The quest to obtain details about enzyme mechanisms is a common goal among a wide range of scientific disciplines. Those goals range from characterizing a variety of chemistries catalyzed, to more applied goals, such as the development of potent inhibitors used to treat disease. In the study of enzymes, a continuum of scrutiny prevails in which the level of atomic detail aids understanding. Often the combination of a high-resolution structure of the enzyme along with detailed chemical and kinetic characterization leads to a working mechanism, or further strengthens an existing one. As the first committed step in cholesterol biosynthesis, the reaction catalyzed by 3-hydroxy-3-methylglutaryl–CoA (HMG-CoA) synthase is a recent success story. The structure of an abortive inhibited complex (1) gives a structural perspective to a wealth of mechanistic data (2–5) and offers a starting point for inhibitor design critical to cholesterol-lowering approaches (6). However, this level of understanding doesn't explain the intricacies of how the protein uses each and every interaction, as well as protein dynamics, in its catalytic role. In several cases, experiments that provide reliable structural models of the enzyme during its mechanism have started to provide the necessary data points to present a more complete and interesting picture, and ultimately may lead an atomic-resolution “movie” of what is going on. The work of Theisen et al. (7) in this issue of PNAS provides two revealing frames of the mechanistic movie catalyzed by HMG-CoA synthase.
In the physiological direction, the mechanism of HMG-CoA synthase begins with acetyl-CoA binding, followed by the straightforward acetylation of Cys111, as depicted in Fig. 1. In ping-pong fashion the second substrate, acetoacetyl-CoA (AcAc-CoA), enters after the release of CoASH, thereby setting up the more challenging condensation reaction. As in the case here, features of enzymes that proceed through a carbon enolate intermediate or in a concerted fashion with carbanion character have long been a mechanistic question for a variety of systems (8–11). In the case of HMG-CoA synthase, Theisen et al. (7) had the good fortune to cryogenically trap protein crystals that had, to a varying extent, two of these mechanistically revealing states.
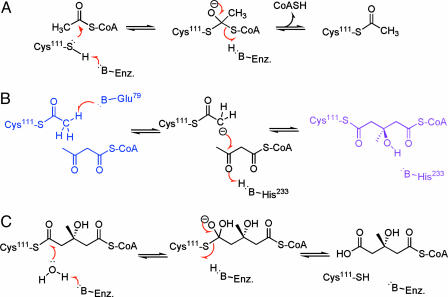
Fig. 1.
The mechanism of HMG-CoA synthase has been elucidated by enzyme intermediate structures of the acetylated-enzyme AcAc-CoA complex (blue) and the covalent HMG-CoA-enzyme complex (purple) (7). The overall reaction of acetyl-CoA and AcAc-CoA to produce HMG-CoA is depicted in three phases. (A) Acetylation/deacetylation. The substrates bind to the enzyme with the acetyl-CoA, forming a covalent acetylated-cysteine intermediate. (B) Condensation/cleavage. Upon dissociation of the CoASH, the second substrate AcAc-CoA enters the active site, forming the acetylated-enzyme AcAc-CoA complex shown in blue. The condensation reaction is shown going through an enolate intermediate to form the covalent HMG-CoA-enzyme complex shown in purple. (C) Hydrolysis/dehydration. The final phase of the overall reaction is the hydrolysis of the covalent HMG-CoA-enzyme complex, which occurs through a tetrahedral intermediate to form HMG-CoA as the sole product of the reaction. The chemically challenging step for this reaction is the condensation step that features the formation of a carbon–carbon bond.
