Abstract
Background: International travel can expose travellers to pathogens not commonly found in their countries of residence, like dengue virus. Travellers and the clinicians who advise and treat them have unique needs for understanding the geographic extent of risk for dengue. Specifically, they should assess the need for prevention measures before travel and ensure appropriate treatment of illness post-travel. Previous dengue-risk maps published in the Centers for Disease Control and Prevention’s Yellow Book lacked specificity, as there was a binary (risk, no risk) classification. We developed a process to compile evidence, evaluate it and apply more informative risk classifications.
Methods: We collected more than 839 observations from official reports, ProMED reports and published scientific research for the period 2005–2014. We classified each location as frequent/continuous risk if there was evidence of more than 10 dengue cases in at least three of the previous 10 years. For locations that did not fit this criterion, we classified locations as sporadic/uncertain risk if the location had evidence of at least one locally acquired dengue case during the last 10 years. We used expert opinion in limited instances to augment available data in areas where data were sparse.
Results: Initial categorizations classified 134 areas as frequent/continuous and 140 areas as sporadic/uncertain. CDC subject matter experts reviewed all initial frequent/continuous and sporadic/uncertain categorizations and the previously uncategorized areas. From this review, most categorizations stayed the same; however, 11 categorizations changed from the initial determinations.
Conclusions: These new risk classifications enable detailed consideration of dengue risk, with clearer meaning and a direct link to the evidence that supports the specific classification. Since many infectious diseases have dynamic risk, strong geographical heterogeneities and varying data quality and availability, using this approach for other diseases can improve the accuracy, clarity and transparency of risk communication.
Keywords: Dengue, clinical guidance, epidemiology, risk maps
Introduction
International travel can expose travellers to pathogens not commonly found in their countries of residence. One example is dengue, a potentially fatal acute illness caused by the mosquito-borne dengue viruses (DENV-1–4). Dengue prevention focuses on avoiding mosquito bites (e.g. using repellent or clothing that covers the skin). In 2010, there were an estimated 280–530 million DENV infections globally and 70–140 million clinically apparent cases. 1 Dengue is characterized by fever, headache and muscle/joint pain, which can be similar to other acute febrile illnesses. Because of this similarity, it can be difficult to identify and properly treat dengue cases. Clinicians need to have up-to-date guidance as to where DENVs may circulate in order to include dengue in differential diagnoses.
An international traveller’s risk for infection with a DENV depends on the local prevalence of infection and exposure to vector mosquitoes. Through websites (www.cdc.gov/travel; www.cdc.gov/dengue) and print material, such as the Health Information for International Travel or “The Yellow Book,” the U.S. Centers for Disease Control and Prevention (CDC) Travelers’ Health and Dengue Branches regularly publish information on dengue for U.S. travellers and clinicians. 2 The Yellow Book is a primary resource for clinicians who are preparing patients for international travel, as well as for those who are evaluating and treating ill patients who have recently returned from international travel. The Yellow Book includes maps to communicate areas where dengue is a risk.
Previous Yellow Book dengue maps used a binary classification of “dengue risk” or “no known dengue risk” to depict risk areas, primarily at the country level; however, this presented several challenges. First, interpretation was difficult because no specific definitions were associated with risk. Second, a binary classification made it impossible to distinguish levels of risk (e.g. areas with sporadic outbreaks vs endemic, year-round DENV transmission). Specifically, countries without effective surveillance systems appeared in the same “no known risk” category as countries known to be free of dengue, even if DENV transmission was suspected or possible in those countries. Finally, data used to classify risk area were not described.
