Abstract
A significant proportion of vulnerable people in sub-Saharan Africa (SSA) remain at risk for contracting diarrhoeal diseases due to the presence of many risk factors facilitating their transmission. A systematic review of published articles from the SSA region was done to determine the prevalence and types of diarrhoeal pathogens in circulation, based on a search of databases, including EBSCO host, PubMed, Scopus, Science Direct, Google scholar and Web of Science was done between September 2009 and December 2010. Data were summarized from 27 studies, with pooled data analysed and reported. Pathogens were isolated from between 26.8–65.6% of cases, with an overall isolation rate of 55.7% (95% CI, 48.2–62.9%). Isolation rates were highest amongst adult cases followed by children, and the odds of isolating a pathogen was greater in diarrhoeal cases (Odds Ratio 4.93 (95% CI, 1.99 to 12.23), than in asymptomatic controls. Overall isolation ranged from 8% to 99%; and heterogeneity testing suggests differences between age groups (Q=5.806; df=2, P=0. 055). Mixed E. coli spp., (29.95%), Cryptosporidium (21.52%), Cyclospora (18%), Entamoeba. (13.8%), Shigella spp. (10.49%), Salmonella spp. (8.36%), and Campylobacter spp. (8.33%), were most commonly reported, and rotavirus was the most common virus isolated. This is the first review to look at the range of enteric pathogens circulating in SSA, and has confirmed high rates of isolation of pathogens from diarrhoeal cases. Public health practitioners can use this information to understanding the challenges related to diarrhoeal illness and set priorities for their prevention and control.
Key words: Enteric pathogens, diarrhoea, gastrointestinal infections, Sub-Saharan Africa, environmental health.
Introduction
The burden of gastrointestinal (GI) illness in developing countries remains significantly high, despite a marked decrease in mortality rates from 4.6 million in 1980 to about 1.5 million annually in 1999.1–3 It is estimated that approximately 1.87 million (CI: 1.56–2.19) children die from diarrhoea before reaching their fifth birthday.4 Diarrhoeal diseases account for 1/5 of all child deaths, 78% of which are concentrated in the African and South East Asian Regions.4,5 Several studies have shown that the burden of GI remain particularly high in the African continent, especially in areas characterized by poverty. Three of the Millennium Development Goals (MDG) are associated with the burden of diarrhoeal diseases. The progress towards achieving the targets of MDG 7- to halve the proportion of people without sustainable access to improved sanitation; MDG 1- to eradicate extreme poverty and hunger and MDG 4- to reduce child deaths by two thirds by 2015, has been slow.6,7
Current trends suggest that many countries in SSA will not reach these target as only about 31% of people have access to improved sanitation,8 leaving a significant proportion of vulnerable people at risk from infectious diseases.7 Infectious organisms can be transmitted through a variety of routes,9,10 the epidemiology of GI illness is influenced by the context in which they are transmitted and differs between developing and developed countries.2,4,11 Recent studies have described a high incidence of pathogenic organisms, especially in children in the SSA region when compared with the rest of the world.2,4,11 Since the African continent is disproportionately affected by a high burden of illness from GI illness, it is important to understand the types and prevalence of pathogens that are responsible in SSA countries and how this can influence planning for prevention and control programmes. The high prevalence and burden of HIV infection in countries in SSA populations have also increased the risk of acute and persistent diarrhoea. There is much value in describing not just a single pathogen, but a range of pathogens, as many are transmitted via similar routes of exposure. The application of prevention and control measures at each route of exposure can impact several pathogens at the same time.12–14 The aim of this paper is to describe the common diarrhoeal pathogens, and discuss their public health implications in the Sub-Saharan African context. A systematic review of studies from the SSA region was done, as it provides empirical information through the appraising and synthesizing of evidence from primary studies, while reducing the reviewer's own bias.15–17
Materials and Methods
Search strategy and selection criteria
A search for studies on diarrhoeal pathogens and associated risk factors conducted in sub-Saharan Africa was performed between September 2009 and December 2010. Several databases including EBSCO host, Academic Search Premier, Scopus, Science Direct, Google scholar and Web of Science, were searched for articles published in the English language. The search strategy used a combination of terms including: infectious intestinal disease, aetiology (etiology) diarrhoea Africa, aetiology (etiology) gastroenteritis Africa, enteric infectious pathogen Africa. Boolean operators (not, and, or) were also used in succession to narrow and widen the searches. Other articles were identified by reviewing the reference list of articles.
Criteria for selecting studies
Inclusion criteria
The primary selection was made based on the major topic of the article. The apriori-decided criteria used were the following:
The age group of the study population must be clearly defined;
The study must define whether the subjects were clinically asymptomatic or symptomatic;
The study must include detailed results of microscopic analysis of stool samples and the number of samples tested must be reported;
The number of study subjects and positive results for both cases/controls must be reported;
Only studies providing adequate information on the actual pathogens identified and prevalence rates for all pathogens identified and tested for three or more (≥3) pathogens were included.
Exclusion criteria
Studies were excluded if they focused on a single pathogen, did not include information on aetiology or did not provide adequate information about methods employed, or was not available in full-text among other reasons presented in Table 1.
Table 1. Records excluded from study and reasons.
| Reason | No. of studies | References |
|---|---|---|
| Full text not available | 9 | 18–26 |
| Non-clinical/non-human specimen tested | 4 | 27–30 |
| Only Travellers | 4 | 31–34 |
| Refugees and asylum seekers | 2 (Full-text excluded) | 35,36 |
| Single pathogen focus | 17 (8 Full-text excluded) | 34,37–52 |
| No aetiology data reported | 4 | 53–56 |
| Inadequate data | 5 | 57–60 |
| Others, Special populations | 3 | 61–63 |
| Reviews | 4 | 64–67 |
Study selection, quality assessment and data extraction
The primary selection included any cross-sectional studies, case controls, and retrospective or prospective cohorts. Studies were selected if they included details of the number of samples tested, laboratory methods, results of analyses, and subjects' HIV status and the period of the study. The outcome of interest was the number and types of pathogens isolated from diarrhoeal and non- diarrhoeal stool specimens. All studies included were screened based on the MOOSE guidelines. Data were summarized based on location, study population and associated risk factors (Table 2). Microbiological analyses of stool specimen are summarized in Table 3.
Table 2. Summary of studies on gastrointestinal pathogens in Sub-Saharan Africa.
| Location, author and date of publication | Setting, HIV prevalence, sample source | Participants age, study design | No. of specimen tested | Overall pathogen isolation rates |
|---|---|---|---|---|
| Guinea-Bissau: Bandim II & Belem of Bissau (2003)68 | Peri-urban, community based | 0–2 yrs; prospective cohort 2 yrs, follow-up | 11987 cases | Pathogens found in 58% of specimen |
| Kenya 2: Kisumu (2009)69 | Urban; HIV sero-prevalence was 1 3.6% amongst cases; hospital based | 0–2 yrs; prospective Cohort 2 yrs, follow-up. | 630 cases | Pathogens found in 32.2% of specimen. |
| Nigeria (1): Abakaliki (2008)70 | Mixed setting; primary health care unit | 0–4 yrs; retrospective study | 150 cases 50 controls | Pathogens found in 81.3% of specimen |
| Nigeria (2): East Central State (1997)71 | Mixed setting; hospital based | 0–5 yrs; retrospective study | 1015 cases 401 controls | Pathogens found in 21.0 % and 3.9% (P<0.001) of cases and control specimen respectively |
| Zambia: Lusaka 1 (1998)72 | Peri-urban; hospital based | 0–5 yrs; retrospective study | 639 | Pathogens found in 29.9% of specimen. |
| Mozambique: Maputo province (2007)73 | Rural, hospital based | 0–5 yrs; retrospective study | 529 cases | Pathogens found in 42.2% of specimen |
| Cameroon: Yaounde (2008)74 | Urban, community based | 0–5 yrs; retrospective study | 3034 cases | pathogens found in 59.5% of specimen |
| Tanzania: Ifakara (2004)75 | Urban; hospital based | 0–5 yrs; retrospective study | 451 cases | Pathogens found in 67.6% of specimen. |
| Ghana: Bulpelia / Tamale (2007)76 | Urban, primary health care unit | 0–11 yrs; case control study | 243 cases, 124 controls | Pathogens found in 76.5 % and 53.2% (P<0.001) of cases and control specimen, respectively |
