Abstract
Background
Pulmonary P. aeruginosa infection is associated with poor outcomes in cystic fibrosis (CF) and early diagnosis is challenging, particularly in those who are unable to expectorate sputum. Specific P. aeruginosa 2-alkyl-4-quinolones are detectable in the sputum, plasma and urine of adults with CF, suggesting that they have potential as biomarkers for P. aeruginosa infection.
Aim
To investigate systemic 2-alkyl-4-quinolones as potential biomarkers for pulmonary P. aeruginosa infection.
Methods
A multicentre observational study of 176 adults and 68 children with CF. Cross-sectionally, comparisons were made between current P. aeruginosa infection using six 2-alkyl-4-quinolones detected in sputum, plasma and urine against hospital microbiological culture results. All participants without P. aeruginosa infection at baseline were followed up for one year to determine if 2-alkyl-4-quinolones were early biomarkers of pulmonary P. aeruginosa infection.
Results
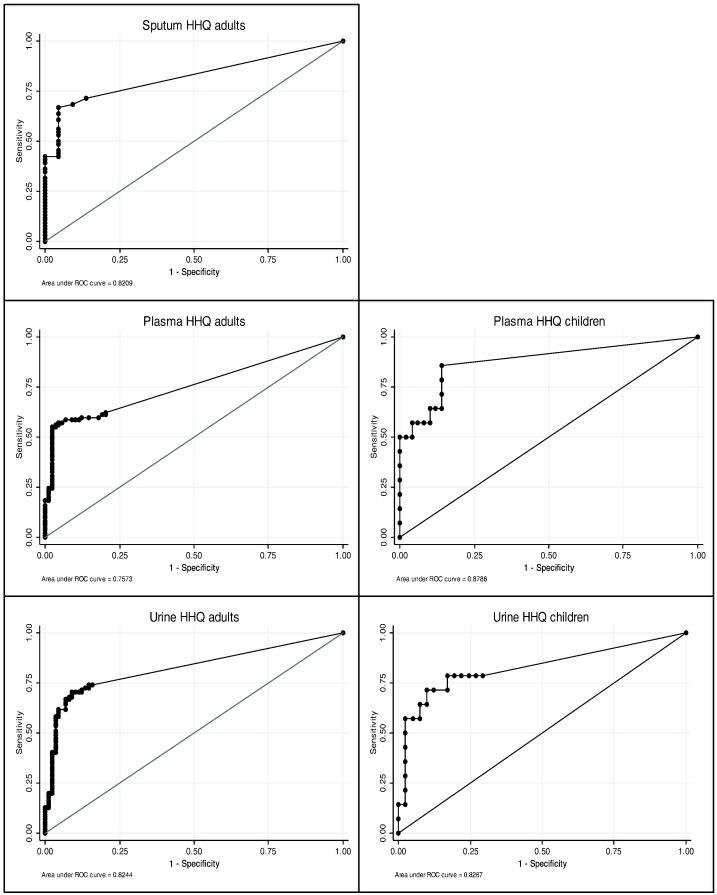
Cross-sectional analysis: the most promising biomarker with the greatest diagnostic accuracy was 2-heptyl-4-hydroxyquinoline (HHQ). In adults, areas under the ROC curves (95% confidence intervals) for HHQ analyses were 0.82 (0.75–0.89) in sputum, 0.76 (0.69–0.82) in plasma and 0.82 (0.77–0.88) in urine. In children, the corresponding values for HHQ analyses were 0.88 (0.77–0.99) in plasma and 0.83 (0.68–0.97) in urine.
Longitudinal analysis: Ten adults and six children had a new positive respiratory culture for P. aeruginosa in follow-up. A positive plasma HHQ test at baseline was significantly associated with a new positive culture for P. aeruginosa in both adults and children in follow-up (odds ratio (OR) = 6.67;-95% CI:-1.48–30.1;-p = 0.01 and OR = 70; 95% CI: 5–956;-p < 0.001 respectively).
Conclusions
AQs measured in sputum, plasma and urine may be used to diagnose current infection with P. aeruginosa in adults and children with CF. These preliminary data show that plasma HHQ may have potential as an early biomarker of pulmonary P. aeruginosa. Further studies are necessary to evaluate if HHQ could be used in clinical practice to aid early diagnosis of P. aeruginosa infection in the future.
1. Introduction
Chronic pulmonary infection with Pseudomonas aeruginosa in patients with cystic fibrosis (CF) is an important risk factor contributing to recurrent hospital admissions [1] and increased mortality [1]. The prevalence of P. aeruginosa infection in CF increases with age, with approximately two thirds of adults either intermittently or chronically colonised with this bacterium [2]. In the early stages of infection, P. aeruginosa may be eradicated from the lungs with targeted antimicrobial therapy [3], [4] which positively affects the long term course of the disease [5].
