Abstract
We propose a conceptual model for the cytoskeletal organization of endothelial cells (ECs) based on a major dichotomy in structure and function at basal and apical aspects of the cells. Intracellular distributions of filamentous actin (F-actin), vinculin, paxillin, ZO-1, and Cx43 were analyzed from confocal micrographs of rat fat-pad ECs after 5 h of shear stress. With intact glycocalyx, there was severe disruption of the dense peripheral actin bands (DPABs) and migration of vinculin to cell borders under a uniform shear stress (10.5 dyne/cm2; 1 dyne = 10 μN). This behavior was augmented in corner flow regions of the flow chamber where high shear stress gradients were present. In striking contrast, no such reorganization was observed if the glycocalyx was compromised. These results are explained in terms of a “bumper-car” model, in which the actin cortical web and DPAB are only loosely connected to basal attachment sites, allowing for two distinct cellular signaling pathways in response to fluid shear stress, one transmitted by glycocalyx core proteins as a torque that acts on the actin cortical web (ACW) and DPAB, and the other emanating from focal adhesions and stress fibers at the basal and apical membranes of the cell.
Keywords: mechanotransduction, actin cortical web, dense peripheral actin band
Hemodynamic shearing stresses on endothelial cells (ECs) are widely recognized as playing a vital role in the regulation of vessel wall remodeling, cellular signaling, mass transport, red and white cell interaction, and atherogenesis (1–3). The possible roles of the endothelial glycocalyx (EG) in this regulation as a molecular sieve, as a barrier and modulator of interactions between blood cells and ECs, and as a mechanotransducer of fluid shear stress have been studied more recently (4–7). Relatively little is known about the specific proteins in the EG, although hyaluronan, chondroitin, and heparan sulfate play a significant role in its assembly (8, 9). In the early 1990s, investigators first observed that the shear-induced dilation of small arteries was abolished when sialic acids were removed from the EG by neuraminidase (10). Florian et al. (11) recently verified the presence of heparan sulfate proteoglycan (HSPG) in the glycocalyx of cultured bovine aortic ECs and demonstrated that partial removal of HSPG with heparinase completely blocked shear-induced NO release. A puzzling and still not understood consequence of EG degradation was the observation that shear-induced NO production was greatly inhibited without apparent effect on shear-dependent vasodilation due to prostaglandin I2 release (12).
Squire et al. (13) showed that the ultrastructural organization of the EG was quasiperiodic, anchored to a geodesic-like scaffold of hexagonally arranged filamentous actin (F-actin) filaments forming an actin cortical web (ACW) (14) just beneath the plasmalemma. A fundamental question addressed in ref. 7 is how fluid shear stresses acting at the surface of the EG are transmitted to this ACW if there is essentially no flow in the EG and hence nearly zero fluid shear stress acting at the level of the cell membrane. This model predicted that the core proteins in the EG serve as stiff bristles (flexural rigidity EI, where E is Young's modulus and I is the moment of inertia of the fiber cross section, is 700 pN·nm2) that transmit the fluid drag on their tips as a bending moment that acts on the ACW.
Although numerous studies have been performed on the intracellular rearrangement of cytoskeletal structural components (microfilaments, intermediate filaments, and microtubules) in EC cultures subject to fluid shear (15, 16), none have compared cytoskeletal reorganization in the absence or presence of the EG. Moreover, all previous parallel plate flow chamber studies have been conducted in the uniform shear stress (USS) region in the central portion of the chamber and have assiduously avoided the disturbed corner flow region associated with high shear stress gradients (HSSGs) because of its complexity. The HSSGs examined in this study vary from 0 to ≈2,500 dyne/cm2 per cm (1 dyne = 10 μN) and are >1 order of magnitude greater than other flow chamber designs (17) and closer to those observed near arterial bifurcations and branch sites (1, 18). These high shear gradients are of special interest in atherogenesis, because it has been proposed that these regions are prone to lesion development (3, 19, 20). The present study attempts to explain these various observations within the context of a new mechanical model for the actin cytoskeleton.
