Abstract
It is unclear if the relationship of total serum IgE with asthma varies with degree of urbanisation. We hypothesised that the relationship of total serum IgE to asthma is more pronounced in an urban versus a rural environment.
We enrolled 1441 children aged 13–15 years in a peri-urban shanty town in Lima, Peru (n=725) and 23 villages in rural Tumbes, Peru (n=716). We asked participants about asthma and allergy symptoms, environmental exposures and sociodemographics; and performed spirometry, and exhaled nitric oxide and allergy skin testing. We obtained blood for total serum IgE in 1143 (79%) participants.
Geometric means for total serum IgE were higher in Lima versus Tumbes (262 versus 192 kU·L−1; p<0.001). The odds of asthma increased by factors of 1.6 (95% CI 1.3–2.0) versus 1.4 (95% CI 0.9–2.1) per log unit increase in total serum in Lima versus Tumbes, respectively. Atopy was an effect modifier of the relationship of total serum IgE on asthma. Among atopics and non-atopics, the odds of asthma increased by a factor of 2.0 (95% CI 1.5–2.7) and 1.0 (95% CI 0.7–1.4) per log unit increase in total serum IgE, respectively.
Total serum IgE was associated with atopic asthma but not with non-atopic asthma. Urbanisation did not appear to be an effect modifier of this relationship.
Keywords: Asthma clinical/basic investigations, atopic asthma, epidemiology of asthma
Asthma is a chronic lung disease that is associated with an inflammatory reaction of the airways, bronchial hyperresponsiveness and increased production of mucus, all which lead to reversible periods of airway obstruction. Over the past several decades, asthma has emerged as one of the most prevalent non-communicable diseases worldwide, particularly among children. It currently affects 300 million individuals around the world, is responsible for 255 000 deaths and results in 15 million disability-adjusted life-years lost each year [1]. The worldwide burden of disease caused by asthma accounts for one per cent of all disability life years lost, and is comparable to that of diabetes mellitus or cirrhosis [2, 3].
Atopic sensitisation is a well-known risk factor for the development of asthma [4], and repeated exposure to high levels of allergens with previous sensitisation can worsen asthma control [5, 6]. The development of an allergic response is mediated by the production of IgE antibodies. Since the discovery of IgE [7], the relationship of total serum IgE with asthma [8–13] has been extensively studied to determine if it could be a useful adjunct in the diagnosis of asthma. However, due to considerable overlap in total serum IgE levels among atopic and non-atopic populations, the diagnostic utility of total serum IgE for asthma has been questioned. Moreover, sensitisation to specific allergens may occur even in the setting of low total serum IgE levels. Interpretation of total IgE in developing countries is complicated even further with a high burden of parasitic infections.
While the relationship of total serum IgE to asthma is well described in developed countries, it is unclear if this relationship varies in different populations according to the types of allergens, levels of indoor and outdoor air pollution, the prevalence of atopy or parasitic infections [14] and other risk factors for asthma, including obesity [15] and degree of urbanisation. In this study, we examined the relationship of total serum IgE with asthma, atopy, airway obstruction and airway inflammation in two regions of a developing country with disparate degrees of urbanisation and a different profile of environmental exposures. We hypothesised that the relationship of total serum IgE to asthma may be more pronounced in an urban than in a rural environment.
METHODS
Study design
The study design is described in detail elsewhere [16–18]. We conducted a population-based, cross-sectional study of asthma prevalence in two regions in Peru. In December 2008, we selected a random sample of children aged 13–15 years from a community census and visited them for enrolment between April 2009 and December 2010. The first site was Lima, the highly urbanised capital of Peru, located at sea level and with a population of 10 million. We conducted our study in Pampas de San Juan de Miraflores, a peri-urban shanty-town located 25 km south of central Lima. The second site was rural Tumbes, also at sea level, located in northern Peru. We asked about asthma and allergy symptoms, sociodemographics and environmental exposures, and obtained anthropometry, a blood sample, allergy skin test, exhaled nitric oxide test, and spirometry before and after bronchodilators. The basis of questionnaire used in this study was a previously validated Spanish version of the International Study of Asthma and Allergies in Childhood study [19]. We conducted spirometry according to American Thoracic Society/European Respiratory Society guidelines [20] with the portable SpiroPro (Jaeger, Hoechberg, Germany). We used the handheld NIOXMINO (Aerocrine, Solna, Sweden) to measure exhaled nitric oxide. We performed allergy tests with the Multi-Test II (Lincoln Diagnostics, Decatur, USA) using 10 common household allergens [17]. Serum specimens were analysed for total serum IgE using an USA Food and Drug Administration cleared fluorescent enzyme immunoassay (ImmunoCAP250, Thermo Fisher Scientific, Kalamazoo, MI, USA). We obtained approval from the ethics committees of A.B. PRISMA in Lima, Peru, and the Johns Hopkins University, Bloomberg School of Public Health, in Baltimore, MD, USA.
Definitions
We defined asthma as wheeze in the past 12 months or use of asthma medications in the past 12 months. We defined atopy as a positive skin response to any of the allergen specificities as previously described [17, 18]. Briefly, an allergy skin test was considered positive if the sum of the vertical and horizontal dimensions of the induration was >3 mm larger than the negative control or if the sum of the vertical and horizontal dimensions of erythema was >5 mm larger than the negative control. We defined reversibility as a 12% increase in post- to pre-forced expiratory volume in 1 s (FEV1).