There is a clinical need for better early markers for pulmonary infection with P. aeruginosa to facilitate early intervention with eradication therapy. In current clinical practice, the main technique used to diagnose pulmonary P. aeruginosa infection is conventional microbiological culture of respiratory specimens [6]. Spontaneous sputum is quick and simple to culture and is widely assumed to accurately diagnose common lower airway pathogens [7]. However, early diagnosis of pulmonary P. aeruginosa infection is more challenging in those who are unable to expectorate sputum, such as young children and adults with well maintained lung function. In these circumstances, diagnostic options include sampling from the upper airways using cough swabs, which have a low sensitivity and may miss lower airways infection [8], or the use of more invasive techniques such as induced sputum [9] or bronchoalveolar lavage [9]. More recently, serological antibody tests against P. aeruginosa have been used to aid diagnosis of infection [10], [11], [12], [13], [14], [15], [16], [17]. Studies have demonstrated that these antibodies can be used as markers of chronic infection with P. aeruginosa [17], but their ability to predict early infection with P. aeruginosa has so far been relatively disappointing [11], [14], [17], [18]. Finally, culture independent techniques using molecular primer sequencing are increasingly being used in a research setting to diagnose infection in the lower airways [19], however these still require direct airway samples and are subjected to false positives due to sample contamination and the high sensitivity of these techniques.
In this study, we use novel LC–MS/MS methodology [20] to directly measure specific quorum sensing signal molecules (alkyl quinolones, AQs) in the systemic circulation of patients with CF. These AQs regulate virulence factor production [21], possess anti-staphylococcal properties [22], and have been detected in infants with CF [23], suggesting that they may play a role in the early stages of P. aeruginosa infection. The aim of this study was to assess the diagnostic and prognostic potential of plasma and urinary AQs as biomarkers for pulmonary P. aeruginosa infection in patients with CF.
2. Methods
2.1. Participants
Patients were recruited from three adult CF centres (Nottingham University hospitals NHS Trust, Heart of England NHS Foundation Trust and Leeds Teaching Hospitals NHS Trust) and one paediatric CF centre (Nottingham University hospitals NHS Trust). Inclusion criteria were a diagnosis of CF and exclusion criteria were any previous isolation of Burkholderia cepacia complex in sputum. Control participants with no known respiratory conditions and no history of P. aeruginosa infection were invited to participate in the study. Informed written consent was obtained from all participants or their legal representative and the study was approved by the Nottingham Research Ethics Committee 1 (09/H0407/1).
2.2. Study design
Adults with CF were recruited at routine CF clinic appointments and were clinically stable at the study visit, having not experienced a pulmonary exacerbation requiring intravenous (IV) antibiotics in the previous 4 weeks. Spontaneous sputum, 8 ml blood and 25 ml urine samples were obtained. Pulmonary function tests were performed according to standardised criteria [24] and validated CFQ quality of life questionnaires were completed [25].
Children with CF were recruited from paediatric CF clinics and samples of plasma and urine were obtained at annual assessment. Sputum collection was not requested from children.
A control population of healthy individuals was recruited by poster advertisement and provided venous blood and urine samples.
2.3. Sputum, blood and urine processing
Spontaneous sputum samples were collected according to standard guidelines [26], [27]. Sputum plugs were harvested and processed for quantitative QSSM analysis [28] and differential cell counts [26], [27]. Sputum plugs for quantitative microbiological analysis were mixed with an equal volume of dithiothreitol and diluted with 0.9% w/v saline and 100 μl of × 10− 2 and × 10− 4 solutions were plated on blood and pseudomonas isolation agar (Difco; BD, Sparks, MD, USA). The plates were incubated at 37 °C and colony counts were performed daily between 24 and 72 h, until maximal growth was achieved. Venous blood samples were centrifuged at 1000g for 15 min at 4 °C and plasma was separated and snap frozen in liquid nitrogen. All biological samples were stored on ice and frozen at − 80 °C within 4 h.
2.4. AQ analysis
Clinical samples for AQ analysis were analysed using LC–MS/MS [20]. Measurement of AQ levels in clinical samples was performed by comparison of extracts with calibration standards. Further details of the sample preparation, LC–MS/MS analysis and quantification can be found in the online data supplement (S1). A total of six AQs were studied: HHQ (2-heptyl-4-hydroxyquinoline), NHQ (2-nonyl-4-hydroxyquinoline), C7-PQS (2-heptyl-3-hydroxy-4(1H)-quinolone), C9-PQS (2-nonyl-3-hydroxy-4(1H)-quinolone), HQNO (2-heptyl-4-hydroxyquinoline-N-oxide) and NQNO (2-nonyl-4-hydroxyquinoline-N-oxide) For 2-alkyl-4(1H)-quinolone (AQ) and 2-alkyl-4-hydroxyquinoline N-oxide molecules, a previously accepted nomenclature has been adopted [29]. A positive AQ test was defined as a measured AQ concentration greater or equal to the lower limit of quantification in the media of interest obtained by LC–MS/MS (see online supplement, E1).
2.5. Diagnosis of P. aeruginosa
Current hospital microbiological culture results were used as the reference standard for a diagnosis of P. aeruginosa and were obtained from cough swabs, spontaneous sputum cultures or bronchoalveolar lavage (BAL) using standard protocols [30]. Patients were categorised into four groups using the Leeds criteria for P. aeruginosa [31]. The four groups were: ‘chronic’ (defined as ≥ 50% of samples positive for P. aeruginosa in the last 12 months); ‘intermittent’ (defined as < 50% of samples positive for P. aeruginosa in the last 12 months); ‘free’ (defined as previous P. aeruginosa infection but not isolated in the last 12 months) and ‘never’ (defined as those who had not previously isolated P. aeruginosa).
