Abstract
Background and Purpose
Obesity and associated co‐morbidities, such as type 2 diabetes and non‐alcoholic fatty liver disease, are major health challenges. Hence, there is an important need to develop weight loss therapies with the ability to reduce the co‐morbidities.
Experimental Approach
The effect of the dual amylin and calcitonin receptor agonist (DACRA), KBP‐089, on body weight, glucose homeostasis and fatty acid accumulation in liver and muscle tissue and on food preference was investigated. Furthermore, we elucidated weight‐independent effects of KBP‐089 using a weight‐matched group.
Key Results
Rats fed a high‐fat diet were treated, s.c., with KBP‐089 0.625, 1.25, 2.5 μg·kg−1 or vehicle. KB‐089 induced in a dose‐dependent and sustained weight loss (~17% by 2.5 μg·kg−1). Moreover, KBP‐089 reduced fat depot size and reduced lipid accumulation in muscle and liver. In Zucker Diabetic Fatty rats, KBP‐089 improved glucose homeostasis through improved insulin action. To obtain a weight‐matched group, significantly less food was offered (9% less than in the KBP‐089 group). Weight matching led to improved glucose homeostasis by reducing plasma insulin; however, these effect were inferior compared to those of KBP‐089. In the food preference test, rats fed a normal diet obtained 74% of their calories from chocolate. KBP‐089 reduced total caloric intake and induced a relative increase in chow consumption while drastically reducing chocolate consumption compared with vehicle.
Conclusions and Implications
The novel DACRA, KBP‐089, induces a sustained weight loss, leading to improved metabolic parameters including food preference, and these are beyond those observed simply by diet‐induced weight loss.
Abbreviations
- DACRA
dual amylin and calcitonin receptor agonist
- FPG
fasting plasma glucose
- HbA1c
glycated haemoglobin
- HFD
high‐fat diet
- HOMA‐IR
homeostatic model assessment for insulin resistance
- IVGTT
i.v. glucose tolerance test
- ND
normal diet
- OGTT
p.o. glucose tolerance test
- PW
pair weighed
- ZDF
Zucker Diabetic Fatty ZDF‐Leprfa/Crl
Tables of Links
| TARGETS |
|---|
| Amylin receptors |
| Calcitonin receptors |
| LIGANDS | |
|---|---|
| Amylin | Insulin |
| Calcitonin | Pramlintide |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016), and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (Alexander et al., 2015).
Introduction
Obesity and associated morbidities, such as diabetes, non‐alcoholic fatty liver disease, cardiovascular disease and cancer, are among this century's greatest health challenges (Pi‐Sunyer, 1999; Cohen et al., 2008; Aballay et al., 2013). The incidence is increasing and the treatment of obesity is in most cases limited to lifestyle interventions. However, when these fail, bariatric surgery and a few pharmacotherapies are available, although these are only used in cases of severe obesity (Fried et al., 2007). Furthermore, due to the potential complications of surgery, novel therapies with an improved efficacy in terms of weight loss and reduction of co‐morbidities are of great interest (Batterham and Cummings, 2016).
The most recently developed therapy for obesity is high‐dose liraglutide, which leads to sustained weight loss at least partially due to a reduction in appetite. Furthermore, liraglutide reduces hyperglycaemia, albeit it is still somewhat limited in terms of efficacy and has challenges on tolerability (Kanoski et al., 2012; Lean et al., 2014). Another molecule that induces weight loss or at least prevents weight gain is the amylin receptor agonist pramlintide (Aronne et al., 2007). Pramlintide, due to its appetite regulating capability, has been shown to reduce insulin‐induced weight gain, while regulating post‐prandial glucose excursions, and therefore has been approved as adjunct therapy to insulin for the treatment of type 2 diabetes (Weyer et al., 2001; Ryan et al., 2009). However, pramlintide use is limited significantly by lack of potency, and hence, more potent amylin receptor agonists are being explored.
Dual amylin and calcitonin receptor agonists (DACRAs) elicit activation not only of the amylin receptor but also of the calcitonin receptor and have been shown to possess superior activity in terms of activation of the amylin receptor, when compared with classical amylin receptor agonists (Andreassen et al., 2014). Interestingly, they also activate the receptors for an extended period of time, when compared with the classical agonists, which appears to increase the in vivo efficacy as well as reducing the dosing frequency (Gydesen et al., 2016).
In vivo studies of DACRAs have recently demonstrated a protection against diet‐induced weight gain, a reduction in overall adiposity, as well as adipocyte hypertrophy (Gydesen et al., 2016). Furthermore, DACRAs have been shown to improve glucose homeostasis in the diabetic Zucker Diabetic Fatty ZDF‐Leprfa/Crl (ZDF) rats, a phenomenon not observed with selective and less potent amylin receptor agonists (Mack et al., 2010, 2011; Andreassen et al., 2014; Hjuler et al., 2015), while alleviating obesity‐derived insulin resistance (Hjuler et al., 2016). Hence, the DACRAs induce amylin receptor‐mediated responses in vivo – reduce food intake, results in weight reduction and suppression of glucagon levels (Roth et al., 2006), and also have additional beneficial effects on fasting blood glucose and insulin sensitivity. With the limited number of DACRAs available, the search for highly potent molecules in this family has continued, resulting in the development of KBP‐089.
In this study, we characterized the effects of KBP‐089 on body weight, glucose homeostasis and fatty acid accumulation in liver and muscle tissue. We then investigated whether KBP‐089 possesses beneficial effects in addition to inducing substantial weight loss, using a weight‐matched group. Finally, we explored the potential effect of KBP‐089 on food preference, by comparing the intake of a highly palatable and energy dense diet (chocolate) with that of regular chow in the presence or absence of KBP‐089.
Methods
Peptide therapy
Synthetic KBP‐089 (American Peptide Company, CA, USA) was dissolved in saline for s.c. delivery. The doses chosen for peptide administration in the current investigations were based on previous comparable DACRA studies in animal models of obesity using potent DACRAs, KBP‐042 and KBP‐088 (Gydesen et al., 2016; Hjuler et al., 2016).
Animal experiments
All animal procedures were performed in accordance with guidelines from the Animal Welfare Division of the Danish Ministry of Justice under the institutional license issued to Nordic Bioscience (2012‐15‐2934‐00094). Male Sprague Dawley rats (Harlan, Venray, The Netherlands) and ZDF (Kingston, NY, USA) were obtained at 6 weeks of age and housed (two rats per cage, standard wood chips enriched with red‐tinted huts, nest material and sticks) at the Nordic Bioscience animal facility (21–23°C, 55–65% relative humidity, 12 h light/dark cycle) with ad libitum access to food and water. Animal studies are reported in compliance with the ARRIVE guidelines (Kilkenny et al., 2010; McGrath & Lilley, 2015).
