Abstract
Purpose
The goal of this study was to identify the effects of amyotrophic lateral sclerosis (ALS) on tongue and jaw control, both cross-sectionally and longitudinally. The data were examined in the context of their utility as a diagnostic marker of bulbar disease.
Method
Tongue and jaw movements were recorded cross-sectionally (n = 33 individuals with ALS, 13 controls) and longitudinally (n = 10 individuals with ALS) using a three-dimensional electromagnetic articulography system during the production of the sentence Buy Bobby a puppy. The movements were examined for evidence of changes in size, speed, and duration and with respect to disease severity and time in the study.
Results
Maximum speed of tongue movements and movement durations were significantly different only at an advanced stage of bulbar ALS compared with the healthy control group. The longitudinal analysis revealed a reduction in tongue movement size and speed with time at early stages of disease, which was not seen cross-sectionally. As speaking rate declined, tongue movements decreased in maximum speed, whereas jaw movements increased in maximum speed.
Conclusions
Longitudinal analyses of sentence-level kinematic data show their sensitivity to early bulbar impairment. A change in articulatory kinematics can serve as a useful diagnostic marker for bulbar ALS and to track bulbar disease progression in a clinical setting.
Amyotrophic lateral sclerosis (ALS) is a rapidly progressive, fatal neurological disease. It affects motor neurons in the brain, brainstem, and spinal cord, resulting in progressive wasting and paralysis of voluntary muscles (Gubbay et al., 1985). When patients exhibit initial symptoms in speech or swallowing, they are diagnosed with bulbar-onset ALS. Although only 30% of patients initially present with bulbar signs and symptoms (Bonduelle, 1975; Dworkin & Hartman, 1979), up to 85% of patients exhibit bulbar disease as ALS progresses (Armon & Moses, 1998; Haverkamp, Appel, & Appel, 1995). Bulbar ALS is arguably the most devastating variant of the disease because it is characterized by fast progression, short survival, impaired speech intelligibility, and reduced quality of life (Goldstein, Atkins, & Leigh, 2002; Mitsumoto & Del Bene, 2000).
A combination of assessment methods is used in a clinic to diagnose bulbar disease and determine its severity. The neurologic cranial nerve examination is one of the main components of a standard clinical assessment. This examination consists of clinical impressions of strength, range, speed of movement, and symmetry of oral musculature. However, this type of assessment is inherently subjective and, consequently, may have low reliability. Needle electromyography of the genioglossus muscle of the tongue plays an important role in documenting bulbar involvement in ALS (Finsterer, Fuglsang-Frederik, & Mamoli, 1997; Lambert & Mulder, 1957). This method, however, is invasive and requires complete relaxation of the tongue, which is often difficult to achieve (Sonoo et al., 2009). As a result, the accuracy and validity of the test is limited (Baek & Desai, 2007; Eisen & Swash, 2001).
Functional changes with disease progression are primarily tracked using the Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised (ALSFRS-R; Cedarbaum et al., 1999), which assesses physical functions needed to accomplish activities of daily living. It includes the assessment of bulbar functions via three questions aimed to judge the integrity of speech, swallowing, and salivation on a scale of 0–4 points. The maximum score of 12 indicates intact bulbar function, whereas values below 12 indicate bulbar impairment. The ALSFRS-R has been used in clinical trials due to its sensitivity to disease progression (Kaufmann et al., 2007; Kimura et al., 2006; Kollewe et al., 2008) and survival (Gordon & Cheung, 2006; Kaufmann et al., 2005). However, this assessment tool is an ordinal scale and is likely insensitive to small differences in disease presentation (Rosenfeld & Jackson, 2013) or early stages of the disease (Voustianiouk et al., 2008). Considering the diagnostic challenge in bulbar ALS, identification of changes in bulbar musculature using measures that are sensitive to bulbar disease onset and progression would be of significant clinical value.
Bulbar ALS is often characterized by a reduction in speaking rate and loss of speech intelligibility (Ball, Willis, Beukelman, & Pattee, 2001; Yorkston, 1993). Speech-language pathologists have traditionally used both of these measures to identify the presence and determine severity of bulbar symptoms. The relationship between intelligibility and speaking rate is well established in ALS. As bulbar symptoms progress, speaking rate declines from expected normal values of 160–200 words per minute (WPM) to an important landmark of approximately 120 WPM (Yorkston, 1993). After this critical point is reached, a rapid deterioration in speech intelligibility may be observed. Because speaking rate declines earlier and in a linear manner as compared with speech intelligibility, it is currently considered the clinical gold standard for monitoring the progression of bulbar disease (Ball et al., 2001; Green, Yunusova, et al., 2013; Yorkston, 1993; Yorkston & Beukelman, 1981). For this reason, speaking rate has often been used for the clinical subgrouping of patients on the basis of their severities of bulbar impairment (Mefferd, Pattee, & Green, 2014; Yorkston & Beukelman, 1981; Yunusova et al., 2010).
Neither speech intelligibility nor speaking rate—the system-level clinical measures of bulbar function—are sensitive to the early phases of bulbar deterioration (Ball, 2002; Green, Yunusova, et al., 2013; Niimi & Nishio, 2000; Yunusova et al., 2010). System-level measures are influenced by all physiological subsystems of speech—respiratory, resonatory, phonatory, and articulatory—at the same time, and numerous compensatory strategies across subsystems can be used to maintain intelligibility and speaking rate (Mefferd et al., 2014). Hence, there is a need to evaluate individual subsystems to identify measures that may be indicative of early changes in the bulbar mechanism.
There is evidence to suggest that the study of the articulatory subsystem (i.e., movements of the tongue, jaw, and lips) may provide the most accurate measures of bulbar disease onset and progression. The articulatory subsystem, particularly the tongue, has been indicated as the primary locus of bulbar involvement in ALS (Carpenter, McDonald, & Howard, 1978; DePaul, Abbs, Caligiuri, Gracco, & Brooks, 1988). In a retrospective study of 441 cases of ALS, Carpenter et al. (1978) reported that the tongue was most commonly described as weak, even in patients clinically characterized as nonbulbar. DePaul et al. (1988) measured maximum force generation in the tongue, jaw, and lip musculature and found that among oral structures the tongue was the most involved, even in patients with symptoms restricted to the limbs. Measures of maximum force may have prognostic value: A study by Weikamp, Schelhaas, Hendriks, De Swart, and Geurts (2012) found that a within-subject decrease in tongue strength was prognostic of short survival in ALS. However, measures of maximum force may not be ideal for diagnostic purposes or as outcome measures for clinical trials (Brooks et al., 1991) due to their large between-subjects variability (Kent, Kent, & Rosenbek, 1987). Other measures investigating the function of the articulatory subsystem need to be explored for their diagnostic utility.
Prior studies suggest that measures of tongue, lip, and jaw movements during speech are sensitive markers for documenting bulbar motor impairment (Rong, Yunusova, Wang, & Green, 2015; Yunusova, Green, Wang, Pattee, & Zinman, 2011). As a group, individuals with ALS present smaller and slower speech movements compared with healthy controls during repetitive alternating motion rate and meaningful syllable tasks (Hirose, Kiritani, & Sawashima, 1982b; Kent, Netsell, & Bauer, 1975; Kuruvilla, Green, Yunusova, & Hanford, 2012; Yunusova, Green, et al., 2012; Yunusova, Weismer, Westbury, & Lindstrom, 2008), especially for the tongue. A number of studies have reported exaggerated jaw movements during these tasks, suggesting that there might be a compensatory mechanism involved to offset tongue impairment (Hirose et al., 1982b; Yunusova et al., 2008). Studies have, however, rarely examined the tongue and jaw together in the same group of individuals (but see Hirose, Kiritani, & Sawashima, 1982a), which is fundamental for an understanding of a relationship between the articulators. Furthermore, studies have rarely investigated articulatory movements at a group level, in relation to the disease severity or at the earliest stage of the disease, and/or in sentence productions. Longitudinal studies, which allow direct within-individual comparisons as well as account for varying disease progression patterns, have also been limited in number and sample size (Niimi & Nishio, 2000; Yunusova et al., 2010).
The overall goal of this study was to identify the effects of ALS on tongue and jaw control during sentence productions, both cross-sectionally and longitudinally. Specific objectives were as follows:
to determine differences in tongue and jaw movements at different severities of bulbar disease as compared to age-matched healthy controls; and
to examine within-subject longitudinal changes in tongue and jaw movements, particularly at an early stage of the disease.