2.6. Statistical analysis
AQ concentrations in sputum, plasma and urine were compared using scatter graphs and Spearman rank correlation co-efficients. Each of the six AQs were compared to hospital microbiological culture results in both adults and children with CF. Sensitivity, specificity, positive predictive values and negative predictive values were calculated for each AQ in sputum, plasma and urine. Non-parametric receiver operator characteristic (ROC) curves [30] and areas under the curves (AUC) were used to compare each AQ to current hospital culture results. Comparisons between P. aeruginosa groups were assessed using Kruskal-Wallis testing. In the longitudinal analysis, logistic regression was used to compare the presence or absence of AQs in plasma and urine of adults and children who had no evidence of P. aeruginosa infection in the previous year with hospital respiratory culture results in the subsequent 12 months.
The association between AQ levels in plasma and clinical variables of interest was explored using Spearman rank correlation co-efficients or Mann Whitney U tests. These explanatory variables were age, gender, forced expiratory volume in 1 s (FEV1) percent predicted, quantitative load of P. aeruginosa measured on pseudomonas isolation agar, sputum neutrophil concentrations and the use of maintenance azithromycin and nebulised antibiotics. All data were analysed using STATA 11 statistical software (Texas, USA).
3. Results
244 patients with CF were recruited, 176 adults and 68 children. Clinical characteristics, P. aeruginosa status of the participants and hospital trust recruited from are shown in Table 1. In addition, 22 control participants were recruited from the general population (11 males, median age = 31.7 years).
Table 1.
Clinical characteristics and P. aeruginosa status of participants with cystic fibrosis.
| Variable | Adults | Children |
|---|---|---|
| Hospital | ||
| Nottingham University Hospitals NHS Trust | 64 | 68 |
| Heart of England NHS Foundation Trust | 76 | |
| Leeds teaching Hospitals NHS Trust | 36 | |
| Age in years: median (range) | 27.8 (17.7 to 65.2) n = 176 |
9.6 (0.8 to 17.0) n = 68 |
| Gender, males (%) | 104 (59) | 31 (46) |
| FEV1 percent predicted: mean (range) | 65 (17 to 110) n = 166 |
79 (41 to 121) n = 35 |
| Absolute FEV1 in L: mean (range) | 2.40 (0.71 to 4.78) n = 165 |
1.70 (0.60 to 3.69) n = 34 |
| P. aeruginosa status at baseline visit: n(%) | ||
| Never | 20 (11) | 19 (28) |
| Free | 39 (22) | 25 (37) |
| Intermittent | 30 (17) | 13 (19) |
| Chronic | 86 (49) n = 175⁎ |
11 (16) n = 68 |
| Maintenance treatment: | ||
| Azithromycin | 105 (60) | 35 (51) |
| Cycled nebulised Colomycin | 83 (48) | 11 (16) |
| Cycled nebulised Tobramycin | 38 (22) | 3 (4) |
| Number of patients with a positive respiratory culture for P. aeruginosa at baseline in the hospital laboratory, n (%) | 84 (48%)^ | 14 (21%) |
n = number of participants with data available.
P. aeruginosa status of patients defined by the Leeds criteria [30].
1 patient had no available respiratory samples in the previous 12 months and P. aeruginosa status could therefore not be assessed.
Data missing for 2 patients.
3.1. AQ concentrations in sputum, plasma and urine are positively correlated between the biofluids
AQ concentrations were positively correlated between sputum, plasma and urine with the strongest correlations between sputum and plasma concentrations of HQNO (r = 0.84, p < 0.0001) and between sputum and urine levels of HHQ (r = 0.79, p < 0.0001; online supplement E2).
3.2. The use of AQs in sputum, plasma and urine to diagnose current pulmonary P. aeruginosa infection
To evaluate the potential of AQs to diagnose current P. aeruginosa infection, AQ results were compared to current hospital respiratory culture results. In adults, the hospital culture results were obtained from 136 spontaneous sputum samples and 40 cough swabs. In children, 46 samples were cough swabs, 20 samples were spontaneous sputum and 2 samples were obtained from BAL washings. Sensitivity and specificity data for all six AQs are shown in the online supplement (E3 and E4). Three patients had a new positive respiratory culture for P. aeruginosa at the baseline visit, having not isolated this bacterium in the preceding year, and AQs were detectable in all 3 patients (online supplement E5).
Area under curve (AUC) results for each of the six AQs in each biofluid are summarised in Table 2.
Table 2.
Area under receiver operating characteristic curves for six quorum sensing signalling molecules in biological samples from patients with cystic fibrosis compared to current hospital respiratory culture results.