Animals
From arrival and throughout the study periods, the high‐fat diet (HFD) rats were fed a 60 kcal% fat diet (#58Y1, TestDiet, London, UK), lean normal diet (ND) age‐matched rats were fed a standard pelleted chow (#5002, LabDiet, St. Louis, MO, USA) and ZDF rats were fed a Purina Formulab diet (#5008, LabDiet, St. Louis, MO, USA). The rats received food and tap water ad libitum. The HFD and ND rats were non‐blindly assigned into experimental groups according to body weight. ZDF rats were non‐blindly assigned into experimental groups according to fasting plasma glucose (FPG), glycated haemoglobin (HbA1c) and body weight, ensuring an equal average value of body weight, FPG and HbA1c in the experimental groups at the start of the study. Body weights are visualized as percentage of initial body weight for comparison to other drugs as previously described (Larsen et al., 2001; Mack et al., 2010). Lean and fat mass data as well as the weight of the different adipose tissues are normalized to the body weight of the individual animal.
Chronic in vivo studies
KBP‐089 in HFD rats
After 10 weeks on a high‐fat diet, HFD rats were assigned to treatment groups receiving either vehicle (saline s.c.) or KBP‐089 (0.625, 1.25 and 2.5 μg·kg−1, s.c.) once daily in the afternoon with a food restricted pair‐fed control group for the highest concentration of peptide (n = 10 rats per group in vehicle and KBP‐089 treatment groups; n = 9 rats in pair‐fed group due to the loss of an animal). Pair‐fed animals received an average of the daily intake of food of the 2.5 μg·kg−1 treatment group every day in the afternoon. Food intake and body weight were monitored daily during the initial 2 weeks and once weekly throughout the entire study period. Following 6 and 7 weeks of treatment, p.o. and i.v. glucose tolerance tests (OGTT and IVGTT respectively) were performed in overnight‐fasted (12 h) rats with blood glucose measured and EDTA‐plasma obtained for hormonal analysis. At the end of the study, animals were killed by being anaesthetized with isoflurane (administered by inhalation) followed by exsanguination and dissection. Retroperitoneal, epididymal and s.c. inguinal fat were surgically removed and weighed. Overnight‐fasted blood samples were collected for basal plasma hormonal analyses.
KBP‐089 in ZDF rats
The day prior to dosing initiation, 20 ZDF rats were assigned to two groups (n = 10 rats per group) receiving either vehicle (saline, s.c.) or KBP‐089 (5 μg·kg−1 for 4 weeks, 20 μg·kg−1 for an additional 4 weeks, s.c.) once daily. The p.o. glucose tolerance test (OGTT) was performed after 4 weeks and the i.p. insulin tolerance test was performed after 7 weeks. At the end of the study end FPG, HbA1c were measured, and the homeostasis model assessment of insulin resistance (HOMA‐IR) analysis was calculated using the formula; HOMA‐IR = fasting insulin (μU·mL−1) × fasting blood glucose (mmol·L−1)/22.5 (Matthews et al., 1985). HOMA‐IR was developed for humans; however, it can be used as a surrogate measurement for insulin resistance in rodents (Cacho et al., 2008; Mather, 2009).
Weight‐matched HFD rats
To address KBP‐089 efficacy independent of weight loss, we did a 6 week study in HFD rats (n = 12) with a weight‐matched group to the 2.5 μg·kg−1 KBP‐089 group. Food intake and body weight were monitored daily throughout the study period, and in order to match the body weights, we estimated the food restriction needed to achieve a comparable weight reduction based on pilot studies (data not shown) and adjusted estimations to body weight on a daily basis. The rats were subjected to an OGTT after 3 weeks of treatment. The rats were weighed and scanned for body composition (EchoMRI‐4in1; EchoMRI, Houston, TX, USA) at study end and killed as for the chronic in vivo studies.
Food preference in ND rats
To assess the effect of KBP‐089 on the preference of diet, ND rats were offered normal chow or chocolate (milk chocolate with hazelnuts) (Marabou, Mondelez Danmark, Brøndby, Denmark). The animals were allowed to accustom to the chocolate for 1 week before injections with 2.5 μg·kg−1 KBP‐809 were initiated. Voluntary food and chocolate intake were monitored for 24 h after treatment for 7 days.
Glucose tolerance tests
HFD rats received glucose by oral gavage (2 g·kg−1) or i.v. in the lateral tail vein (0.5 g·kg−1) and ZDF p.o. (1 g·kg−1). Blood samples were collected from the tail vein before glucose challenge (0 min) in both tests and 5, 15, 30 and 60 min post‐glucose challenge in the IVGTT, and 15, 30, 60 and 120 min post‐glucose challenge in the OGTT.
Insulin tolerance test
ZDF rats (fasted for 6 h) were administered with KBP‐089 at t = −30 and received intraperitoneal insulin (1.0 U·kg−1) at t = 0, and blood glucose was measured subsequently at t = 0, 30, 60 and 120 min after insulin injection. The data are visualized as percentage of initial blood glucose for simplicity.
Fat accumulation in liver and muscle tissue
To address tissue fat accumulation, the liver and gastrocnemius muscle were surgically removed for optimal cutting temperature compound embedding, snap frozen on ice/ethanol, stored at −80°C until cryosectioning. Tissue sections were stained with oil red O stain, and images were captured with a light microscope (magnification of ×40 for gastrocnemius and ×20 for liver, nine images per animal; three pictures per depth) and quantified using ImageJ capable of calculating the amount of red pixels in relation to μm2 as previously described (Mehlem et al., 2013) and, for simplicity, were visualized as the fold‐induction from lean rats.
Biochemical analysis
Blood samples were collected in EDTA tubes and centrifuged at 1850 × g for 10 min at 4°C. Blood glucose was monitored by Accu‐Check® Avia monitoring system (Roche Diagnostics, Rotkreuz, Switzerland). HbA1c was measured using an automatized DCA Vantage Analyzer (Siemens AG, Erlangen, Germany). Plasma levels of insulin (Mercodia Rat Insulin ELISA, Mercodia AB, Uppsala, Sweden) were analysed according to the manufacturer's instruction.
Statistical analysis
All data are presented as means ± SEM. The statistical analysis of various drug effects were conducted using one‐way ANOVA followed by Tukey's post test for multiple comparison for parametric data and Kruskal–Wallis test with Dunn's post test for non‐parametric data if F achieved the necessary level of statistical significance (P < 0.05). Lean age‐matched controls are compared with HFD vehicle, and ZDF vehicle with ZDF KBP‐089, using Student's t‐test. All analyses were performed using GraphPad Prism software (GraphPad Prism, San Diego, CA, USA). A value of P < 0.05 was considered statistically significant. The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015).
Results
KBP‐089 potently reduces appetite, body weight and fat depots
High‐fat feeding resulted in a phenotype with significantly increased body weight (596 ± 12 vs. 545 ± 8 g, P < 0.05), hyperinsulinaemia (1.9 ± 0.1 vs. 0.9 ± 0.1 ng·mL−1, P < 0.05), impaired glucose control without hyperglycaemia (OGTT tAUC 1343 ± 18.1 vs. 1259 ± 17.1, P < 0.05) and impaired insulin sensitivity (HOMA‐IR) (11.3 ± 0.3 vs. 4.3 ± 0.3, P < 0.05), compared with the lean age‐matched controls. Thus, the HFD rats resembled an obese and pre‐diabetic phenotype as expected from previous studies (Hjuler et al., 2015; Gydesen et al., 2016).