We hypothesized that there would be a decrease in size and speed of tongue movements and an increase in size and speed of jaw movements with increasing severity and with disease progression over time. We also hypothesized that tongue and jaw movement durations would increase with worsening bulbar disease. Characterizing the pattern of disease progression via movements of the tongue and jaw may contribute to staging of bulbar ALS, which has significant clinical value because it contributes to providing earlier diagnosis and more targeted approaches to disease management.
Method
Participants
All participants signed informed consent according to the requirements of the Declaration of Helsinki. A neurologist diagnosed all participants in the patient group with possible, probable, or definite ALS as defined by the El Escorial Criteria for the World Federation of Neurology (Brooks, Miller, Swash, & Munsat, 2000). All participants were native speakers of English. Only those with normal hearing and without evidence of cognitive impairment, as determined by a minimum score of 26 of 30 on the Montreal Cognitive Assessment (Nasreddine et al., 2005), participated.
Thirty-three participants (23 men and 10 women) with ALS were assessed cross-sectionally. They were selected from a larger group of individuals (n = 143) who underwent a 5-year longitudinal study of bulbar deterioration in ALS. The patients were selected on the basis of the completeness of the data—both tongue and jaw movements had to be present and a minimum of five repetitions that were free of movement-tracking artifacts had to be present for the participant to be included in the cross-sectional analysis. If multiple sessions for a participant were available, the first session was included. Missing data typically occurred due to participant fatigue and dropout from the study as well as instances of equipment/software malfunction. The control group comprised four men and nine women (n = 13). Healthy participants had no history of significant health or neurologic conditions, cognitive, or sensory problems. Demographic and disease-related information is reported in Table 1.
Table 1.
Participant demographic and disease-related information.
| Group | n (M/F) | Age, years | ALSFRS-R total, /48 | Disease duration, months |
|---|---|---|---|---|
| ALS | 23/10 | 62.65 (±6.87) | 33.21 (± 9.41) | 56.35 (± 24.92) |
| Control | 4/9 | 69.38 (± 3.20) |
Note. Numbers are means with standard deviations in parentheses. Disease duration is estimated from time of symptom onset. ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised; ALS = amyotrophic lateral sclerosis.
Participants with ALS were grouped into three severity groups on the basis of speaking rate, which was measured using the Speech Intelligibility Test (Yorkston, 1993; Yorkston, Dowden, & Beukelman, 1992). The mild group included patients with speaking rates greater than 160 WPM; the moderate group included patients with speaking rates between 120 and 160 WPM; and the severe group of participants had a speaking rate less than 120 WPM. The healthy controls group had intact intelligibility and an average speaking rate of 221.37 WPM (SD = 19.04). Group differences in intelligibility were determined using a one-way analysis of variance, F(3) = 4.276, p < .010, η2 = .238. Post hoc comparisons using the Tukey's honestly significant difference (HSD) test determined significant differences between controls and severe ALS: mean differences = 23.07, p < .015; and mild ALS versus severe ALS: mean differences = 22.42, p < .017. The measure of speaking rate was significantly different between all four groups as determined by a one-way analysis of variance, F(3) = 101.548, p < .001, η2 = .881, with post hoc comparisons using the Tukey's HSD test: Controls versus mild ALS: mean difference = 35.15 WPM, p < .001; controls versus moderate ALS: mean difference = 79.022 WPM, p < .001; controls versus severe ALS: mean difference = 131.961 WPM, p < .001; mild ALS versus moderate ALS: mean difference = 43.87 WPM, p < .001; mild ALS versus severe ALS: mean difference = 96.808 WPM, p < .001; moderate ALS versus severe ALS: mean difference = 52.938 WPM, p < .001. Table 2 shows descriptive statistics for the clinical measures of bulbar disease by subgroup.
Table 2.
Summary statistics for the clinical measures of bulbar disease for mild, moderate, and severe ALS subgroups.
| Group | n | Speaking rate (WPM) | Intelligibility (%) | ALSFRS-R bulbar subscore a |
|---|---|---|---|---|
| Mild | 14 | 186.22 (± 19.39) | 99.4 (± 0.75) | 11.18 (± 0.87) |
| Moderate | 8 | 142.35 (± 11.71) | 97.4 (± 3.61) | 10.63 (± 0.89) |
| Severe | 11 | 89.41 (± 22.17) | 74.93 (± 36.93) | 9.33 (± 0.57) |
Note. Numbers are means with standard deviations in parentheses. WPM = words per minute; ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised.
Maximum score = 12.
A subset of 10 participants (six men and four women) was selected for a longitudinal analysis. The participants were selected on the basis of the completeness of the data—tongue and jaw movements both had to be present, and a minimum of five repetitions free of movement-tracking artifacts had to be present for two or more sessions. Because our primary goal was to identify measures suitable for early detection of the bulbar disease, we focused on those participants with a speaking rate greater than 120 WPM, a hallmark at which bulbar disease is perceptually obvious and speech intelligibility is affected (Mefferd, Green, & Pattee, 2012; Yorkston, 1993). A summary of participant information for the longitudinal analysis is reported in Table 3.
Table 3.
Summary statistics and session information for participants in the longitudinal subgroup.
| n (M/F) | Number of sessions | Time between sessions (months) | ALSFRS-R bulbar subscore at time = 0 a |
|---|---|---|---|
| 10 (6/4) | 3 (± 0.66) | 5.32 (± 2.01) | 11.1 (± 1.16) |
Note. Numbers are means with standard deviations in parentheses. ALS = amyotrophic lateral sclerosis; ALSFRS-R = Amyotrophic Lateral Sclerosis Functional Rating Scale–Revised.
Maximum score = 12.
Measures of Bulbar Dysfunction
Speech intelligibility and speaking rate were obtained for each speaker and session using the Speech Intelligibility Test (Yorkston, Beukelman, & Tice, 1996). A single judge, an unfamiliar listener, performed the analysis. The listener was a native speaker of English, with no history of speech or language disorders and naïve to dysarthric speech. Speech intelligibility was expressed as the percentage of total words transcribed correctly. Speaking rate was calculated as the number of WPM. The effect of overall motor and bulbar motor impairment on daily functions was assessed using the ALSFRS-R (total and bulbar subscores).
Speech Sample
The recorded task consisted of a sentence, Buy Bobby a puppy, read at a comfortable reading rate and loudness and repeated 10 times. This sentence was chosen in order to elicit large jaw movements and complex tongue movements (i.e., the diphthong /ai/ in buy; Kleinow, Smith, & Ramig, 2001; McHenry, 2003; A. Smith & Kleinow, 2000; Yunusova et al., 2011).
Instrumentation and Data Acquisition
Articulatory movements of the tongue and jaw were collected using an electromagnetic tracking device (the Wave Speech Research System; NDI, Waterloo, Ontario, Canada) with acceptable accuracy of < 0.5 mm (Berry, 2011). Movements were captured at a sampling rate of 400 Hz. A 6 degrees of freedom (DOF) reference sensor was attached to a headband and securely positioned on the head in order to collect head movements. The reference sensor was used to subtract the head movements from articulator movements. Tongue movements were obtained by attaching a 5 DOF sensor on the midsagittal plane of the tongue approximately 20 mm from the tongue tip. PeriAcryl Oral Tissue Adhesive (Glustitch Inc., Delta, British Columbia, Canada), a nontoxic dental glue, was used for sensor attachment. Jaw movements were obtained by attaching two 5 DOF sensors to the mandibular gumline between the canine and incisor teeth on the right and left side, using stoma adhesive (Stomahesive, ConvaTec, Greensboro, NC). The left sensor was used to represent jaw movements for all analyses.
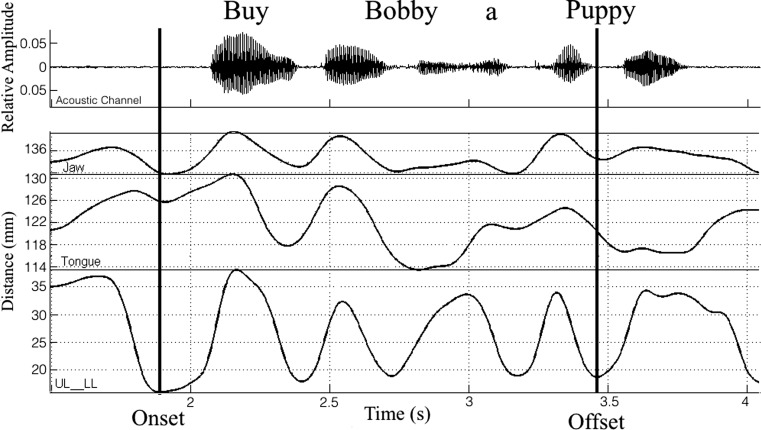
Post-acquisition, the data were manually checked for tracking errors, and a low-pass filter at 15 Hz using a zero-phase shift forward, and a reverse digital filter (8-pole Butterworth) was used to remove high-frequency noise from the signals. The low-pass frequency cutoff was determined by applying fast Fourier transforms to the relevant signals. Data were parsed for measurements using custom-made MATLAB routines in SMASH (Green, Wang, & Wilson, 2013). Upper and lower lip aperture time history was used for parsing movement traces. A custom-built MATLAB program algorithmically marked the minimum aperture in the vicinity of the acoustic onset in buy. Offset of the sentence was marked as the minimum aperture following the last consonant-vowel-consonant syllable in puppy. Three-dimensional (3D) Euclidean distance traces were calculated for tongue and jaw movements and used for measurements.