Recent efforts to compile diverse datasets have led to new opportunities to connect evidence to risk maps. In 2012, Brady et al. 3 compiled an extensive database of global dengue epidemiology and used a novel algorithm to produce a global map with nine levels of evidence consensus from “complete absence” to “complete presence”. Though scientifically rigorous, this map was not intended for direct interpretation by clinicians or travellers faced with travel health-related decisions. In developing the 2016 Yellow Book dengue maps, we recognized the need to improve the accuracy, clarity and transparency of the risk classifications. Therefore, we sought to develop risk classifications that would be easily interpretable by clinicians and travellers. Further, we sought to provide evidence-based definitions for each risk classification, and used those definitions to translate the data into the new risk map. Finally, we sought to construct a map that would clearly represent the dengue-risk areas for clinicians and travellers.
Methods
Data Collection
The global dengue epidemiology database, with records from 1960 to 2012, is more extensively described elsewhere 3 , 4 ; however, we also included the database’s records from 2012 through 2014 for this assessment. In brief, database sources included dengue surveillance data, official country reports, ProMED reports and published research. Sources reported record(s) of cases due to local DENV transmission, with each situation representing a unique time and location. Geographic locations were recorded at the finest administrative area reported: country (administrative level-0), state (administrative level-1) or county (administrative level-2). The boundaries for these administrative areas are based on the 2014 Global Administrative Unit Layer (GAUL) dataset. 5
Risk Classifications
We designed risk classifications to account for the immediacy of risk but to also allow for the potential scarcity of data. Therefore, we included only data from the past 10 years (2005–2014). First, we aimed to identify areas where dengue is always considered a risk (endemic areas with periodic epidemics). Although dengue may be a constant risk in these areas, cases may only be reported during epidemics. Since dengue epidemics typically occur every 3–5 years, 6 we assumed that endemic areas would be most likely to report more than 10 dengue cases in at least three distinct years over the most recent 10-year period. We classified these areas as frequent/continuous risk. For areas that did not meet the frequent/continuous definition, we sought to classify areas with at least some, or sporadic, risk. This was defined as any area with at least one reported, locally acquired case in the previous 10 years. Therefore, we included areas with either sporadic DENV transmission or sparse information about more frequent transmission, recognizing that distinguishing between these two possibilities is difficult. We called this risk level sporadic/uncertain. Areas with no reports of DENV transmission were classified as no evidence of risk.
Assigning Geographic Areas into Risk Classifications
We assigned risk classifications to geographic locations relative to the finest administrative area reported. Differences due to reporting at the administrative level were attributable primarily to the type of report from which the information came (e.g. outbreaks reported at the county level). To identify areas of potential misclassification, subject matter experts at CDC reviewed all classifications of areas and compared maps with previous Yellow Book maps. Expert opinion was used to change the classification of select areas that (a) were adjacent to areas classified as frequent/continuous and had similar climate, (b) had reports of dengue cases more than 10 years earlier that experts felt were relevant to current classifications, (c) had seroprevalence data indicative of transmission in the absence of incidence data or (d) had reports of dengue-like illness in the absence of diagnostic testing.
Results
The database contained 839 unique records for the years 2005–2014. Initial categorizations classified 134 areas as frequent/continuous, of which 59 were admin-0, 73 were admin-1 and 2 were admin-2 levels. Further, 140 areas were initially classified as sporadic/uncertain (70 admin-0; 52 admin-1; 18 admin-2). Three potential risk areas were included on previous Yellow Book maps but were not included in the global database. This was primarily due to differences in geographic administrative areas between the global database and previous CDC maps.
