulation would be expected to result in the oxidation of only those PTEN molecules located nearby, possibly explaining the small proportion of PTEN molecules that undergo oxidative inactivation in growth factor-stimulated cells.
Given that H2O2 is readily converted to the toxic hydroxyl radical in the presence of Fe and Cu ions, localized production of H2O2 only where needed for intracellular signaling would appear to be desirable, as is the destruction of H2O2 molecules that diffuse away from this site of action. The removal of H2O2 in cells is mediated predominantly by catalase, glutathione peroxidase (GPx), and Prx. Catalase is localized exclusively in peroxisomes, so that elimination of cytosolic H2O2 by catalase requires its diffusion into these organelles. The major isoform of GPx, GPx1, is largely restricted to the cytosol but is also present in mitochondria (26). The Prx family of peroxidases includes at least six isoforms in mammalian cells (27). Among these isoforms, Prx I and Prx II are cytosolic enzymes and are abundant, typically constituting 0.1–0.8% of total soluble protein, but their catalytic efficiency is less than that of GPx or catalase by one to three orders of magnitude (27). However, Prx I and Prx II have been shown to be able to eliminate H2O2 produced in cells stimulated with EGF or PDGF (23, 27).
In the catalytic cycle of Prx I and Prx II, the sulfhydryl group of the essential cysteine residue (Cys–SH) is selectively oxidized by H2O2 to Cys–SOH, which then reacts with a neighboring cysteine to form a disulfide. The disulfide is subsequently and specifically reduced by thioredoxin (27). However, the sulfenic intermediate (Cys–SOH) is occasionally further oxidized to sulfinic acid (Cys–SO2H), resulting in inactivation of peroxidase activity (28). The oxidation to sulfinic acid was recently found to be a reversible step, with the back reaction being catalyzed by each of two ATP-dependent reductases designated sulfiredoxin and sestrin (29–31). Consistent with the fact that the prokaryotic orthologs of Prx I and Prx II are insensitive to oxidative inactivation, prokaryotes contain neither sulfiredoxin nor sestrin (30–32). Therefore, the sulfinylation-dependent inactivation of Prx I and Prx II has been suggested to be the result of structural features acquired during evolution to accommodate the intracellular messenger function of H2O2 (32). The messenger function of H2O2 likely requires that its concentration increase rapidly above a certain threshold level, a requirement that is likely met both through the production of H2O2 at specific locations by a mechanism that involves a positive feedback loop and through protection of the generated H2O2 molecules from destruction by Prx. Such protection is needed because of the large amounts of Prx I and Prx II that are present in the cytosol to remove the low levels of H2O2 produced as a result of normal cellular metabolism. This protection is probably transiently provided by the built-in mechanism of Prx inactivation mediated by H2O2.
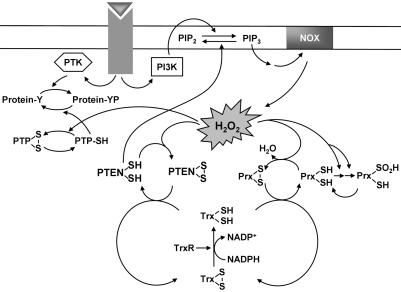
The scheme presented in Fig. 6 depicts the growth factor-induced generation of H2O2 by Nox, the participation of H2O2 in intracellular signaling by targeting of PTEN, and regulation of the concentration of H2O2 by Prx. Our results suggest that the activation of PI 3-kinase by some growth factors might not be sufficient to induce the accumulation of PIP3 because of the opposing activity of constitutively active PTEN. The concomitant local inactivation of PTEN by H2O2 might thus be necessary to increase the abundance of PIP3 to a level sufficient for it to trigger downstream signaling events. The constitutive activity of PTEN is likely required to prevent accumulation of PIP3 under resting conditions and thereby to suppress unwanted mitogenic signaling. Therefore, the oxidative inactivation of PTEN in response to H2O2 generation might be an important determinant of the timing and localization of the production of PIP3 triggered by cell stimulation. This notion, together with the H2O2-induced inactivation of PTPs, is consistent with the previous observations that H2O2 generation and accumulation are necessary for downstream actions of growth factors (1, 3, 4).
Fig. 6.
