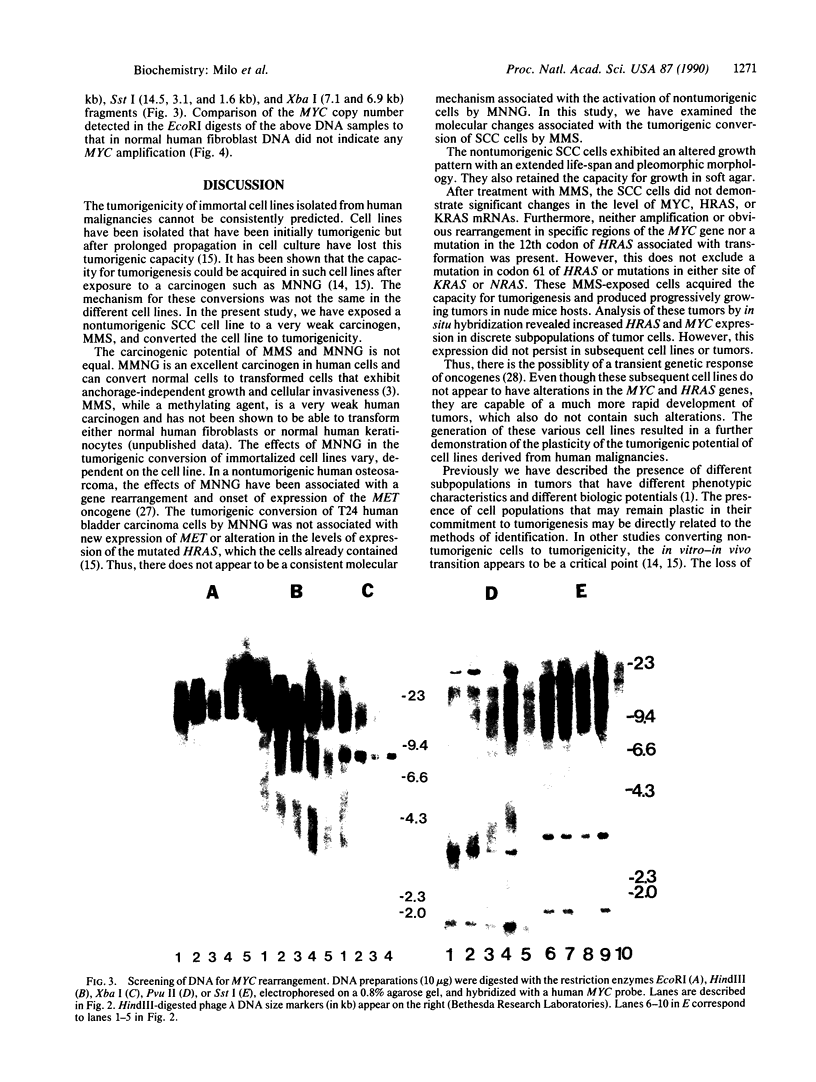
Abstract
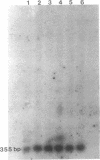
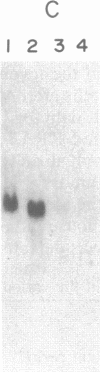
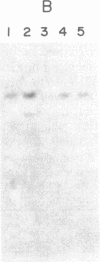
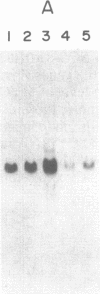
Plasticity of human tumor populations could account for the reason why many tumorigenic human cell lines lose this feature when grown in culture. Methyl methanesulfonate (MMS) was used to convert premalignant squamous cell carcinoma (SCC) cell line SCC-83-01-82 to a malignant phenotype. The MMS-treated SCC-83-01-82 cells (MMS-SCC-83-01-82) produced progressively growing tumors in 5 of 11 splenectomized BALB/c nude mice within 3-5 months. A cell line, designated SCC-83-01-82 CA, was established in vitro from one of the mouse tumors and was repassaged successively. This SCC-83-01-82 CA cell line was aggressively tumorigenic. A tumor greater than or equal to 2.0 cm in size was present within a month, as opposed to the 3-5 months required for the tumors produced by the MMS-SCC-83-01-82 cells. Examination of frozen cross sections by in situ hybridization revealed that focal areas of the tumor produced by the MMS-SCC-83-01-82 cells expressed MYC and HRAS mRNA. However, by the third passage in vivo, the levels of expression of the corresponding genes in the mouse tumors were undetectable. Blot-hybridization analysis of the RNA from the MMS-SCC-83-01-82 cells and the subsequently derived tumors and cells did not indicate any consistent overexpression of MYC, HRAS, or KRAS. Restriction fragment length polymorphism analysis of both MYC and HRAS genes revealed neither rearrangement nor amplification of MYC nor point mutation in the 11th or 12th codon of HRAS. The data suggest that alterations in MYC and HRAS were not directly involved in either the initial transformation or MMS-induced tumorigenic conversion of the SCC-83-01-82 cell line. Persistence of tumorigenicity after reisolation of the MMS-converted premalignant SCC-83-01-82 cells did not disappear immediately following the treatment with MMS.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amstad P., Reddel R. R., Pfeifer A., Malan-Shibley L., Mark G. E., 3rd, Harris C. C. Neoplastic transformation of a human bronchial epithelial cell line by a recombinant retrovirus encoding viral Harvey ras. Mol Carcinog. 1988;1(3):151–160. doi: 10.1002/mc.2940010303. [DOI] [PubMed] [Google Scholar]
- Barbacid M. ras genes. Annu Rev Biochem. 1987;56:779–827. doi: 10.1146/annurev.bi.56.070187.004023. [DOI] [PubMed] [Google Scholar]