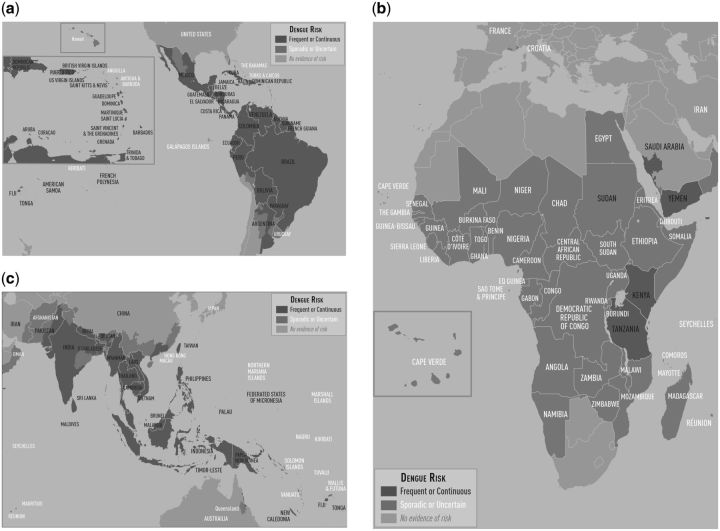
CDC subject matter experts reviewed all initial frequent/continuous and sporadic/uncertain categorizations and the previously uncategorized areas. From this review, most categorizations stayed the same; however, 11 categorizations changed from the initial determinations (Table 1). Of these 11, 5 moved from sporadic/uncertain to frequent/continuous, and 3 moved from sporadic/uncertain to no evidence of risk. For example, Kenya was initially classified as sporadic/uncertain; however, expert review determined it should be classified as frequent/continuous based on recent reports. 7–9 Similarly, Haiti was initially classified as sporadic/uncertain; however, it was categorized as frequent/continuous after review on the basis of its adjacency to the Dominican Republic, strong historical record of dengue in the 1990s, and observations that both surveillance and case reporting is inconsistent. 10 , 11 Of the previously uncategorized areas, three states in northern India were determined to be sporadic/uncertain based on their proximity to other frequent/continuous areas. Final classifications categorized 139 frequent/continuous areas, of which 63 were admin-0, 73 were admin-1 and 3 were admin-2 levels. Further, 136 areas were categorized as sporadic/uncertain (65 admin-0, 54 admin-1 and 17 admin-2) (Figure 1, Supplementary File).
Table 1.
Classification changes based on CDC SME review
| Location name | Global database classification | Classification after CDC SME review | Justification |
|---|---|---|---|
| Papua New Guinea | Sporadic/Uncertain | Frequent/Continuous | Data for Papua New Guinea were sparse, but its proximity to Indonesia, where dengue is endemic suggests risk is likely higher |
| Haiti | Sporadic/Uncertain | Frequent/Continuous | Dengue surveillance in Haiti is inconsistent. However, dengue is endemic in Hispaniola and outbreaks were reported in the 1990s 10 , 11 |
| Kenya | Sporadic/Uncertain | Frequent/Continuous | Recent evidence of dengue in Kenya suggests it is more common than has been reported. 7 , 8 , 9 |
| Tanzania | Sporadic/Uncertain | Frequent/Continuous | Recent evidence of dengue in Tanzania suggests it is more common than has been reported 13 , 14 |
| US Virgin Islands | Sporadic/Uncertain | Frequent/Continuous | Surveillance data in the USVI suggests dengue is more common than has been reported |
| Santa Fe, New Mexico | Sporadic/Uncertain | No evidence of risk | The single case associated with travel to New Mexico 15 was an atypical case in which the case–patient immunosuppressed. Although the case–patient was in New Mexico for the 14 days preceding illness, little is known about the incubation period of DENV in immunosuppressed individuals. It was not confirmed that the case-patient had been infected in New Mexico |
| South Gyeongsang, South Korea | Sporadic/Uncertain | No evidence of risk | All cases reported in the following manuscript were travel-associated 16 |
| Kordestan Province, Iran | Sporadic/Uncertain | No evidence of risk | Evidence from the single report identified did not conclusively establish local DENV transmission 17 |
| States in India: Meghalaya, Mizoram, and Tripura | No data | Sporadic/Uncertain | These states are surrounded by areas where dengue-risk is classified as Frequent/Continuous |
Figure 1.