| Central African Rep. Bangui (1994)77 | Urban, hospital based | 0–15 yrs; retrospective study | 1197 cases | Pathogens found in 49.4% of specimen |
| Zaire: Kinshasa (1994)78 | Urban; hospital and health centre based | 0–5 years; matched case control | 173 cases, 155 controls | Pathogens found in 100% and 94% of cases and control specimen respectively |
| Zaire: Kivu (1983)79 | Peri-urban; hospital based | 0–5 years; case control | 355 cases; 320 controls | Pathogens found in 40.3% and 14.1% of cases and control specimen respectively. |
| Nigeria: Lagos (1994)80 | Urban; hospital and health centre based | 0–5 years; case control | 215 cases, 100 controls | Pathogens found in 74.9% and 28% of cases and control specimen respectively |
| Nigeria: Osun State (2003)81 | Urban; hospital based | 0–5 years; retrospective study | 135 | Pathogens found in 100% of cases. |
| Nigeria: Abuja (2008)82 | Peri-urban; hospital based | 0–5 years; retrospective study | 404 | Pathogens found in 68.5% of cases. |
| Burkina Faso: Ouagadougou (2007)83 | Peri-urban; HIV sero-prevalence = approx 10.6% amongst cases health centre based | 0–5 years; retrospective study | 66 | Pathogens found in 42.4% of cases. |
| Ghana: Tamale (2008)84 | Peri-urban; health centre based | 0–11 years; case control | 243 cases; 124 controls | Pathogens found in 92.6% and 86.3% of cases and control specimen, respectively. |
| Uganda: Kampala (2009)85 | Peri-urban; HIV sero-prevalence = approx 24.7% amongst cases hospital based | 0–5 years; retrospective study | 190 | Pathogens found in 24.7% of specimen. |
| Meta-analysis: random effects mean isolation rate in children: 58.1% (95% CI; 50.1–65.6%); heterogeneity P<0.046; | ||||
| Malawi: Lilongwe (1996)86 | Urban; HIV sero-prevalence = approx 60% amongst controls; hospital based | ≥12 yrs; case control study. | 132 cases 73 controls | Pathogens found in 48.3% and 2% of cases and control specimen respectively. |
| Uganda: Entebbe (2002)87 | Semi-urban; HIV sero-prevalence = approx 100% amongst cases and controls; community based | Adults (IQR = 26–36 yrs) Prospective Cohort, 2 yrs, follow-up | 357 cases, 127 controls | Pathogens found in 49% and 39% of cases and control specimen, respectively |
| Zambia: Lusaka (2) (1996)88 | Urban; HIV sero-prevalence = approx 97% amongst cases; community based. | 18–79 yrs; retrospective study | 77 | Pathogens found in 78% of specimen |
| Zambia, Misisi, Lusaka (3) (2009)89 | Urban; HIV sero-prevalence was 31% amongst cases; hospital based | 18–79 yrs; prospective Cohort, 3 yrs, follow-up | 4780 | Pathogens found in 99% of specimen |
| Central African Republic, Bangui (1998)90 | HIV sero-prevalence = approx 74% and 52% amongst cases and controls, respectively; hospital based | >18 years; case control | 290 cases; 140 controls | Pathogens found in 55.5% and 61.4% of cases and control specimen, respectively |
| Meta-analysis: random effects mean isolation rate in adults: 65.6% (95% CI, 26.0–91.2%); heterogeneity P<0.454 | ||||
| South Africa: Venda region (2003)91 | Rural; community based | All age groups; retrospective study | 401 cases | Pathogens found in >95.3% of specimen (totals not given) |
| Burkina Fasa: Ouagadougou (2002)92 | Rural; hospital based | All age groups; retrospective study | 4131 (protozoa) 826 (bacteria) | Pathogens found in 8% of specimen, respectively |
| Kenya 1: Asembo Bay (2006)93 | Rural, community based | 0–70+ years; retrospective surveillance type | 3445 cases | Pathogens found in 31.7% of specimen |
| Kenya 1: Asembo Bay (2003)94 | Rural, health centre based | 0–70+ years; retrospective surveillance type | 451 cases | Pathogens found in 51.% of specimen |
Meta-analysis: random effects mean isolation rate in mixed-ages: 26.8% (95% CI, 11.3–51.3%); heterogeneity P<0.063. Overall random effects mean isolation rate for all age groups: 55.7% (95% CI, 48.2–62.9%); heterogeneity P>0.05. Q=5.806; df =2, P=0.055.
Table 3. Summary of microbiological tests done by studies on GI pathogens in sub-Saharan Africa.
| Location and date of publication | Bacteriology methods | Virology methods | Parasitological methods |
|---|---|---|---|
| Guinea-Bissau: Bandim II & Belem of Bissau (2003)68 | Standard culture methods, DNA-DNA hybridization | ELISA | Microscopy |
| Kenya 2: Kisumu (2009)69 | Standard culture methods & bright-field microscopy | ELISA | Microscopy & IFA |
| Nigeria (1): Abakaliki (2008)70 | Standard culture methods & direct microscopy | ELISA | Microscopy |
| Nigeria (2): East Central State (1997)71 | Standard culture methods | N/A | N/A |
| Zambia: Lusaka 1 (1998)72 | Standard culture methods | N/A | N/A |
| Mozambique: Maputo Province (2007)73 | Standard culture methods & direct microscopy | N/A | Direct observation & microscopy |
| Cameroon: Yaounde (2008)74 | Standard culture methods | ELISA | Microscopy |
| Tanzania: Ifakara (2004)75 | Standard culture methods & direct microscopy | Agglutination test | Direct observation |
| Ghana: Bulpelia / Tamale (2007)76 | Standard culture methods | RT-PCR | Microscopy |
| Central African Rep. Bangui (1994)77 | Standard culture methods | ELISA | Microscopy |
| Zaire: Kinshasa (1994)78 | Standard culture & direct microscopy | Latex agglutination test | Direct observation & microscopy |
| Zaire: Kivu (1983)79 | Standard culture methods | ELISA | Direct microscopy. |
| Nigeria: Lagos (1994)80 | Standard culture methods | ELISA | Direct microscopy + iron haemotoxylin staining for Cryptosporidium sp. |
| Nigeria: Osun State (2003)81 | Standard culture methods and plate dilution technique for antibiotic susceptibility testing. | N/A | N/A |
| Nigeria: Abuja (2008)82 | Standard culture and slide agglutination technique; modified disc diffusion technique for antibiotic susceptibility testing. | EIA | Light microscopy & Ziehl-Neelsen (Kinyoun's) stain for Cryptosporidium. |
| Burkina Faso: Ouagadougou (2007)83 | N/A | Immunochromatographic tests. | Direct microscopy |
| Ghana: Tamale (2008)84 | Standard culture methods and breakpoint microdilution test for antibiotic susceptibility. | N/A | N/A |
| Uganda: Kampala (2009)85 | Standard culture methods and disc diffusion technique for antibiotic susceptibility. | N/A | N/A |
| Malawi: Lilongwe (1996)86 | Standard culture methods & direct microscopy. | N/A | N/A |
| Uganda: Entebbe (2002)87 | Standard culture methods & direct microscopy. | N/A | Microscopy |
| Zambia: Lusaka (2) (1996)88 | N/A | N/A | Light microscopy, electron microscopy and PCR |
| Zambia, Misisi, Lusaka (3) (2009)89 | Standard culture methods & direct microscopy. | N/A | Microscopy |
| Central African Republic, Bangui (1998)90 | Standard culture methods and disc diffusion technique for antibiotic susceptibility. | Latex agglutination test | Dark-field microscopy with staining |
| South Africa: Venda region (2003)91 | Standard culture. | ELISA | N/A |
| Burkina Faso: Ouagadougou (2002)92 | Standard culture methods & direct microscopy. | N/A | N/A |
| Kenya 1: Asembo Bay (2006)93 | Standard culture and PCR for E. coli sp. | ELISA | Microscopy |
| Kenya 1: Asembo Bay (2003)94 | Standard culture methods and disc diffusion technique for antibiotic susceptibility. | N/A | N/A |
ELISA, Enzyme-linked immnuno-assay; EIA, enzyme immnuno assay; PCR, polymerase chain reaction; IFA, immunofluorescence assay; N/A, not available.
Pooled data was analysed using the Comprehensive Meta-analysis programme,95 based on the random effects (RE) model. This model assumes that the impact of covariates capture some but not all of the true variation among effects, hence the RE model is designed to take these differences into account and makes the assumption that the effect size (pooled prevalence) is the mean of the true effect sizes for all studies with a given value of the co-variates.96
Pooled data was stratified by age groups for analysis. Prevalence was reported with 95% confidence interval (CI), and odds ratios (OR) given where applicable. The random-effect method97 was used in meta-analysis and heterogeneity between studies was calculated on the basis of the Cochran's Q-test. Heterogeneity among studies was considered significant if the P value of Cochran's Q-test was less than 0.05. The findings were interpreted in light of current knowledge and practice based on the previously outlined aims of the study.
Results
Studies identified
The initial search identified 198 articles, 66 of which were reviewed for inclusion, with 39 rejected for various reasons (Figure 1 and Table 1). After critical review, 27 articles were selected for this review. The methodology and summary of findings for each study,68–94,98–101 are summarized in Table 2. The studies represented 14 countries in SSA. Different study designs were employed in the studies, including cross-sectional, case control and prospective follow-up cohort designs. The samples were obtained from persons seen in hospitals, primary health care centres or recruited in community cohorts, in urban, peri-urban and rural settings. Seventeen of the studies looked at children, six looked at adults (12–80+ years) and four looked at mixed age groups.
Figure 1.
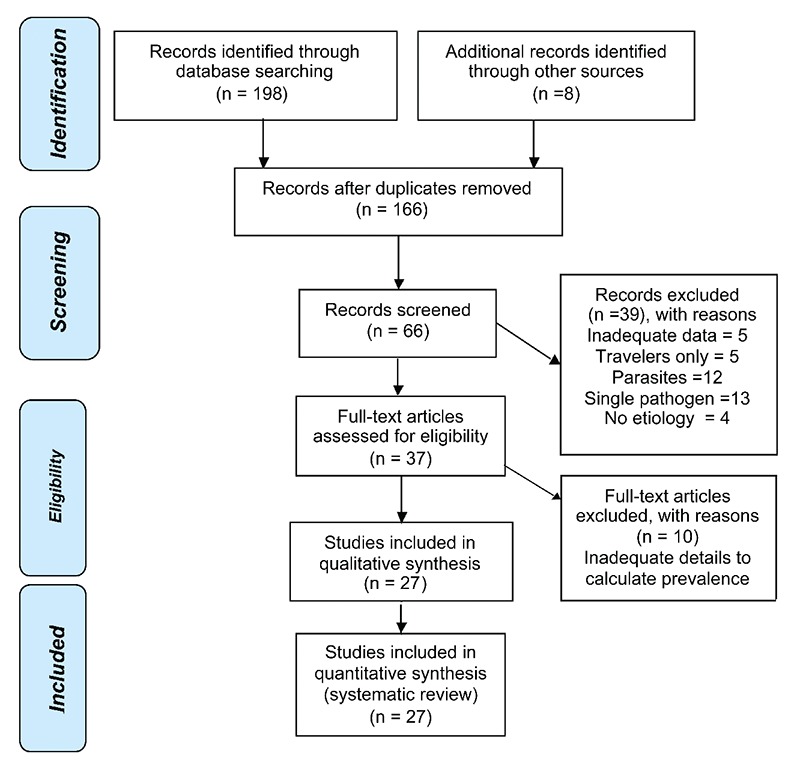
Identification and screening of studies for systematic review.