Biostatistical methods
Our primary objective was to study the relationship of total serum IgE to asthma. We used multivariable logistic regression stratified by site and by atopic status and adjusted for age, sex, body mass index (>25 kg·m−2), personal history of tobacco smoke, second-hand tobacco smoke and sociodemographics including maternal education (<6 years), monthly household income (<175 USD), household density (more than six people per household) and concrete floor. The distribution of total serum IgE was skewed left, with values that ranged from 2 to 9420 kU·L−1. Thus, we log transformed values of total IgE for statistical analyses. In exploratory analyses, we found that the relationship of log total IgE to the log odds of asthma was approximately linear. We also studied the relationship of total IgE to airway obstruction as measured by the ratio of FEV1 to forced vital capacity (FVC). We conducted multivariable linear regression stratified by site and asthma status and adjusted for the same sociodemographic variables. Secondary objectives included analyses of the relationship of total IgE to both atopy and airways inflammation as measured by an exhaled nitric oxide greater than 40 ppm. We used multivariable logistic regression adjusted by sociodemographics for these analyses. Finally, we used t-tests to compare the log transformed values of total IgE and chi-squared tests for differences in proportions across strata. Analyses were restricted to the subgroup of 1143 participants for which we had a total serum IgE measurement. We used R (www.r-project.org) for statistical analyses.
RESULTS
Baseline characteristics
Of 1441 children who agreed to participate in the study, we obtained blood samples for total serum IgE levels from 1143 (79%). There were no differences observed in the age (p=0.97), sex (p=0.70), prevalence of asthma (p=0.72) or atopy (p=0.24), pre-FEV1/FVC (p=0.69), exhaled nitric oxide (p=0.35), body mass index (p =0.40), income (p = 0.66) and maternal education (p=0.73) between participants with and without a blood sample for total serum IgE levels. Children living in the urban environment were less likely to live in households with a monthly income <175 USD (25% versus 63%; p<0.001), were more likely to live in households with uninterrupted water services (92% versus 6%; p<0.001) or electricity (100% versus 85%; p<0.001), were more likely to have an indoor sewage connection (92% versus 27%; p<0.001), and were less likely to live in households with regular use of biomass fuels (9% versus 42%; p<0.001) or farm animals (72% versus 23%; p<0.001) than those in the rural environment.
The mean ± SD total serum IgE was 588 ± 883 kU·L−1 in Lima and 407 ± 679 kU·L−1 in Tumbes (p<0.001; t-test of log IgE); however, there was considerable overlap in the range of total IgEs between sites (fig. 1). Corresponding geometric means were 262 kU·L−1 and 192 kU·L−1, respectively. Average mean ± SD age at the time of enrolment was comparable in both study groups, 14.8 ± 0.9 years in Lima versus 14.9 ± 0.9 years in Tumbes (p=0.44). In Lima, total IgE levels were higher in boys than in girls and higher in overweight participants (body mass index >25 kg·m−2) than in those who were not overweight (table 1). While these differences were not statistically significant in Tumbes, they trended in the same direction. We did not find significant differences in average total serum IgE levels across select socioeconomic indicators such as maternal education, monthly income or household density in either site (table 1).
FIGURE 1.
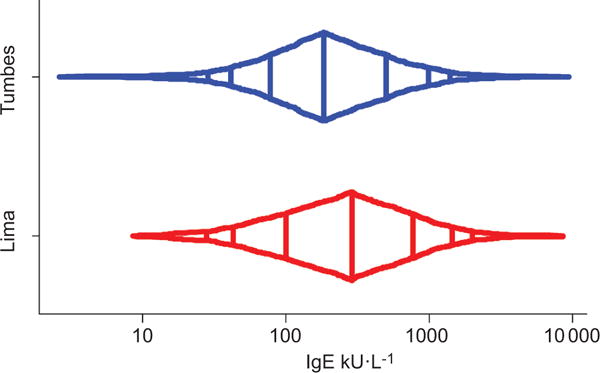
Distribution of total serum IgE stratified by site; Peru, 2009–2010. x-axis is in log-scale. Vertical lines inside the box-percentile plot represent, from left to right, the 5th, 10th, 25th, 50th, 75th, 90th and 95th percentiles, respectively.
TABLE 1.