- Campo M. S., Moar M. H., Sartirana M. L., Kennedy I. M., Jarrett W. F. The presence of bovine papillomavirus type 4 DNA is not required for the progression to, or the maintenance of, the malignant state in cancers of the alimentary canal in cattle. EMBO J. 1985 Jul;4(7):1819–1825. doi: 10.1002/j.1460-2075.1985.tb03856.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y. H., Marzluf G. A. Characterization of nit-2, the major nitrogen regulatory gene of Neurospora crassa. Mol Cell Biol. 1987 May;7(5):1691–1696. doi: 10.1128/mcb.7.5.1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horowitz J. M., Yandell D. W., Park S. H., Canning S., Whyte P., Buchkovich K., Harlow E., Weinberg R. A., Dryja T. P. Point mutational inactivation of the retinoblastoma antioncogene. Science. 1989 Feb 17;243(4893):937–940. doi: 10.1126/science.2521957. [DOI] [PubMed] [Google Scholar]
- Hurlin P. J., Fry D. G., Maher V. M., McCormick J. J. Morphological transformation, focus formation, and anchorage independence induced in diploid human fibroblasts by expression of a transfected H-ras oncogene. Cancer Res. 1987 Nov 1;47(21):5752–5757. [PubMed] [Google Scholar]
- Hurlin P. J., Maher V. M., McCormick J. J. Malignant transformation of human fibroblasts caused by expression of a transfected T24 HRAS oncogene. Proc Natl Acad Sci U S A. 1989 Jan;86(1):187–191. doi: 10.1073/pnas.86.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl T., Sedgwick B., Sekiguchi M., Nakabeppu Y. Regulation and expression of the adaptive response to alkylating agents. Annu Rev Biochem. 1988;57:133–157. doi: 10.1146/annurev.bi.57.070188.001025. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Casto B., Ferrone S. Comparison of features of carcinogen-transformed human cells in vitro with sarcoma-derived cells. Mutat Res. 1988 Jun;199(2):387–398. doi: 10.1016/0027-5107(88)90216-3. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Jr, DiPaolo J. A. Neoplastic transformation of human diploid cells in vitro after chemical carcinogen treatment. Nature. 1978 Sep 14;275(5676):130–132. doi: 10.1038/275130a0. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Oldham J. W., Zimmerman R., Hatch G. G., Weisbrode S. A. Characterization of human cells transformed by chemical and physical carcinogens in vitro. In Vitro. 1981 Aug;17(8):719–729. doi: 10.1007/BF02628409. [DOI] [PubMed] [Google Scholar]
- Milo G. E., Yohn J., Schuller D., Noyes I., Lehman T. Comparative stages of expression of human squamous carcinoma cells and carcinogen transformed keratinocytes. J Invest Dermatol. 1989 Jun;92(6):848–853. doi: 10.1111/1523-1747.ep12696872. [DOI] [PubMed] [Google Scholar]
- Nishimura S., Sekiya T. Human cancer and cellular oncogenes. Biochem J. 1987 Apr 15;243(2):313–327. doi: 10.1042/bj2430313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M., Dean M., Cooper C. S., Schmidt M., O'Brien S. J., Blair D. G., Vande Woude G. F. Mechanism of met oncogene activation. Cell. 1986 Jun 20;45(6):895–904. doi: 10.1016/0092-8674(86)90564-7. [DOI] [PubMed] [Google Scholar]