| 2-alkyl-4-quinolone tested | Adults |
Children |
|||
|---|---|---|---|---|---|
| Sputum n = 88 |
Plasma n = 171 | Urine n = 173 | Plasma n = 64 |
Urine n = 55 |
|
| AUC (95% CI) |
AUC (95% CI) |
AUC (95% CI) |
AUC (95% CI) |
AUC (95% CI) |
|
| HHQ | 0.82 (0.75–0.89) |
0.76 (0.69–0.82) |
0.82 (0.77–0.88) |
0.88 (0.77–0.99) |
0.83 (0.68–0.97) |
| HQNO | 0.86 (0.79–0.93) |
0.73 (0.67–0.78) |
0.73 (0.67–0.79) |
0.78 (0.64–0.92) |
0.83 (0.70–0.96) |
| NHQ | 0.82 (0.75–0.90) |
0.58 (0.51–0.64) |
0.63 (0.56–0.70) |
0.65 (0.52–0.78) |
0.69 (0.52–0.85) |
| NQNO | 0.84 (0.76–0.91) |
0.67 (0.62–0.73) |
0.56 (0.52–0.59) |
0.71 (0.57–0.84) |
0.62 (0.49–0.74) |
| C7-PQS | 0.71 (0.61–0.81) |
0.66 (0.60–0.71) |
0.66 (0.61–0.72) |
0.71 (0.58–0.85) |
0.72 (0.57–0.87) |
| C9-PQS | 0.73 (0.62–0.85) |
0.55 (0.51–0.58) |
0.53 (0.49–0.57) |
0.63 (0.51–0.75) |
0.61 (0.47–0.75) |
| Prevalence of current PA infection using respiratory culture results (%) | 75 | 48 | 49 | 22 | 25 |
AUC = Area under Receiver Operating Characteristics curve.
PA = Pseudomonas aeruginosa.
HHQ = 2-heptyl-4-hydroxyquinoline.
HQNO = 2-heptyl-4-hydroxyquinoline-N-oxide.
NHQ = 2-nonyl-4-hydroxyquinoline.
NQNO = 2-nonyl-4-hydroxyquinoline-N-oxide.
C7-PQS = 2-heptyl-3-hydroxy-4(1H)-quinolone.
C9-PQS = 2-nonyl-3-hydroxy-4(1H)-quinolone.
n = number of samples available for analysis.
Of the six AQs studied, the most promising biomarkers (with the highest AUCs and therefore greatest diagnostic accuracy) were HHQ (Fig. 1) and HQNO, and further analyses focused on these two molecules. In adults, the sensitivity and specificity of HHQ in plasma were 62% (95% CI: 51–73) and 80% (95% CI: 70–88) respectively and the corresponding values in urine were 74% (95% CI: 63–83) and 84% (95% CI: 75–91) respectively (Table 3). In children, the presence of HHQ in plasma had a sensitivity of 86% (95% CI: 57–98) and specificity of 86% (95% CI: 73–94), and the corresponding values in urine were 79% (95% CI: 49–95) and 71% (95% CI: 55–84) respectively (Table 3). The prevalence of HHQ and HQNO in each media is shown in the on line supplement (E6).
Fig. 1.
Receiver operator curves for 2-heptyl-4-hydroxyquinoline (HHQ) in sputum, plasma and urine in adults and children with cystic fibrosis compared to hospital microbiological culture results.
Table 3.
Evaluation of 2-heptyl-4-hydroxyquinoline (HHQ) for the diagnosis of P. aeruginosa compared to standard microbiological culture in adults and children.
| Test (95% CI) | Adults |
Children |
|||
|---|---|---|---|---|---|
| Sputum n = 88 |
Plasma n = 171 |
Urine n = 173 |
Plasma n = 64 |
Urine n = 55 |
|
| Sensitivity, % (95% CI) |
71 (59–82) |
62 (51–73) |
74 (63–83) |
86 (57–98) |
79 (49–95) |
| Specificity, % (95% CI) |
86 (65–97) |
80 (70–88) |
84 (75–91) |
86 (73–94) |
71 (55–84) |
| PPV, % (95% CI) |
94 (84–99) |
74 (62–84) |
82 (71–90) |
63 (38–84) |
48 (27–69) |
| NPV, % (95% CI) |
50 (33–67) |
70 (60–78) |
77 (68–85) |
96 (85–99) |
91 (75–98) |
| Prevalence of PA using respiratory cultures results, % | 75 | 48 | 49 | 22 | 25 |
PPV = positive predictive value.
NPV = negative predictive value.
PA = Pseudomonas aeruginosa.
A positive test was defined as an HHQ concentration greater or equal to the lower level of quantification in the sample type (see online supplement S1).
n = number of samples available for analysis.
3.3. Relationship between plasma HHQ and HQNO with P. aeruginosa infection status
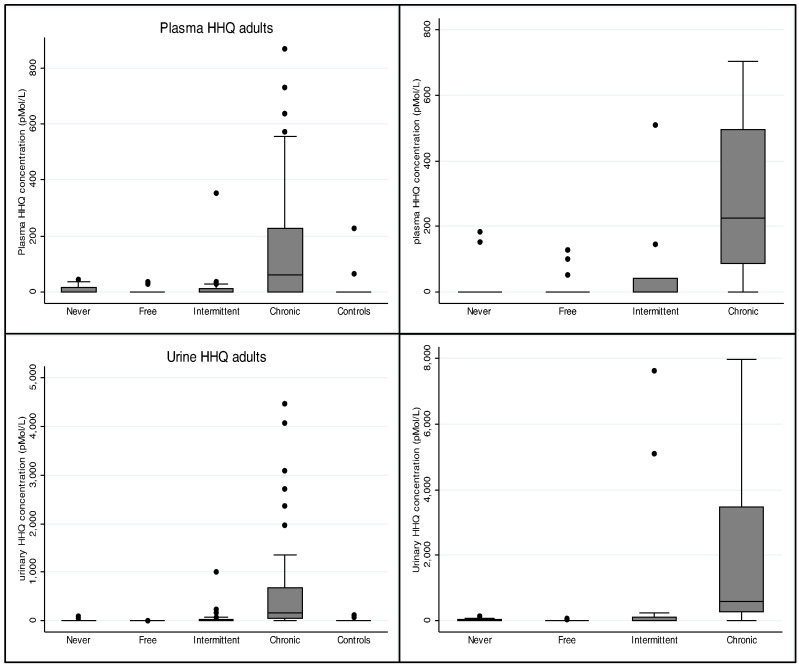
Clinical samples from all patients with CF, stratified by P. aeruginosa status were analysed, focusing on the two most promising biomarkers, HHQ and HQNO. There were significant differences in AQ concentrations between samples obtained from adults with different categories of P. aeruginosa infection and the control participants in all biofluids (Kruskal-Wallis test: p ≤ 0.003 for both HHQ and HQNO in sputum, plasma and urine; Fig. 2). Similar results were observed in plasma and urine samples obtained from children with CF (Fig. 2, p < 0.02).