To investigate the anti‐obesity potential of KBP‐089 in vivo, we treated HFD rats for 8 weeks. Previously, DACRAs have been shown to induce hypophagia (Hjuler et al., 2015; Gydesen et al., 2016); therefore, a pair‐fed group to the highest concentration of KBP‐089 was included to explore the impact of food restriction on body weight. KBP‐089 was s.c. administered in three doses (0.625, 1.25 and 2.5 μg·kg−1) for 56 days. Food intake was transiently attenuated by KBP‐089 (Figure 1A), albeit cumulative food intake after the initial 2 weeks of treatment was not significantly different in 2.5 μg·kg−1 treated rats compared with vehicle rats (504 ± 35 vs. 408 ± 19 g per animal). 8 weeks of KBP‐089 treatment resulted in a dose‐dependent and sustained 17 ± 1.7 % weight loss in the 2.5 μg·kg−1 group (Figure 1B), while pair‐feeding resulted in a 4 ± 2.0 % body weight reduction. Based on food intake and body weight change, food efficiency was calculated. Expectedly, treatment with KBP‐089 markedly attenuated the food efficiency compared with vehicle and the pair‐fed group (Figure 1C).
Figure 1.
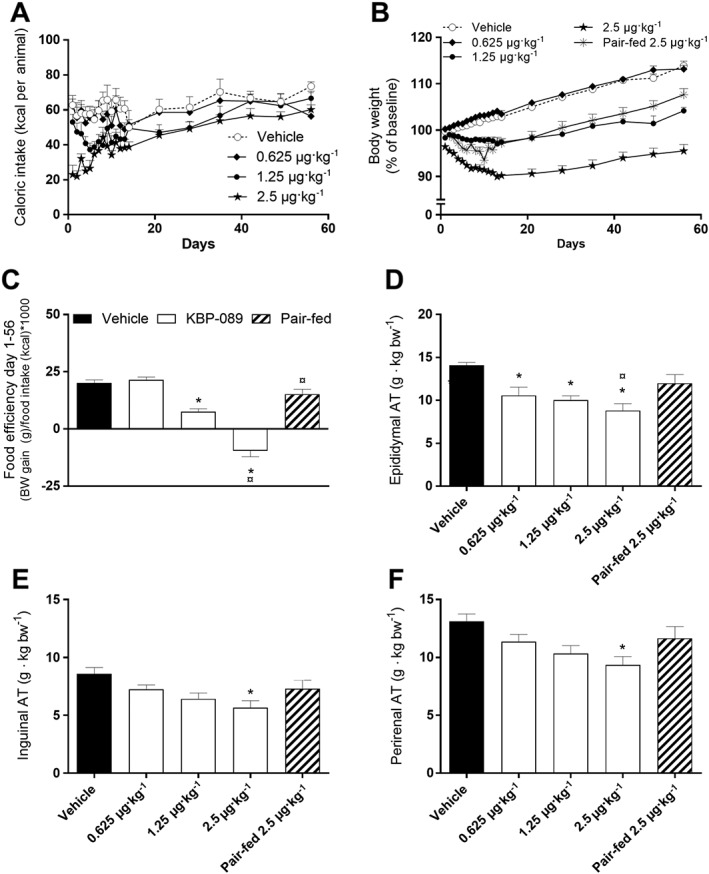
KBP‐089 potently reduces appetite, body weight and fat depots in HFD rats. (A) Caloric intake monitored daily initially, and then weekly in high‐fat diet fed rats. Expressed as daily intake per animal. (B) Body weight expressed as percentage from baseline. (C) Food efficiency day 1–56 in rats dosed with KBP‐089 (0.625, 1.25 and 2.5 μg·kg−1). Relative weight of (D) epididymal, (E) inguinal and (F) peritoneal adipose tissue at study end. (n = 10 rats per group in vehicle and KBP‐089 treatment groups; n = 9 rats in pair‐fed group). AT, adipose tissue. Statistical analysis between groups was evaluated by an ordinary one‐way ANOVA with Tukey's multiple comparisons test. *P < 0.05. compared with vehicle, ¤P<0.05 compared with pair‐fed.
Epididymal, inguinal and perirenal fat pads were weighed, and in conjunction with the significant body weight reduction, the weight of the adipose tissues was significantly reduced after treatment with KBP‐089 (Figure 1D–F). This reduction was not observed in the pair‐fed control rats.
KBP‐089 enhances glucose tolerance and potentially insulin sensitivity
An OGTT was performed after 6 weeks of treatment and followed by an IVGTT allowing circumvention of the influence of the gastrointestinal tract and thereby assessment of peripheral glucose tolerance after 7 weeks of treatment (Figure 2). In contrast to previous DACRA studies (Hjuler et al., 2015, 2016; Gydesen et al., 2016), the rats were not dosed 30 min prior to the glucose challenge to avoid the strong effect on gastric emptying. In both tests, all treatment groups showed a trend towards lower blood glucose levels compared with vehicle and pair‐fed controls 5 min (IVGTT) and 15 min (OGTT) after glucose administration (Figure 2A, D). However, tAUC was not significantly changed for either test when compared with vehicle or pair‐fed controls (Figure 2B, E). Interestingly, the glucose‐induced insulin hyper secretion observed in vehicle and pair‐fed groups was markedly and dose‐dependently suppressed by KBP‐089 during both OGTT and IVGTT, which resulted in significantly reduced insulin AUC values in KBP‐089 treated rats (Figure 2C, F). Pair feeding did not improve glucose tolerance or hyperinsulinaemia in either test.
Figure 2.