A high-quality earset microphone (Countryman E6, Countryman Associates, Inc., Menlo Park, CA) was positioned approximately 5 cm from the mouth during the recordings. Acoustic signals were recorded simultaneously with kinematic signals at a sampling rate of 22.05 KHz and 16-bit resolution onto the hard drive of a computer.
Kinematic Measures
Kinematic measures were obtained for tongue and jaw (T+J) and jaw (J) movement signals. Movement measures that previously demonstrated their sensitivity to bulbar dysfunction in ALS (Hirose et al., 1982a; Kent et al., 1975; Kuruvilla et al., 2012; Mefferd et al., 2012; Yunusova, Rosenthal, Rudy, Baljko, & Daskalogiannakis, 2012) were selected for analyses:
Range (mm) of movement, a measure representative of movement size, was calculated as the difference between the maximum and minimum values of the 3D Euclidean distance trace.
Maximum speed (mm/s) of movement was calculated as the maximum absolute value of the first derivative of the 3D Euclidean distance trace.
Movement duration was calculated as the time (s) between movement onset and offset as shown in Figure 1 (same measure for both tongue and jaw).
Figure 1.
Upper and lower lip aperture (UL-LL) time history was used for identification of the onset and offset of movement traces in the sentence Buy Bobby a puppy.
Statistical Analyses
All objectives were addressed by using a linear mixed effects (LME) method (SPSS Statistics, Version 20, IBM, Armonk, NY). To examine cross-sectional group differences, LME models predicted the articulatory measures as a function of group (healthy controls vs. mild, moderate, and severe ALS groups). Separate models were set for T+J versus J, for a total of five LME models per set (2 [Articulators] × 2 [Measures]) + 1 (Duration). Because the data consisted of a mix of within-subject (i.e., repetitions) and between-subjects observations, the inclusion of a subject-dependent intercept as a random effect in the LME model accounted for the intersubject variations in articulatory measures. The equivalent jaw measure was included as a covariate in all models that predicted tongue kinematics to control for the contribution of jaw movements to tongue movements (i.e., jaw range was added as a covariate for models predicting a change in tongue range). Sex was included as a covariate for all models because previous findings have reported sex-specific differences in tongue movements, specifically during the production of vowels (Simpson, 2001, 2002). An α level of .05 was chosen for all main effects. Post hoc pairwise comparisons between groups were conducted using Tukey's HSD.
To model longitudinal changes in articulatory movements, multiple LME models with random subject intercepts and slopes were used. Separate models were run for T+J and J data. Similar to the cross-sectional analysis, the equivalent jaw measure was included as a covariate in all T+J models. Gender was included as a covariate for all models. An α level of .05 was chosen for all main effects. To estimate changes in articulatory movements as a function of bulbar disease severity, the first set of LME models examined speaking rate—a clinical measure of bulbar disease severity—as a predictor. The second set of LME models was a basic longitudinal model treating time in study (months) as the predictor. With the ultimate goal of identifying sensitive markers of bulbar disease beyond the clinical measure of speaking rate, the last set of LME models examined changes in articulatory movement measures with time in study (months) as a predictor when controlling for speaking rate as a fixed covariate. All longitudinal models included sex as a covariate. An α level of .05 was chosen for all models.
Results
Group Differences in Speech Movements
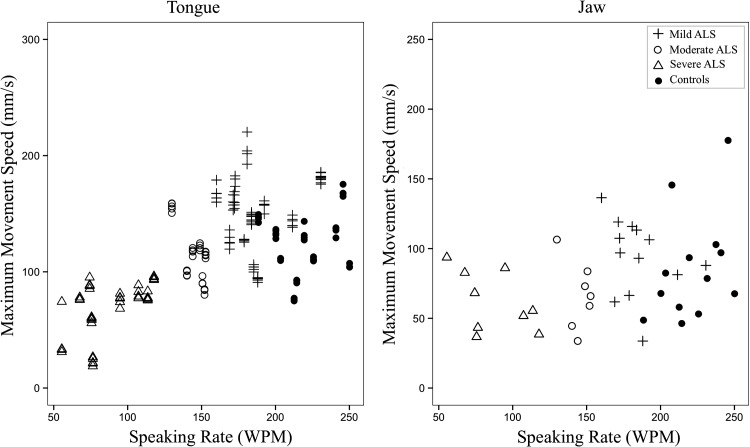
Summary statistics of each kinematic measure for each group and the results from the corresponding statistical analyses are summarized in Table 4. Participants in the severe group presented with significantly smaller maximum speed of tongue movements and larger movement durations compared with healthy controls (see Figures 2 and 3).
Table 4.
Means and SD (in parentheses) per group for each kinematic measure.
| Kinematic measure | ALS |
Healthy controls | Omnibus significance | ||
|---|---|---|---|---|---|
| Mild | Moderate | Severe | |||
| Tongue | |||||
| Movement range (mm) | 16.22 (± 3.91) | 12.75 (± 3.34) | 12.56 (± 4.44) | 14.18 (± 3.25) | ns |
| Maximum movement speed (mm/s) | 151.31 (± 36.03) | 114.81 (± 25.10) | 68.29 (± 25.55)*** | 121.79 (± 29.92) | *** |
| Jaw | |||||
| Movement range (mm) | 8.55 (± 2.40) | 6.73 (± 2.68) | 8.28 (± 3.99) | 7.14 (± 3.28) | ns |
| Maximum movement speed (mm/s) | 97.08 (± 30.59) | 67.27 (± 25.54) | 60.83 (± 27.77) | 86.07 (± 40.13) | ns |
| Movement duration (s) | 1.09 (± .35) | 1.14 (± .44) | 2.05 (± .68)*** | 1.18 (± .25) | *** |
Note. ALS = amyotrophic lateral sclerosis; ns = not significant.
Significantly impaired relative to healthy controls at p < .001.
Figure 2.
Maximum speeds (mm/s) (predicted values) of tongue movements across amyotrophic lateral sclerosis (ALS) groups and healthy controls as a function of speaking rate in words per minute (WPM).
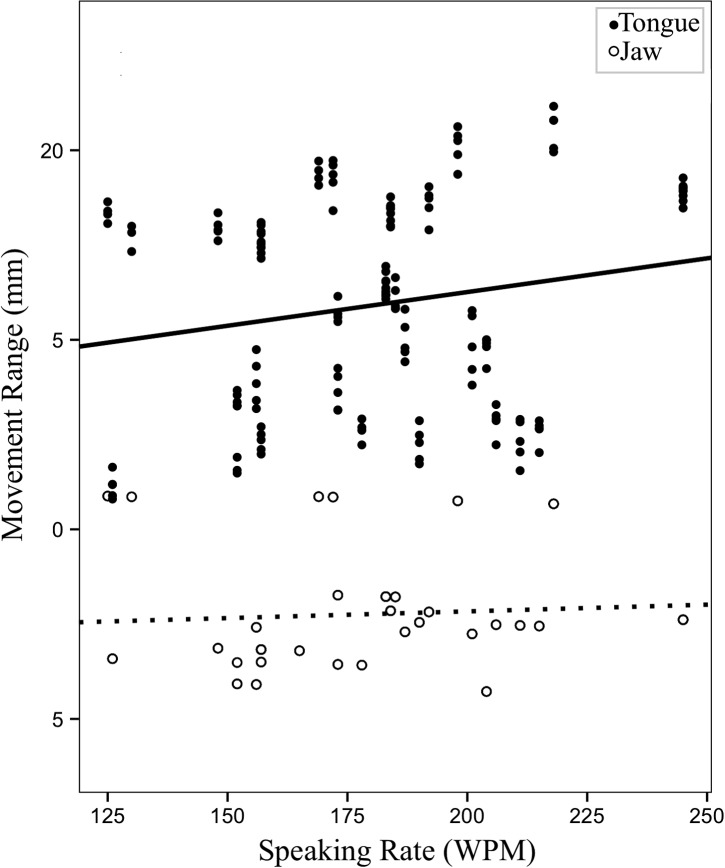
Figure 3.