2016 Yellow Book Dengue Maps. (a) The Caribbean and Central and South America. (b) Africa and the Middle East. (c) Asia and Oceania
To make the classifications easier to visualize, we produced three regional maps (Americas and the Caribbean; Africa and the Middle East; and Asia and Oceania) instead of a single global map. The labels help viewers interpret the classification of small island nations that are not be visible given the regional scale of the map. The high-resolution maps in colour are also available on the CDC website (http://wwwnc.cdc.gov/travel/yellowbook/2016/infectious-diseases-related-to-travel/dengue).
Discussion
We sought to provide clinicians and international travellers with an easily interpretable dengue-risk map based on up-to-date epidemiological evidence of DENV transmission. Although dengue-risk areas change over time, this revised dengue map incorporates a larger body of direct evidence and a more detailed assessment of risk to improve the information available to clinicians and travellers who consult CDC’s travel health resources, including the 2016 Yellow Book. 2 The final map provides more information on where travellers should take steps to prevent mosquito bites and where dengue should be considered in the differential diagnoses of ill travellers.
Using a defined set of risk classifications, we have also provided more transparency as to the rationale behind how we categorized areas. Furthermore, the data used to inform the classifications are publicly available. 3 , 4 This standardization provides specific definitions associated with risk that are current, flexible and relevant.
By using a map with three levels of dengue risk (frequent/continuous, sporadic/uncertain, no evidence of risk) rather than two (dengue risk, no known dengue risk), we have increased the amount of information provided and indicated the gradation of risk. Specifically, including the sporadic/uncertain risk classification, which increased the geographic range of potential dengue-risk areas, has reduced the confusion of the previous map. In the prior map, areas were only designated as dengue-risk areas if previous outbreaks or cases had been documented there. If no data were available for a particular area, it was classified as “no known dengue risk.” Although this approach was evidence based, it presented confusing messages regarding areas where DENV may have circulated but evidence was limited. For example, Uruguay was categorized as “no known dengue risk;” however, it is surrounded by areas with dengue risk. Because virus circulation is not influenced by political boundaries, travellers and clinicians advising them may have had misconceptions about dengue risk in this country. The new classifications take into account the potential scarcity of documentation and provide a clearer understanding, in plain language, as to where to expect frequent/continuous risk, sporadic/uncertain risk and no known risk.
Our process did have some limitations. Data used inevitably depends on what dengue information are reported from countries with very different reporting systems. There are some clear biases at the global level, with particularly poor reporting in Africa. 12 Future dengue surveillance priorities should focus on understanding the full geographic extent of the disease in these areas, especially in areas with only one reported transmission event. The analysis conducted by Brady et al. 3 and Bhatt et al. 1 described and quantified the gaps in evidence that create uncertainties in the current distribution of dengue. Although our map used evidence available to create risk categories, it does not address the gaps in existing evidence and was written for easy interpretability for clinicians and travellers. Therefore, some areas of high risk may not have been appropriately classified. In addition, we assumed that endemic areas would be likely to report cases in at least three distinct years over the most recent 10-year period. A more liberal approach would have only used two distinct years.
Conclusion
Travellers and the clinicians who advise and treat them need to understand the geographic extent of risk for dengue and other diseases. Using the revised dengue map and the aforementioned definitions, clinicians conducting pre-travel health consultations can advise travellers to protect themselves by preventing mosquito bites in frequent/continuous or sporadic/uncertain areas. In addition, the map also provides valuable information for clinicians where dengue should be considered in the differential diagnoses of ill travellers. Previous dengue-risk maps lacked specificity, as there was a single, binary risk classification with no clear link between data and the risk classification. To address these challenges, we collected evidence and applied it to develop more informative risk classifications that are reflected in these new maps. Such an approach could be used for other travel-related diseases, particularly those without effective vaccines or chemoprophylaxis for prevention.
Supplementary Data
Supplementary data are available at JTM online.