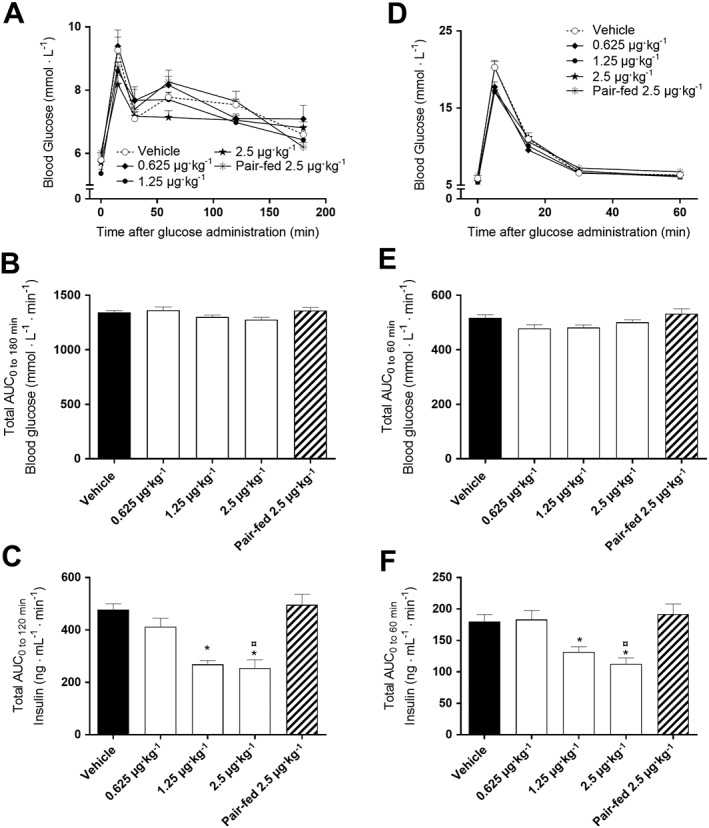
KBP‐089 enhances glucose tolerance and potentially insulin sensitivity in HFD rats. (A, D) Plasma glucose during OGTT and IVGTT in high‐fat diet fed rats treated with KBP‐089 (0.625, 1.25 and 2.5 μg·kg−1) for 6 and 7 weeks respectively. Total AUC for (B, E) glucose and (C, F) plasma insulin during OGTT and IVGTT after 6 and 7 weeks respectively (n = 10 rats per group in vehicle and KBP‐089 treatment groups; n = 9 rats in pair‐fed group). Statistical analysis between groups was evaluated by (B, C, F) an ordinary one‐way ANOVA with Tukey's multiple comparisons test and (E) Kruskal–Wallis test with Dunn's multiple comparisons test. *P < 0.05 compared with vehicle, ¤P < 0.05compared with pair‐fed.
KBP‐089 reduces the accumulation of lipids in both muscle and liver
After treatment with KBP‐089 for 56 days, lipid accumulation was assessed in liver and muscle tissue. As seen in Figure 3, high‐fat feeding led to increased lipid accumulation in both liver and muscle compared with lean age‐matched controls. This inappropriate storage of lipids was completely eliminated by treatment with 2.5 μg·kg−1 KBP‐089, despite the rats having been on HFD for 10 weeks prior to initiation of therapy. Importantly, this effect was not obtained by pair‐feeding.
Figure 3.
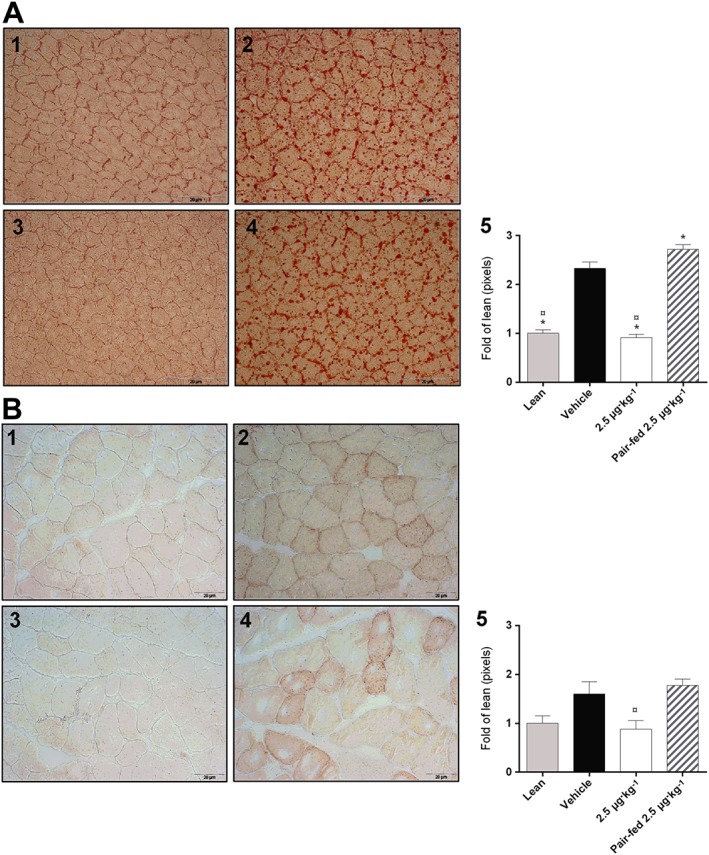
KBP‐089 reduces the accumulation of lipids in both muscle and liver in HFD rats. Oil Red O stained frozen (A) liver sections and (B) gastrocnemius muscle (magnification of ×20 for liver and ×40 for gastrocnemius, nine images per animal; three pictures per depth) in (1) ND rats, (2) vehicle HFD rats, (3) 2.5 μg·kg−1 KBP‐089, (4) pair‐fed to 2.5 μg·kg−1 KBP‐089 and (5) quantification of the results (n = 10 rats per group in vehicle and KBP‐089 treatment groups; n = 9 rats in pair‐fed group). Data are expressed as fold of lean. Statistical analysis between groups was evaluated by a Kruskal–Wallis test with Dunn's multiple comparisons test. *P < 0.05 compared with vehicle, ¤P < 0.05 compared with pair‐fed.
KBP‐089 lowers glycaemia and increases glucose tolerance and insulin action in ZDF rats
We tested the anti‐hyperglycaemic efficacy of KBP‐089 in vivo in ZDF rats for 8 weeks (5 μg·kg−1 for 4 weeks, 20 μg·kg−1 for additional 4 weeks, s.c.). In ZDF rats, fasting blood glucose levels were decreased significantly (6.9 ± 0.7 mM, P < 0.05) over 7 weeks by KBP‐089 treatment compared with vehicle, resulting in HbA1c reduction by ~2.5 ± 0.2% compared with vehicle at the end of the study (Figure 4A, B). Glucose tolerance was tested by an OGTT where treatment with KBP‐089 resulted in a moderate glucose reduction compared with vehicle. The tAUC was lowered significantly (~30%, P < 0.05). Insulin action was assessed in an insulin tolerance test which manifested in a significant larger drop in blood glucose in response to insulin in KBP‐089‐treated rats compared with vehicle (tAUC ~19%, P < 0.05), supporting increased insulin sensitivity.
Figure 4.