Model for the production, signaling role, and removal of H2O2 in growth factor-stimulated cells. Stimulation of cells with a growth factor induces the activation of PI 3-kinase (PI3K), which catalyzes the conversion of PI(4,5)P2 to PIP3. PIP3 activates the NADPH oxidase (NOX) complex, resulting in the production of H2O2. The H2O2 so generated likely mediates inactivation of cytosolic Prx molecules located nearby through a two-step oxidation of the active site Cys–SH to Cys–SO2H. The inactivation of Prx in turn promotes local accumulation of H2O2. The results of the present study suggest that the accumulated H2O2 molecules inactivate PTEN by oxidizing the catalytic cysteine residue. This inactivation of PTEN increases the abundance of PIP3 sufficiently to trigger downstream signaling events. The H2O2 signal is likely terminated by the reactivation of sulfinylated Prx and the consequent removal of H2O2. As the local concentration of H2O2 decreases, oxidized PTEN is reactivated by thioredoxin (Trx), which in turn receives reducing equivalents from NADPH by means of thioredoxin reductase (TrxR).
Acknowledgments
We thank J. D. Lambeth for NIH 3T3 cells overexpressing Nox1 and N. R. Leslie for PI 3-kinase γ. This work was supported by Korea Research Foundation Grant KRF-2003-041-C20173 (to S.-R.L.).
Author contributions: J.K., S.-R.L., K.-S.Y., Y.A., and Y.J.K. performed research; S.G.R. wrote the paper; and E.R.S. provided general knowledge for oxidative stress-related research and revised the manuscript.
Abbreviations: EGF, epidermal growth factor; PDGF, platelet-derived growth factor; PTP, protein tyrosine phosphatase; PI, phosphatidylinositol; PIP3, PI 3,4,5-trisphosphate; Nox1, NADPH oxidase 1; DN, dominant negative mutant; Prx, peroxiredoxin; HA, hemagglutinin epitope; NEM, N-ethylmaleimide.
References
- 1.Rhee, S. G., Bae, Y. S., Lee, S.-R. & Kwon, J. (2000) Sci. STKE 2000, pe1. [DOI] [PubMed] [Google Scholar]
- 2.Lambeth, J. D. (2004) Nat. Rev. Immunol. 4, 181–189. [DOI] [PubMed] [Google Scholar]
- 3.Sundaresan, M., Yu, Z. X., Ferrans, V. J., Irani, K. & Finkel, T. (1995) Science 270, 296–299. [DOI] [PubMed] [Google Scholar]
- 4.Bae, Y. S., Kang, S. W., Seo, M. S., Baines, I. C., Tekle, E., Chock, P. B. & Rhee, S. G. (1997) J. Biol. Chem. 272, 217–221. [PubMed] [Google Scholar]
- 5.Burdon, R. H. (1995) Free Radical Biol. Med. 18, 775–794. [DOI] [PubMed] [Google Scholar]
- 6.Szatrowski, T. P. & Nathan, C. F. (1991) Cancer Res. 51, 794–798. [PubMed] [Google Scholar]
- 7.Lee, S. R., Kwon, K. S., Kim, S. R. & Rhee, S. G. (1998) J. Biol. Chem. 273, 15366–15372. [DOI] [PubMed] [Google Scholar]
- 8.Meng, T. C., Fukada, T. & Tonks, N. K. (2002) Mol. Cell 9, 387–399. [DOI] [PubMed] [Google Scholar]
- 9.Mahadev, K., Zilbering, A., Zhu, L. & Goldstein, B. J. (2001) J. Biol. Chem. 276, 21938–21942. [DOI] [PubMed] [Google Scholar]
- 10.Maehama, T., Taylor, G. E. & Dixon, J. E. (2001) Annu. Rev. Biochem. 70, 247–279. [DOI] [PubMed] [Google Scholar]
- 11.Lee, S.-R., Yang, K.-S., Kwon, J., Lee, C., Jeong, W. & Rhee, S. G. (2002) J. Biol. Chem. 277, 20336–20342. [DOI] [PubMed] [Google Scholar]
- 12.Barrett, W. C., DeGnore, J. P., Keng, Y. F., Zhang, Z. Y., Yim, M. B. & Chock, P. B. (1999) J. Biol. Chem. 274, 34543–34546. [DOI] [PubMed] [Google Scholar]