Changes in range (mm) of tongue and jaw movements (predicted values) as a function of speaking rate in words per minute (WPM).
Longitudinal Changes in Articulatory Movements as a Function of Bulbar Disease Severity
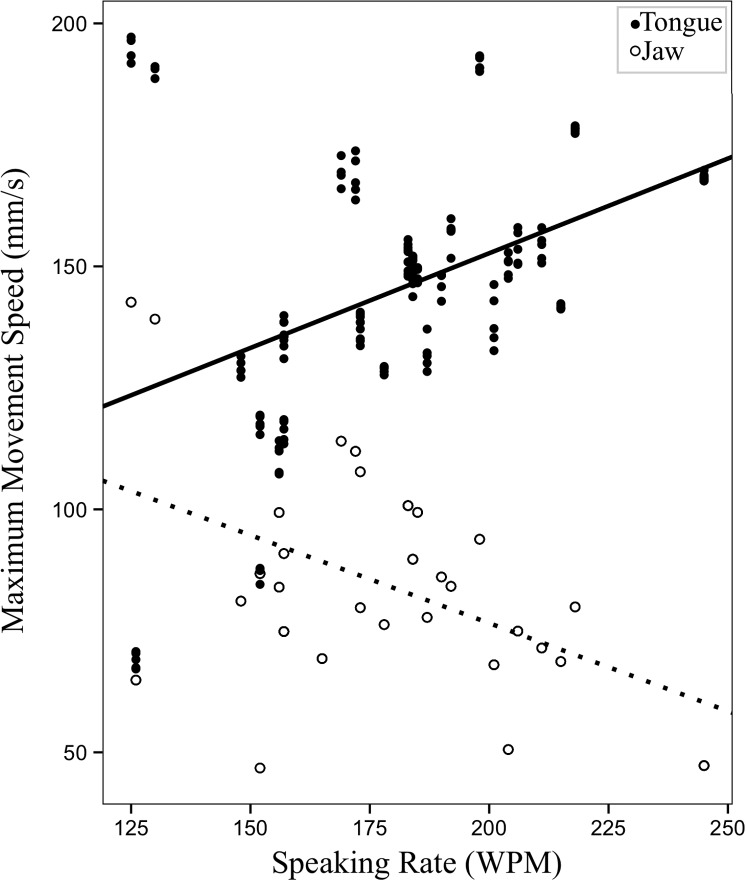
The first set of longitudinal models investigated speaking rate—an index of disease severity—as a predictor of change in articulatory movements. Of the five LME models, a significant effect of speaking rate was observed on range and maximum speed of tongue movements, F(1, 146.98) = 7.01, p = .009; F(1, 146.88) = 43.05, p < .001, respectively, and maximum speed of jaw movements, F(1, 126.81) = 46.00, p < .001. Decreased speaking rate was associated with decreased range and maximum speed of tongue movements and increased maximum speed of jaw movements. Neither range of jaw movements nor movement durations showed a significant relationship with speaking rate. Unstandardized beta coefficients and p values for the five LME models with speaking rate as a predictor are shown in Table 5.
Table 5.
Unstandardized beta coefficients and p values for LME models investigating the changes in articulatory measures, with speaking rate as a predictor.
| Articulator | Outcome measure | Predictor | Fixed covariates | b | p |
|---|---|---|---|---|---|
| T+J | Movement range (mm) | Speaking rate (WPM) | Sex; J range (mm) | 0.02 | .009* |
| Maximum movement speed (mm/s) | Speaking rate (WPM) | Sex; J maximum speed (mm/s) | 0.59 | < .001** | |
| J | Movement range (mm) | Speaking rate (WPM) | Sex | −0.003 | .465 |
| Maximum movement speed (mm/s) | Speaking rate (WPM) | Sex | −0.70 | < .001** | |
| Movement duration (s) | Speaking rate (WPM) | Sex | −0.001 | .095 |
Note. LME = linear mixed effects; T+J = tongue and jaw; J = jaw; WPM = words per minute.
Significant at alpha = .05.
Significant at alpha = .01.
Figure 4 shows changes in movement range. Figure 5 shows changes in maximum speed of tongue and jaw movements with respect to changes in speaking rate.
Figure 4.
Changes in range (mm) of tongue and jaw movements (predicted values) as a function of speaking rate in words per minute (WPM).
Figure 5.
Changes in maximum speed (mm/s) of tongue and jaw movements (predicted values) as a function of speaking rate in words per minute (WPM).
Longitudinal Changes in Articulatory Movements With Time
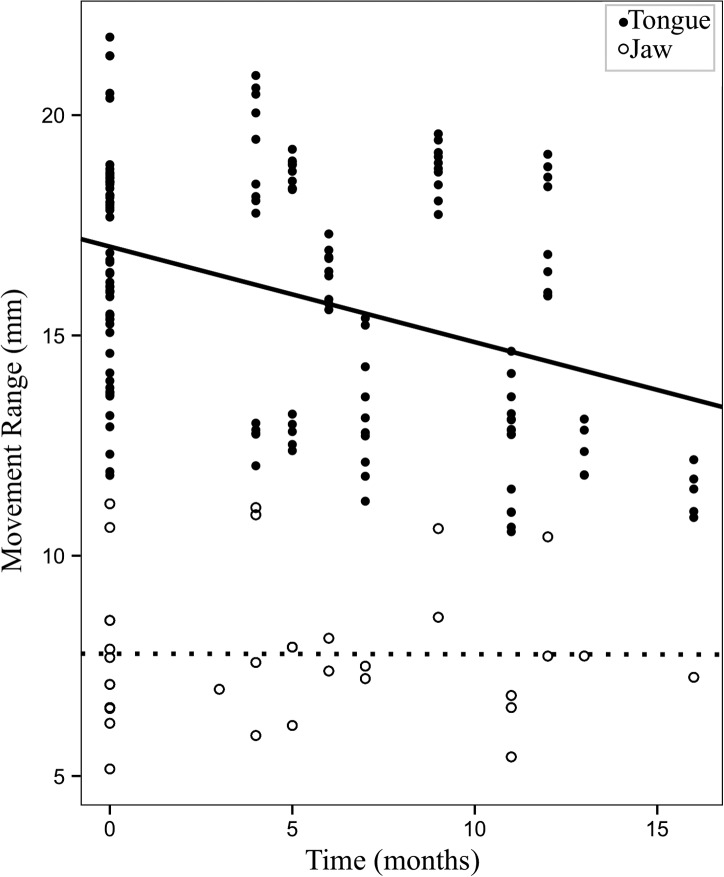
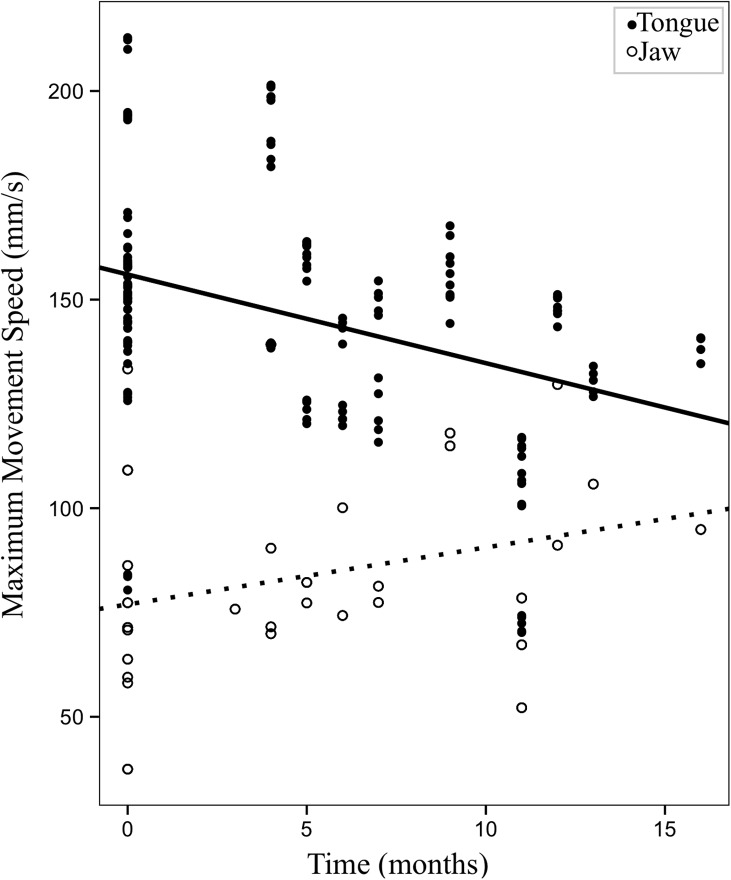
The next set of models investigated the change in articulatory movements over time. For three of the five LME models, time in the study was a significant predictor of articulatory movements. Specifically, time in the study was a significant predictor for range and maximum speed of tongue movements, when statistically controlling for J, F(1, 4.87) = 10.12, p = .025; F(1, 5.55) = 10.367, p = .02, respectively; and movement durations, F(1, 8.44) = 5.77, p = .042. As time passed, tongue movements decreased in range and maximum speed, and movement durations increased. Neither range nor maximum speed of jaw movements showed a significant change over time. Unstandardized beta coefficients and p values for LME models with time as the predictor are shown in Table 6.