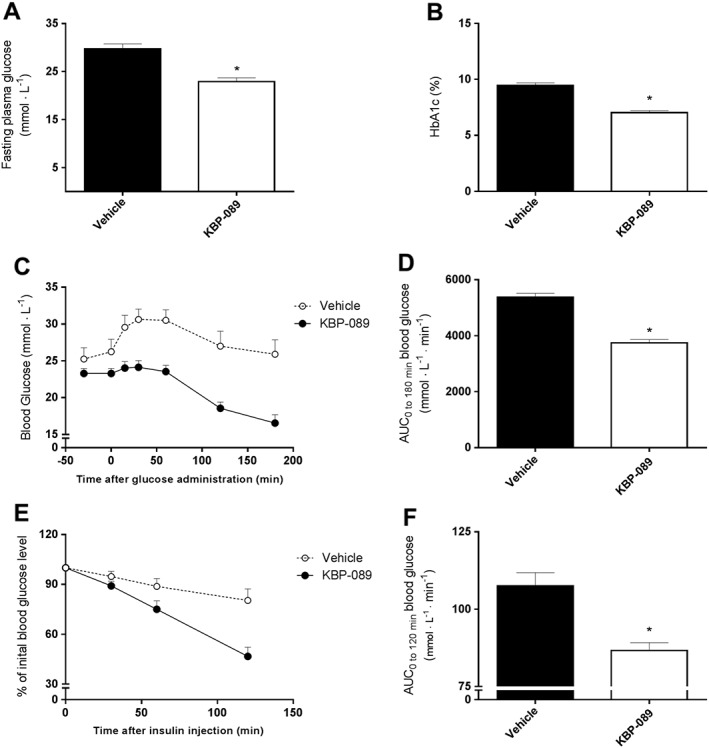
KBP‐089 lowers glycaemia and increases glucose tolerance and insulin action in ZDF rats. (A, B) Fasting plasma glucose and HbA1c levels respectively in ZDF treated with KBP‐089 or saline (vehicle) for 8 weeks. (C) Plasma glucose during OGTT, (D) total AUC of the OGTT displayed in (C). (E) Plasma glucose during insulin tolerance test displayed as % of initial blood glucose value. (F) Total AUC of the insulin tolerance test displayed in (E). (n = 10 rats per group). Statistical analysis between groups was evaluated by t‐test. P < 0.05.
KBP‐089 induces metabolic improvements in addition to those induced by weight loss through food restriction
In order to evaluate drug‐induced metabolic improvements beyond what a weight loss can do, we performed a study with a weight‐matched control in which weight reductions were induced either by KBP‐089 administration (‘KBP‐089’) or by food restriction alone (‘Pair weighed’/‘PW’). In order to match the body weights, the pair‐weighed rats received significantly less food compared with the KBP‐089‐treated rats (Figure 5A). As in the previous study, body weight was significantly reduced by KBP‐089 administration, and this was matched during the study in the pair‐weighed group (Figure 5B). There was no significant difference between the groups in body weight at study start (vehicle: 409 ± 3 g, KBP‐089: 410 ± 3 g and PW: 408 ± 4 g). During the study, the body weight was significantly reduced in KBP‐089 and pair‐weighed rats, albeit there was no difference between KBP‐089‐treated rats and the pair‐weighed rats (vehicle: 462 ± 6 g, KBP‐089: 398 ± 4 g and PW: 403 ± 6 g). Interestingly, the epididymal and perirenal adipose tissues, which are directly associated with visceral adiposity and insulin resistance (Gabriely et al., 2002), were significantly lower in the KBP‐089 and the pair‐weighed group compared with vehicle (Figure 5C). There was no significant difference between the surgically removed adipose tissues in KBP‐089‐treated rats and the pair‐weighed rats; however, there was a trend towards a more distinct reduction in adipose tissue in KBP‐089‐treated rats compared with pair‐weighed rats. Using MR, we found a slight increase in lean body mass in KBP‐089‐treated rats compared with vehicle rats, and a reduced amount of whole body fat mass in KBP‐089‐treated and pair‐weighed rats compared with vehicle (Figure 5D), indicating that the weight loss occurs primarily in fat tissue. As expected, KBP‐089 again caused improved glucose tolerance with significantly lowered plasma insulin levels (Figure 5D, E, G, H) compared with vehicle. Surprisingly, the pair‐weighed group did not show a marked improvement in glucose tolerance despite the significant weight reduction. Food restriction alone had significantly ameliorated hyperinsulinaemia during OGTT (Figure 5H); however, it was still significantly higher compared with KBP‐089. Finally, KBP‐089‐treated rats had a reduced rate of gastric emptying compared with vehicle and pair‐weighed rats (data not shown) as previously observed with DACRA treatment (Hjuler et al., 2016).
Figure 5.
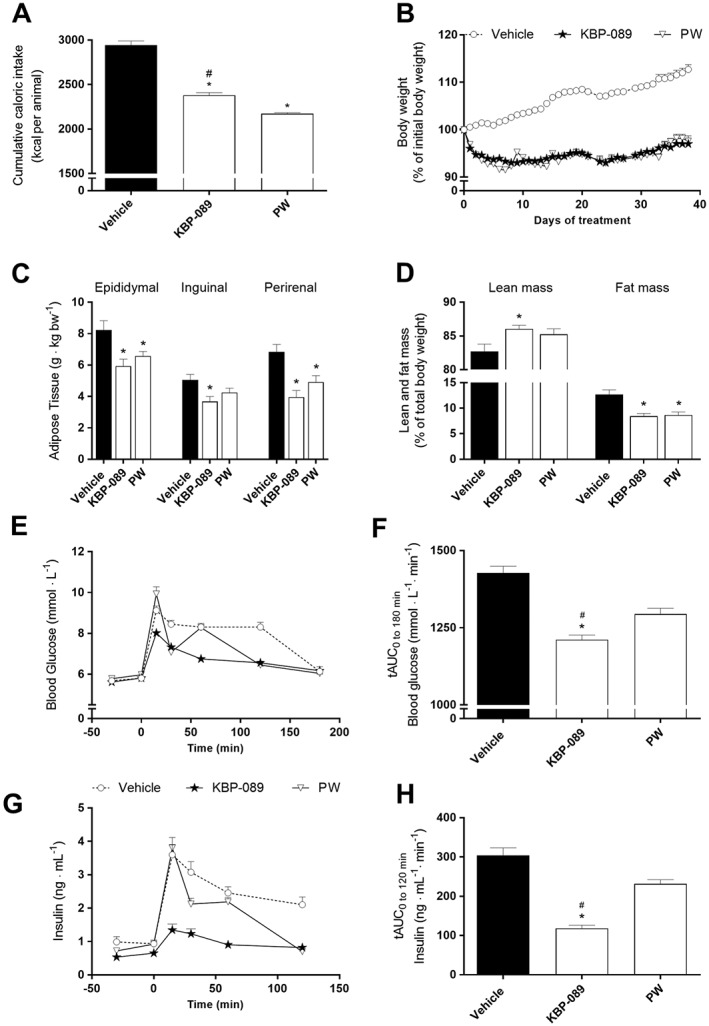
KBP‐089 and weight‐matched rats. (A) Cumulative caloric intake at study end. (B) Daily body weight and (C) relative weight of epididymal, inguinal and retroperitoneal adipose tissue at study end in HFD rats treated with KBP‐089 (2.5 μg·kg−1) and weight‐matched rats. (E, G) Plasma glucose and plasma insulin respectively and (F, H) total AUC of glucose and insulin respectively during OGTT after 3 weeks (n = 12 rats per group). Statistical analysis between the groups was evaluated by an ordinary one‐way ANOVA with Tukey's multiple comparisons test (C, F, H) or (D) Kruskal–Wallis with Dunn's multiple comparisons test. *P < 0.05 compared with vehicle, # P < 0.05 compared with PW.