Microbiology
Stool samples were examined using standard parasitological (microscopy or direct observation), bacteriology (cultures), and virology techniques [mainly the enzyme-linked immunosorbent assay (ELISA) for rotavirus screening], presented in Table 3.
Pathogen isolation rates
The rate of isolating pathogens from cases varied widely between countries and between age groups, with an overall isolation rate of 55.7% (95% CI, 48.2–62.9%). Isolation of pathogens was highest amongst adult cases (mean 65.6%; 95% CI, 26.0%–91.2%) followed by children, (mean 58.1%; 95% CI, 50.1–65.6%) and mixed aged groups showed the lowest rates (26.8% (95% CI, 11.3%–51.3%) (Figure 2). Heterogeneity testing suggests slight differences between age groups (Q=5.806; df=2), but this was not significant (P=0.055). Eight studies reported HIV sero-prevalence rates ranging from 10.6%–100% amongst adult participants. When these studies were removed from the analysis the overall isolation rate was not significantly different (54.3%; 95% CI, 43.5%–64.7%). In ten studies where comparable asymptomatic controls were tested, there were significantly more pathogens isolated from cases than controls with a mean overall odds ratio (OR) of 4.93 (95% CI, 1.99–12.23), ranging from 0.52 to 72.05. Very large differences between cases and controls of over 20 times higher isolation rates were observed in Malawi (39), OR 20.52 (95% CI, 2.73–154.24, Nigeria (42), OR 50.0 (95% CI, 16.63–150.36), and Central Africa Rep. (31) OR 72.05 (95% CI, 38.21–135.85).
Figure 2.
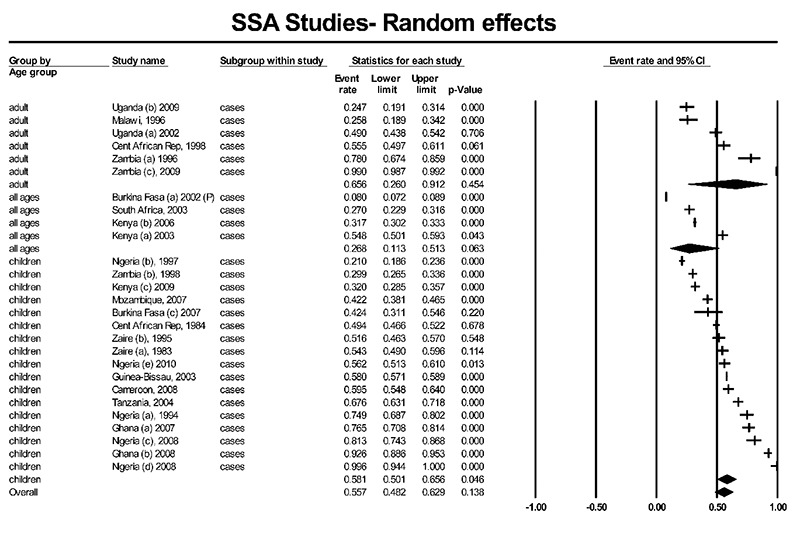
Rates of isolation of enteric pathogens in twenty seven Sub-Saharan African countries.
Etiology data by category was collated from the details reported in each study (Figure 3). Bacterial pathogens (39.82%) were the most common group isolated in a majority of studies followed by parasites (27.11%) and viruses (21.95%).
Figure 3.
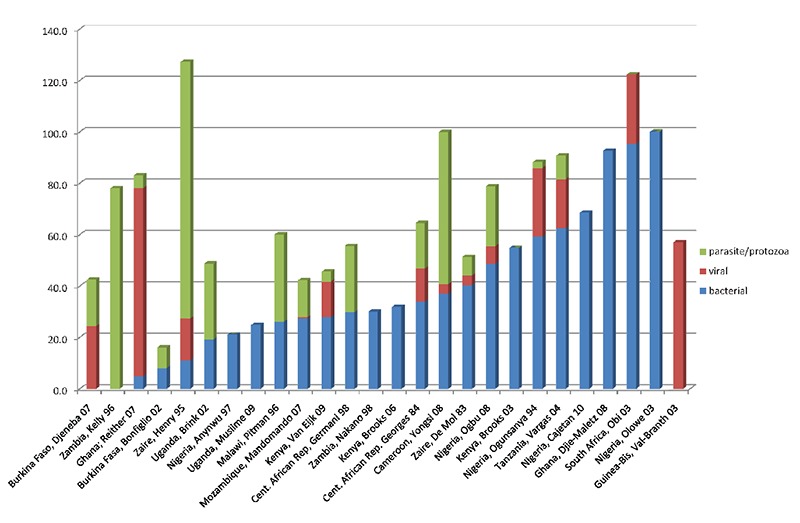
Prevalence of pathogenic bacteria, virus and parasites isolated in twenty seven Sub-Saharan African countries.
On average, other diarrheagenic E. coli spp., (29.95%), ETEC (15.37%), Shigella spp. (10.49%), Salmonella spp. (8.36%), and Campylobacter spp. (8.33%), were the most common bacterial pathogens reported by 12, 9, 22, 21 and 17 studies, respectively. Non-cholera Vibrio spp., Staphylococcus aureus and C. difficile were reported by one study each (Table 4).
Table 4. Rates of isolation of bacterial pathogens in diarrhoea cases in sub-Saharan African countries.
| Countries | Aeromonas spp. | C. jejuni | Enteropathogenic E. coli | Enterotoxigenic E. Coli | Other E. coli sp. | P. shigelloies | Salmonella spp. | Shigella spp. | Vibrio cholerae spp. | Yersinia spp. |
|---|---|---|---|---|---|---|---|---|---|---|
| Ghana, 2007 | N/R | 0.82% | N/R | N/R | N/R | N/R | 2.47% | 1.65% | N/R | N/R |
| Cameroon, 2008 | N/R | 5.72% | 1.37% | 2.50% | 2.75% | N/R | 5.72% | 4.35% | N/R | N/R |
| Cent. African Rep. 1984 | N/R | 10.69% | 3.43% | 12.10% | N/R | N/R | 4.85% | 2.67% | N/R | N/R |
| Malawi, 1996 | N/R | N/R | N/R | N/R | N/R | N/R | N/R | 24.24% | N/R | N/R |
| Tanzania, 2004 | 0.67% | 2.00% | N/R | N/R | 35.70% | 0.67% | 1.11% | 21.51% | N/R | N/R |
| Nigeria, 2008 | N/R | 2.67% | 15.33% | 11.33% | 3.33% | 3.00% | ||||
| Burkina Faso, 2002 | N/R | N/R | N/R | N/R | 34.02% | N/R | N/R | 10.05% | N/R | 4.00% |
| Kenya, 2003 | N/R | 8.65% | N/R | N/R | N/R | N/R | 5.25% | 15.94% | 4.00% | N/R |
| South Africa, 2003 | 8.48% | 19.70% | 2.49% | 7.50% | 10.22% | 10.72% | 14.46% | 12.47% | 4.00% | 5.00% |
| Mozambique, 2007 | N/R | 1.70% | 6.81% | 4.30% | 11.53% | N/R | 2.46% | 0.19% | N/R | N/R |
| Zambia, 2009 | 0.36% | N/R | N/R | N/R | N/R | N/R | 1.21% | 0.38% | N/R | N/R |
| Uganda, 2002 | N/R | 4.76% | N/R | N/R | N/R | N/R | 10.92% | 12.89% | N/R | N/R |
| Zambia, 1996 | N/R | N/R | N/R | N/R | 14.87% | N/R | 1.41% | 10.17% | 3.00% | N/R |
| Guinea-Bis, 2003 | N/R | 35.18% | 95.02% | N/R | N/R | N/R | 56.76% | 29.05% | N/R | N/R |
| Nigeria, 1997 | N/R | 2.46% | N/R | 12.00% | N/R | N/R | 3.05% | 2.07% | 1.00% | N/R |
| Kenya, 2009 | N/R | 20.79% | N/R | N/R | N/R | N/R | 3.49% | 5.40% | N/R | N/R |
| Zaire, 1983 | N/R | 16.06% | 9.01% | 3.40% | N/R | N/R | 2.54% | 1.97% | 6.00% | 1.00% |
| Zaire, 1995 | N/R | 0.58% | N/R | 6.90% | N/R | N/R | 3.47% | N/R | N/R | N/R |
| Cent. African Rep, 1998 | N/R | 1.72% | 1.03% | N/R | 10.00% | N/R | 10.00% | N/R | N/R | N/R |
| Ghana, 2008 | N/R | 0.82% | N/R | N/R | 87.65% | N/R | 2.47% | 1.65% | N/R | N/R |
| Nigeria, 1994 | 1.40% | N/R | 14.42% | 10.70% | 14.42% | N/R | 3.26% | 5.12% | N/R | 1.00% |
| Kenya, 2003 | N/R | 7.32% | N/R | N/R | N/R | N/R | 3.33% | 43.90% | N/R | N/R |
| Nigeria, 2003 | N/R | N/R | N/R | N/R | 77.78% | N/R | N/R | 16.30% | 1.00% | N/R |
| Nigeria, 2010 | N/R | N/R | N/R | N/R | 43.07% | 2.72% | 2.23% | N/R | N/R | N/R |
| Uganda, 2009 | N/R | N/R | 4.74% | 8.90% | 2.11% | N/R | 5.79% | 3.16% | N/R | N/R |
N/R, not reported.