Table 6.
Unstandardized beta coefficients and p values for LME models investigating the changes in articulator measures over time.
| Articulator | Outcome measure | Predictor | Fixed covariates | b | p |
|---|---|---|---|---|---|
| T+J | Movement range (mm) | Time in study (months) | Sex; J range (mm) | −0.152 | .025* |
| Maximum movement speed (mm/s) | Time in study (months) | Sex; J max speed (mm/s) | −3.10 | .02* | |
| J | Movement range (mm) | Time in study (months) | Sex | 0.03 | .54 |
| Maximum movement speed (mm/s) | Time in study (months) | Sex | 1.72 | .18 | |
| Movement duration (s) | Time in study (months) | Sex | 0.012 | .042* |
Note. LME = linear mixed effects; T+J = tongue and jaw; J = jaw.
Significant at alpha = .05.
Figure 6 shows changes in range (mm) of tongue and jaw movements with respect to time (in months). Figure 7 shows changes in maximum speed (mm/s) of tongue and jaw movements over time.
Figure 6.
Changes in range (mm) of tongue and jaw movements (predicted values) over time in the study (in months).
Figure 7.
Changes in maximum speed (mm/s) of tongue and jaw movements (predicted values) over time in the study (in months).
Longitudinal Changes in Articulatory Movements With Time, Beyond Speaking Rate
The third set of models considered changes in articulatory movements over time while controlling for disease severity via the measure of speaking rate. When controlling for the effect of speaking rate, range, F(1, 7.18) = 9.01, p = .019, and maximum speed, F(1, 5.73) = 7.59, p = .035, of tongue movements showed significant changes over time. Specifically, over the course of the disease, range and maximum speed of tongue movements decreased independently of speaking rate. Unstandardized beta coefficients and p values for the LME models with time as the predictor and speaking rate as a covariate are shown in Table 7. Neither of the jaw measures nor movement durations showed significance.
Table 7.
Unstandardized beta coefficients and p values for LME models investigating the changes in articulator measures over time, beyond speaking rate.
| Articulator | Outcome measure | Predictor | Fixed covariates | b | p |
|---|---|---|---|---|---|
| T+J | Movement range (mm) | Time in study (months) | Sex; J range (mm); speaking rate (WPM) | −0.162 | .019* |
| Maximum movement speed (mm/s) | Time in study (months) | Sex; J maximum speed (mm/s); speaking rate (WPM) | −2.61 | .035* | |
| J | Movement range (mm) | Time in study (months) | Sex; speaking rate (WPM) | 0.011 | .821 |
| Maximum movement speed (mm/s) | Time in study (months) | Sex; speaking rate (WPM) | 0.69 | .547 | |
| Movement duration (s) | Time in study (months) | Sex; speaking rate (WPM) | 0.01 | .065 |
Note. LME = linear mixed effects; T+J = tongue and jaw; J = jaw.
Significant at alpha = .05.
Discussion
This study investigated cross-sectional group and longitudinal within-individual changes in tongue and jaw movements due to disease progression; this was done by comparing productions of a sentence by individuals diagnosed with ALS and exhibiting varying severity of bulbar disease with those of healthy controls. The movements were examined for evidence of changes in size, speed, and duration. The data were examined in the context of their utility as a diagnostic marker of bulbar disease. Overall, the results suggest that speech movements show a distinct pattern of change relatively early in the course of the disease, which has potential diagnostic value.
Tongue and Jaw Movements: Cross-Sectional Group Comparisons
A cross-sectional analysis revealed significantly smaller maximum speeds of tongue movements and larger movement durations for the patients at the severe stage of the disease (speaking rate < 120 WPM) as compared with the healthy controls. On average, tongue movements were approximately twice as slow, and movement durations were twice as large in the severe group. These findings are similar to the existing reports of kinematic changes in dysarthria, particularly in ALS, reporting slower than normal tongue movements and longer than normal movement durations (Green, Yunusova, et al., 2013; Hirose et al., 1982b; Kent et al., 1975; Kuruvilla et al., 2012; Yunusova, Green, et al., 2012; Yunusova et al., 2008).
Group differences, however, were not seen in articulatory movements in the mild and moderate ALS groups, as defined by the clinical measure of speaking rate, when compared with healthy controls. These findings differ from existing studies that reported reduced maximum speeds and larger movements of the jaw during the early stages of the disease (Mefferd et al., 2012). This discrepancy in the findings is most likely due to differences in the speech tasks used in the studies. Mefferd et al. (2012) examined movements during a rapid metronome-paced syllable repetition task, which in its nature is close to a maximum performance task and, thus, might be expected to show breakdown earlier in the disease than a sentence production task. Our findings suggest that the present sentence task may not have been an ideal choice for detecting group differences at the movement level, especially at a subclinical phase of bulbar disease, and therefore may not be useful in detecting the onset of bulbar disease. A study by Yunusova et al. (2008) reported that productions requiring larger and faster movements of the tongue were more sensitive to disease states than productions requiring smaller and slower movements. Thus, more complex sentences requiring larger movements may be more sensitive to early bulbar deterioration. However, ultimately all sentence tasks may be insensitive to relatively mild impairment, and this may primarily be due to compensatory mechanisms acting to preserve speech intelligibility (Green, Yunusova, et al., 2013; Netsell & Rosenbek, 1985), making speech measures largely insensitive to subtle changes in muscle physiology. Maximum performance tasks, such as alternating motion rates, may be more sensitive in capturing early impairment in the bulbar system (Rong et al., 2015).
Sentence-length movement durations were longer in the severe ALS group as compared with age-matched healthy controls. This finding agrees with existing literature that reports segmental and phrase lengthening with ALS (Tjaden & Turner, 2000; Turner & Weismer, 1993). It is interesting that significant differences in durations were not observed for mild and moderate stages of bulbar disease, even though speaking rate was significantly lower in both of these groups as compared with the healthy controls. This suggests that speaking rate estimates at early stages of the disease may be affected by factors other than the articulatory movement alone. These factors may include pause durations and frequencies (Green, 2004; Turner & Weismer, 1993).
Although significant group effects were observed in the cross-sectional analyses for the severe ALS group, kinematic measures yielded poor sensitivity at the early stages of disease, most likely due to the large variability between speakers in speech movement measures obtained during speech (Gracco & Abbs, 1986). This between-subject variability could arise from numerous factors, including anatomical differences (Rudy & Yunusova, 2013; Shiller, Laboissière, & Ostry, 2002), differences in habitual speaking rate and style (A. Smith, Goffman, Zelaznik, Ying, & McGillem, 1995; Westbury, Hashi, & Lindstrom, 1998), and different control strategies (i.e., motor equivalence; Hertrich & Ackermann, 2000; Perkell, Matthies, Svirsky, & Jordan, 1993; B. L. Smith & McLean-Muse, 1987). Our models for the cross-sectional analyses did not include a random slope coefficient, which would account for the between-subject variability in each measure, due to the absence of speaking rate variation between sentence repetitions. A large portion of the unexplained variance in our models may be due to other secondary factors that were not captured in the study. Thus, there is a need to continue groupwide investigations of speech movements that capture multiple sources of within-speaker variability.