KBP‐089 induces changes in food preference
To examine the effect of the reduced food intake in detail, a food preference test was performed (Figure 6). When the rats had access to ad libitum chow and chocolate (as compared with chow alone), caloric intake was significantly increased. Furthermore, chow intake was significantly reduced as the rats preferred the chocolate and obtained 74% of their calories from chocolate [caloric intake vehicle/chow: 143 ± 3.0 kcal, caloric intake of vehicle/chow + chocolate: 173 ± 8.1 kcal (chow = 46.1 ± 2.9 kcal and chocolate = 127.3 ± 9.8 kcal)]. KBP‐089 administration was associated with a significantly reduced caloric intake, – 34% compared with vehicle treatment; caloric intake of KBP‐089/chow + chocolate: 115 ± 10.7 kcal (chow =74 ± 6.6 kcal, chocolate =41.4 ± 9.4 kcal), accompanied by a relative increase in chow consumption and a drastic reduction in chocolate consumption (127.3 ± 9.8 kcal vs. 41.4 ± 9.4 kcal).
Figure 6.
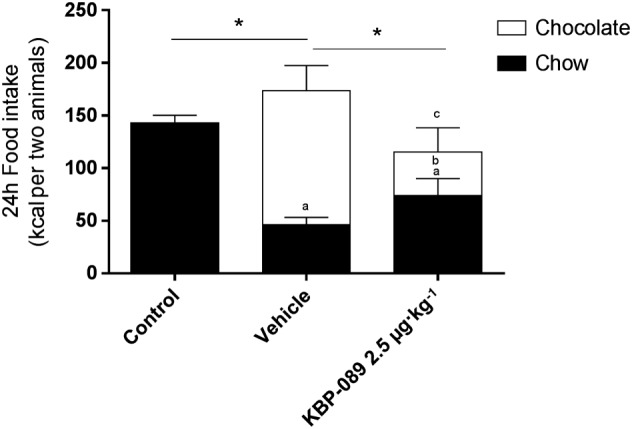
KBP‐089 induces changes in food preference. Voluntary food (chow) and chocolate intake was monitored for 24 h after 7 days of 2.5 μg·kg−1 KBP‐089 treatment. Statistical analysis between groups and diets was evaluated by two‐way ANOVA with Tukey's multiple comparisons test. *P < 0.05 treatment groups total caloric comparison; a P < 0.05 compared with control (chow); b P < 0.05 compared with vehicle (chow); and c P < 0.05 compared with vehicle (chocolate).
Discussion
The present study describes a novel DACRA, called KBP‐089, which is able to induce and sustain a significant weight loss irrespective of food intake. Importantly, KBP‐089 possesses the ability to improve glucose tolerance above what can be obtained with weight loss alone.
Treatment with KBP‐089 reduced food intake initially. However, this effect was reduced during the course of the study, and the effects obtained with KBP‐089 treatment on weight, glucose tolerance, adipose tissue reduction and removal of ectopic lipid depositions in liver and muscle were not achieved with pair‐feeding, clearly demonstrating effects of KBP‐089 beyond appetite restriction. The weight reducing effect and the effect on food intake can most likely be attributed to central amylin receptor activation. It has previously been demonstrated that amylin facilitates a reduction in body weight that cannot only be attributed to suppression of food intake (Isaksson et al., 2005; Roth et al., 2006). An interesting aspect of the reduction in fatty acid accumulation in the liver is the known relationship between liver fat, insulin resistance and non‐alcoholic steatohepatitis (Cusi, 2009; Milić et al., 2014), and these data indicate that KBP‐089, at least due to its weight reducing capacity, could be a novel treatment candidate for liver steatosis.
The KBP‐089‐mediated changes in adiposity were confirmed in the pair‐weight study where KBP‐089‐treated rats had significantly lower epididymal, inguinal and perirenal adipose tissues compared with vehicle‐treated rats, and trends towards lowered adiposity compared with pair‐weighed adipose tissues – despite the same body weight. This could indicate that KBP‐089 treatment results in a loss of fat mass rather than lean body mass. Food restriction alone is however not sufficient to obtain the same reduction in adipose depots. As mentioned above, amylin agonism has long been associated with an increase in respiratory quotient, which is associated with a preferential oxidation of fat (Wielinga et al., 2010). Moreover, other studies have also associated activation of amylin receptors with a specific reduction in fat mass rather than lean mass (Roth et al., 2006, 2007), whereas inhibiting amylin signalling centrally increases fat mass (Rushing et al., 2001), thus potentially explaining the difference in fat depots in this study. This was tested using MR scanning. There was no difference in whole body fat mass between the KBP‐089‐treated and pair‐weighed rats; however, in support of the limited loss of lean mass, we found a slight – albeit significant – increase in lean mass in KBP‐089‐treated rats compared with vehicle rats, underscoring that the weight loss is primarily mediated through a reduction in adipose tissue weight. Furthermore, the slight increase in lean mass is a positive effect, as heavy weight loss in many cases is associated with loss in lean body mass in humans (Garthe et al., 2011; Weiss et al., 2016).
In terms of hyperglycaemia and insulin resistance, amylin analogues have shown promise (Ratner et al., 2004; Mack et al., 2011); however, they do not possess the intrinsic ability to reduce fasting plasma glucose levels and insulin tolerance, in contrast to KBP‐089 as shown here in both ZDF and HFD rats, or other DACRAs (Hjuler et al., 2015). These data are further corroborated by the pair‐weight study where substantially lower glucose and insulin levels were observed during glucose tolerance tests, in the KBP‐089 treatment group when compared with the weight‐matched group. The improvement in insulin levels also manifested as an improvement in glucose control too; however, the possibility that the PW animals had an ‘artificial’ increase in glucose intolerance due to prolonged fasting (or significant food restriction) cannot be out ruled, although they did not show any signs of malnutrition or ill behaviour. The lowering of insulin levels could be attributed to the lack of improvement in glucose tolerance when compared with the improvement observed in KBP‐089‐treated rats, which would have been likely after a significant weight loss (Horton and Hill, 2001; Lafontan and Langin, 2009; Karpe et al., 2011). The KBP‐089‐induced improvement in glucose tolerance is partly mediated through the lowering of gastric emptying rate, as previously observed with amylin agonism (Young et al., 1995; Young, 2005). In an p.o. glucose tolerance test without dosing prior to the glucose challenge (data not shown), glucose tolerance was slightly improved in KBP‐089‐treated rats, and insulin levels were significantly lowered compared with vehicle rats, while PW animals mimic glucose tolerance and insulin levels as demonstrated in the OGTT. These data further indicate the strong insulinostatic effect of KBP‐089.
Another important way to regulate body weight could be to manipulate volunteer food intake/composition of food in the brain. This was hypothesized to be relevant for KBP‐089 due to a known effect of amylin agonism on the release of dopamine in the hypothalamus (Brunetti et al., 2002) and alterations in the melanocortigenic system, (Roth et al., 2012) both of which are mediators of the reward/pleasure circuits known to affect feeding patterns (Pandit et al., 2016). Normally, amylin does not produce conditioned taste aversion (Lutz et al., 1995; Rushing et al., 2002); hence, this is not normally used to explain the alterations in food intake. Alternatively, the reduced impulse to consume sugar instead of normal chow could be explained in other ways. In humans, patients treated with pramlintide also experience a voluntary shift in eating behaviour and ‘binge eating’ (Smith et al., 2007). A change of food intake towards a more healthy diet (less energy dense and sweet) is also observed in patients after surgical weight intervention (Mathes and Spector, 2012). The mechanisms behind this are not clear; however, alterations in food reward or taste functions have been suggested as possible explanations (Miras and le Roux, 2014). From the food preference study presented here, it could be speculated that dosing with KBP‐089 offers some of the effects obtained by surgical interventions, making KBP‐089 a relevant option for treating severely obese patients and thereby aiding a significant weight loss along with a change in lifestyle, which might improve the results even further.