IgE levels across demographic variables, asthma status, atopic status, airways inflammation and socioeconomic variables, stratified by study site; Peru, 2009–2010
| Characteristic | Total serum IgE kU·L−1
|
|||||
|---|---|---|---|---|---|---|
| Lima#
|
Tumbes¶
|
|||||
| Geometric mean | Mean ± SD | p-value | Geometric mean | Mean ± SD | p-value | |
|
Overall Demographics |
262 | 588 ± 883 | 192 | 407 ± 679 | ||
| Sex | ||||||
| Male | 326 | 692 ± 962 | 204 | 416 ± 725 | ||
| Female | 210 | 484 ± 784 | <0.001 | 178 | 397 ± 623 | 0.20 |
| Body mass index >25 kg·m−2 | ||||||
| No | 240 | 566 +921 | 189 | 396 +652 | ||
| Yes | 432 | 712 ± 630 | <0.001 | 235 | 549 ± 951 | 0.28 |
| Asthma and atopy | ||||||
| Asthma status | ||||||
| No | 238 | 546 ± 891 | 188 | 397 ± 666 | ||
| Yes | 516 | 894 ± 760 | <0.001 | 338 | 743 ± 1006 | 0.12 |
| Atopy status | ||||||
| No | 182 | 440 ± 745 | 147 | 355 ± 773 | ||
| Yes | 351 | 716 ± 992 | <0.001 | 294 | 479 ± 463 | <0.001 |
| Asthma phenotype | ||||||
| Atopic | 761 | 1080 ± 794 | 472 | 662 ± 438 | ||
| Non-atopic | 136 | 302 ± 290 | <0.01 | 251 | 816 ± 1358 (Median = 119) | 0.38 |
| Exhaled nitric oxide >40 ppm | ||||||
| No | 217 | 478 ± 764 | 172 | 367 ± 666 | ||
| Yes | 1142 | 1404 ± 877 | <0.001 | 611 | 838 ± 709 | <0.001 |
| Socioeconomic indicators | ||||||
| Maternal education ⩾6 years | ||||||
| No | 308 | 589 ± 680 | 182 | 380 ± 507 | ||
| Yes | 249 | 590 ± 943 | 0.11 | 194 | 419 ± 747 | 0.60 |
| Income <175 USD per month | ||||||
| No | 274 | 616 ± 946 | 189 | 429 ± 672 | ||
| Yes | 229 | 509 ± 668 | 0.17 | 193 | 394 ± 685 | 0.85 |
| 6+ people per house | ||||||
| No | 255 | 595 ± 925 | 185 | 400 ± 731 | ||
| Yes | 268 | 581 ± 841 | 0.68 | 212 | 428 ± 519 | 0.26 |
| Concrete floor | ||||||
| No | 255 | 537 ± 691 | 216 | 485 ± 876 | ||
| Yes | 273 | 673 ± 1132 | 0.59 | 175 | 347 ± 468 | 0.05 |
| Pets in household | ||||||
| Dogs | ||||||
| No | 279 | 571 ± 834 | 201 | 433 ± 629 | ||
| Yes | 249 | 601 ± 920 | 0.32 | 188 | 397 ± 698 | 0.56 |
| Cats | ||||||
| No | 267 | 632 ± 978 | 192 | 413 ± 730 | ||
| Yes | 255 | 535 ± 750 | 0.69 | 191 | 399 ± 607 | 0.98 |
| Chickens | ||||||
| No | 250 | 552 ± 771 | 175 | 345 ± 487 | ||
| Yes | 311 | 728 ± 1214 | 0.12 | 203 | 446 ± 775 | 0.16 |
| Ducks | ||||||
| No | 257 | 581 ± 886 | 191 | 385 ± 528 | ||
| Yes | 324 | 665 ± 848 | 0.23 | 192 | 434 ± 828 | 0.97 |
| Turkeys | ||||||
| No | 262 | 590 ± 886 | 190 | 413 ± 714 | ||
| Yes | 177 | 353 ± 244 | 0.71 | 204 | 374 ± 413 | 0.61 |
| Doves | ||||||
| No | 257 | 564 ± 808 | 190 | 412 ± 701 | ||
| Yes | 287 | 719 ± 1205 | 0.50 | 204 | 370 ± 444 | 0.65 |
| Guinea pigs | ||||||
| No | 261 | 582 ± 830 | 191 | 411 ± 691 | ||
| Yes | 266 | 639 ± 1224 | 0.91 | 196 | 352 ± 441 | 0.90 |
n= 566;
n=577.
Pets and farm animals were commonly found in households at both sites. There was a greater proportion of households with dogs (72% versus 57%; p<0.001), chickens (61% versus 21%; p<0.001), ducks (45% versus 9%; p<0.001) and turkeys (14% versus <1%; p<0.001) in Tumbes than in Lima. There was a similar proportion of households with cats (43% versus 45%; p=0.52) in Tumbes and Lima. There was a greater proportion of households with doves (16% versus 10%; p<0.01) and guinea pigs (12% versus 6%; p<0.001) in Lima than in Tumbes. Total serum IgE levels were not affected by the presence of pets or farm animals at either site (table 1). Dogs (38% versus 13%; p<0.001) and cats (76% versus 32%; p<0.001) were more likely to be exclusively indoors in Lima than in Tumbes. The prevalence of atopic sensitisation was significantly greater in Lima than in Tumbes for all tested allergens. While the rate of atopic sensitisation was slightly greater for cockroach (57% versus 42%; p<0.001), mite (58% versus 35%; p<0.001) and cat (55% versus 32%; p<0.001), it was substantially greater for dog (52% versus 13%; p<0.001), mouse (52% versus 19%; p<0.001) and mould (53% versus 15%; p<0.001).
Relationship of total serum IgE to asthma, atopy and airways inflammation
Among the 1143 participants with a total serum IgE measurement, the prevalence of atopy as assessed by skin testing was 55% (292 out of 527) in Lima and 37% (206 out of 550) in Tumbes. The prevalences of atopic and non-atopic asthma in Lima were 17% (50 out of 292) and 6% (13 out of 235), respectively; in Tumbes, the corresponding prevalences of atopic and non-atopic asthma were 4% (eight out of 206) and 3% (nine out of 344), respectively.