Longitudinal Changes at an Early Stage of Bulbar Disease
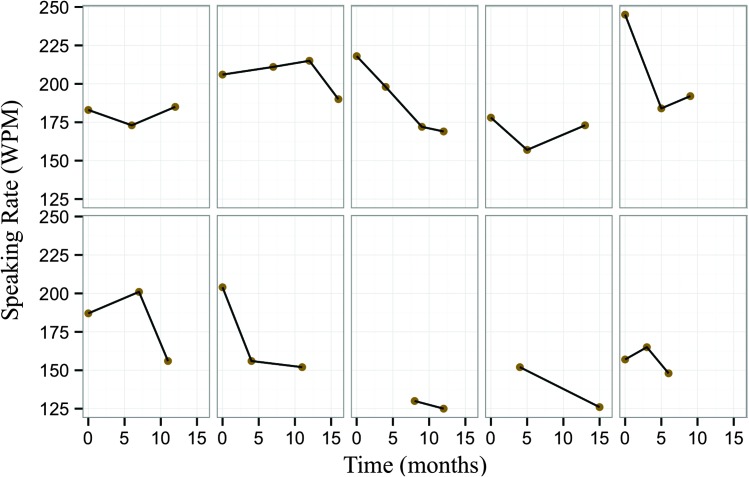
Figure 8 shows longitudinal profiles of speaking rate over their time in the study for the 10 participants whose movement data were analyzed. As seen in the figure, there is evidence of large variation in the severity and rate of disease progression between participants, as captured by the different patterns of speaking rate decline over time. This is typical of heterogeneous populations, such as that of ALS, for whom the course of disease and time of entry into research studies vary greatly. In order to address this heterogeneity, first, speech movements were assessed as a function of severity of bulbar symptoms, characterized by speaking rate. Results showed that as bulbar disease progressed, tongue movements showed a decrease in maximum speed and size; meanwhile, jaw movements showed a significant increase in maximum speed (see Figures 4 and 5). This finding agrees with a preliminary longitudinal report by Yunusova et al. (2010), who found that jaw movements increased in speed at a stage immediately prior to a loss of intelligibility, which might be highly associated with changes in the tongue (Kent, 1989).
Figure 8.
Speaking rate in words per minute (WPM) over time (in months) for the 10 participants in the longitudinal set; time represents time in the study.
Are Tongue and Jaw Movements Compensatory?
Netsell and Rosenbek (1985) were among the first to have stressed the importance of evaluating each physiological component of the complex speech production system, particularly in ALS, which affects each structure differently (Carpenter et al., 1978; DePaul et al., 1988; Dworkin, 1980; Dworkin & Hartman, 1979; Langmore & Lehman, 1994; Lawyer & Netsky, 1953). Speech kinematic studies in ALS suggest that differential impairment might lead to the existence of compensations (i.e., an unaffected articulator helps the affected articulator to reach a set speech target). The smaller and slower than normal tongue movements and larger than normal jaw movements have been viewed as an example of compensatory behavior in ALS (Mefferd et al., 2012; Yunusova et al., 2008). However, there is an alternative explanation. Mefferd et al. (2014) reported highly variable patterns of jaw movements during sentence productions at a mild stage of ALS and suggested that an increase in jaw speed may simply reflect an initial loss of articulatory control. As hypothesized, however, our data showed that, when speaking rate declined, the tongue and jaw showed an opposing pattern of movements—the tongue decreased in speed, whereas the jaw increased in speed. This finding seems to corroborate the presence of compensation in ALS. Further examination on the tongue–jaw relation is needed to understand whether the increased jaw speed is in response to the decline in tongue function.
One of the limitations of our approach is that we were not able to decouple the tongue movements from the jaw movements, and thus, the analyses were restricted to jaw decoupling by a statistical method. As a result, examining correlations between the tongue and jaw signals directly was not possible because a large portion of the tongue movements would be determined by the jaw (Kiritani, 1986). Previous studies have decoupled tongue movements from the jaw using a linear subtraction method (Westbury, Lindstrom, & McClean, 2002). However, this method does not account for jaw rotation and thus can introduce movement error of up to 5 mm (Henriques & Van Lieshout, 2013). The ideal decoupling method pertains to the mathematical reexpression of tongue positions relative to the jaw (Westbury et al., 2002), which requires all six dimensions of positions and rotations to be available. The technical development of this jaw correction algorithm is currently in progress.
Speech Movements Over Time: Markers of Bulbar Disease
There is increasing research and clinical emphasis on finding markers to diagnose changes in bulbar function as early as possible and to track disease progression. Multiple measures have been considered, including nonspeech tasks such as maximum voluntary contractions, as well as system-level speech tasks such as intelligibility and speaking rate (Weismer, 2006). The system-level speech tasks have consistently been criticized for their insensitivity to impairment at the subclinical and early stages of disease (Ball, 2002; Green, Wang, & Wilson, 2013; Yorkston, 1993). Kinematic measures have emerged only recently as candidate markers for diagnosis and disease monitoring. Sensitivity of these measures to change over time, particularly early in the course of the disease, has not been established.
We hypothesized that tongue and jaw kinematics would show a distinct pattern of change at an early stage of disease: Tongue movements would decrease in size and speed and jaw movements would increase in size and speed as the disease progressed. Our longitudinal models revealed that tongue movements do indeed decrease in size and speed, beyond the change observed in the current clinical gold standard of speaking rate. This finding validates the use of movement measures in tracking bulbar disease in a clinical setting. When defining bulbar progression as a decrease in speaking rate, the hypothesis that jaw movements would increase in speed was also well supported, as seen in our speaking rate models. However, a distinct pattern of change in jaw movements over time was not observed.
Conclusions
The current study investigated the tongue and jaw articulatory movement measures, in both cross-sectional and longitudinal analyses. The overall goal of the investigation was to assess the role of articulatory measures in a diagnostic context. The study demonstrated the role of kinematic measures in the assessment of neurogenic speech disorders. The clinical assessment of bulbar ALS would benefit from including movement-based measures because speech movements show observable changes at very early stages of disease.
Findings from the study suggest that tongue movement measures may be more suitable for tracking early changes in bulbar function than the clinical gold standard of speaking rate. A decrease in tongue movement size with disease progression may serve as a potential diagnostic marker for early detection of bulbar involvement. In contrast with other speech measures, such as intelligibility and speaking rate, kinematic measures are able to evaluate independent structures of the speech mechanism, allowing for a more comprehensive assessment of the motor speech disorder due to ALS. Movement measures can also contribute to our understanding of interactions between structures during speech production, which are clearly observable in our data. Continued work is required, however, to develop a sensitive protocol for detection of early signs of bulbar ALS and for documenting the disease progression over time in the clinic and for clinical trials.
Acknowledgments
This research was supported by the National Institutes of Health R01 DC009890 (awarded to Jordan R. Green and Yana Yunusova) and R01 DC0135470 (awarded to Jordan R. Green). We are grateful for the patients and their families for participating in this project. We also thank Danielle Thomas, Lori Synhorst, and Vincci Tau for their assistance with this project.
Funding Statement
This research was supported by the National Institutes of Health R01 DC009890 (awarded to Jordan R. Green and Yana Yunusova) and R01 DC0135470 (awarded to Jordan R. Green).