Fig. 2.
2-heptyl-4-hydroxyquinoline (HHQ) concentration as a function of P. aeruginosa status in adults and children with cystic fibrosis and adult control participants measured in the plasma and urine.
Prevalence of HHQ in plasma above the lower limit of quantification using LC–MS/MS stratified by P. aeruginosa status in adults: 25% (5/20) categorised as ‘never’, 18% (7/38) categorised as ‘free’, 27% (8/30) categorised as ‘intermittent’ and 60% (50/84) categorised as ‘chronic’ and 9% (2/22) in healthy controls.
In children, the prevalence of HHQ in plasma, stratified by P. aeruginosa status was: 11% (2/18) in the ‘never’ category, 165 (4/25) in ‘free’, 30% (3/10) in ‘intermittent’, 91% (10/11) in ‘chronic’ category. HHQ was detectable in the plasma of 3 controls and urine of 4 controls.
3.4. Relationship between plasma HHQ and HQNO and clinical status in adults (n = 176)
Overall, HHQ and HQNO levels in the plasma of adults were negatively correlated with percent-predicted FEV1 (Spearman rank correlation coefficients, r = − 0.19; p = 0.01 and r = − 0.25; p = 0.001 respectively) and this association was not significantly affected by adjustment for age. HHQ in plasma was positively correlated with quantitative load of P. aeruginosa in sputum (Spearman rank correlation co-efficient: r = 0.28, p = 0.02) and negatively correlated with the use of maintenance azithromycin, with higher values in those who were receiving maintenance azithromycin therapy compared to those who were not (Mann–Whitney U test: p = 0.004). No significant associations were observed between plasma HQNO and P. aeruginosa load or plasma HQNO and the use of azithromycin. There were no significant associations between plasma HHQ and HQNO levels in adults and sputum neutrophil concentration, age, gender, the use of maintenance nebulised antibiotics or respiratory quality of life responses (data not shown).
3.5. Longitudinal analysis: use of plasma and urinary AQs to detect early P. aeruginosa infection
In the year following QSSM testing, a new positive respiratory tract culture for P. aeruginosa was reported in and 6 (14%) children and 10 (18%) adults who were categorised as ‘free’ or ‘never’ having isolated P. aeruginosa at baseline (Table 4 and online supplement E7).
Table 4.
Relationships between baseline plasma 2-heptyl-4-hydroxyquinoline (HHQ) and respiratory culture results for P. aeruginosa in the 12 month follow up period for participants with CF who were classified as ‘free’ or ‘never’ having isolated P. aeruginosa at baseline^.
| P. aeruginosa culture ‘free’ or ‘never’ at baseline^ | Plasma HHQ result at baseline visit# | Remained P. aeruginosa culture negative during follow up | New positive culture for P. aeruginosa during follow up |
|---|---|---|---|
| Adults n = 56⁎ |
HHQ negative | 40 | 5 |
| HHQ positive | 6 | 5 | |
| Children n = 42♦ |
HHQ negative | 35 | 2 |
| HHQ positive | 1 | 4 |
n = number of samples available for analysis.
A positive test was defined as an HHQ concentration greater or equal to the lower level of quantification (see online supplement S1).
P. aeruginosa status of patients defined by the Leeds criteria [32].
3 adults had no respiratory cultures reported by the hospital laboratory during 1-year follow up.
2 children did not provide plasma samples at the baseline visit.
The presence of HHQ in plasma at baseline was significantly associated with a new positive respiratory tract P. aeruginosa culture in the following year in both adults and children with CF (OR = 6.67; 95% CI 1.48 to 30.1; p = 0.01 and OR = 70; 95% CI: 5 to 956; p < 0.001 respectively; Table 4 and online supplement E7).
The presence of HHQ in urine or HQNO in plasma or urine at baseline was not significantly associated with a new positive P. aeruginosa respiratory tract culture in the following year in children or adults (online supplement E7).