Funding
S.I.H. was funded by a Senior Research Fellowship from the Wellcome Trust (#095066), and grants from the Bill & Melinda Gates Foundation (OPP1119467, OPP1093011, OPP1106023 and OPP1132415). S.I.H. would also like to acknowledge funding support from the International Research Consortium on Dengue Risk Assessment Management and Surveillance (IDAMS; European Commission 7th Framework Programme (21803)). O.J.B. is also supported by funding from the Bill & Melinda Gates Foundation (OPP1119467).
Conflicts of interest: None declared.
References
- 1. Bhatt S, Brady OJ, Messina JP, et al. The global distribution and burden of dengue. Nature 2013; 496: 504–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tomashek KM, Sharp TM, Margolis HS. Dengue In: Brunette GW, Kozarsky PE, O'Sullivan MC. (eds). Health Information for International Travel. New York, NY: Oxford University Press, 2016, pp. 171–7. [Google Scholar]
- 3. Brady OJ, Bhatt S, Messina JP, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS Negl Trop Dis 2012; 6: e1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Messina JP, Pigott DM, Brownstein JS, et al. A global compendium of human dengue virus occurrence. Sci Data 2014; 1: 140004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. EC-FAO Food Security Program. The Global Administrative Unit Layers (GAUL): Technical Aspects. Food and Agricultural Organization of the United Nations, 2008. [Google Scholar]
- 6. Johansson MA, Glass GE. Multiyear climate variability and dengue–el Niño Southern oscillation, weather, and dengue incidence in Puerto Rico, Mexico, and Thailand: a longitudinal data analysis. PLoS Med 2009; 6: e1000168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blaylock JM, Bauer K, Nyakoe N, et al. The seroprevalence and seroincidence of dengue virus infection in western Kenya. Travel Med Infect Dis 2011; 9: 246–8. [DOI] [PubMed] [Google Scholar]
- 8. Sutherland LJ, Huang YJ, Sang RC, et al. Serologic evidence of arboviral infections among humans in Kenya. Am J Trop Med Hyg 2011; 85: 158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ellis EM, Delorey M, Ochieng M, et al. A household serosurvey to estimate the magnitude of a dengue outbreak in Mombasa, Kenya, 2013. PLoS Negl Trop Dis 2015; 9: e0003733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeFraites R, Smoak B, Trofa A, et al. Dengue fever among U.S. Military personnel–Haiti, September–November, 1994. MMWR Morb Mortal Wkly Rep 1994; 43: 845–8. [PubMed] [Google Scholar]
- 11. Rossi CA, Drabick JJ, Gambel JM, et al. Laboratory diagnosis of acute dengue fever during the United Nations mission in Haiti, 1995–1996. Am J Trop Med Hyg 1998; 59: 275–8. [DOI] [PubMed] [Google Scholar]
- 12. Jaenisch T, Wills B, Brady OJ, et al. Dengue expansion in Africa—not recognized or not happening? Emerg Infect Dis 2014; 20: e140487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ippolito G. ProMed, 2010. http://www.promedmail.org/direct.php?id=463005 (9 July 2015, date last accessed).
- 14. Amarasinghe A, Letson GW, Margolis HS. Dengue virus infection in Africa. Emerg Infect Dis 2011; 17: 1349–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sharp TM, Muehlenbachs A, Hunsperger E, et al. Fatal hemophagocytic lymphohistiocytosis associated with locally acquired dengue virus infection—New Mexico and Texas, 2012. MMWR Morb Mortal Wkly Rep 2014; 63: 49–54. [PMC free article] [PubMed] [Google Scholar]
- 16. Park J-H. Dengue fever in South Korea, 2006–2010. Emerg Infect Dis 2012; 18: 1525–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Chinikar S, Shah-Hosseini N, Mostafavi E, et al. Preliminary study of dengue virus infection in Iran. Travel Med Infect Dis 2013; 11: 166–9. [DOI] [PubMed] [Google Scholar]