Rotavirus was by far the most common viral agent isolated in an average of 19.51% of cases, in 13 studies; with nearly half of these studies isolating it from 20% or more of cases (Figure 4). Adenovirus (9.2%) was reported by five studies, while astroviruses (5%) and norovirus (9.5%) were only reported in the Ghana 2005–2006 study. Intestinal parasites were isolated from an average of 25.9% of cases. The enteric protozoa Cryptosporidium spp. (21.52%), Cyclospora spp. (18%), Entamoeba spp. (13.8%), Blastocystis hominis (11.0%) were the most common. Ascaris lumbricoides was the most common helminthic infection reported in an average of 9.14% of cases, while several other pathogenic and non-pathogenic parasites reported in a few studies. In the study from Zambia (1994) which only tested for parasites in HIV positive adults, up to 78% of specimen were positive with a high prevalence of intracellular protozoa (Cryptosporidium parvum, Cystoisospora (isospora) belli and microsporidia).
Figure 4.
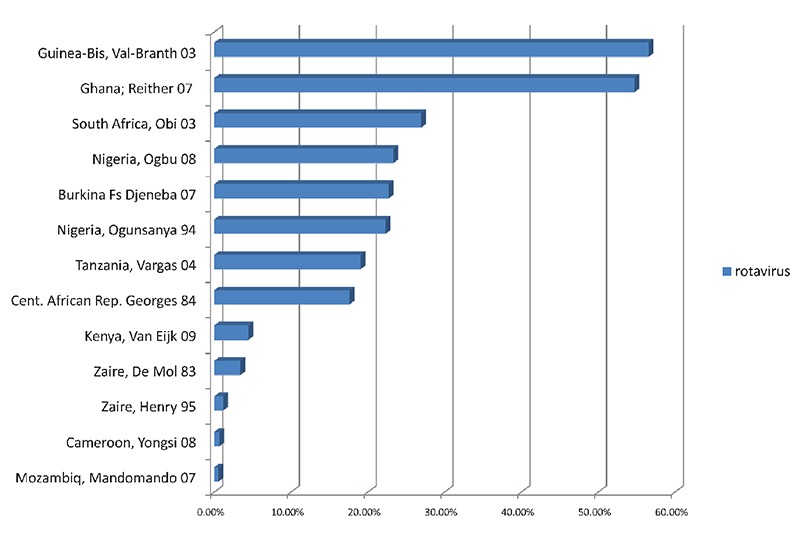
Rates of isolation of rotavirus in thirteen subSub-Saharan African Countriescountries.
Discussion
Diarrhoea continues to affect children and adults in African countries.2,4,11 Increases in diarrhoea rates over the period 1967 to 1997 have been seen in Kenya (6%–18%) and Uganda (16–21%), but reduction seen in Tanzania (11–8%)12 and Malawi (20–14%) from 1992–2000.56 While some countries have attempted to determine the prevalence and etiology of GI, most have looked at single pathogens.102 This review has confirmed and strengthened the view that there is a high rate of illness due to pathogenic enteric microorganisms affecting the region.
Eight of the studies reported HIV seroprevalence rates amongst patients. The HIV sero-prevalence in some SSA countries is high and infected persons may have been included in some studies, but was not reported. All adult studies included HIV positive cases, but when these were removed from the analysis, there was no apparent difference in prevalence. While immuno-compromised persons are more susceptible to diarrhoeal illness, the pathogens are similar to those in immunocompetent persons, with differences mainly seen with opportunistic parasitic infections.87,103,104 Where higher rates are seen in immuno-compromised patients this may be due to the higher likelihood of seeking medical attention for their symptoms, and are more likely to be tested for pathogens.105
Bacteria were the main pathogens identified by a majority of studies, and the high rates of diarrhoeagenic E. coli spp., Shigella, Salmonella spp., and Campylobacter spp., is consistent with the prevailing risk factors,9,11,106,107 and likely reflects the availability of bacteriological diagnostic techniques. Our study like many others confirm that pathogenic E. coli and Shigella dominate in developing countries,68,72,77,108 compared with Salmonella, Shigella, and Campylobacter in industrialized countries.109–111 These pathogens are transmitted through mainly fecal oral route,112–115 and contaminated food or water.115–123 In eleven studies, only one category of pathogen was investigated, which is a limitation of such studies in estimating the overall burden of infectious pathogens.71,72,81,82,84,85,88,92–94,117
Rotavirus was the most common virus isolated and reported in SSA. Worldwide, norovirus, rotavirus, and other caliciviruses represent over 80% of acute GI from known causes, affecting young children and the elderly.107,115,124–127 In both developing and developed countries rotavirus is the leading cause of viral gastroenteritis.68,74,77,108–110 A majority of children become infected with rotavirus by their third birthday,68,76,77,112,125,126,128 with the average age of onset in developing countries being lower than in children in developed countries.112 Waterborne transmission is common, but risk of nosocomial infections in infants and newborns and in childcare settings increases once infection occurs.108,115 The epidemiology of norovirus on the other hand is different as it affects persons of all age groups and has become notorious for causing epidemics on ships, hotels and large gatherings.125,126,129
High rates of parasitic infections have been found in SSA with Cryptosporidium, Cyclospora spp., and Entamoeba spp implicated as common parasites, and is consistent with findings from other developing settings, where sanitation and access to clean water is compromised.25,60,102,130 Blastocystis hominis was frequently isolated from diarrhoeal stools, but there are conflicting views about its role as a pathogen, and some laboratories may not place priority on looking for this parasite.131–133 In addition, limited diagnostic capacity for protozoan pathogens may have influenced what is reported, and the true prevalence may be higher.25,133,134 Advanced biotechnological methods such as PCR will improve the diagnosis and understanding of intestinal parasites.132,134,135 The presence of multiple parasite species is also common and this has further implications for diarrhoea related malnutrition and stunting.23,60,136–138
Prevention and control
Many enteric pathogens are transmitted through similar exposures routes, hence community based multi-stage prevention measures will control several at the same time. Improved sanitation, drinking water quality and hygiene measures have proven to decrease the incidence of diarrhoeal disease by at least 1/3.8,139 There is evidence that hand hygiene alone can reduce incidence by 31–47%,140,141 while low cost household treatment, safe storage and improved water quality can reduce incidence by about 25–35%.139 Except some protozoa, the enteric pathogens are easily controlled by chlorination of water, which can be done at the household level,14,142 supplemented by safe storage and use.139
Public Health significance
The high prevalence of gastrointestinal pathogens in SSA suggests the presence and high risk of exposure to environmental risk factors. Risk factors must therefore be tackled head-on, if countries in SSA are to achieve the target of reducing child deaths by two thirds by the year 2015. Public health programmes must therefore be given priority by governments, with policies supported by sufficiently well trained personnel, modern equipment and legislation. Many countries have the will, but with diarrheal diseases competing with other programmes for scarce resources, the degree of the impact of small interventions can hardly be felt. Currently, less than 5% of funding for research and development goes into diarrhoeal disease.143–145
Conclusions
This review is the first looking at the range of infectious enteric pathogens circulating in SSA, and confirms high rates of isolation of pathogens from diarrhoeal cases; while age related differences were observed and some looked at one category of pathogen, the quality of those included was assured by the peer review process.
Further studies are needed to quantify the prevalence and types of pathogens in circulation in SSA. Public health practitioners can use this information to understanding the challenges related to GI pathogens and set priorities for prevention programmes, and develop multi-stage prevention strategies for increased overall effectiveness.
References
- 1.Victora CG, Bryce J, Fontaine O, Monasch R. Reducing deaths from diarrhoea through oral rehydration therapy. Bull World Health Organ. 2000;78:1246–55. Available from: http://www.scielosp.org/scielo.php?script=sci_arttext&pid=S0042-96862000001000010&nrm=iso. [PMC free article] [PubMed] [Google Scholar]
- 2.Kosek M, Bern C, Guerrant R. The global burden of diarrhoeal disease, as estimated from studies published between 1992 and 2000. Bull World Health Organ. 2003;81:197–204. [PMC free article] [PubMed] [Google Scholar]
- 3.Guerrant RL, Kosek M, Moore S, et al. Magnitude and Impact of Diarrheal Diseases. Arch Med Res. 2002;33:351–5. doi: 10.1016/s0188-4409(02)00379-x. [DOI] [PubMed] [Google Scholar]
- 4.Boschi-Pinto C, Velebit L, Shibuya K. Estimating mortality due to diarrhoea indeveloping countries. Bull World Health Organ. 2008;86:710–7. doi: 10.2471/BLT.07.050054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.United Nations. Millennium Development Goals Report 2008. Available from: http://www.un.org/millenniumgoals/pdf/The%20Millennium%20Development%20Goals%20Report%202008.pdf.