Materials and Methods
Flow Chamber Design and Flow Characteristics. We have modified the gasket of the conventional parallel plate flow chamber (Cytodyne, La Jolla, CA) to obtain a very narrow flow space (4 × 60 mm × 220-μm gap height), which has enabled us to analyze the effects of USS as well as spatial HSSGs in the corner flow region. The flow loop consisted of a parallel plate flow chamber and a recirculating flow circuit, as described in ref. 21. Spatial variations of shear stress gradients in the entire chamber cross section were calculated by using an infinite series solution of the 2D steady Navier–Stokes equation. (see Appendix A, which is published as supporting information on the PNAS web site).
Cell Culture and Flow Experiments. Rat fat-pad ECs (passage 14–20, obtained from Anthony Ashton, Albert Einstein College of Medicine) were cultured in DMEM containing 1% penicillin–streptomycin and 10% FBS. Cells were grown on glass slides until confluency and transferred to the flow chamber for experiments. Cells were exposed to USS of 10.5 dyne/cm2 for 5 h with different perfusion media (DMEM, DMEM + 10%FBS, and DMEM + 1% BSA). Sham controls were the no-flow condition for each perfusion medium. Each set of experiments was repeated four times.
Immunofluorescence and Confocal Microscopy. To detect the changes in stress fibers (SFs) and dense peripheral actin bands (DPABs) [F-actin rhodamine-labeled phalloidin (Sigma)], focal adhesions (FAs) [Vinculin and Paxillin (Chemicon)], and tight junction [TJ; ZO-1 (Zymed)] and gap junction complexes (Cx43, courtesy of E. Hertzberg, Albert Einstein College of Medicine), immunofluorescence analyses were performed on both controls and shear stress-exposed cells (21). Fifteen milliunits per milliliter of heparinase III (Sigma) was used to digest the glycocalyx (11), and HSPG antibody (US Biological, Swampscott, MA) and CellTracker orange (Molecular Probes) were used to assess heparan sulfate removal. Image stacks were collected by using an Olympus FluoView FV500 Laser Scanning Confocal Microscope (Olympus, Melville, NY). Four images from each flow treatment and eight images from HSPG control treatments were taken for each set of experiments. For detailed methods, see Appendix B, which is published as supporting information on the PNAS web site.
Protein Distribution and Statistical Analysis. Overall average density profiles of 10 cell pairs from each stacked image were plotted by using scionimage (Scion, Frederick, MD), and changes in protein distribution were detected by using kurtosis analysis. HSPG reduction was measured and analyzed by using scionimage and imaris (Bitplane, St. Paul). For detailed analysis, see Appendix B. Statistical comparisons were performed by using one-way ANOVA (sigmastat). Asterisks in figures indicate significant difference compared with controls (*, P < 0.05).
Results
Presence and Absence of Glycocalyx. Previous studies have suggested the existence of a structured EG in the presence of BSA or plasma in the lumen and its absence or collapse in simple Ringer's solution (11, 22). Thus, our in vitro flow experiments compared different perfusion media: DMEM, DMEM + 10% FBS, and DMEM + 1% BSA. Fig. 1 shows the expression of HSPG under various control conditions. The surface of ECs in the presence of FBS was abundantly decorated with HSPG (Fig. 1 A, XZ view). When serum proteins were removed, 27.4 ± 1.1% reduction of cell surface heparan sulfate was observed (Fig. 1B, XZ view and graph). This finding, together with previous observations of the EG closely adherent to ECs in protein-free Ringer's solution (22), suggested that the EG collapsed and the core proteins of HSPGs clumped in the absence of serum proteins. In addition, enzymatic removal of HSPG by 2 h of heparinase III showed 53.2 ± 2.4% reduction, and this removal was not greatly changed during 5 h of incubation with 1% BSA + DMEM (Fig. 1C, XZ view and graph).
Fig. 1.
Confocal analysis of cell surface HSPG in the EG layer. Cells were cultured in DMEM with (A) and without (B) 10% FBS for 5 h, with 15 milliunits/ml heparinase III (Hep III) (C) for 2 h, and postcultured in DMEM + 1% BSA for 5 h (graph). To visualize cell-surface HSPG, cells were stained with primary antibody for HSPG (green) and with CellTracker orange dye. XZ views from the highlighted boxes of various control conditions show different degrees of cell surface HSPG distributions. Expression of HSPG was quantified and plotted by using scion image (Scion, Frederick, MD). Eight images per experiment, for a total of four experiments, were taken for each treatment condition. All data are presented as mean ± SEM, n = 32 (*, P < 0.05). (Bar, 20 μm.)