Structures of Intermediates That Frame the Enolate Intermediate Get to the Heart of Catalysis
The x-ray diffraction data were of high enough resolution and quality to unequivocally model these two intermediates. The protein crystallized with multiple views of the active site in the crystallographic repeating asymmetric unit. One might typically expect to see the same structure repeated in each “copy” of the structure. However, in this case, each active site in a given asymmetric unit gave a slightly different balance of the two intermediates modeled as alternate conformations. Structures solved were the acetylated-enzyme AcAc-CoA complex and the covalent HMG-CoA-enzyme complex depicted in Fig. 1B in blue and purple, respectively. Although the overall equilibrium constant favors the physiological formation of HMG-CoA, previous studies had suggested that each step is reversible and “the backward” reaction occurs at a slow, but detectable, extent. Solution studies by Theisen et al. (7) included H2O18 exchange data that further supported this model of a reversible reaction. Additionally, MALDI MS data cleanly showed a detectable amount of the covalent HMG-CoA-enzyme complex in solution. The intermediate crystal structures were determined by using crystals, which were grown in the presence of the physiological product HMG-CoA. Crystals that had thermodynamically equilibrated via the slow backward reaction over 5, 14, or 31 days were cryogenically trapped, and the structures were determined. Because of microscopic reversibility, we can take stock that the crystal structures of intermediates and interpretations discussed here are entirely relevant to the physiological direction.
The use of cryogenics to trap an enzyme structure at a particular enzyme–substrate or enzyme–intermediate complex has had considerable success in offering mechanistic details of enzymes (10, 12–15). For example, studies by Lahiri et al. (14) have presented crystallographic evidence of a pentacovalent phosphorus intermediate for the reaction catalyzed by β-phosphoglucomutase. A possibility that was raised in further discussion of this work (16) was that the cryogenic conditions that were used to freeze the crystals (77°K) and sustain them at cryogenic temperature during data collection (93°K) had stabilized what at physiological temperature would have been the transition state. The same consideration should be made regarding the physiological relevance of the intermediate structures presented in the work by Theisen et al. (7). The crystals used for the structure of HMG-CoA synthase intermediates were grown at 22°C in the presence of HMG-CoA. Crystals at days 5, 14, and 31 after the initiation of vapor diffusion were flash-frozen. It is noteworthy that each crystal, and each independent view of the active site within a single crystal, gave a mixture of intermediate complexes. This finding strongly suggests that the observation of these intermediates is not an anomaly of the freezing process but represents an equilibrium attained during crystal growth. The more reasonable conclusion is that, at least in the crystalline form, the acetylated-enzyme AcAc-CoA complex and the covalent HMG-CoA-enzyme complex are nearly isoenergetic and stable enough to be seen as the main thermodynamically equilibrated forms. Small variations in the extent of reaction and the protein crystal contacts in the three separated crystals gave rise to a slightly shifted balance of these thermodynamically stable ground-state species.
The observation of the intermediates represents an equilibrium attained during crystal growth.
The Groundwork Has Been Laid to Film the Rest of the HMG-CoA Synthase Movie
The consistency of the multiple views and resulting reported errors of atomic coordinates for each of the intermediates reported by Theisen et al. (7) strengthens the validity of these structural models. Furthermore, it is noteworthy that these structures present not only the substrate and intermediate atomic positions but also the entire protein structure. Variations of the entire protein structure between these two intermediates and the ligand-free form of the enzyme (1) offers the first glimpse of protein motions that must be occurring during the reaction trajectory of HMG-CoA synthase. As shown in Fig. 1, there are several other enzyme species that the mechanistic trajectory must pass through during each catalytic turnover. Ideally, theoretical modeling, using the condensation intermediates as ground-state boundary conditions, will lead to a dynamic mechanism of the enzyme, as has been attempted in other cases (17–19). As discussed above, the observation of a mixture of intermediates in the HMG-CoA synthase crystals suggests nearly matched energies for the ground-state species bridging the putative enolate intermediate of the condensation reaction. The formation of a carbon–carbon bond offers a unique catalytic challenge. It will be interesting to see whether this apparent stabilization only exists in the HMG-CoA synthase crystals, or whether it likewise exists for the solution form of the enzyme. Computational approaches need to be pursued that take into account protein crystal contacts and crystallographic symmetry and allow a comparison of the relative energy levels of the solution versus the crystal forms of these intermediates.