4. Discussion
This is the first study to test the diagnostic and prognostic significance of systemic AQ quorum sensing signal molecules as biomarkers for pulmonary P. aeruginosa in adults and children with CF. Of the QSSMs measured, the diagnostic accuracy of HHQ and HQNO were highest, suggesting these molecules have potential as biomarkers of infection with P. aeruginosa. The follow up data also permitted identification of HHQ in plasma as a potential early biomarker of P. aeruginosa. The consistency in these observations in both adults and children with CF suggests that this finding may be generalizable across all age groups. If externally validated, these findings would be particularly important for young children with CF, who stand to benefit the most from an early diagnostic test for P. aeruginosa. This study also showed that (1) higher concentrations of HHQ in plasma were associated with lower percent-predicted FEV1 and higher bacterial load and (2) systemic AQs concentrations were elevated in patients with newly acquired P. aeruginosa infection, suggesting that these molecules may be associated with an adverse prognosis. These data also support our previous findings that systemic levels of some AQs are elevated during pulmonary exacerbations in adults with CF [33].
Strengths of the study include the large study population, who were recruited from three specialist CF centres, with a wide range of disease severities, which may be generalizable to the broader UK CF population. The direct comparison of sputum, plasma and urinary AQ levels in 87 adults demonstrated that these were highly correlated, providing confidence that the lower airways were the source of the AQs. We used routine hospital culture results as our reference standard for P. aeruginosa, thus comparing our test with current clinical practice, and this was a necessary part of the study design. However, this may underestimate the true value of the new tests, as the diagnostic accuracy of both cough swabs and sputum cultures is lower than BAL [6], [32], which is considered the ‘gold standard’ for defining lower airway microbiology.
There are several factors that require consideration when interpreting these data. Firstly, multiple hypotheses were tested and some of the associations observed may have occurred by chance, although as most of the p values are small, this is unlikely. Secondly, interpreting the clinical significance of low levels of AQs is challenging. Low concentrations of several AQs were detected in some adults who were classified as ‘free’ or ‘never’ having had P. aeruginosa infection and also in a minority of the control population. This may represent environmental exposure resulting in subclinical infection and further investigation into these findings is warranted. Thirdly, the numbers of patients who were negative for P. aeruginosa at recruitment and subsequently developed infection was small. This is reflected in the wide confidence intervals of the ORs, which should be taken into account when interpreting these data. Fourthly, we observed that patients with higher AQ concentrations were more likely to be currently prescribed oral azithromycin. There is currently no evidence that azithromycin affects AQ production, although it inhibits the in vitro production of some quorum sensing signal molecules from the N-acyl-L-homoserine lactone class [34]. We suggest that the use of azithromycin may be a confounding factor in this study, as patients with chronic P. aeruginosa infection were more likely to be prescribed azithromycin and chronic P. aeruginosa infection was also associated with higher AQ levels. In cross sectional analysis, the use of maintenance nebulised antibiotics was not associated with differences in AQ concentrations in plasma and urine. However, further studies would be necessary to assess the impact of antibiotic therapy on AQ levels at an individual level. Fifthly, the predictive values for AQ tests should be interpreted in the context of disease prevalence, as a higher disease prevalence is associated with higher positive predictive values (PPVs) and lower negative predictive values (NPVs) [35]. Thus, AQ testing in children be used to confidently rule out infection (high NPV) but there is a higher false positive rate (low PPV) compared to the adult population, who had a higher prevalence of P. aeruginosa infection.
Recently, serological antibody tests have been used to aid the diagnosis of P. aeruginosa infection. Studies report varying accuracies for serological tests, depending on the antigens tested, the defined cut points, patient cohort and the test standard used [11], [14], [17], [36]. Whilst direct comparisons between these studies are not possible, the diagnostic accuracy of AQ tests in this study is broadly similar to serological test results. However, there are some limitations of serological antigen testing. Serological tests rely on detecting the host immune response to infection and titres can be affected by co-infection in the lungs [37] and by the concurrent use of antibiotics [13], [16], [37], [38] or oral steroids [39]. Potential advantages of AQ testing compared to serological testing include the direct detection of bacterial products themselves and the ability to detect these molecules in the urine, which would allow non-invasive testing in children.
We acknowledge that this study represents an early step to determine if AQs could aid clinical decision-making in CF in future. One area for further investigation includes the testing of AQs in control populations, in particular, the paediatric population and also in patients with Burkholderia cepacia complex. Another challenge is determining the clinical significance of low levels of AQs. This is particularly important, as low concentrations of AQs were detectable in the plasma and urine of a minority of the control population. Understanding the role of AQs in early infection with P. aeruginosa in the CF population is another area that deserves attention, as in vivo data are limited [40], [41] and most of our current knowledge comes from animal models or in vitro studies [20]. Other areas that warrant investigation are the effects of antibiotics, including eradication therapy, on systemic AQ levels and understanding the metabolism and excretion of AQs. Further steps include an external validation study, optimization of cut points and comparisons of AQ tests to other tests for P. aeruginosa used in clinical practice such as BAL, serological tests and PCR. Finally, to bring this test to routine clinical practice would also require development of a widely available laboratory or point of care test, such as a biosensor for AQs [42], which could be developed into a quick and cost effective bedside test for P. aeruginosa.
In summary, these data suggest that AQs can be used to determine current infection with P. aeruginosa. Limited data also show that HHQ detected in the plasma may be an early biomarker for pulmonary P. aeruginosa infection. If validated, this finding would be particularly relevant in young children, where diagnosis of pulmonary infection with P. aeruginosa is challenging and early detection of P. aeruginosa is sought to initiate timely eradication therapy. The high negative predictive levels of plasma and urinary AQs in children suggest that these tests may be useful in ruling out P. aeruginosa infection, thus supporting robust infection control measures and risk stratification of patients in CF clinics.