- 6.UNECA. Assessing Progress in Africa towards the Millennium Development Goals Report 2008. Addis Ababa, Ethiopia 2008. Available from: http://www.uneca.org/cfm/2008/docs/AssessingProgressinAfricaMDGs.pdf.
- 7.United Nations. Millennium Development Goals Report 2009. Available from: http://www.un.org/millenniumgoals/pdf/MDG_Report_2009_ENG.pdf.
- 8.Burki T. Slow progress towards sanitation goal. Lancet Infect Dis. 2009;9:531–2. [Google Scholar]
- 9.Schmidt RH, Goodrich RM, Archer DL, Schneider KR. General Overview of the Causative Agents of Foodborne Illness 2003. Available from: http://edis.ifas.ufl.edu/pdffiles/FS/FS09900.pdf. [Google Scholar]
- 10.Gilbert GL. Improving foodborne disease surveillance in NSW. NSW Public Health Bull. 2008;19:1–2. doi: 10.1071/nb07127. [DOI] [PubMed] [Google Scholar]
- 11.Fletcher SM, Li Y, Stark D, Ellis J. Infectious Gastrointestinal Pathogens in Developed and Developing Countries: a Meta-Analysis. Communicable Disease Control Conference 2011; Canberra, ACT, Australia. [Google Scholar]
- 12.Tumwine JK, Thompson J, Katua-Katua M, et al. Diarrhoea and effects of different water sources, sanitation and hygiene behaviour in East Africa. Trop Med Int Health. 2002;7:750–6. doi: 10.1046/j.1365-3156.2002.00927.x. [DOI] [PubMed] [Google Scholar]
- 13.Tiwari S-SK, Schmidt W-P, Darby J, et al. Intermittent slow sand filtration for preventing diarrhoea among children in Kenyan households using unimproved water sources: randomized controlled trial. Trop Med Int Health. 2009;14:1374–82. doi: 10.1111/j.1365-3156.2009.02381.x. [DOI] [PubMed] [Google Scholar]
- 14.Crump JA, Otieno PO, Slutsker L, et al. Household based treatment of drinking water with flocculant-disinfectant for preventing diarrhoea in areas with turbid source water in rural western Kenya: cluster randomised controlled trial. BMJ. 2005;331:478–81. doi: 10.1136/bmj.38512.618681.E0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White A, Schmidt K. Systematic literature reviews. Complement Ther Med. 2005;13:54–60. doi: 10.1016/j.ctim.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Armstrong R, Waters E, Roberts H, et al. International Encyclopedia of Public Health. Academic Press; Oxford, UK: 2008. Systematic Reviews in Public Health; pp. 297–301. [Google Scholar]
- 17.Whiting P, Westwood M, Burke M, et al. Systematic reviews of test accuracy should search a range of databases to identify primary studies. J Clin Epidemiol. 2008;61:357–64. doi: 10.1016/j.jclinepi.2007.05.013. [DOI] [PubMed] [Google Scholar]
- 18.Jarmey-Swan C, Bailey IW, Howgrave-Graham AR. Ubiquity of the water-borne pathogens, Cryptosporidium and Giardia, in KwaZulu-Natal populations. Water SA. 2001;27:57–64. [Google Scholar]
- 19.Baxter E, Rose PD, Kirby R. Age and population group-related distribution of enteropathogens in the Eastern Cape, South-Africa. Letters in Applied Microbiology. 1994;19:442–5. [Google Scholar]
- 20.Chunge RN, Nagelkerke N, Karumba PN, et al. Longitudinal study of young children in Kenya: Intestinal parasitic infection with special reference to Giardia lamblia, its prevalence, incidence and duration, and its association with diarrhoea and with other parasites. Acta Trop. 1991;50:39–49. doi: 10.1016/0001-706x(91)90071-q. [DOI] [PubMed] [Google Scholar]
- 21.Chunge RN, Wamola IA, Kinoti SN, et al. Mixed infections in childhood diarrhoea: results of a community study in Kiambu District, Kenya. East Afr Med J. 1989;66:715–23. [PubMed] [Google Scholar]
- 22.Mutanda LN. Epidemiology of acute gastroenteritis in early childhood in Kenya. III. Distribution of the aetological agents. East Afr Med J. 1980;57:317–26. [PubMed] [Google Scholar]
- 23.Creek TL, Kim A, Lu L, et al. Hospitalization and Mortality Among Primarily Nonbreastfed Children During a Large Outbreak of Diarrhea and Malnutrition in Botswana, 2006. J Acquir Immune Defic Syndr. 2006;53:14–9. doi: 10.1097/QAI.0b013e3181bdf676. [DOI] [PubMed] [Google Scholar]
- 24.Kusiluka LJM, Karimuribo ED, Mdegela RH, et al. Prevalence and impact of water-borne zoonotic pathogens in water, cattle and humans in selected villages in Dodoma Rural and Bagamoyo districts, Tanzania. Phys Chem Earth, Pt A/B/C. 2005;30:818–25. [Google Scholar]
- 25.Roche J, Benito A. Prevalence of intestinal parasite infections with special reference to Entamoeba histolytica on the island of Bioko (Equatorial Guinea) Am J Trop Med Hyg. 1999;60:257–62. doi: 10.4269/ajtmh.1999.60.257. [DOI] [PubMed] [Google Scholar]
- 26.Goh Rowland SGJ, Lloyd-Evans N, Williams K, Rowland MGM. The etiology of diarrhoea studied in the community in young urban Gambian children. J Diarrhoeal Dis Res. 1985;3:7–13. [PubMed] [Google Scholar]
- 27.Shapiro RL, Otieno MR, Adcock PM, et al. Transmission of epidemic Vibrio cholerae O1 in rural western Kenya associated with drinking water from Lake Victoria: An environmental reservoir for cholera? Am J Trop Med Hyg. 1999;60:271–6. doi: 10.4269/ajtmh.1999.60.271. [DOI] [PubMed] [Google Scholar]
- 28.Kaddu-Mulindwa DH, Aisu T, Gleier K, et al. Occurrence of Shiga toxin-producing Escherichia coli in fecal samples from children with diarrhea and from healthy zebu cattle in Uganda. Int J Food Microbiol. 2001;66:95–101. doi: 10.1016/s0168-1605(00)00493-1. [DOI] [PubMed] [Google Scholar]
- 29.Akpede GO, Adeyemi O, Ambe JP. Trends in the susceptibility to antimicrobial drugs of common pathogens in childhood septicaemia in Nigeria: experience at the University of Maiduguri Teaching Hospital, Nigeria, 1991–1994. Int J Antimicrob Agents. 1995;6:91–7. doi: 10.1016/0924-8579(95)00023-9. [DOI] [PubMed] [Google Scholar]
- 30.Dromigny JA, Ndoye B, Macondo EA, et al. Increasing prevalence of antimicrobial resistance among Enterobacteriaceae uropathogens in Dakar, Senegal: a multi-center study. Diagn Microbiol Infect Dis. 2003;47:595–600. doi: 10.1016/s0732-8893(03)00155-x. [DOI] [PubMed] [Google Scholar]
- 31.Shaheen HI, Kamal KA, Wasfy MO, et al. Phenotypic diversity of enterotoxigenic Escherichia coli (ETEC) isolated from cases of travelers' diarrhea in Kenya. Int J Infect Dis. 2003;7:35–41. doi: 10.1016/s1201-9712(03)90040-3. [DOI] [PubMed] [Google Scholar]
- 32.Bourgeois AL, Gardiner CH, Thornton SA, et al. Etiology of acute diarrhea among united-states military personnel deployed to South-America and West-Africa. Am J Trop Med Hyg. 1993;48:243–8. doi: 10.4269/ajtmh.1993.48.243. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Z-D, Brett L, Verenkar MP, Ashley Dea. Prevalence of enteric pathogens among international travelers with diarrhea acquired in Kenya (Mombasa), India (Goa), or Jamaica (Montego Bay) J Infect Dis. 2002;185:497–497. doi: 10.1086/338834. [DOI] [PubMed] [Google Scholar]
- 34.Onyango AO, Kenya EU, Mbithi JJN, Ng'ayo MO. Pathogenic Escherichia coli and food handlers in luxury hotels in Nairobi, Kenya. Travel Med Infect Dis. 2009;7:359–66. doi: 10.1016/j.tmaid.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 35.Posey Drew L, Blackburn B G, Weinberg M, et al. High Prevalence and Presumptive Treatment of Schistosomiasis and Strongyloidiasis among African Refugees. Clin Infect Dis. 2007;45:1310–5. doi: 10.1086/522529. [DOI] [PubMed] [Google Scholar]
- 36.Shultz A, Omollo JO, Burke H, et al. Cholera Outbreak in Kenyan Refugee Camp: Risk Factors for Illness and Importance of Sanitation. Am J Trop Med Hyg. 2009;80:640–5. [PubMed] [Google Scholar]
- 37.Abong'o BO, Momba MNB. Prevalence and potential link between E. coli O157:H7 isolated from drinking water, meat and vegetables and stools of diarrhoeic confirmed and non-confirmed HIV/AIDS patients in the Amathole District - South Africa. J Appl Microbiol. 2008;105:424–31. doi: 10.1111/j.1365-2672.2008.03756.x. [DOI] [PubMed] [Google Scholar]
- 38.Birmingham ME, Lee LA, Ndayimirije N, et al. Epidemic cholera in Burundi: patterns of transmission in the Great Rift Valley Lake region. Lancet. 1997;349:981–5. doi: 10.1016/S0140-6736(96)08478-4. [DOI] [PubMed] [Google Scholar]