References
- Armon C., & Moses D. (1998). Linear estimates of rates of disease progression as predictors of survival in patients with ALS entering clinical trials. Journal of the Neurological Sciences, 160, S37–S41. [DOI] [PubMed] [Google Scholar]
- Baek W. S., & Desai N. P. (2007). ALS: Pitfalls in the diagnosis. Practical Neurology, 7, 74–81. [PubMed] [Google Scholar]
- Ball L. J. (2002). Timing of speech deterioration in people with amyotrophic lateral sclerosis. Journal of Medical Speech-Language Pathology, 10, 231–235. [Google Scholar]
- Ball L. J., Willis A., Beukelman D. R., & Pattee G. L. (2001). A protocol for identification of early bulbar signs in amyotrophic lateral sclerosis. Journal of the Neurological Sciences, 191, 43–53. doi:S0022510X01006232 11676991 [Google Scholar]
- Berry J. J. (2011). Accuracy of the NDI Wave speech research system. Journal of Speech, Language, and Hearing Research, 54, 1295–1301. [DOI] [PubMed] [Google Scholar]
- Bonduelle V. M. (1975). The familial forms of amyotrophic lateral sclerosis [in German]. Wiener Medizinische Wochenschrift, 125, 330–331. [PubMed] [Google Scholar]
- Brooks B. R., Miller R. G., Swash M., & Munsat T. L. (2000). El Escorial revisited: Revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1, 293–299. [DOI] [PubMed] [Google Scholar]
- Brooks B. R., Sufit R. L., DePaul R., Tan Y. D., Sanjak M., & Robbins J. (1991). Design of clinical therapeutic trials in amyotrophic lateral sclerosis. Advances in Neurology, 56, 521–546. [PubMed] [Google Scholar]
- Carpenter R. J. III, McDonald T. J., & Howard F. M. Jr. (1978). The otolaryngologic presentation of amyotrophic lateral sclerosis. Otolaryngology, 86(3, Pt. 1), ORL479–ORL484. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J. M., Stambler N., Malta E., Fuller C., Hilt D., Thurmond B., & Nakanishi A. (1999). The ALSFRS-R: A revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS Study Group (Phase III). Journal of the Neurological Sciences, 169, 13–21. [DOI] [PubMed] [Google Scholar]
- DePaul R., Abbs J. H., Caligiuri M., Gracco V. L., & Brooks B. R. (1988). Hypoglossal, trigeminal, and facial motoneuron involvement in amyotrophic lateral sclerosis. Neurology, 38, 281–283. [DOI] [PubMed] [Google Scholar]
- Dworkin J. P. (1980). Tongue strength measurement in patients with amyotrophic lateral sclerosis: Qualitative vs. quantitative procedures. Archives of Physical Medicine and Rehabilitation, 61, 422–424. [PubMed] [Google Scholar]
- Dworkin J. P., & Hartman D. E. (1979). Progressive speech deterioration and dysphagia in amyotrophic lateral sclerosis: Case report. Archives of Physical Medicine and Rehabilitation, 60, 423–425. [PubMed] [Google Scholar]
- Eisen A., & Swash M. (2001). Clinical neurophysiology of ALS. Clinical Neurophysiology, 112, 2190–2201. doi:S1388-2457(01)00692-7 [DOI] [PubMed] [Google Scholar]
- Finsterer J., Fuglsang-Frederik A., & Mamoli B. (1997). Needle EMG of the tongue: Motor unit action potential versus peak ratio analysis in limb and bulbar onset amyotrophic lateral sclerosis. Journal of Neurology, Neurosurgery, and Psychiatry, 63, 175–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein L. H., Atkins L., & Leigh P. N. (2002). Correlates of quality of life in people with motor neuron disease (MND). Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 3, 123–129. doi:10.1080/146608202760834120 [DOI] [PubMed] [Google Scholar]
- Gordon P. H., & Cheung Y. K. (2006). Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology, 67, 1314–1315. [DOI] [PubMed] [Google Scholar]
- Gracco V. L., & Abbs J. H. (1986). Variant and invariant characteristics of speech movements. Experimental Brain Research, 65, 156–166. [DOI] [PubMed] [Google Scholar]
- Green J. R. (2004). Algorithmic estimation of pauses in extended speech samples of dysarthric and typical speech. Journal of Medical Speech-Language Pathology, 12, 149–154. [PMC free article] [PubMed] [Google Scholar]
- Green J. R., Wang J., & Wilson D. L. (2013). SMASH: A tool for articulatory data processing and analysis. In Bimbot F., Cerisara C., Fougeron C., Gravier G., Lamel L., Pellegrino F., & Perrier P. (Eds.), Interspeech 2013 (pp. 1331–1335). Lyon, France: International Speech Communication Association. [Google Scholar]
- Green J. R., Yunusova Y., Kuruvilla M. S., Wang J., Pattee G. L., Synhorst L., … Berry J. D. (2013). Bulbar and speech motor assessment in ALS: Challenges and future directions. Amyotrophic Lateral Sclerosis Frontotemporal Degeneration, 14, 494–500. doi:10.3109/21678421.2013.817585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubbay S. S., Kahana E., Zilber N., Cooper G., Pintov S., & Leibowitz Y. (1985). Amyotrophic lateral sclerosis. A study of its presentation and prognosis. Journal of Neurology, 232, 295–300. [DOI] [PubMed] [Google Scholar]
- Haverkamp L. J., Appel V., & Appel S. H. (1995). Natural history of amyotrophic lateral sclerosis in a database population. Validation of a scoring system and a model for survival prediction. Brain, 118(Pt. 3), 707–719. [DOI] [PubMed] [Google Scholar]
- Henriques R. N., & Van Lieshout P. (2013). A comparison of methods for decoupling tongue and lower lip from jaw movements in 3D articulography. Journal of Speech, Language, and Hearing Research, 56, 1503–1516. [DOI] [PubMed] [Google Scholar]
- Hertrich I., & Ackermann H. (2000). Lip-jaw and tongue-jaw coordination during rate-controlled syllable repetitions. The Journal of the Acoustical Society of America, 107, 2236–2247. [DOI] [PubMed] [Google Scholar]
- Hirose H., Kiritani S., & Sawashima M. (1982a). Patterns of dysarthric movement in patients with amyotrophic lateral sclerosis and pseudobulbar palsy. Folia Phoniatrica et Logopaedica (Basel), 34, 106–112. [DOI] [PubMed] [Google Scholar]
- Hirose H., Kiritani S., & Sawashima M. (1982b). Velocity of articulatory movements in normal and dysarthric subjects. Folia Phoniatrica et Logopaedica (Basel), 34, 210–215. [DOI] [PubMed] [Google Scholar]
- Kaufmann P., Levy G., Montes J., Buchsbaum R., Barsdorf A. I., Battista V., … Thompson J. L. (2007). Excellent inter-rater, intra-rater, and telephone-administered reliability of the ALSFRS-R in a multicenter clinical trial. Amyotrophic Lateral Sclerosis, 8, 42–46. doi:10.1080/17482960600888156 [DOI] [PubMed] [Google Scholar]
- Kaufmann P., Levy G., Thompson J. L., Delbene M. L., Battista V., Gordon P. H., … Mitsumoto H. (2005). The ALSFRS-R predicts survival time in an ALS clinic population. Neurology, 64, 38–43. doi:10.1212/01.wnl.0000148648.38313.64 [DOI] [PubMed] [Google Scholar]
- Kent R. D. (1989). Relationships between speech intelligibility and the slope of second-formant transitions in dysarthric subjects. Clinical Linguistics & Phonetics, 3, 347–358. [Google Scholar]
- Kent R. D., Kent J. F., & Rosenbek J. C. (1987). Maximum performance tests of speech production. Journal of Speech and Hearing Disorders, 52, 367–387. [DOI] [PubMed] [Google Scholar]
- Kent R. D., Netsell R., & Bauer L. L. (1975). Cineradiographic assessment of articulatory mobility in the dysarthrias. Journal of Speech and Hearing Disorders, 40, 467–480. [DOI] [PubMed] [Google Scholar]
- Kimura F., Fujimura C., Ishida S., Nakajima H., Furutama D., Uehara H., … Hanafusa T. (2006). Progression rate of ALSFRS-R at time of diagnosis predicts survival time in ALS. Neurology, 66, 265–267. doi:10.1212/01.wnl.0000194316.91908.8a [DOI] [PubMed] [Google Scholar]
- Kiritani S. (1986). X-ray microbeam method for measurement of articulatory dynamics—Techniques and results Speech Communication, 5, 119–140. [Google Scholar]
- Kleinow J., Smith A., & Ramig L. O. (2001). Speech motor stability in IPD: Effects of rate and loudness manipulations. Journal of Speech, Language, and Hearing Research, 44, 1041–1051. [DOI] [PubMed] [Google Scholar]
- Kollewe K., Mauss U., Krampfl K., Petri S., Dengler R., & Mohammadi B. (2008). ALSFRS-R score and its ratio: A useful predictor for ALS-progression. Journal of the Neurological Sciences, 275, 69–73. doi:10.1016/j.jns.2008.07.016 [DOI] [PubMed] [Google Scholar]
- Kuruvilla M. S., Green J. R., Yunusova Y., & Hanford K. (2012). Spatiotemporal coupling of the tongue in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 55, 1897–1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert E. H., & Mulder D. W. (1957). Electromyographic studies in amyotrophic lateral sclerosis. Mayo Clinic Proceedings, 32, 441–446. [PubMed] [Google Scholar]