In conclusion, the novel DACRA KBP‐089 induces and sustains a substantial weight loss in obese rats and reduces overall adiposity and ectopic lipid accumulation in the liver. In addition, KBP‐089 improved glucose tolerance and indirectly improved insulin action independent of food intake and body weight, hence revealing the potential of KBP‐089 as an anti‐obesity agent with additional benefits on glucose control and liver steatosis.
Author contributions
S.G., S.T.H and K.H designed the study. S.G., S.T.H. performed the study. S.G., S.T.H., Z.F and N.S. analysed the data. S.G. and S.T.H. drafted the manuscript. S.G., S.T.H., K.A.N., L.I.H., M.A.K. and K.H revised the manuscript. S.G., S.T.H., Z.F., K.A.N., N.S., L.I.H., M.A.K. and K.H approved the final version of the manuscript.
Conflict of interest
M.A.K. and K.H. own stock in Nordic Bioscience. All other authors disclose no conflict of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Acknowledgements
We would like to acknowledge funding grants from the Danish Agency for Science, Technology and Innovation and the Danish Research Foundation (Den Danske Forskningsfond). Furthermore, we would like to thank Professor Jørgen Wojtaszewski and Christian Frøsig from Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark, for valuable guidance and assistance with the MR scans.
Gydesen, S. , Hjuler, S. T. , Freving, Z. , Andreassen, K. V. , Sonne, N. , Hellgren, L. I. , Karsdal, M. A. , and Henriksen, K. (2017) A novel dual amylin and calcitonin receptor agonist, KBP‐089, induces weight loss through a reduction in fat, but not lean mass, while improving food preference. British Journal of Pharmacology, 174: 591–602. doi: 10.1111/bph.13723.
References
- Aballay LR, Eynard AR, Díaz Mdel P, Navarro A, Muñoz SE (2013). Overweight and obesity: a review of their relationship to metabolic syndrome, cardiovascular disease, and cancer in South America. Nutr Rev 71: 168–179. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015). The Concise Guide to PHARMACOLOGY 2015/16: G protein‐coupled receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreassen KV, Feigh M, Hjuler ST, Gydesen S, Henriksen JE, Beck‐Nielsen H et al. (2014). A novel oral dual amylin and calcitonin receptor agonist (KBP‐042) exerts anti‐obesity and anti‐diabetic effects in rats. Am J Physiol Endocrinol Metab 307: E24–33. [DOI] [PubMed] [Google Scholar]
- Aronne L, Fujioka K, Aroda V, Chen K, Halseth A, Kesty NC et al. (2007). Progressive reduction in body weight after treatment with the amylin analog pramlintide in obese subjects: a phase 2, randomized, placebo‐controlled, dose‐escalation study. J Clin Endocrinol Metab 92: 2977–2983. [DOI] [PubMed] [Google Scholar]
- Batterham RL, Cummings DE (2016). Mechanisms of diabetes improvement following bariatric/metabolic surgery. Diabetes Care 39: 893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunetti L, Recinella L, Orlando G, Michelotto B, Nisio CD, Vacca M (2002). Effects of ghrelin and amylin on dopamine, norepinephrine and serotonin release in the hypothalamus. Eur J Pharmacol 454: 189–192. [DOI] [PubMed] [Google Scholar]
- Cacho J, Sevillano J, de Castro J, Herrera E, Ramos MP (2008). Validation of simple indexes to assess insulin sensitivity during pregnancy in Wistar and Sprague–Dawley rats. Am J 295: E1269–1276. [DOI] [PubMed] [Google Scholar]
- Cohen SS, Palmieri RT, Nyante SJ, Koralek DO, Kim S, Bradshaw P et al. (2008). Obesity and screening for breast, cervical, and colorectal cancer in women: a review. Cancer 112: 1892–1904. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SPA, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusi K (2009). Role of insulin resistance and lipotoxicity in non‐alcoholic steatohepatitis. Clin Liver Dis 13: 545–563. [DOI] [PubMed] [Google Scholar]
- Fried M, Hainer V, Basdevant A, Buchwald H, Deitel M, Finer N et al. (2007). Inter‐disciplinary European guidelines on surgery of severe obesity. Int J Obes (Lond) 31: 569–577. [DOI] [PubMed] [Google Scholar]
- Gabriely I, Ma XH, Yang XM, Atzmon G, Rajala MW, Berg AH et al. (2002). Removal of visceral fat prevents insulin resistance and glucose intolerance of aging: an adipokine‐mediated process? Diabetes 51: 2951–2958. [DOI] [PubMed] [Google Scholar]
- Garthe I, Raastad T, Refsnes PE, Koivisto A, Sundgot‐Borgen J (2011). Effect of two different weight‐loss rates on body composition and strength and power‐related performance in elite athletes. Int J Sport Nutr Exerc Metab 21: 97–104. [DOI] [PubMed] [Google Scholar]
- Gydesen S, Andreassen KV, Hjuler ST, Christensen JM, Karsdal MA, Henriksen K (2016). KBP‐088, a novel DACRA with prolonged receptor activation, is superior to davalintide in terms of efficacy on body weight. Am J Physiol Endocrinol Metab . doi:10.1152/ajpendo.00514.2015. [DOI] [PubMed] [Google Scholar]
- Hjuler ST, Andreassen KV, Gydesen S, Karsdal MA, Henriksen K (2015). KBP‐042 improves bodyweight and glucose homeostasis with indices of increased insulin sensitivity irrespective of route of administration. Eur J Pharmacol 762: 229–238. [DOI] [PubMed] [Google Scholar]
- Hjuler ST, Gydesen S, Andreassen KV, Pedersen SL, Hellgren LI, Karsdal MA et al (2016). The dual amylin‐ and calcitonin‐receptor agonist KBP‐042 increases insulin sensitivity and induces weight loss in rats with obesity. 24: 1712–1722. [DOI] [PubMed] [Google Scholar]
- Horton TJ, Hill JO (2001). Prolonged fasting significantly changes nutrient oxidation and glucose tolerance after a normal mixed meal. J Appl Physiol 90: 155–163. [DOI] [PubMed] [Google Scholar]
- Isaksson B, Wang F, Permert J, Olsson M, Fruin B, Herrington MK et al. (2005). Chronically administered islet amyloid polypeptide in rats serves as an adiposity inhibitor and regulates energy homeostasis. Pancreatology 5: 29–36. [DOI] [PubMed] [Google Scholar]
- Kanoski SE, Rupprecht LE, Fortin SM, Jonghe BCD, Hayes MR (2012). The role of nausea in food intake and body weight suppression by peripheral GLP‐1 receptor agonists, exendin‐4 and liraglutide. Neuropharmacology 62: 1916–1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karpe F, Dickmann JR, Frayn KN (2011). Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes 60: 2441–2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontan M, Langin D (2009). Lipolysis and lipid mobilization in human adipose tissue. Prog Lipid Res 48: 275–297. [DOI] [PubMed] [Google Scholar]