Cytoskeleton Organization. To study the participation of the EG in the process of transmitting fluid shear stress to the ACW, distribution of F-actin fibers was analyzed among samples that were exposed to various perfusion media and no-flow controls. In controls, F-actin fibers were distributed mostly at the peripheral cell borders as a DPAB, with some randomly distributed SFs linking basal and apical adhesion plaques (thin arrows, Fig. 2A). Exposure of ECs to both USS and HSSG with DMEM alone caused no noticeable changes in F-actin organization compared with control (Fig. 2 A, graph). Similarly, for cells with digested glycocalyx (heparinase III), there was insignificant F-actin redistribution in both USS and HSSG regions compared with controls. Exposure of the cells to USS with 1% BSA or 10% FBS perfusion media resulted in dramatic redistribution of F-actin (Fig. 2 A, arrowheads), as noted in previous studies (15, 23). As shown in Fig. 2 A [graph (Inset)], this behavior was augmented when ECs were exposed to HSSG. Kurtosis analyses suggested that there was increased SF formation near cell borders and throughout the cell (Fig. 2 A).
Fig. 2.
Reorganization of EC cytoskeleton and FAs in response to fluid shear stress with various flow media. Cells were exposed to USS of 10.5 dyne/cm2 and HSSG of 0 ≈ 2,500 dyne/cm2 per cm for 5 h. Effects of USS and HSSG on distribution of F-actin (A) and vinculin (B) were analyzed by using confocal microscopy. Overall average protein-density profiles from stacked images of different treatments were plotted by using scion image. Changes in the distributions of F-actin and vinculin were detected by using kurtosis analysis. Four images per experiment, for a total of four experiments, were taken from USS and HSSG regions. All data are presented as mean ± SEM, n = 160 (*, P < 0.05). Flow direction, arrows; transverse SFs, thin arrows; redistribution of F-actin (A) and vinculin (B), arrowheads; HepIII, heparinase III. (Bar, 20 μm.)
FAs. To investigate the effects of USS and HSSG in the presence of EG on FA remodeling, we studied changes of vinculin and paxillin localization in ECs. In no-flow controls, vinculin distribution was mostly random, with some localization around cell borders, and mainly situated near the basal membrane (Fig. 2B). Exposure to fluid shear stress with DMEM perfusion medium led to no apparent changes in vinculin localization (Fig. 2B, graph). As for heparinase III-treated samples there was a slight increase in localization of vinculin at cell–cell borders (Fig. 2B, graph). With either 1% BSA or 10% FBS in the perfusion media, vinculin became notably redistributed toward appositional membranes (Fig. 2B, arrowheads). Paxillin was distributed randomly throughout control ECs, with slightly more near appositional membranes (data not shown). Unlike vinculin, the presence of the EG did not affect paxillin distribution.
Junctional Complexes. To examine the role of the EG in tight and gap junction distribution, we analyzed the TJ-associated protein ZO-1 and the gap junction protein Cx43. In controls, ZO-1 expression was mostly continuous and occasionally brush-strokelike at TJ, and Cx43 was abundantly punctate with linear appositional staining at cell borders. Immunoreactivity in perinuclear regions was also observed (Fig. 3B, thin arrows). Greater disruption of ZO-1 and Cx43 at cell borders was observed in the HSSG region when glycocalyx was present (Fig. 3 A and B, arrowheads).
Fig. 3.
Junctional adaptation of EC in response to fluid shear stress with various flow media. Cells were exposed to USS of 10.5 dyne/cm2 and HSSG of 0 ≈ 2,500 dyne/cm2 per cm for 5 h. Effects of USS and HSSG on the distribution of ZO-1 (A) and Cx43 (B) were analyzed by using confocal microscopy. Overall average protein density profiles from stacked images of different treatments were plotted by using scion image. Disruption and distribution of ZO-1 and Cx43 were detected by using kurtosis analysis. Four images per experiment, for a total of four experiments, were taken from USS and HSSG regions. All data are presented as mean ± SEM, n = 160 (*, P < 0.05). Flow direction, arrow; perinuclear Cx43, thin arrows; disruption of ZO-1 (A) and Cx43 (B) at cell–cell borders, arrowheads. HepIII, heparinase III. (Bar, 20 μm.)