Total serum IgE levels were higher in asthmatics than in non-asthmatics (table 1). The prevalence of asthma across our sample increased with total serum IgE (fig. 2). This increase was more apparent in Lima than in Tumbes. In multivariable analyses, the odds of asthma increased by factors of 1.6 (95% CI 1.3–2.0) and 1.4 (95% CI 0.9–2.1) per log unit increase in total serum IgE in Lima and in Tumbes, respectively. In pooled analysis, the odds of asthma increased by a factor of 1.6 (95% CI 1.3–1.9) per log unit increase in total serum IgE. Further stratified analyses revealed that atopy was an effect modifier of the relationship of total IgE to asthma (table 2). More specifically, total serum IgE was associated with asthma only among those who were atopic.
FIGURE 2.
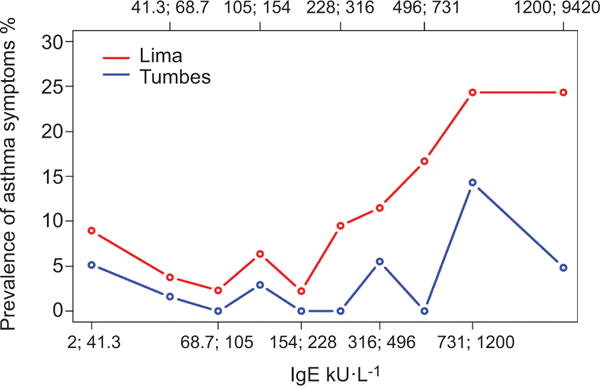
Prevalence of asthma by deciles of total serum IgE stratified by study site; Peru, 2009–2010. Decile interval values are indicated as x;y corresponding to values greater than x and including y.
TABLE 2.
Multiple variable regression of predictors of asthma stratified by atopic status; Peru, 2009–2010
| Variable | OR (95% CI)
|
|||
|---|---|---|---|---|
| Atopic asthma# | p-value | Non-atopic asthma¶ | p-value | |
| log total serum IgE kU·L−1 | 1.9 (1.4–2.6) | <0.001 | 1.0 (0.7–1.4) | 0.91 |
| Body mass index >25 kg·m−2 | 3.5 (1.7–7.1) | 0.001 | 2.4 (0.8–7.3) | 0.11 |
| Maternal education ⩾6 years | 1.4 (0.6–2.9) | 0.43 | 0.9 (0.3–2.3) | 0.79 |
| 6+ people per house | 1.8 (1.0–3.4) | 0.07 | 0.9 (0.4–2.2) | 0.83 |
| Income <175 USD per month | 0.6 (0.3–1.3) | 0.20 | 0.6 (0.2–1.3) | 0.22 |
| Concrete floor | 0.6 (0.3–1.2) | 0.13 | 0.8 (0.3–1.8) | 0.59 |
| Female sex | 0.7 (0.4–1.2) | 0.24 | 1.6 (0.6–3.6) | 0.32 |
| Age | 1.0 (0.7–1.4) | 0.99 | 0.9 (0.5–1.4) | 0.59 |
| Personal history of tobacco smoke | 2.1 (0.7–6.4) | 0.20 | 1.6 (0.2–13.9) | 0.67 |
| Second-hand tobacco smoke | 0.5 (0.2–1.3) | 0.18 | 0.5 (0.1–2.1) | 0.32 |
n=461;
n= 541.
Total serum IgE levels were also higher in atopics than in non-atopics, and in participants with exhaled nitric oxide > 40 ppm versus those with lower values (table 1). The prevalence of atopy across our sample increased with total serum IgE, and this increase was consistent across sites (fig. 3). In multivariable analyses, the odds of atopy increased by factors of 1.5 (95% CI 1.3–1.7) and 1.6 (95% CI 1.4–1.9) per log unit increase in total serum IgE in Lima and in Tumbes, respectively. Mean exhaled nitric oxide increased with total serum IgE, and this increase was consistent across sites (fig. 4). The odds of exhaled nitric oxide >40 ppm increased by factors of 4.0 (95% CI 2.7–5.8) and 2.5 (95% CI 1.9–3.4) per log unit increase in total serum IgE in Lima and in Tumbes, respectively. In pooled analysis, the odds of having exhaled nitric oxide >40 ppm increased by a factor of 3.0 (95% CI 2.4–3.8) per log unit increase in total serum IgE. When stratified by atopic status, the odds of having exhaled nitric oxide >40 ppm increased by a factor of 2.3 (95% CI 1.6–3.3) in non-atopics and 3.7 (95% CI 2.6–5.1) in atopics. This relationship remained significant even among those without asthma. Among non-asthmatics, the odds of having exhaled nitric oxide >40 ppm increased by factors of 3.6 (95% CI 2.3–5.5) and 2.5 (95% CI 1.8– 3.3) per log unit increase in total serum IgE in Lima and in Tumbes, respectively.
FIGURE 3.