Author statement
HLB/MC/DB/NH/DLF/AS/AWF contributed to the concept, design, acquisition of data, interpretation of data, drafting of manuscript and approval of final version of manuscript. AJK/PW contributed to the concept, design, interpretation of data, drafting of manuscript and approval of final version of manuscript. KW/DP/DH/JW/EN/AC contributed to the acquisition of data, interpretation of data, drafting of manuscript and approval of final version of manuscript.
Competing interests
The University of Nottingham has a patent pending for the use of alkyl quinolones as biomarkers for P. aeruginosa infection (PCT/GB2014/051458).
Funding
Medical Research Council (G0801558); Doug Forrester is funded by the Wellcome Trust, fellow number: WT088614.
Footnotes
Supplementary data to this article can be found online at doi:10.1016/j.jcf.2016.10.005.
Appendix A. Supplementary data
Supplementary material
References
- 1.Emerson J., Rosenfeld M., McNamara S., Ramsey B., Gibson R.L. Pseudomonas aeruginosa and other predictors of mortality and morbidity in young children with cystic fibrosis. Pediatr Pulmonol. 2002;34(2):91–100. doi: 10.1002/ppul.10127. [Epub 2002/07/12] [DOI] [PubMed] [Google Scholar]
- 2.Smith D.L., Freeman W., Cayton R.M., Stableforth D.E. Nocturnal hypoxaemia in cystic fibrosis: relationship to pulmonary function tests. Respir Med. 1994;88(7):537–539. doi: 10.1016/s0954-6111(05)80338-5. [Epub 1994/08/01] [DOI] [PubMed] [Google Scholar]
- 3.Valerius N.H., Koch C., Hoiby N. Prevention of chronic Pseudomonas aeruginosa colonisation in cystic fibrosis by early treatment. Lancet. 1991;338(8769):725–726. doi: 10.1016/0140-6736(91)91446-2. [Epub 1991/09/21] [DOI] [PubMed] [Google Scholar]
- 4.Ratjen F., Doring G., Nikolaizik W.H. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonisation in patients with cystic fibrosis. Lancet. 2001;358(9286):983–984. doi: 10.1016/S0140-6736(01)06124-4. [Epub 2001/10/05] [DOI] [PubMed] [Google Scholar]
- 5.Standards for the clinical care of children and adults with cystic fibrosis in the UK. 2011. Cystic fibrosis trust. [Google Scholar]
- 6.Gilljam H., Malmborg A.S., Strandvik B. Conformity of bacterial growth in sputum and contamination free endobronchial samples in patients with cystic fibrosis. Thorax. 1986;41(8):641–646. doi: 10.1136/thx.41.8.641. [Epub 1986/08/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosenfeld M., Emerson J., Accurso F., Armstrong D., Castile R., Grimwood K. Diagnostic accuracy of oropharyngeal cultures in infants and young children with cystic fibrosis. Pediatr Pulmonol. 1999;28(5):321–328. doi: 10.1002/(sici)1099-0496(199911)28:5<321::aid-ppul3>3.0.co;2-v. [Epub 1999/10/27] [DOI] [PubMed] [Google Scholar]
- 8.De Boeck K., Alifier M., Vandeputte S. Sputum induction in young cystic fibrosis patients. Eur Respir J. 2000;16(1):91–94. doi: 10.1034/j.1399-3003.2000.16a16.x. [Epub 2000/08/10] [DOI] [PubMed] [Google Scholar]
- 9.Wainwright C.E., Vidmar S., Armstrong D.S., Byrnes C.A., Carlin J.B., Cheney J. Effect of bronchoalveolar lavage-directed therapy on Pseudomonas aeruginosa infection and structural lung injury in children with cystic fibrosis: a randomized trial. JAMA. 2011;306(2):163–171. doi: 10.1001/jama.2011.954. [Epub 2011/07/14] [DOI] [PubMed] [Google Scholar]
- 10.Pedersen S.S., Espersen F., Hoiby N. Diagnosis of chronic Pseudomonas aeruginosa infection in cystic fibrosis by enzyme-linked immunosorbent assay. J Clin Microbiol. 1987;25(10):1830–1836. doi: 10.1128/jcm.25.10.1830-1836.1987. [Epub 1987/10/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alton E.W., Stern M., Farley R., Jaffe A., Chadwick S.L., Phillips J. Cationic lipid-mediated CFTR gene transfer to the lungs and nose of patients with cystic fibrosis: a double-blind placebo-controlled trial. Lancet. 1999;353(9157):947–954. doi: 10.1016/s0140-6736(98)06532-5. [Epub 1999/08/25] [DOI] [PubMed] [Google Scholar]
- 12.Xu J., Moore J.E., Murphy P.G., Millar B.C., Elborn J.S. Early detection of Pseudomonas aeruginosa–comparison of conventional versus molecular (PCR) detection directly from adult patients with cystic fibrosis (CF) Ann Clin Microbiol Antimicrob. 2004;3:21. doi: 10.1186/1476-0711-3-21. [Epub 2004/10/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Burns J.L., Gibson R.L., McNamara S., Yim D., Emerson J., Rosenfeld M. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J Infect Dis. 2001;183(3):444–452. doi: 10.1086/318075. [Epub 2001/01/03] [DOI] [PubMed] [Google Scholar]