Discussion
Previous studies have reported that fluid shear stress disrupts DPABs (15, 23, 24) and leads to the formation of basal SFs and FAs (23, 25), presumably due to the activation of signaling pathways (26) and autacoid release (10, 12). The possibility that the EG might serve as a mechanotransducer for fluid shear stress was raised by the findings that the presence of cell surface proteoglycans is intimately linked to the ability of cultured ECs to release NO in response to shear (11).
In the present study, we have manipulated the EG through the presence or absence of BSA and FBS in perfusion solution and digestion of glycocalyx proteins with heparinase, which was reported to completely abolish NO release (11) and to attenuate agonist-mediated reorganization of actin (27). The efficacy of heparinase is presumably due to the high ratio of heparan to chondroitin sulfate (≈4:1) at the apical EC membrane (28).
Under conditions manipulating the EG, we have determined the relationship of the ACW to the two other actin filament structures in the EC, the DPAB and the α-actinin-bundled SFs, and their associated binding partners, vinculin, paxillin, ZO-1, and Cx43. Our observation that there is virtually no actin cytoskeletal reorganization or vinculin redistribution in DMEM, and that the redistribution in serum or BSA can be largely arrested by heparinase treatment, strikingly demonstrates that the transmission of fluid shear stress to the actin filament system of the EC is very different when the shear stress is applied at the level of the apical plasmalemma, when the EG is collapsed, or at the edge of the EG. A key insight into the mechanism that might allow for such behavior is predicted in ref. 7, where it is shown that the core proteins of the EG proteoglycans behave much like stiff fibers that are exquisitely designed to function as mechanotransducers at physiological flow rates. Syndecan I is the logical candidate for this mechanosensor, because it has an extracellular domain of variable length and a cytoplasmic tail that links to F-actin (29). In contrast, the plasmalemma is a viscous bilayer that can easily flow around membrane proteins (30). The principal restriction to this diffusion is the TJ, where serial section electron microscopy has revealed one or more nearly continuous junction strands with occasional breaks (31). Therefore, the plasmalemma might ripple under flow but should be incapable of transmitting fluid shear stress to the ACW beneath it because it has little structural rigidity. Although Weinbaum et al. (7) predict a fundamentally different transmission of the fluid shear stress at the apical surface with and without the EG, the traction force on the FAs at the basal surface will be the same for a given shear stress, because the total reaction force at the base of the EC is identical.
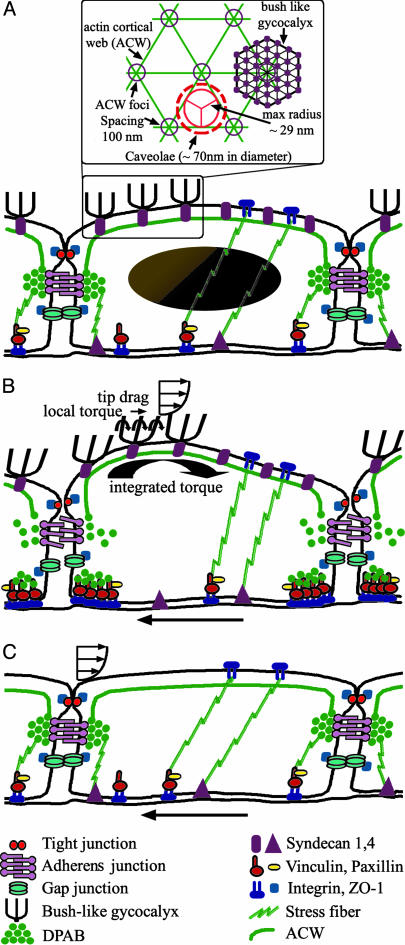
Our hypothesis for the organization and function of the three basic actin structures (ACW, DPAB, and SF) and their coordinated response to fluid shear stress with and without an EG are illustrated in Fig. 4. The hypothesized essential features of the DPAB (Fig. 4A) are the following. (i) It is a nearly free-floating rim that is flexibly attached by SFs to FAs at the basal surface and ACW. It functions much like a rubber fender on a bumper car that is constantly undergoing small collisions with its neighbors. (ii) Under equilibrium or low shear flow conditions the DPAB is kept in lateral register with the DPAB in neighboring cells by the weak VE-cadherin linkages in the adherens junction (2). These linkages suffice for small flow disturbances where the DPAB bumper prevents collisions between neighboring cells in unprotected regions above and below the DPAB. (iii) The DPAB and the ACW function as a single unit that can either move laterally or undergo small rotations about axes parallel to the cell surface, similar to what one would observe if one jumped on the bumper of a car. The SFs that tether the DPAB to the basal adhesions are weak compression elements that support tension. (iv) When forces and torques exceed the weak bonds of the VE-cadherins, these bonds rupture, and the DPAB gradually breaks up into fragments that are recruited for the formation of new SFs in the rest of the cell. These changes are needed to stabilize the cell during a transient state in which the shape of the cell is changing dramatically.