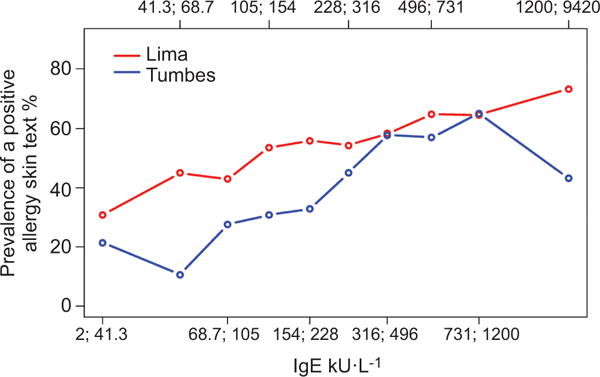
Prevalence of atopy by deciles of total serum IgE stratified by study site; Peru, 2009–2010. Decile interval values are indicated as x;y corresponding to values greater than x and including y.
FIGURE 4.
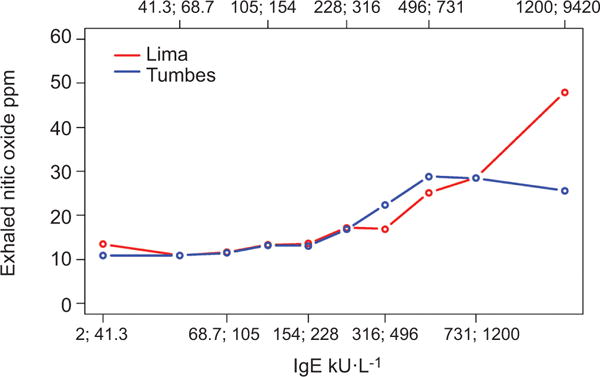
Mean levels of exhaled nitric oxide ppm by deciles of total serum IgE stratified by study site; Peru, 2009–2010. Decile interval values are indicated as x;y corresponding to values greater than x and including y.
Relationship of total serum IgE to airway obstruction and airway reversibility
Pre- and post-FEV1/FVC were inversely related to total serum IgE levels in Lima but not in Tumbes (fig. 5). In Lima, mean pre- and post-FEV1/FVC decreased by 0.5% (95% CI −0.9%– −0.1%) and 0.3% (95% CI −0.7%–0.0%) per log unit increase in total serum IgE, respectively. In a similar analysis in the subset of children without asthma, higher total serum IgE levels were also associated with lower pre- and post-FEV1/FVC. Specifically, among non-asthmatics, mean pre- and post-FEV1/FVC decreased by 0.5% (95% CI −0.9%– −0.1%) and 0.4% (95% CI −0.8%–0.0%) per log unit increase in total serum IgE, respectively. There were too few children with asthma and a total serum IgE (n=66) to adequately examine dose response relationships between total serum IgE measurements and FEVl/FVC. In Tumbes, the mean pre-FEVl/FVC (p=0.65) and post-FEVl/FVC (p=0.75) was not associated with total serum IgE levels.
FIGURE 5.
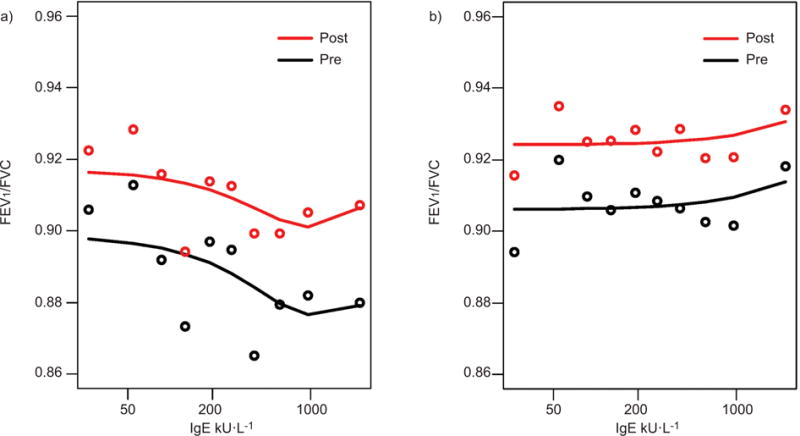
Mean pre- and post-forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) by deciles of total serum IgE stratified by study site; a) Lima and b) Tumbes, Peru, 2009–2010.
Higher levels of total serum IgE were also associated with more bronchodilator reversibility. More specifically, in multivariable analysis, the odds of airway reversibility increased by factors of 1.4 (95% CI 1.0–2.1) and 1.4 (95% CI 0.9–2.1) per log unit increase in total IgE in Lima and in Tumbes, respectively. In pooled analysis, the odds of airway reversibility increased by a factor of 1.4 (9% CI 1.1–1.9) per log unit increase in total IgE.
DISCUSSION
Among our sample of Peruvian children aged 13 to 15 years, total serum IgE levels were higher compared to children in the USA or Europe and directly related to the prevalence of atopic asthma but not to that of non-atopic asthma. The relationship of total serum IgE to atopic asthma was consistent across two regions with disparate degrees of urbanisation. Airway inflammation, as measured by exhaled nitric oxide, and bronchodilator reversibility was also greater with higher levels of total serum IgE. The association between total serum IgE and exhaled nitric oxide remained significant in the subset of children without asthma. In addition, the degree of airways obstruction as measured by FEV1/FVC was worse with increasing total serum IgE in Lima but not in Tumbes. This inverse association of total serum IgE and FEV1/FVC remained significant in the subset of children without asthma living in the urban environment of Lima.