- Langmore S. E., & Lehman M. E. (1994). Physiologic deficits in the orofacial system underlying dysarthria in amyotrophic lateral sclerosis. Journal of Speech and Hearing Research, 37, 28–37. [DOI] [PubMed] [Google Scholar]
- Lawyer T. Jr., & Netsky M. G. (1953). Amyotrophic lateral sclerosis. AMA Archives of Neurology and Psychiatry, 69, 171–192. [DOI] [PubMed] [Google Scholar]
- McHenry M. A. (2003). The effect of pacing strategies on the variability of speech movement sequences in dysarthria. Journal of Speech, Language, and Hearing Research, 46, 702–710. doi:10.1044/1092-4388(2003/055) [DOI] [PubMed] [Google Scholar]
- Mefferd A. S., Green J. R., & Pattee G. L. (2012). A novel fixed-target task to determine articulatory speed constraints in persons with amyotrophic lateral sclerosis. Journal of Communication Disorders, 45, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mefferd A. S., Pattee G. L., & Green J. R. (2014). Speaking rate effects on articulatory pattern consistency in talkers with mild ALS. Clinical Linguistics & Phonetics, 28, 799–811. doi:10.3109/02699206.2014.908239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsumoto H., & Del Bene M. (2000). Improving the quality of life for people with ALS: The challenge ahead. Amyotrophic Lateral Sclerosis and Other Motor Neuron Disorders, 1, 329–336. [DOI] [PubMed] [Google Scholar]
- Nasreddine Z. S., Phillips N. A., Beédirian V., Charbonneaus S., Whitehead V., Collin I., … Chertkow H. (2005). The Montreal Cognitive Assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatric Society, 53, 695–699. [DOI] [PubMed] [Google Scholar]
- Netsell R., & Rosenbek J. (1985). Treating the dysarthrias. In Darby J. K. (Ed.), Speech and language evaluation in neurology: Adult disorders (pp. 363–392). Orlando, FL: Grune & Stratton. [Google Scholar]
- Niimi M., & Nishio S. (2000). Changes over time in dysarthric patients with amyotrophic lateral sclerosis (ALS): A study of changes in speaking rate and maximum repetition rate (MRR). Clinical Linguistics & Phonetics, 14, 485–497. [Google Scholar]
- Perkell J. S., Matthies M. L., Svirsky M. A., & Jordan M. I. (1993). Trading relations between tongue-body raising and lip rounding in production of the vowel /u/: A pilot “motor equivalence” study. The Journal of the Acoustical Society of America, 93, 2948–2961. [DOI] [PubMed] [Google Scholar]
- Rong P., Yunusova Y., Wang J., & Green J. R. (2015). Predicting early bulbar decline in amyotrophic lateral sclerosis—A speech subsystem approach. Behavioural Neurology, 2015, 1–11. Retrieved from http://dx.doi.org/10.1155/2015/183027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J., & Jackson C. E. (2013). Outcome measures and clinical assessment instruments. In Katirji B., Kaminski H. J., & Ruff R. L. (Eds.), Neuromuscular Disorders in Clinical Practice (pp. 287–326). New York, NY: Springer. [Google Scholar]
- Rudy K., & Yunusova Y. (2013). The effect of anatomic factors on tongue position variability during consonants. Journal of Speech, Language, and Hearing Research, 56, 137–149. [DOI] [PubMed] [Google Scholar]
- Shiller D. M., Laboissière R., & Ostry D. J. (2002). Relationship between jaw stiffness and kinematic variability in speech. Journal of Neurophysiology, 88, 2329–2340. doi:10.1152/jn.00286.2002 [DOI] [PubMed] [Google Scholar]
- Simpson A. P. (2001). Dynamic consequences of differences in male and female vocal tract dimensions. The Journal of the Acoustical Society of America, 109(5, Pt. 1), 2153–2164. [DOI] [PubMed] [Google Scholar]
- Simpson A. P. (2002). Gender-specific articulatory–acoustic relations in vowel sequences. Journal of Phonetics, 30, 417–435. doi:10.1006/jpho.2002.0171 [Google Scholar]
- Smith A., Goffman L., Zelaznik H. N., Ying G., & McGillem C. (1995). Spatiotemporal stability and patterning of speech movement sequences. Experimental Brain Research, 104, 493–501. [DOI] [PubMed] [Google Scholar]
- Smith A., & Kleinow J. (2000). Kinematic correlates of speaking rate changes in stuttering and normally fluent adults. Journal of Speech, Language, and Hearing Research, 43, 521–536. [DOI] [PubMed] [Google Scholar]
- Smith B. L., & McLean-Muse A. (1987). An investigation of motor equivalence in the speech of children and adults. The Journal of the Acoustical Society of America, 82, 837–842. [DOI] [PubMed] [Google Scholar]
- Sonoo M., Kuwabara S., Shimizu T., Komori T., Hirashima F., Inaba A., … Kugio Y. (2009). Utility of trapezius EMG for diagnosis of amyotrophic lateral sclerosis. Muscle & Nerve, 39, 63–70. doi:10.1002/mus.21196 [DOI] [PubMed] [Google Scholar]
- Tjaden K., & Turner G. (2000). Segmental timing in amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 43, 683–696. [DOI] [PubMed] [Google Scholar]
- Turner G. S., & Weismer G. (1993). Characteristics of speaking rate in the dysarthria associated with amyotrophic lateral sclerosis. Journal of Speech, Language, and Hearing Research, 36, 1134–1144. [DOI] [PubMed] [Google Scholar]
- Voustianiouk A., Seidel G., Panchal J., Sivak M., Czaplinski A., Yen A., … Lange D. J. (2008). ALSFRS and Appel ALS scores: Discordance with disease progression. Muscle & Nerve, 37, 668–672. [DOI] [PubMed] [Google Scholar]
- Weikamp J., Schelhaas H., Hendriks J., De Swart B., & Geurts A. (2012). Prognostic value of decreased tongue strength on survival time in patients with amyotrophic lateral sclerosis. Journal of Neurology, 259, 2360–2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weismer G. (2006). Philosophy of research in motor speech disorders. Clinical Linguistics & Phonetics, 20, 315–349. doi:10.1080/02699200400024806 [DOI] [PubMed] [Google Scholar]
- Westbury J. R., Hashi M., & Lindstrom M. J. (1998). Differences among speakers in lingual articulation for American English /ɹ/. Speech Communication, 26, 203–226. doi:10.1016/S0167-6393(98)00058-2 [Google Scholar]
- Westbury J. R., Lindstrom M. J., & McClean M. D. (2002). Tongues and lips without jaws: A comparison of methods for decoupling speech movements. Journal of Speech, Language, and Hearing Research, 45, 651–662. [DOI] [PubMed] [Google Scholar]
- Yorkston K. M. (1993). Speech deterioration in amyotrophic lateral sclerosis: Implications for the timing of intervention. Journal of Medical Speech-Language Pathology, 1, 35–46. [Google Scholar]
- Yorkston K. M., & Beukelman D. R. (1981). Communication efficiency of dysarthric speakers as measured by sentence intelligibility and speaking rate. Journal of Speech and Hearing Disorders, 46, 296–301. [DOI] [PubMed] [Google Scholar]
- Yorkston K., Beukelman D., & Tice R. (1996). Speech Intelligibility Test. Lincoln, NE: Madonna Rehabilitation Hospital, Institute for Rehabilitation Science and Engineering. [Google Scholar]
- Yorkston K. M., Dowden P. A., & Beukelman D. R. (1992). Intelligibility measurement as a tool in the clinical management of dysarthric speakers. In Kent R. D. (Ed.), Intelligibility in speech disorders: Theory, measurement, and management (pp. 265–286). Amsterdam, the Netherlands: John Benjamins. [Google Scholar]
- Yunusova Y., Green J. R., Greenwood L., Wang J., Pattee G. L., & Zinman L. (2012). Tongue movements and their acoustic consequences in amyotrophic lateral sclerosis. Folia Phoniatrica et Logopaedica, 64, 94–102. doi:10.1159/000336890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y., Green J. R., Lindstrom M. J., Ball L. J., Pattee G. L., & Zinman L. (2010). Kinematics of disease progression in bulbar ALS. Journal of Communication Disorders, 43, 6–20. doi:10.1016/j.jcomdis.2009.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y., Green J. R., Wang J., Pattee G., & Zinman L. (2011). A protocol for comprehensive assessment of bulbar dysfunction in amyotrophic lateral sclerosis (ALS). Journal of Visualized Experiments, 48, e2422 doi:10.3791/2422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunusova Y., Rosenthal J. S., Rudy K., Baljko M., & Daskalogiannakis J. (2012). Positional targets for lingual consonants defined using electromagnetic articulography. The Journal of the Acoustical Society of America, 132, 1027–1038. doi:10.1121/1.4733542 [DOI] [PubMed] [Google Scholar]
- Yunusova Y., Weismer G., Westbury J. R., & Lindstrom M. J. (2008). Articulatory movements during vowels in speakers with dysarthria and healthy controls. Journal of Speech, Language, and Hearing Research, 51, 596–611. doi:10.1044/1092-4388(2008/043) [DOI] [PubMed] [Google Scholar]