- 39.Rappelli P, Folgosa E, Solinas ML, et al. Pathogenic enteric Escherichia coli in children with and without diarrhea in Maputo, Mozambique. FEMS Immunol Med Microbiol. 2005;43:67–72. doi: 10.1016/j.femsim.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 40.Samie A, Bessong PO, Obi CL, et al. Cryptosporidium species: Preliminary descriptions of the prevalence and genotype distribution among school children and hospital patients in the Venda region, Limpopo Province, South Africa. Exp Parasitol. 2006;114:314–22. doi: 10.1016/j.exppara.2006.04.007. [DOI] [PubMed] [Google Scholar]
- 41.Steinsland H, Valentiner-Branth P, Perch M, et al. Enterotoxigenic Escherichia coli Infections and Diarrhea in a Cohort of Young Children in Guinea-Bissau. J Infect Dis. 2002;186:1740–1740. doi: 10.1086/345817. [DOI] [PubMed] [Google Scholar]
- 42.Binka FN, Anto FK, Oduro AR, et al. Incidence and risk factors of paediatric rotavirus diarrhoea in northern Ghana. Trop Med Int Health. 2003;8:840–6. doi: 10.1046/j.1365-3156.2003.01097.x. [DOI] [PubMed] [Google Scholar]
- 43.Usha G, Chunderika M, Prashini M, et al. Characterization of extended-spectrum [beta]-lactamases in Salmonella spp. at a tertiary hospital in Durban, South Africa. Diagn Microbiol Infect Dis. 2008;62:86–91. doi: 10.1016/j.diagmicrobio.2008.04.014. [DOI] [PubMed] [Google Scholar]
- 44.Aminu M, Esona M, Geyer A, Steele AD. Epidemiology of rotavirus and astrovirus infections in children in Northwestern Nigeria. Ann Afr Med. 2008;7:168–74. doi: 10.4103/1596-3519.55658. [DOI] [PubMed] [Google Scholar]
- 45.Fodha I, Chouikha A, Fredj MBH, et al. PVII-9 Evolution of group A rotavirus strains circulating in Tunisia over a 13-years period (1995–2007) J Clin Virol. 2009;46:S39–S40. [Google Scholar]
- 46.Graham SM, Walsh AL, Molyneux EM, et al. Clinical presentation of non-typhoidal Salmonella bacteraemia in Malawian children. Trans R Soc Trop Med Hyg. 2000;94:310–4. doi: 10.1016/s0035-9203(00)90337-7. [DOI] [PubMed] [Google Scholar]
- 47.Modjarrad K, Zulu I, Redden DT, et al. Prevalence and predictors of intestinal helminth infections among human immunodeficiency virus type 1-infected adults in an urban African setting. Am J Trop Med Hyg. 2005;73:777–82. [PMC free article] [PubMed] [Google Scholar]
- 48.Kasule M, Sebunya TK, Gashe BA, et al. Detection and characterization of human rotavirus among children with diarrhoea in Botswana. Trop Med Int Health. 2003;8:1137–42. doi: 10.1046/j.1360-2276.2003.01141.x. [DOI] [PubMed] [Google Scholar]
- 49.van Gool T, Luderhoff E, Nathoo KJ, et al. High prevalence of Enterocytozoon bieneusi infections among HIV-positive individuals with persistent diarrhoea in Harare, Zimbabwe. Trans R Soc Trop Med Hyg. 1995;89:478–80. doi: 10.1016/0035-9203(95)90073-x. [DOI] [PubMed] [Google Scholar]
- 50.Malakooti MA, Alaii J, Shanks GD, Phillips-Howard PA. Epidemic dysentery in western Kenya. Trans R Soc Trop Med Hyg. 1997;91:541–3. doi: 10.1016/s0035-9203(97)90018-3. [DOI] [PubMed] [Google Scholar]
- 51.Nicholas MK, Rose K, Grace I, et al. The Epidemiology of Human Rotavirus Associated with Diarrhoea in Kenyan Children: A Review. J Trop Pediatr. 2008;54:401–5. doi: 10.1093/tropej/fmn052. [DOI] [PubMed] [Google Scholar]
- 52.Nkinin SW, Asonganyi T, Didier ES, Kaneshiro ES. Microsporidian Infection Is Prevalent in Healthy People in Cameroon. J Clin Microbiol. 2007;45:2841–6. doi: 10.1128/JCM.00328-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kandala N-B, Emina JB, Nzita PDK, Cappuccio FP. Diarrhoea, acute respiratory infection, and fever among children in the Democratic Republic of Congo. Soc Sci Med. 2009;68:1728–36. doi: 10.1016/j.socscimed.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Sodemann M, Jakobsen MS, Mølbak K, et al. Episode-specific risk factors for progression of acute diarrhoea to persistent diarrhoea in West African children. Trans R Soc Trop Med Hyg. 1999;93:65–8. doi: 10.1016/s0035-9203(99)90183-9. [DOI] [PubMed] [Google Scholar]
- 55.Tornheim JA, Manya AS, Oyando N, et al. The epidemiology of hospitalization with diarrhea in rural Kenya: the utility of existing health facility data in developing countries. Int J Infect Dis. 2010;16:e499–505. doi: 10.1016/j.ijid.2009.07.021. [DOI] [PubMed] [Google Scholar]
- 56.Kandala N-B, Magadi MA, Madise NJ. An investigation of district spatial variations of childhood diarrhoea and fever morbidity in Malawi. Soc Sci Med. 2006;62:1138–52. doi: 10.1016/j.socscimed.2005.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ahmed AA, Osman H, Mansour AM, et al. Antimicrobial agent resistance in bacterial isolates from patients with diarrhea and urinary tract infection in the Sudan. Am J Trop Med Hyg. 2000;63:259–63. [PubMed] [Google Scholar]
- 58.Areeshi M, Dove W, Papaventsis D, et al. Cryptosporidium species causing acute diarrhoea in children in Antananarivo, Madagascar. Ann Trop Med Parasitol. 2008;102:309–15. doi: 10.1179/136485908X278793. [DOI] [PubMed] [Google Scholar]
- 59.Colebunders R, Francis H, Mann JM, et al. Persistent diarrhea, strongly associated with HIV infection in Kinshasa, Zaire. Am J Gastroenterol. 1987;82:859–64. [PubMed] [Google Scholar]
- 60.Raso G, Luginbuhl A, Adjoua CA, et al. Multiple parasite infections and their relationship to self-reported morbidity in a community of rural Cote d'Ivoire. Int J Epidemiol. 2004;33:1092–102. doi: 10.1093/ije/dyh241. [DOI] [PubMed] [Google Scholar]
- 61.Haberberger RL, Lissner CR, Podgore JK, et al. Etiology of acute diarrhea among United-States Embassy personnel and dependents in Cairo, Egypt. Am J Trop Med Hyg. 1994;51:870–4. doi: 10.4269/ajtmh.1994.51.870. [DOI] [PubMed] [Google Scholar]
- 62.El-Sharoud WM. Prevalence and survival of Campylobacter in Egyptian dairy products. Food Res Int. 2009;42:622–6. [Google Scholar]
- 63.Khoury H, Ogilvie I, El Khoury A, et al. Burden of rotavirus gastroenteritis in the Middle Eastern and North African pediatric population. BMC Infect Dis. 2011;11:9–9. doi: 10.1186/1471-2334-11-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okeke IN. Diarrheagenic Escherichia coli in sub-Saharan Africa: Status, Uncertainties and Necessities. J Infect Dev Ctries. 2009;3:817–42. doi: 10.3855/jidc.586. [DOI] [PubMed] [Google Scholar]
- 65.Cunliffe NA, Kilgore PE. Epidemiology of rotavirus diarrhoea in Africa: A review to assess the need for rotavirus. Bull World Health Organ. 1998;76:525–37. [PMC free article] [PubMed] [Google Scholar]
- 66.Sanchez-Padilla E, Grais RF, Guerin PJ, et al. Burden of disease and circulating serotypes of rotavirus infection in sub-Saharan Africa: systematic review and meta-analysis. Lancet Infect Dis. 2009;9:567–76. doi: 10.1016/S1473-3099(09)70179-3. [DOI] [PubMed] [Google Scholar]
- 67.Vlieghe E, Phoba MF, Tamfun JJM, Jacobs J. Antibiotic resistance among bacterial pathogens in Central Africa: a review of the published literature between 1955 and 2008. Int J Antimicrob Agents. 2009;34:295–303. doi: 10.1016/j.ijantimicag.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 68.Valentiner-Branth P, Steinsland H, Fischer T, et al. Cohort Study of Guinea Children: Incidence, Pathogenicity, Conferred Protection, and Attributable Risk for Enteropathogens during the First 2 Years of Life. J Clin Microbiol. 2003;41:4238–45. doi: 10.1128/JCM.41.9.4238-4245.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.van Eijk AM, Brooks JT, Adcock PM, et al. Diarrhea in children less than two years of age with known HIV status in Kisumu, Kenya. Int J Infect Dis. 2010;14:e220–5. doi: 10.1016/j.ijid.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 70.Ogbu O, Agumadu N, Uneke CJ, Amadi ES. Aetiology of Acute Infantile Diarrhoea in the south-Eastern Nigeria: An Assessment Of Microbiological And Antibiotic Sensitivity Profile. Internet Journal of Third World Medicine. 2008;7:1–1. [Google Scholar]
- 71.Anyanwu BN. The aetiologic agents of bacterial diarrhoea in the children of the former East Central State of Nigeria. Int J Environ Health Res. 1997;7:215–32. [Google Scholar]