Our findings on the relationship of total IgE to asthma by atopic status were consistent with those of a recent survey conducted by the US National Health and Nutrition Examination Study (NHANES) between 2005 and 2006 [12]. Our data provide complementary information to the NHANES analysis in that we further examined the relationship of total IgE to quantitative measures of airways obstruction and airway inflammation. An important difference between our study and the NHANES study was that the geometric mean of total serum IgE of our study was approximately four times greater than that of children aged 12 to 15 years in the NHANES sample; however, the relationship of total IgE to atopic asthma persisted despite these elevated levels. There are several reasons why total serum IgE may be higher in our Peruvian population than in the general population in the USA. One possibility for this increase is the higher prevalence of enteric infections, including protozoans [21, 22], and soil-based helminths [14] in Peru than in the USA. However, the levels of total serum IgE in our sample of Peruvian children were not as high as in other regions with high levels of parasitism [14]. The geometric means between asthmatics and non-asthmatics in our study were similar in magnitude to those identified in a smaller, cross-sectional study of 198 children aged 10 to 13 years conducted in Costa Rica [23]. As with the study in Costa Rica, we did not examine stool samples for ova and parasites in our study children. However, the prevalence of soil-based helminthic infections in children of similar age in our study areas is low (online supplementary material) and therefore unlikely to explain the higher levels of total serum IgE when compared with values of similarly aged children in the NHANES sample.
On the other hand, our findings were different from those of a large general population study of 2657 subjects in Tucson, USA [8], 1916 young adults in five areas of Spain [10] and 1219 consecutive pulmonary patients to a pulmonary practice in Frankfurt, Germany [11], in that we did not find a direct relationship between total serum IgE and non-atopic asthma. The Tucson study tested 18 allergens by prick test; however, this study did not include allergens for common household pets such as cats and dogs, and household pests such as cockroaches and mice. In contrast, our study and the NHANES study [12] tested for these common household allergens. It is possible that several asthmatics in the Tucson study were misclassified as non-atopic and this could explain why there was a linear relationship of total IgE to non-atopic asthma. In our study, geometric means for total IgE for atopic asthmatics were 754 kU·L−1 in Lima and 472 kU·L−1 in Tumbes, higher than those in the Tucson study. The geometric means for total IgE for non-atopic asthmatics were 137 kU·L−1 in Lima and 251 kU·L−1 in Tumbes, also higher than those in the Tucson study. The Spanish study also tested fewer (i.e. five) allergen specificities than ours (i.e. 10) and the NHANES study, and as a result may have had a similar degree of misclassification. The Frankfurt study, unlike our study and others [8, 10, 12], was not a population study but included a large range of 14 common allergens, that did not include cockroach or mouse, and eight food allergens. In this study, the odds of asthma increased by a factor of 5.1 (95% CI 2.6–10.1) when total serum IgE was greater than 150 kU·L−1. In contrast, in our study, there was no single cut-off for total serum IgE between 100 kU·L−1 and 800 kU·L−1 that was associated with non-atopic asthma. Possible explanations for an effect are that the Frankfurt study included a more select group of asthmatics that sought attention for respiratory complaints or follow-up, and their sample of non-atopic asthmatics was larger than ours. However, the Frankfurt study did not include normal healthy controls from the general population.
Our study also identified a greater chance of airway reversibility and greater levels of airways inflammation at higher levels of total serum IgE. This finding is consistent with previous studies that found a relationship between IgE and more labile airways [24, 25]. These studies, however, used methacholine for bronchoprovocation. Hence, our study provides complementary information on quantitative measures of airway effects in the setting of higher levels of total serum IgE. We also found a greater degree of airway obstruction as measured by FEV1/FVC with higher levels of total serum IgE in Lima and not in Tumbes. This could be explained in part by the higher prevalence of asthma and atopy in Lima; however, in subset analyses, we observed that this relationship persisted among non-asthmatics in Lima. Our findings of an inverse relationship of total serum IgE to FEV1/FVC are consistent with those of previous studies of asthmatics [26, 27].
Our study has some potential shortcomings. First, our study design was cross-sectional. Therefore, we were not able to characterise the effects of early life exposures (including respiratory infections or environmental exposures in early childhood) on our observed relationships. Secondly, we did not conduct an evaluation of parasitic infections in our study children; however, previous population-based evaluations by our team on the burden of soil-based helminths in our study areas were found to be low (online supplementary material). Nonetheless, this low burden of parasitic infections, in light of elevated total serum IgE levels, needs to be further confirmed in future studies. Thirdly, our study did not include an evaluation of dietary habits or micronutrients, which may also have an effect on our observed relationships. However, our findings are largely consistent with a large, population-based study in the USA [12].