- 13.Salmeen, A., Andersen, J. N., Myers, M. P., Meng, T. C., Hinks, J. A., Tonks, N. K. & Barford, D. (2003) Nature 423, 769–773. [DOI] [PubMed] [Google Scholar]
- 14.Lee, J. O., Yang, H., Georgescu, M. M., Di Cristofano, A., Maehama, T., Shi, Y., Dixon, J. E., Pandolfi, P. & Pavletich, N. P. (1999) Cell 99, 323–334. [DOI] [PubMed] [Google Scholar]
- 15.Leslie, N. R., Bennett, D., Lindsay, Y. E., Stewart, H., Gray, A. & Downes, C. P. (2003) EMBO J. 22, 5501–5510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arnold, R. S., Shi, J., Murad, E., Whalen, A. M., Sun, C. Q., Polavarapu, R., Parthasarathy, S., Petros, J. A. & Lambeth, J. D. (2001) Proc. Natl. Acad. Sci. USA 98, 5550–5555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang, S. W., Chang, T. S., Lee, T. H., Kim, E. S., Yu, D. Y. & Rhee, S. G. (2004) J. Biol. Chem. 279, 2535–2543. [DOI] [PubMed] [Google Scholar]
- 18.Zhang, L. & Buxton, I. L. (1998) Methods Mol. Biol. 105, 47–63. [DOI] [PubMed] [Google Scholar]
- 19.Lassegue, B., Sorescu, D., Szocs, K., Yin, Q., Akers, M., Zhang, Y., Grant, S. L., Lambeth, J. D. & Griendling, K. K. (2001) Circ. Res. 88, 888–894. [DOI] [PubMed] [Google Scholar]
- 20.Park, H. S., Lee, S. H., Park, D., Lee, J. S., Ryu, S. H., Lee, W. J., Rhee, S. G. & Bae, Y. S. (2004) Mol. Cell. Biol. 24, 4384–4394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mahadev, K., Motoshima, H., Wu, X., Ruddy, J. M., Arnold, R. S., Cheng, G., Lambeth, J. D. & Goldstein, B. J. (2004) Mol. Cell. Biol. 24, 1844–1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chae, H. Z., Kim, I. H., Kim, K. & Rhee, S. G. (1993) J. Biol. Chem. 268, 16815–16821. [PubMed] [Google Scholar]
- 23.Kang, S. W., Chae, H. Z., Seo, M. S., Kim, K., Baines, I. C. & Rhee, S. G. (1998) J. Biol. Chem. 273, 6297–6302. [DOI] [PubMed] [Google Scholar]
- 24.Myers, M. P., Pass, I., Batty, I. H., Van der Kaay, J., Stolarov, J. P., Hemmings, B. A., Wigler, M. H., Downes, C. P. & Tonks, N. K. (1998) Proc. Natl. Acad. Sci. USA 95, 13513–13518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae, Y. S., Sung, J. Y., Kim, O. S., Kim, Y. J., Hur, K. C., Kazlauskas, A. & Rhee, S. G. (2000) J. Biol. Chem. 275, 10527–10531. [DOI] [PubMed] [Google Scholar]
- 26.Panfili, E., Sandri, G. & Ernster, L. (1991) FEBS Lett. 290, 35–37. [DOI] [PubMed] [Google Scholar]
- 27.Rhee, S. G., Kang, S. W., Chang, T. S., Jeong, W. & Kim, K. (2001) IUBMB Life 52, 35–41. [DOI] [PubMed] [Google Scholar]
- 28.Yang, K. S., Kang, S. W., Woo, H. A., Hwang, S. C., Chae, H. Z., Kim, K. & Rhee, S. G. (2002) J. Biol. Chem. 277, 38029–38036. [DOI] [PubMed] [Google Scholar]
- 29.Woo, H. A., Chae, H. Z., Hwang, S. C., Yang, K. S., Kang, S. W., Kim, K. & Rhee, S. G. (2003) Science 300, 653–656. [DOI] [PubMed] [Google Scholar]
- 30.Biteau, B., Labarre, J. & Toledano, M. B. (2003) Nature 425, 980–984. [DOI] [PubMed] [Google Scholar]
- 31.Budanov, A. V., Sablina, A. A., Feinstein, E., Koonin, E. V. & Chumakov, P. M. (2004) Science 304, 596–600. [DOI] [PubMed] [Google Scholar]
- 32.Wood, Z. A., Poole, L. B. & Karplus, P. A. (2003) Science 300, 650–653. [DOI] [PubMed] [Google Scholar]
- 33.Whitman, M., Downes, C. P., Keeler, M., Keller, T. & Cantley, L. (1988) Nature 332, 644–646. [DOI] [PubMed] [Google Scholar]