- 72.Nakano T, Kamiya H, Matsubayashi N, et al. Diagnosis of bacterial enteric infections in children in Zambia. Pediatr Int. 1998;40:259–63. doi: 10.1111/j.1442-200x.1998.tb01924.x. [DOI] [PubMed] [Google Scholar]
- 73.Mandomando IM, Macete EV, Ruiz J, et al. Etiology Of Diarrhea In Children Younger Than 5 Years Of Age Admitted In A Rural Hospital Of Southern Mozambique. Am J Trop Med Hyg. 2007;76:522–7. [PubMed] [Google Scholar]
- 74.Yongsi HBN. Pathogenic Microorganisms Associated with Childhood Diarrhoea in Low-and-Middle Income Countries: Case Study of Youande-Cameroon. Int J Environ Res Public Health. 2008;5:213–29. doi: 10.3390/ijerph5040213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Vargas M, Gascon J, Casals C, et al. Etiology Of Diarrhea In Children Less Than Five Years Of Age In Ifakara, Tanzania. Am J Trop Med Hyg. 2004;70:536–9. [PubMed] [Google Scholar]
- 76.Reither K, Ignatius R, Weitzel T, et al. Acute childhood diarrhoea in northern Ghana: epidemiological, clinical and microbiological characteristics. BMC Infect Dis. 2007;7:104–104. doi: 10.1186/1471-2334-7-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Georges MC, Wachsmuth IK, Meunier DMV, Nebout N. Parasitic, Bacterial and Viral Enteric Pathogens Associated with Diarrhoea in Central African Republic. J Clin Microbiol. 1984;19:571–5. doi: 10.1128/jcm.19.5.571-575.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Henry MC, Alary M, Desmet P, et al. Community survey of Diarrhooea in children under five years in Kinshasa Zaire. Ann Soc Belge Med Trop. 1995;75:105–14. [PubMed] [Google Scholar]
- 79.De Mol P, Hemelhof W, Butzler JP, Brasseur D, Kalala T, Vis HL. Enteropathogenic agents in children with diarrhoea in rural Zaire. Lancet. 1983;321:516–8. doi: 10.1016/s0140-6736(83)92202-x. [DOI] [PubMed] [Google Scholar]
- 80.Ogunsanya TI, Rotimi VO, Adenuga A. A study of the aetiological agents of childhood diarrhoea in Lagos, Nigeria. J Med Microbiol. 1994;40:10–4. doi: 10.1099/00222615-40-1-10. [DOI] [PubMed] [Google Scholar]
- 81.Olowe OA, Olayemi AB, Eniola KIT, Adeyeba OA. Aetiology agents of diarrhoea in children under five years of age in Osogbo, Osun State. Afr J Clin Exper Microbiol. 2003;4:62–6. [Google Scholar]
- 82.Cajetan ICI, Nnennaya IR, Casmir AA, Florence IN. Enteric Bacteria Pathogens Associated With Diarrhoea of Children in the Federal Capital Territory Abuja, Nigeria. New York Science Journal. 2010;3:62–9. [Google Scholar]
- 83.Djeneba O, Damintoti K, Denise I, et al. Prevalence of rotavirus adenovirus and enteric parasites amongst paediatric patients attending Saint Camille Medical Centre in Ouagadougou. Pak J Biol Sci. 2007;10:4266–70. doi: 10.3923/pjbs.2007.4266.4270. [DOI] [PubMed] [Google Scholar]
- 84.Djie-Maletz A, Reither K, Danour S, et al. High rate of resistance to locally used antibiotics among enteric bacteria from children in Northern Ghana. J Antimicrob Chemother. 2008;61:1315–8. doi: 10.1093/jac/dkn108. [DOI] [PubMed] [Google Scholar]
- 85.Musiime V, Kalyesubula I, Kaddu-Mulindwa D, Byarugaba J. Enteric Bacterial Pathogens in HIV-Infected Children With Acute Diarrhea in Mulago Referral and Teaching Hospital, Kampala, Uganda. J Int Assoc Physicians AIDS Care (Chic) 2009;8:185–90. doi: 10.1177/1545109709333082. [DOI] [PubMed] [Google Scholar]
- 86.Pitman C, Amali R, Kanyerere H, et al. Bloody diarrhoea of adults in Malawi: clinical features, infectious agents, and antimicrobial sensitivities. Trans R Soc Trop Med Hyg. 1996;90:284–7. doi: 10.1016/s0035-9203(96)90251-5. [DOI] [PubMed] [Google Scholar]
- 87.Brink AK, Mahé C, Watera C, et al. Diarrhoea, CD4 Counts and Enteric Infections in a Community-Based Cohort of HIV-Infected Adults in Uganda. J Infect. 2002;45:99–106. doi: 10.1053/jinf.2002.1002. [DOI] [PubMed] [Google Scholar]
- 88.Kelly P, Baboo Ks, Wolff M, et al. The prevalence and aetiology of persistent diarrhoea in adults in urban Zambia. Acta Tropica. 1996;61:183–90. doi: 10.1016/0001-706x(95)00142-2. [DOI] [PubMed] [Google Scholar]
- 89.Kelly P, Todd J, Sianongo S, et al. Susceptibility to intestinal infection and diarrhoea in Zambian adults in relation to HIV status and CD4 count. BMC Gastroenterol. 2009;9:7–7. doi: 10.1186/1471-230X-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Germani Y, Minssart P, Vohito M, et al. Etiologies of acute, persistent, and dysenteric diarrheas in adults in Bangui, Central African Republic, in relation to human immunodeficiency virus serostatus. Am J Trop Med Hyg. 1998;59:1008–14. doi: 10.4269/ajtmh.1998.59.1008. [DOI] [PubMed] [Google Scholar]
- 91.Obi CL, Potgieter N, Bessong PO, et al. Prevalence of pathogenic bacteria and rotaviruses in stools of patients presenting with diarrhoea from rural communities in Venda, South Africa. South Afr J Sci. 2003;99:589–92. [Google Scholar]
- 92.Bonfiglio G, Simporè J, Pignatelli S, et al. Epidemiology of bacterial resistance in gastro-intestinal pathogens in a tropical area. Int J Antimicrob Agents. 2002;20:387–9. doi: 10.1016/s0924-8579(02)00208-x. [DOI] [PubMed] [Google Scholar]
- 93.Brooks JT, Ochieng JB, Kumar L, et al. Surveillance for Bacterial Diarrhea and Antimicrobial Resistance in Rural Western Kenya, 1997–2003. Clin Infect Dis. 2006;43:393–401. doi: 10.1086/505866. [DOI] [PubMed] [Google Scholar]
- 94.Brooks JT, Shapiro RL, Kumar L, et al. Epidemiology of sporadic bloody diarrhea in rural Western Kenya. Am J Trop Med Hyg. 2003;68:671–7. [PubMed] [Google Scholar]
- 95.Borenstein M, Hedges L, Higgins J, Rothstein H. Comprehensive Meta-analysis. Version 2. Biostat Inc.; Englewood, NJ, USA: 2005. [Google Scholar]
- 96.Borenstein M, Hedges L, Higgins J, Rothstein H. Introduction to Meta-Analysis. John Wiley and Sons, Ltd.; West Sussex, UK: 2009. [Google Scholar]
- 97.Jackson D, Bowden J, Baker R. How does the DerSimonian and Laird procedure for random effects meta-analysis compare with its more efficient but harder to compute counterparts? J Stat Plann Infer. 2010;140:961–70. [Google Scholar]
- 98.Bonfiglio G, Simporè J, Pignatelli S, Musumeci S, Solinas ML. Epidemiology of bacterial resistance in gastro-intestinal pathogens in a tropical area. Int J Antimicrob Agents. 2002;20:387–9. doi: 10.1016/s0924-8579(02)00208-x. [DOI] [PubMed] [Google Scholar]
- 99.Brooks JT, Ochieng JB, Kumar L, et al. Surveillance for Bacterial Diarrhea and Antimicrobial Resistance in Rural Western Kenya, 1997–2003. Clin Infect Dis. 2006;43:393–401. doi: 10.1086/505866. [DOI] [PubMed] [Google Scholar]
- 100.Anyanwu BN. The aetiologic agents of bacterial diarrhoea in the children of the former East Central State of Nigeria. Int J Environ Health Res. 1993;7:215–32. [Google Scholar]
- 101.Brink AK, Mahé C, Watera C, et al. Diarrhoea, CD4 Counts and Enteric Infections in a Community-Based Cohort of HIV-Infected Adults in Uganda. J Infect. 2002;45:99–106. doi: 10.1053/jinf.2002.1002. [DOI] [PubMed] [Google Scholar]
- 102.O'Ryan M, Prado V, Pickering LK. A millennium update on pediatric diarrheal illness in the developing world. Semin Pediatr Infect Dis. 2005;16:125–36. doi: 10.1053/j.spid.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 103.Rossit AR, de Almeida MT, Nogueira CA, et al. Bacterial, yeast, parasitic, and viral enteropathogens in HIV-infected children from São Paulo State, Southeastern Brazil. Diagn Microbiol Infect Dis. 2007;57:59–66. doi: 10.1016/j.diagmicrobio.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 104.Chhin S, Harwell JI, Bell JD, et al. Etiology of Chronic Diarrhea in Antiretroviral Naive Patients with HIV Infection Admitted to Norodom Sihanouk Hospital, Phnom Penh, Cambodia. Clin Infect Dis. 2006;43:925–32. doi: 10.1086/507531. [DOI] [PubMed] [Google Scholar]
- 105.Day L, Langley J, Voaklander D, et al. Minimizing Bias in a Case-Control Study of Farm Injury. J Agric Saf Health. 2005;11:175–84. doi: 10.13031/2013.18184. [DOI] [PubMed] [Google Scholar]
- 106.Hall G, Kirk M. Foodborne illness in Australia. Annual incidence circa 2000. Report: Australian Government, Department of Health and Ageing. 2005