The relationship of total serum IgE to severity of airway obstruction is poorly understood. One possible explanation is that higher total serum IgE may reflect more severe asthma due to elevations in allergen-specific IgE levels. Another possibility is that elevated total serum IgE may also contribute indirectly to airway inflammation, which may help explain why we observed an inverse relationship of total serum IgE to FEV1/FVC among non-asthmatics living in Lima. In the Normative Aging Study, a longitudinal study of 2280 healthy adult volunteers in Boston without a history of asthma, skin test reactivity to four common aeroallergens was a significant predictor of decline in both FEV1 and FEV1/FVC [28]. Persistent exposure in sensitised individuals may be associated with chronic bronchial inflammation, which may help explain the findings of a reduced lung function over time in the Normative Aging Study. In the longitudinal Tucson Epidemiological Study of Airways Obstructive Disease, total serum IgE was not associated with a FEV1 decline in ever-smokers without asthma [29]. Studies have shown that allergen exposure affects cytokine and anti-inflammatory production. Tumour necrosis factor-alpha, interleukin (IL)-6 and IL-8 are upregulated in airway epithelium after exposure to allergens [30]. A recent study comparing inflammatory markers in atopic versus non-atopic patients with chronic obstructive pulmonary disease found that IL-8 was upregulated in patients with mite allergies [31].
In summary, total serum IgE was associated with atopic asthma in a developing country setting despite high background levels of total serum IgE. Urbanisation did not appear to be an effect modifier of this relationship. Our study also confirms the lack of association between total serum IgE and non-atopic asthma. Despite the lack of association with non-atopic asthma, we found that total serum IgE was associated with markers of airway inflammation and lung function even among non-asthmatics living in an urban environment. Markers of airway obstruction such as response to bronchodilators, exhaled nitric oxide and FEV1/FVC were also associated with total serum IgE.
Supplementary Material
Acknowledgments
SUPPORT STATEMENT
This study was supported by a Johns Hopkins Center for Global Health Award and the Fogarty International Center Training Grant (Grant R24 TW007988). W. Checkley was supported by a Clinician Scientist Award from the Johns Hopkins University, a K99/R00 Pathway to Independence Award (R00HL096955) from the National Heart, Lung and Blood Institute, National Institutes of Health and by a contract (HHSN268200900033C) with the National Heart, Lung and Blood Institute, National Institutes of Health. N. Hansel and W. Checkley were further supported by a R01 grant from the National Institutes of Environmental Health Sciences (R01ES018845). C.L. Robinson was a Fogarty International Clinical Research Scholar during the time of this work and was further supported by Tufts University School of Medicine. L.M. Baumann was supported by a pre-doctoral NIH T35 Training Grant (T35AI065385).
Footnotes
STATEMENT OF INTEREST
Conflict of interest information can be found alongside the online version of this article at www.erj.ersjournals.com
References
- 1.Braman SS. The global burden of asthma. Chest. 2006;130:4–12. doi: 10.1378/chest.130.1_suppl.4S. [DOI] [PubMed] [Google Scholar]
- 2.Masoli M, Fabian D, Holt S, et al. The global burden of asthma: executive summary of the GINA Dissemination Committee Report. Allergy. 2004;59:469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- 3.Bousquet J, Bousquet PJ, Godard P, et al. The public health implications of asthma. Bull World Health Organ. 2005;83:548–554. [PMC free article] [PubMed] [Google Scholar]
- 4.Pearce N, Pekkanen K, Beasley R. How much asthma is really attributable to atopy? Thorax. 1999;54:268–272. doi: 10.1136/thx.54.3.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gruchalla RS, Pongracic J, Plaut M, et al. Inner City Asthma Study: relationships among sensitivity, allergen exposure, and asthma morbidity. J Allergy Clin Immunol. 2005;115:478–485. doi: 10.1016/j.jaci.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 6.Rosenstreich DL, Eggleston P, Kattan M, et al. The role of cockroach allergy and exposure to cockroach allergen in causing morbidity among inner-city children with asthma. N Engl J Med. 1997;336:1356–1363. doi: 10.1056/NEJM199705083361904. [DOI] [PubMed] [Google Scholar]
- 7.Bennich HH, Ishizaka HK, Johansson SG, et al. Immunoglobulin E: a new class of human immunoglobulin. Immunology. 1968;3:323–324. [PMC free article] [PubMed] [Google Scholar]
- 8.Burrows B, Martinez FD, Halonen M, et al. Association of asthma with serum IgE levels and skin-test reactivity to allergens. N Engl J Med. 1989;320:271–277. doi: 10.1056/NEJM198902023200502. [DOI] [PubMed] [Google Scholar]
- 9.Burrows B, Halonen M, Lebowitz MD, et al. The relationship of serum immunoglobulin E, allergy skin tests, and smoking to respiratory disorders. J Allergy Clin Immunol. 1982;70:199–204. doi: 10.1016/0091-6749(82)90042-2. [DOI] [PubMed] [Google Scholar]
- 10.Sunyer J, Anto JM, Soriano JB, et al. Total serum IgE is associated with asthma independently of specific IgE levels. Eur Respir J. 1996;9:1880–1884. doi: 10.1183/09031936.96.09091880. [DOI] [PubMed] [Google Scholar]