Fig. 4.
A conceptual bumper-car model for the structural organization of the EC in response to fluid shear stress. In its confluent control state (A), ECs display an intact DPAB that is localized to the adherens junction, where it serves as the base for the ACW that we hypothesize is the underlying cortical scaffold for the entire apical surface. The ACW is invisible in immunofluorescence studies, because it is comprised of a geodesic-like network of individual actin filaments in contrast to SFs and the DPAB, which are bundles of hundreds of α-actinin crosslinked antiparallel microfilaments. The polygonal nature of the ACW was first reported in optical tweezer experiments (30). Recent freeze-fracture EMs by Squire et al. (13) in regions close to the plasmalemma have revealed a highly ordered hexagonal lattice with a characteristic spacing of 100 nm between junctional nodes (Inset). Schematic diagrams show adaptation steps for confluent ECs predicted by the bumper-car model for intact (B) and compromised (C) EG in response to fluid shear stress; see text for detailed discussion.
The mechanism that causes fragmentation of the DPAB when the ECs are subject to fluid shear stresses above a critical magnitude and the EG is intact is illustrated in Fig. 4B. Drag on the tips of the core proteins at the edge of the EG causes a torque on the ACW that produces a clockwise rotation as shown in Fig. 4B. The bending moment on each core protein is small, but the collective behavior of all of the core proteins acts to produce a clockwise rotation of the DPAB. This creates a disjoining torque that is resisted by the VE-cadherins in the adherens junction. The quantitative feasibility of this hypothesis is demonstrated in Appendix C, which is published as supporting information on the PNAS web site, where a simple model for the disjoining torque on the VE-cadherins predicts a disjoining force (≈70 pN) that closely agrees with the 70- to 120-pN higher-order unbinding force for the unzippering of the adherens junction estimated in Baumgartner et al. (32). Further evidence in support of this prediction is provided by an additional experiment in which rat fat-pad ECs were exposed to a fluid shear stress of 5 dyne/cm2 for 5h in 10% FBS. For this lower shear stress where our model predicts a disjoining force of only ≈35 pN, little reorganization of the DPAB was observed.
We hypothesize that when this torque exceeds a threshold, the adherens junction ruptures and the DPAB starts to fragment, as seen in Fig. 2 A. The EC is now in an unstable configuration at its lateral margins, and for the ECs to remain confluent, new FAs and SFs need to be formed in the junctional region at the basal margins of the ECs. This requires the migration of vinculin to the EC borders seen in Fig. 2B to form new basal peripheral adhesions. This is a transient reorganization that, given sufficient time, will approach a new stable configuration provided the cells are in a region of USS. In this scenario, new DPABs form, and the vinculin at the cell borders is dispersed once the basal SFs in the junctional region are no longer needed.
The sequence of events just described never occurs if the EG is compromised, as shown in Fig. 4C. The fluid shear stress now acts directly on the apical plasmalemma, and the transmission of fluid shear stress is largely from the apical membrane of one EC to the next via the TJ complex, because the plasma membrane cannot flow through these nearly continuous junction strands. This type of shear stress transmission is described in ref. 33. The important observation is that when the EG is collapsed, the fluid shear stress is never transmitted to the ACW, and hence no disjoining torque acts on the DPAB. The adherens junction is stable and the DPAB remains intact, consistent with Fig. 2 A.