- Larsen PJ, Fledelius C, Knudsen LB, Tang‐Christensen M (2001). Systemic administration of the long‐acting GLP‐1 derivative NN2211 induces lasting and reversible weight loss in both normal and obese rats. Diabetes 50: 2530–2539. [DOI] [PubMed] [Google Scholar]
- Lean M, Carraro R, Finer N, Hartvig H, Lindegaard M, Rossner S et al. (2014). Tolerability of nausea and vomiting and associations with weight loss in a randomized trial of liraglutide in obese, non‐diabetic adults. Int J Obes (Lond) 38: 689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz TA, Geary N, Szabady MM, Prete ED, Scharrer E (1995). Amylin decreases meal size in rats. Physiol Behav 58: 1197–1202. [DOI] [PubMed] [Google Scholar]
- Mack CM, Soares CJ, Wilson JK, Athanacio JR, Turek VF, Trevaskis JL et al. (2010). Davalintide (AC2307), a novel amylin‐mimetic peptide: enhanced pharmacological properties over native amylin to reduce food intake and body weight. Int J Obes (Lond) 34: 385–395. [DOI] [PubMed] [Google Scholar]
- Mack CM, Smith P a, Athanacio JR, Xu K, Wilson JK, Reynolds JM et al. (2011). Glucoregulatory effects and prolonged duration of action of davalintide: a novel amylinomimetic peptide. Diabetes Obes Metabol 13: 1105–1113. [DOI] [PubMed] [Google Scholar]
- Mather K (2009). Surrogate measures of insulin resistance: of rats, mice, and men. Am J Physiol Endocrinol Metab 296: E398–399. [DOI] [PubMed] [Google Scholar]
- Mathes CM, Spector AC (2012). Food selection and taste changes in humans after Roux‐en‐Y gastric bypass surgery: a direct‐measures approach. Physiol Behav 107: 476–483. [DOI] [PubMed] [Google Scholar]
- Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985). Homeostasis model assessment: insulin resistance and β‐cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28: 412–419. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehlem A, Hagberg CE, Muhl L, Eriksson U, Falkevall A (2013). Imaging of neutral lipids by oil red O for analyzing the metabolic status in health and disease. Nat Protoc 8: 1149–1154. [DOI] [PubMed] [Google Scholar]
- Milić S, Lulić D, Štimac D (2014). Non‐alcoholic fatty liver disease and obesity: biochemical, metabolic and clinical presentations. World J Gastroenterol 20: 9330–9337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miras AD, le Roux CW (2014). Can medical therapy mimic the clinical efficacy or physiological effects of bariatric surgery? Int J Obes (Lond) 38: 325–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit R, Omrani A, Luijendijk MC, de Vrind VA, Van Rozen AJ, Ophuis RJ et al. (2016). Melanocortin 3 receptor signaling in midbrain dopamine neurons increases the motivation for food reward. Neuropsychopharmacology 41: 2241–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pi‐Sunyer FX (1999). Comorbidities of overweight and obesity: current evidence and research issues. Med Sci Sport Exerc 31: S602–S608. [DOI] [PubMed] [Google Scholar]
- Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA et al. (2004). Amylin replacement with pramlintide as an adjunct to insulin therapy improves long‐term glycaemic and weight control in Type 1 diabetes mellitus: a 1‐year, randomized controlled trial. Diabet Med 21: 1204–1212. [DOI] [PubMed] [Google Scholar]
- Roth JD, Hughes H, Kendall E, Baron AD, Anderson CM (2006). Antiobesity effects of the beta‐cell hormone amylin in diet‐induced obese rats: effects on food intake, body weight, composition, energy expenditure, and gene expression. Endocrinology 147: 5855–5864. [DOI] [PubMed] [Google Scholar]
- Roth JD, Hughes H, Coffey T, Maier H, Trevaskis JL, Anderson CM (2007). Effects of prior or concurrent food restriction on amylin‐induced changes in body weight and body composition in high‐fat‐fed female rats. Am J Physiol Endocrinol Metab 293: E1112–E1117. [DOI] [PubMed] [Google Scholar]
- Roth JD, D'Souza L, Griffin PS, Athanacio J, Trevaskis JL, Nazarbaghi R et al. (2012). Interactions of amylinergic and melanocortinergic systems in the control of food intake and body weight in rodents. Diabetes Obes Metab 14: 608–615. [DOI] [PubMed] [Google Scholar]
- Rushing PA, Hagan MM, Seeley RJ, Lutz TA, D'Alessio DA, Air EL et al. (2001). Inhibition of central amylin signaling increases food intake and body adiposity in rats. Endocrinology 142: 5035. [DOI] [PubMed] [Google Scholar]
- Rushing PA, Seeley RJ, Air EL, Lutz TA, Woods SC (2002). Acute 3rd‐ventricular amylin infusion potently reduces food intake but does not produce aversive consequences. Peptides 23: 985–988. [DOI] [PubMed] [Google Scholar]
- Ryan G, Briscoe TA, Jobe L (2009). Review of pramlintide as adjunctive therapy in treatment of type 1 and type 2 diabetes. Drug Des Devel Ther 2: 203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SR, Blundell JE, Burns C, Ellero C, Schroeder BE, Kesty NC et al. (2007). Pramlintide treatment reduces 24‐h caloric intake and meal sizes and improves control of eating in obese subjects: a 6‐wk translational research study. Am J Physiol Endocrinol Metab 293: E620–E627. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SP et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44: D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss EP, Jordan RC, Frese EM, Albert SG, Villareal DT (2016). Effects of weight loss on lean mass, strength, bone, and aerobic capacity. Med Sci Sport Exerc 49: 206–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer C, Maggs DG, Young AA, Kolterman OG (2001). Amylin replacement with pramlintide as an adjunct to insulin therapy in type 1 and type 2 diabetes mellitus: a physiological approach toward improved metabolic control. Curr Pharm Des 7: 1353–1373. [DOI] [PubMed] [Google Scholar]
- Wielinga PY, Lowenstein C, Muff S, Munz M, Woods SC, Lutz TA (2010). Central amylin acts as an adiposity signal to control body weight and energy expenditure. Physiol Behav 101: 45–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young A (2005). Inhibition of gastric emptying. Adv Pharmacol 52: 99–121. [DOI] [PubMed] [Google Scholar]
- Young AA, Gedulin B, Vine W, Percy A, Rink TJ (1995). Gastric emptying is accelerated in diabetic BB rats and is slowed by subcutaneous injections of amylin. Diabetologia 38: 642–648. [DOI] [PubMed] [Google Scholar]