- 107.Mead P, Slutsker L, Dietz V, et al. Food-related illness and death in the United States. Emerg Infect Dis. 1999;5:607–25. doi: 10.3201/eid0505.990502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dutta P, Lahiri M, Sen D, Pal S. Prospective hospital based study on persistent diarhoea. Gut. 1991;32:787–90. doi: 10.1136/gut.32.7.787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Cohen MB. Etiology and mechanisms of acute infectious diarrhea in infants in the United States. J Pediatr. 1991;118:S34–9. doi: 10.1016/s0022-3476(05)81423-4. [DOI] [PubMed] [Google Scholar]
- 110.Boga JA, Melon S, Nicieza I, et al. Etiology of Sporadic cases of pediatric Acute Gastroenteritis in Asturia, Spain, and Genotyping and Characterization of Norovirus Strains Involved. J Clin Microbiol. 2004;42:2668–74. doi: 10.1128/JCM.42.6.2668-2674.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hellard ME, Sinclair MI, Hogg GG, Fairley CK. Prevalence of enteric pathogens among community based asymptomatic individuals. J Gastroenterol Hepatol. 2000;15:290–3. doi: 10.1046/j.1440-1746.2000.02089.x. [DOI] [PubMed] [Google Scholar]
- 112.Podewils LJ, Mintz ED, Nataro JP, Parashar UD. Acute, infectious diarrhea among children in developing countries. Semin Pediatr Infect Dis. 2004;15:155–68. doi: 10.1053/j.spid.2004.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kuntz TB, Kuntz ST. Enterohemorrhagic E Coli infection. Primary Care Update for OB/GYNS. 1999;6:192–6. [Google Scholar]
- 114.Wanke C, Sears CL. International Encyclopedia of Public Health. Academic Press; Oxford, UK: 2008. Escherichia coli; pp. 452–59. [Google Scholar]
- 115.Heymann DL. 19 ed. American Public Health Association; Washington, DC, USA: 2008. Control of Communicable Diseases Manual. [Google Scholar]
- 116.Nguyen TV, Le Van P, Le Huy C, et al. Etiology and epidemiology of diarrhea in children in Hanoi, Vietnam. Int J Infect Dis. 2006;10:298–308. doi: 10.1016/j.ijid.2005.05.009. [DOI] [PubMed] [Google Scholar]
- 117.Lamps LW. Infective disorders of the gastrointestinal tract. Histopathology. 2007;50:55–63. doi: 10.1111/j.1365-2559.2006.02544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Okeke IN, Nataro JP. Enteroaggregative Escherichia coli. Lancet Infect Dis. 2001;1:304–13. doi: 10.1016/S1473-3099(01)00144-X. [DOI] [PubMed] [Google Scholar]
- 119.Huang DB, DuPont HL. Enteroaggregative Escherichia coli: An emerging pathogen in children. Semin Pediatr Infect Dis. 2004;15:266–71. doi: 10.1053/j.spid.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 120.Gross RJ. Escherichia coli diarrhoea. J Infect. 1983;7:177–92. doi: 10.1016/s0163-4453(83)96953-0. [DOI] [PubMed] [Google Scholar]
- 121.Olsvik Ø, Wasteson Y, Lund A, Hornes E. Pathogenic Escherichia coli found in food. Int J Food Microbiol. 1991;12:103–13. doi: 10.1016/0168-1605(91)90051-p. [DOI] [PubMed] [Google Scholar]
- 122.Bahrani-Mougeot FK, Scobey MW, Sansonetti PJ, Moselio S. Encyclopedia of Microbiology. Academic Press; Oxford, UK: 2009. Enteropa-thogenic Infections; pp. 329–43. [Google Scholar]
- 123.McIver CJ. Australian Society for Microbiology Publ.; Melbourne, Asutralia: 2005. A compendium of laboratory diagnostic methods for common and unusual enteric pathogens: an Australian perspective. [Google Scholar]
- 124.Kang G, Kris H. International Encyclopedia of Public Health. Academic Press; Oxford, UK: 2008. Viral Diarrhea; pp. 518–26. [Google Scholar]
- 125.Schlenker C, Surawicz CM. Emerging infections of the Gastrointestinal tract. Best Pract Res Clin Gastroenterol. 2009;23:89–99. doi: 10.1016/j.bpg.2008.11.014. [DOI] [PubMed] [Google Scholar]
- 126.Hamer DH, Gorbach SL, Kris H. International Encyclopedia of Public Health. Academic Press; Oxford, UK: 2008. Intestinal Infections: Overview; pp. 683–95. [Google Scholar]
- 127.Fabiana A, Donia D, Gabrieli R, et al. Influence of enteric viruses on gastroenteritis in Albania: Epidemiological and molecular analysis. J Med Virol. 2007;79:1844–9. doi: 10.1002/jmv.21001. [DOI] [PubMed] [Google Scholar]
- 128.Bereciartu A, Bok K, Gómez J. Identification of viral agents causing gastroenteritis among children in Buenos Aires, Argentina. J Clin Virol. 2002;25:197–203. doi: 10.1016/s1386-6532(02)00010-0. [DOI] [PubMed] [Google Scholar]
- 129.Butt AA, Aldridge KE, Sanders CV. Infections related to the ingestion of seafood Part I: viral and bacterial infections. Lancet Infect Dis. 2004;4:201–12. doi: 10.1016/S1473-3099(04)00969-7. [DOI] [PubMed] [Google Scholar]
- 130.Mor Siobhan M, Tzipori S. Cryptosporidiosis in Children in Sub-Saharan Africa: A Lingering Challenge. Clin Infect Dis. 2008;47:915–21. doi: 10.1086/591539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Stark D, Barratt JLN, van Hal S, et al. Clinical Significance of Enteric Protozoa in the Immunosuppressed Human Population. Clin Microbiol Rev. 2009;22:634–50. doi: 10.1128/CMR.00017-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Jones M, Whipps C, Ganac R, et al. Association of Blastocystis subtype 3 and 1 with patients from an Oregon community presenting with chronic gastrointestinal illness. Parasitol Res. 2009;104:341–5. doi: 10.1007/s00436-008-1198-7. [DOI] [PubMed] [Google Scholar]
- 133.Boorom K, Smith H, Nimri L, Viscogliosi E, Spanakos G, Parkar U, et al. Oh my aching gut: irritable bowel syndrome, Blastocystis, and asymptomatic infection. Parasit Vectors. 2008;1:40–40. doi: 10.1186/1756-3305-1-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Stark D, Al-Qassab SE, Barratt JLN, et al. Evaluation of Multiplex Tandem Real-Time PCR for Detection of Cryptosporidium spp., Dientamoeba fragRilis, Entamoeba histolytica, and Giardia intestinalis in Clinical Stool Samples. J Clin Microbiol. 2011;49:257–62. doi: 10.1128/JCM.01796-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Özyurt M, Kurt Ö, Mølbak K, et al. Molecular epidemiology of Blastocystis infections in Turkey. Parasitol Int. 2008;57:300–6. doi: 10.1016/j.parint.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 136.Checkley W, Buckley G, Gilman RH, et al. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol. 2008;37:816–30. doi: 10.1093/ije/dyn099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Baqui AH, Ahmed T. Diarrhoea and malnutrition in children. BMJ. 2006;332:378–378. doi: 10.1136/bmj.332.7538.378. doi: 10.1136/bmj.332.7538.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dillingham RA, Lima AA, Guerrant RL. Cryptosporidiosis: epidemiology and impact. Microbes Infect. 2002;4:1059–66. doi: 10.1016/s1286-4579(02)01630-1. [DOI] [PubMed] [Google Scholar]
- 139.Fewtrell L, Kaufmann RB, Kay D, et al. Water, sanitation, and hygiene interventions to reduce diarrhoea in less developed countries: a systematic review and meta-analysis. Lancet Infect Dis. 2005;5:42–52. doi: 10.1016/S1473-3099(04)01253-8. [DOI] [PubMed] [Google Scholar]
- 140.Curtis V, Cairncross S. Effect of washing hands with soap on diarrhoea risk in the community: a systematic review. Lancet Infect Dis. 2003;3:275–81. doi: 10.1016/s1473-3099(03)00606-6. [DOI] [PubMed] [Google Scholar]
- 141.Aiello AE, Coulborn RM, Perez V, Larson EL. Effect of hand hygiene on infectious disease risk in the community setting: a meta-analysis. Am J Public Health. 2008;98:1372–81. doi: 10.2105/AJPH.2007.124610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Ashbolt NJ. Microbial contamination of drinking water and disease outcomes in developing regions. Toxicology. 2004;198:229–38. doi: 10.1016/j.tox.2004.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Moran M, Guzman J, Henderson K, et al. Neglected Disease Research & Development: New Times, New Trends. The George Institute for International Health, Division HP. 2009 Available from: http://www.ghtcoalition.org/files/gfinder_dec2009.pdf.
- 144.[No authors listed]. More research needed into childhood diarrhoea. Indian J Med Sci. 2009;63:126–7. [PubMed] [Google Scholar]
- 145.Rudan I, El Arifeen S, Black RE, Campbell H. Childhood pneumonia and diarrhoea: setting our priorities right. Lancet Infect Dis. 2007;7:56–61. doi: 10.1016/S1473-3099(06)70687-9. [DOI] [PubMed] [Google Scholar]