The question of whether there is a torque threshold, and whether the torque acts locally on individual core protein clusters or globally over the entire geodesic canopy in stimulating mechanical signaling, is subtle. Weinbaum et al. (7) predict that the torque on each core protein cluster can produce forces on the microfilaments in the ACW of the order of 0.1 pN. Forces of this magnitude have been shown to produce significant deformations in the microfilaments comprising the ACW (30). Although this highly localized mechanosignaling is possible, it is not consistent with the observations in Florian et al. (11). These investigators noticed that the release of NO was completely inhibited after a heparinase treatment that removed only 45% of HSPG. Our data also suggested that 30% reduction of HSPG (collapsed EG) was sufficient to inhibit cytoskeletal reorganization. Such behavior suggests a threshold response that could be mediated by a minimum rotation and torque on the DPAB in our bumper-car model. This threshold would be difficult to explain if transmission occurred through each core protein cluster.
There are numerous signaling cascades that are activated by FAs on the basal surface, leading to protein kinase C activation, tyrosine phosphorylation, and the phosphorylation of FA kinase (FAK) (34–36). Paxillin is a marker for FAK in the Src kinase pathway (37). Thus, it is striking that the present results indicate virtually no change in the distribution of paxillin whether the EG is present or not. This strongly suggests a weak coupling between SFs at the basal membrane and the ACW associated with the DPAB. The key to this behavior would seem to be the fact that the total traction force at the basal surface does not depend on the existence of the EG. As pointed out previously, the total reaction force that is carried by the FAs at the basal surface is equal and opposite to total fluid shear force acting at the apical plasmalemma, whether the EG is removed or intact. These forces are transmitted to basal FAs via SFs that attach at apical plaques (38) and via tensile forces that are transmitted to the basal membrane through the TJ complexes.
In the second part of this study, we examined the effect of HSSGs on actin filament organization, vinculin distribution, and junction complex formation. For F-actin, regions of HSSG exhibited behavior similar to USS regions, except that the fragmentation of the DPAB was more accentuated, and there was a wider dispersion of the actin filaments. There was no distinguishable difference in vinculin distribution between HSSG and USS regions, whether the EG was present or not. These results suggest that HSSGs play only a secondary role in the reorganization of actin filaments and their associated linker protein vinculin. Previous studies (17, 21) have shown that ZO-1 and Cx43 expression is sensitive to fluid shear stress in USS and HSSG regions, because stable junctions are not able to form. Recent studies suggest that, in addition to organizing TJ proteins, such as occludin and ZO-2, and enabling Cx43 to dock at the cell surface, ZO-1 serves as a linker between TJ proteins and F-actin (39, 40). Thus, we further speculate that it might also serve as a tertiary mechanotransducer at cell–cell junctions. In general, ECs in the HSSG region experience more significant junctional disruption than those in the USS region. Therefore, we conclude that apparent differences in distributions of F-actin and vinculin, as well as severe ZO-1 and Cx43 disruption commonly associated with ECs in the HSSG region, demonstrate cell–cell inability to stabilize and establish steady-state adhesion and hence permanent contact inhibition. Although our studies were performed in a 5-h period, we predict that changes corresponding to USS regions are transient only until the cells are fully adapted to the flow, whereas changes corresponding to HSSG regions are permanent, as shown by others with 24- to 30-h studies (17, 19, 23).
Conclusion
The model in Fig. 4 suggests a dichotomy of structure and function, with the DPAB and the ACW acting nearly independently of the SFs associated with the integrin complexes at the basal and apical membranes. This could account for the observation that degradation of the EG blocks NO formation (10–12) but has no influence on prostaglandin I2 production (12). In addition, the bumper-car model predicts that a partially deformed ACW, which is triggered by the disruption of the adherens junction and its associated DPAB, is needed for caveolae (≈70-nm diameter) to invaginate in caveolin-rich areas. Fig. 4A Inset shows that the intact ACW may be expected to inhibit caveolae formation, because their diameters exceed the largest sphere (≈60-nm diameter) that can easily fit through an ordered ACW. This may explain why shear stress increases caveolae density at the luminal surface of ECs, as observed between 6 h and 3 days after application of shear (41, 42).