- Reddel R. R., Ke Y., Kaighn M. E., Malan-Shibley L., Lechner J. F., Rhim J. S., Harris C. C. Human bronchial epithelial cells neoplastically transformed by v-Ki-ras: altered response to inducers of terminal squamous differentiation. Oncogene Res. 1988;3(4):401–408. [PubMed] [Google Scholar]
- Rhim J. S., Park D. K., Arnstein P., Huebner R. J., Weisburger E. K., Nelson-Rees W. A. Transformation of human cells in culture by N-methyl-N'-nitro-N-nitrosoguanidine. Nature. 1975 Aug 28;256(5520):751–753. doi: 10.1038/256751a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rose P. G., Koolemans-Beynen A., Boutselis J. G., Minton J. P., Milo G. E. An improved human tumor stem cell assay in ovarian cancer. Am J Obstet Gynecol. 1987 Mar;156(3):730–734. doi: 10.1016/0002-9378(87)90088-3. [DOI] [PubMed] [Google Scholar]
- Senger D. R., Perruzzi C. A., Ali I. U. T24 human bladder carcinoma cells with activated Ha-ras protooncogene: nontumorigenic cells susceptible to malignant transformation with carcinogen. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5107–5111. doi: 10.1073/pnas.85.14.5107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silinskas K. C., Kateley S. A., Tower J. E., Maher V. M., McCormick J. J. Induction of anchorage-independent growth in human fibroblasts by propane sultone. Cancer Res. 1981 May;41(5):1620–1627. [PubMed] [Google Scholar]
- Sutherland B. M., Bennett P. V., Freeman A. G., Moore S. P., Strickland P. T. Transformation of human cells by DNAs ineffective in transformation of NIH 3T3 cells. Proc Natl Acad Sci U S A. 1985 Apr;82(8):2399–2403. doi: 10.1073/pnas.82.8.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker C., Nettesheim P., Barrett J. C., Jirik F. R., Sorge J., Joyce M., Gilmer T. Mouse retroviral sequences acquired by cell lines after passaging through nude mice detected by hybridization of the fms probe pSM3. Cancer Res. 1989 Feb 1;49(3):625–628. [PubMed] [Google Scholar]
- Walker C., Nettesheim P., Barrett J. C., Jirik F. R., Sorge J., Joyce M., Gilmer T. Mouse retroviral sequences acquired by cell lines after passaging through nude mice detected by hybridization of the fms probe pSM3. Cancer Res. 1989 Feb 1;49(3):625–628. [PubMed] [Google Scholar]
- Wilson D. M., Fry D. G., Maher V. M., McCormick J. J. Transformation of diploid human fibroblasts by transfection of N-ras-oncogenes. Carcinogenesis. 1989 Apr;10(4):635–640. doi: 10.1093/carcin/10.4.635. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H., Lechner J. F., Gabrielson E. W., Korba B. E., Malan-Shibley L., Willey J. C., Valerio M. G., Shamsuddin A. M., Trump B. F., Harris C. C. Transformation of human bronchial epithelial cells transfected by Harvey ras oncogene. Science. 1985 Mar 8;227(4691):1174–1179. doi: 10.1126/science.3975607. [DOI] [PubMed] [Google Scholar]
- Zimmerman R. J., Little J. B. Characteristics of human diploid fibroblasts transformed in vitro by chemical carcinogens. Cancer Res. 1983 May;43(5):2183–2189. [PubMed] [Google Scholar]