- 11.Beeh KM, Ksoll M, Buhl R. Elevation of total serum immunoglobulin E is associated with asthma in nonallergic individuals. Eur Respir J. 2000;16:609–614. doi: 10.1034/j.1399-3003.2000.16d07.x. [DOI] [PubMed] [Google Scholar]
- 12.Gergen PJ, Arbes SJ, Jr, Calatroni A, et al. Total IgE levels and asthma prevalence in the US population: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2009;124:447–453. doi: 10.1016/j.jaci.2009.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Barron-Casella EA, Strunk RC, Hamilton RG, et al. Elevation of IgE in children with sickle cell disease is associated with doctor diagnosis of asthma and increased morbidity. J Allergy Clin Immunol. 2011;127:1440–1446. doi: 10.1016/j.jaci.2010.12.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Houba V, Rowe DS. A comparison of African and European serum levels of immunoglobulin E. Bull World Health Organ. 1973;49:539–545. [PMC free article] [PubMed] [Google Scholar]
- 15.Visness CM, London SJ, Daniels JL, et al. Association of obesity with IgE levels and allergy symptoms in children and adolescents: results from the National Health and Nutrition Examination Survey 2005–2006. J Allergy Clin Immunol. 2009;123:1163–1169. doi: 10.1016/j.jaci.2008.12.1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Robinson CL, Baumann LM, Gilman RH, et al. Effects of varying degrees of urbanization on asthma, allergy and airways inflammation in a developing country setting. Thorax. 2011;66:1051–1057. doi: 10.1136/thx.2011.158956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baumann LM, Robinson CL, Combe JM, et al. Effects of distance from a heavily transited avenue on asthma and atopy in a peri-urban shanty-town in Lima, Peru. J Allergy Clin Immunol. 2011;127:875–882. doi: 10.1016/j.jaci.2010.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Robinson CL, Baumann LM, Gilman RH, et al. The Peru Urban versus Rural Asthma (PURA) Study: methods and baseline quality control data for a cross-sectional investigation into the prevalence, severity, genetics, immunology, and environmental factors affecting adolescent asthma in Peru. BMJ Open. 2012;2:e000421. doi: 10.1136/bmjopen-2011-000421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mata Fernández C, Fernández-Benítez M, Pérez Miranda M, et al. Validation of the Spanish version of the Phase III ISAAC questionnaire on asthma. J Investig Allergol Clin Immunol. 2005;15:201–210. [PubMed] [Google Scholar]
- 20.Miller MR, Hankinson JL, Brusasco V, et al. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 21.Jiménez JC, Fontaine J, Grzych JM, et al. Systemic and mucosal responses to oral administration of excretory and secretory antigens from Giardia intestinalis. Clin Diagn Lab Immunol. 2004;11:152–160. doi: 10.1128/CDLI.11.1.152-160.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pérez O, Lastre M, Bandera F, et al. Evaluation of the immune response in symptomatic and asymptomatic human giardiasis. Arch Med Res. 1994;25:171–177. [PubMed] [Google Scholar]
- 23.Celedon JC, Soto-Quiros ME, Hanson LA, et al. The relationship among markers of allergy, asthma, allergic rhinitis, and eczema in Costa Rica. Pediatr Allergy Immunol. 2002;13:91–97. doi: 10.1034/j.1399-3038.2002.00083.x. [DOI] [PubMed] [Google Scholar]
- 24.Sunyer J, Antó JM, Sabrià J, et al. Relationship between serum IgE and airway responsiveness in adults with asthma. J Allergy Clin Immunol. 1995;95:699–706. doi: 10.1016/s0091-6749(95)70175-3. [DOI] [PubMed] [Google Scholar]
- 25.Sears MR, Burrows B, Flannery EM, et al. Relation between airway responsiveness and serum IgE in children with asthma and in apparently normal children. N Engl J Med. 1991;325:1067–1071. doi: 10.1056/NEJM199110103251504. [DOI] [PubMed] [Google Scholar]
- 26.Carroll WD, Lenney W, Child F, et al. Asthma severity and atopy: how clear is the relationship? Arch Dis Child. 2006;91:405–409. doi: 10.1136/adc.2005.088278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haselkorn T, Szefler SJ, Simons FER, et al. Allergy, total serum immunoglobulin E, and airflow in children and adolescents in TENOR. Pediatr Allergy Immunol. 2010;21:1157–1165. doi: 10.1111/j.1399-3038.2010.01065.x. [DOI] [PubMed] [Google Scholar]
- 28.Gottlieb DJ, Sparrow D, O’Connor GT, et al. Skin test reactivity to common aeroallergens and decline of lung function: The Normative Aging Study. Am J Respir Crit Care Med. 1996;153:561–566. doi: 10.1164/ajrccm.153.2.8564098. [DOI] [PubMed] [Google Scholar]
- 29.Sherrill DL, Lebowitz MD, Halonen M, et al. Longitudinal evaluation of the association between pulmonary function and total serum IgE. Am J Respir Crit Care Med. 1995;152:98–102. doi: 10.1164/ajrccm.152.1.7599870. [DOI] [PubMed] [Google Scholar]
- 30.Vroling AB, Duinsbergen D, Fokkens WF, et al. Allergen induced gene expression of airway epithelial cells shows a possible role for TNF-α. Allergy. 2007;62:1310–1319. doi: 10.1111/j.1398-9995.2007.01495.x. [DOI] [PubMed] [Google Scholar]
- 31.Tsai JJ, Liao EC, Hsu JY, et al. The differences of eosinophil- and neutrophil-related inflammation in elderly allergic and nonallergic chronic obstructive pulmonary disease. J Asthma. 2010;47:1040–1044. doi: 10.1080/02770903.2010.491145. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