See companion article on page 16442.
References
- 1.Campobasso, N., Patel, M., Wilding, I. E., Kallender, H., Rosenberg, M. & Gwynn, M. N. (2004) J. Biol. Chem. 279, 44883–44888. [DOI] [PubMed] [Google Scholar]
- 2.Chun, K. Y., Vinarov, D. A., Zajicek, J. & Miziorko, H. M. (2000) J. Biol. Chem. 275, 17946–17953. [DOI] [PubMed] [Google Scholar]
- 3.Chun, K. Y., Vinarov, D. A. & Miziorko, H. M. (2000) Biochemistry 39, 14670–14681. [DOI] [PubMed] [Google Scholar]
- 4.Greenspan, M. D., Yudkovitz, J. B., Lo, C. Y., Chen, J. S., Alberts, A. W., Hunt, V. M., Chang, M. N., Yang, S. S., Thompson, K. L. & Chiang, Y. C. (1987) Proc. Natl. Acad. Sci. USA 84, 7488–7492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miziorko, H. M. & Lane, M. D. (1977) J. Biol. Chem. 252, 1414–1420. [PubMed] [Google Scholar]
- 6.Teo, K. K. & Burton, J. R. (2002) Drugs 62, 1707–1715. [DOI] [PubMed] [Google Scholar]
- 7.Theisen, M. J., Misra, I., Saadat, D., Campobasso, N., Miziorko, H. M. & Harrison, D. H. T. (2004) Proc. Natl. Acad. Sci. USA 101, 16442–16447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gerlt, J. A. & Gassman, P. G. (1993) Biochemistry 32, 11943–11952. [DOI] [PubMed] [Google Scholar]
- 9.Anderson, V. E., Bahnson, B. J., Wlassics, I. D. & Walsh, C. T. (1990) J. Biol. Chem. 265, 6255–6261. [PubMed] [Google Scholar]
- 10.Bahnson, B. J., Anderson, V. E. & Petsko, G. A. (2002) Biochemistry 41, 2621–2629. [DOI] [PubMed] [Google Scholar]
- 11.Wlassics, I. D. & Anderson, V. E. (1989) Biochemistry 28, 1627–1633. [DOI] [PubMed] [Google Scholar]
- 12.Stoddard, B. L. (1999) Biochem. Soc. Trans. 27, 42–48. [DOI] [PubMed] [Google Scholar]
- 13.Schlichting, I., Berendzen, J., Chu, K., Stock, A. M., Maves, S. A., Benson, D. E., Sweet, R. M., Ringe, D., Petsko, G. A. & Sligar, S. G. (2000) Science 287, 1615–1622. [DOI] [PubMed] [Google Scholar]
- 14.Lahiri, S. D., Zhang, G., Dunaway-Mariano, D. & Allen, K. N. (2003) Science 299, 2067–2071. [DOI] [PubMed] [Google Scholar]
- 15.Bolduc, J. M., Dyer, D. H., Scott, W. G., Singer, P., Sweet, R. M., Koshland, D. E., Jr. & Stoddard, B. L. (1995) Science 268, 1312–1318. [DOI] [PubMed] [Google Scholar]
- 16.Knowles, J. (2003) Science 299, 2002–2003. [DOI] [PubMed] [Google Scholar]
- 17.Karplus, M. & McCammon, J. A. (2002) Nat. Struct. Biol. 9, 646–652. [DOI] [PubMed] [Google Scholar]
- 18.Luo, J. & Bruice, T. C. (2004) Proc. Natl. Acad. Sci. USA 101, 13152–13156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoddard, B. L., Dean, A. & Bash, P. A. (1996) Nat. Struct. Biol. 3, 590–595. [DOI] [PubMed] [Google Scholar]