Supplementary Material
Acknowledgments
We thank Drs. Andrei Iacobas, Alejandra Bosco, and Karen Cusato for advice on confocal image analysis; Dr. Elliot Hertzberg (Albert Einstein College of Medicine) for the generous supply of Cx43 antibody; and Dr. Anthony Ashton (Albert Einstein College of Medicine) for providing the rat fat-pad EC cell line. This research was supported by Program Project National Institute of Diabetes and Digestive and Kidney Disease Grant PO1 (DK06037); National Institute of Neurological Disorders and Stroke Grant NS41282; and National Heart, Lung, and Blood Institute Grant HL35549.
Author contributions: M.M.T., S.W., and D.C.S. designed research; M.M.T. performed research; M.M.T. analyzed data; and M.M.T., S.W., and D.C.S. wrote the paper.
Abbreviations: EC, endothelial cell; EG, endothelial glycocalyx; HSPG, heparan sulfate proteoglycan; ACW, actin cortical web; DPAB, dense peripheral actin band; SF, stress fiber; TJ, tight junction; USS, uniform shear stress; HSSG, high shear stress gradient; F-actin, filamentous actin; FA, focal adhesion.
References
- 1.Davies, P. F. (1995) Physiol. Rev. 75, 519–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogunrinade, O., Kameya, G. T. & Truskey, G. A. (2002) Ann. Biomed. Eng 30, 430–446. [DOI] [PubMed] [Google Scholar]
- 3.Tarbell, J. M. (2003) Annu. Rev. Biomed. Eng. 5, 79–118. [DOI] [PubMed] [Google Scholar]
- 4.Vink, H. & Duling, B. R. (1996) Circ. Res. 79, 581–589. [DOI] [PubMed] [Google Scholar]
- 5.Hu, X. & Weinbaum, S. (1999) Microvasc. Res. 58, 281–304. [DOI] [PubMed] [Google Scholar]
- 6.Mulivor, A. W. & Lipowsky, H. H. (2002) Am. J. Physiol. 283, H1282–H1291. [DOI] [PubMed] [Google Scholar]
- 7.Weinbaum, S., Zhang, X., Han, Y., Vink, H. & Cowin, S. C. (2003) Proc. Natl. Acad. Sci. USA 100, 7988–7995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ihrcke, N. S., Wrenshall, L. E., Lindman, B. J. & Platt, J. L. (1993) Immunol. Today 14, 500–505. [DOI] [PubMed] [Google Scholar]
- 9.Henry, C. B. & Duling, B. R. (1999) Am. J. Physiol. 277, H508–H514. [DOI] [PubMed] [Google Scholar]
- 10.Pohl, U., Herlan, K., Huang, A. & Bassenge, E. (1991) Am. J. Physiol. 261, H2016–H2023. [DOI] [PubMed] [Google Scholar]
- 11.Florian, J. A., Kosky, J. R., Ainslie, K., Pang, Z., Dull, R. O. & Tarbell, J. M. (2003) Circ. Res. 93, E136–E142. [DOI] [PubMed] [Google Scholar]
- 12.Hecker, M., Mulsch, A., Bassenge, E. & Busse, R. (1993) Am. J. Physiol. 265, H828–H833. [DOI] [PubMed] [Google Scholar]
- 13.Squire, J. M., Chew, M., Nneji, G., Neal, C., Barry, J. & Michel, C. (2001) J. Struct. Biol. 136, 239–255. [DOI] [PubMed] [Google Scholar]
- 14.Drenckhahn, D. & Ness, W. (1997) Vasc. Endothel. Physiol. Pathol. Ther. Opp. 3, 1–25. [Google Scholar]
- 15.Galbraith, C. G., Skalak, R. & Chien, S. (1998) Cell Motil. Cytoskeleton 40, 317–330. [DOI] [PubMed] [Google Scholar]
- 16.Helmke, B. P., Goldman, R. D. & Davies, P. F. (2000) Circ. Res. 86, 745–752. [DOI] [PubMed] [Google Scholar]
- 17.Depaola, N., Davies, P. F., Pritchard, W. F., Jr., Florez, L., Harbeck, N. & Polacek, D. C. (1999) Proc. Natl. Acad. Sci. USA 96, 3154–3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ku, D. N., Giddens, D. P., Zarins, C. K. & Glagov, S. (1985) Arteriosclerosis 5, 293–302. [DOI] [PubMed] [Google Scholar]
- 19.Davies, P. F., Remuzzi, A., Gordon, E. J., Dewey, C. F., Jr., & Gimbrone, M. A., Jr. (1986) Proc. Natl. Acad. Sci. USA 83, 2114–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White, C. R., Haidekker, M., Bao, X. & Frangos, J. A. (2001) Circulation 103, 2508–2513. [DOI] [PubMed] [Google Scholar]
- 21.Thi, M. M., Kojima, T., Cowin, S. C., Weinbaum, S. & Spray, D. C. (2003) Am. J. Physiol. 284, C389–C403. [DOI] [PubMed] [Google Scholar]
- 22.Adamson, R. H. & Clough, G. (1992) J. Physiol. 445, 473–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Girard, P. R. & Nerem, R. M. (1995) J. Cell Physiol. 163, 179–193. [DOI] [PubMed] [Google Scholar]
- 24.Dewey, C. F., Jr., Bussolari, S. R., Gimbrone, M. A., Jr., & Davies, P. F. (1981) J. Biomech. Eng. 103, 177–185. [DOI] [PubMed] [Google Scholar]
- 25.Davies, P. F., Robotewskyj, A. & Griem, M. L. (1994) J. Clin. Invest. 93, 2031–2038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuchan, M. J. & Frangos, J. A. (1994) Am. J. Physiol. 266, C628–C636. [DOI] [PubMed] [Google Scholar]
- 27.Dull, R. O., Dinavahi, R., Schwartz, L., Humphries, D. E., Berry, D., Sasisekharan, R. & Garcia, J. G. (2003) Am. J. Physiol 285, L986–L995. [DOI] [PubMed] [Google Scholar]
- 28.Mulivor, A. W. & Lipowsky, H. H. (2004) Am. J. Physiol 286, H1672–H1680. [DOI] [PubMed] [Google Scholar]
- 29.Bass, M. D. & Humphries, M. J. (2002) Biochem. J. 368, 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sako, Y. & Kusumi, A. (1995) J. Cell Biol. 129, 1559–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adamson, R. H. & Michel, C. C. (1993) J. Physiol. 466, 303–327. [PMC free article] [PubMed] [Google Scholar]
- 32.Baumgartner, W., Hinterdorfer, P., Ness, W., Raab, A., Vestweber, D., Schindler, H. & Drenckhahn, D. (2000) Proc. Natl. Acad. Sci. USA 97, 4005–4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fung, Y. C. & Liu, S. Q. (1993) J. Biomech. Eng. 115, 1–12. [DOI] [PubMed] [Google Scholar]
- 34.Burridge, K., Turner, C. E. & Romer, L. H. (1992) J. Cell Biol. 119, 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hu, Y. L. & Chien, S. (1997) J. Histochem. Cytochem. 45, 237–249. [DOI] [PubMed] [Google Scholar]
- 36.Li, S., Kim, M., Hu, Y. L., Jalali, S., Schlaepfer, D. D., Hunter, T., Chien, S. & Shyy, J. Y. (1997) J. Biol. Chem. 272, 30455–30462. [DOI] [PubMed] [Google Scholar]
- 37.Turner, C. E. (2000) Nat. Cell Biol. 2, E231–E236. [DOI] [PubMed] [Google Scholar]
- 38.Kano, Y., Katoh, K. & Fujiwara, K. (2000) Circ. Res. 86, 425–433. [DOI] [PubMed] [Google Scholar]
- 39.Fanning, A. S., Jameson, B. J., Jesaitis, L. A. & Anderson, J. M. (1998) J. Biol. Chem. 273, 29745–29753. [DOI] [PubMed] [Google Scholar]
- 40.Giepmans, B. N. & Moolenaar, W. H. (1998) Curr. Biol. 8, 931–934. [DOI] [PubMed] [Google Scholar]
- 41.Boyd, N. L., Park, H., Yi, H., Boo, Y. C., Sorescu, G. P., Sykes, M. & Jo, H. (2003) Am. J. Physiol. 285, H1113–H1122. [DOI] [PubMed] [Google Scholar]
- 42.Rizzo, V., Morton, C., Depaola, N., Schnitzer, J. E. & Davies, P. F. (2003) Am. J. Physiol. 285, H1720–H1729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.