- 14.Cohn L.A., Weber A., Phillips T., Lory S., Kaplan M., Smith A. Pseudomonas aeruginosa infection of respiratory epithelium in a cystic fibrosis xenograft model. J Infect Dis. 2001;183(6):919–927. doi: 10.1086/319245. [Epub 2001/03/10] [DOI] [PubMed] [Google Scholar]
- 15.Ramsey B.W., Pepe M.S., Quan J.M., Otto K.L., Montgomery A.B., Williams-Warren J. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. Cystic fibrosis inhaled tobramycin study group. N Engl J Med. 1999;340(1):23–30. doi: 10.1056/NEJM199901073400104. [Epub 1999/01/08] [DOI] [PubMed] [Google Scholar]
- 16.Ratjen F., Walter H., Haug M., Meisner C., Grasemann H., Doring G. Diagnostic value of serum antibodies in early Pseudomonas aeruginosa infection in cystic fibrosis patients. Pediatr Pulmonol. 2007;42(3):249–255. doi: 10.1002/ppul.20562. [Epub 2007/01/24] [DOI] [PubMed] [Google Scholar]
- 17.Ling B.N., Zuckerman J.B., Lin C., Harte B.J., McNulty K.A., Smith P.R. Expression of the cystic fibrosis phenotype in a renal amphibian epithelial cell line. J Biol Chem. 1997;272(1):594–600. doi: 10.1074/jbc.272.1.594. [Epub 1997/01/03] [DOI] [PubMed] [Google Scholar]
- 18.Smith S.N., Delaney S.J., Dorin J.R., Farley R., Geddes D.M., Porteous D.J. Effect of IBMX and alkaline phosphatase inhibitors on Cl- secretion in G551D cystic fibrosis mutant mice. Am J Physiol. 1998;274(2 Pt 1):C492–C499. doi: 10.1152/ajpcell.1998.274.2.C492. [Epub 1998/03/05] [DOI] [PubMed] [Google Scholar]
- 19.Ramsey B.W., Davies J., McElvaney N.G., Tullis E., Bell S.C., Drevinek P. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365(18):1663–1672. doi: 10.1056/NEJMoa1105185. [Epub 2011/11/04] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Heeb S., Fletcher M.P., Chhabra S.R., Diggle S.P., Williams P., Camara M. Quinolones: from antibiotics to autoinducers. FEMS Microbiol Rev. 2011;35(2):247–274. doi: 10.1111/j.1574-6976.2010.00247.x. [Epub 2010/08/27] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Machan Z.A., Taylor G.W., Pitt T.L., Cole P.J., Wilson R. 2-Heptyl-4-hydroxyquinoline N-oxide, an antistaphylococcal agent produced by Pseudomonas aeruginosa. J Antimicrob Chemother. 1992;30(5):615–623. doi: 10.1093/jac/30.5.615. [Epub 1992/11/01] [DOI] [PubMed] [Google Scholar]
- 22.Diggle S.P., Matthijs S., Wright V.J., Fletcher M.P., Chhabra S.R., Lamont I.L. The Pseudomonas aeruginosa 4-quinolone signal molecules HHQ and PQS play multifunctional roles in quorum sensing and iron entrapment. Chem Biol. 2007;14(1):87–96. doi: 10.1016/j.chembiol.2006.11.014. [Epub 2007/01/27] [DOI] [PubMed] [Google Scholar]
- 23.Miller M.R., Crapo R., Hankinson J., Brusasco V., Burgos F., Casaburi R. General considerations for lung function testing. Eur Respir J. 2005;26(1):153–161. doi: 10.1183/09031936.05.00034505. [Epub 2005/07/05] [DOI] [PubMed] [Google Scholar]
- 24.Quittner A.L., Buu A., Messer M.A., Modi A.C., Watrous M. Development and validation of the cystic fibrosis questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest. 2005;128(4):2347–2354. doi: 10.1378/chest.128.4.2347. [Epub 2005/10/21] [DOI] [PubMed] [Google Scholar]
- 25.Standards unit PHE. UK standards for microbiology investigations.: Public Health England 2014. https://http://www.gov.uk/government/uploads/system/uploads/attachment_data/file/343994/B_57i2.5.pdf Available from:
- 26.Ortori C.A., Dubern J.F., Chhabra S.R., Camara M., Hardie K., Williams P. Simultaneous quantitative profiling of N-acyl-L-homoserine lactone and 2-alkyl-4(1H)-quinolone families of quorum-sensing signaling molecules using LC–MS/MS. Anal Bioanal Chem. 2011;399(2):839–850. doi: 10.1007/s00216-010-4341-0. [Epub 2010/11/04] [DOI] [PubMed] [Google Scholar]
- 27.Ortori C.A., Atkinson S., Chhabra S.R., Camara M., Williams P., Barrett D.A. Comprehensive profiling of N-acylhomoserine lactones produced by Yersinia pseudotuberculosis using liquid chromatography coupled to hybrid quadrupole-linear ion trap mass spectrometry. Anal Bioanal Chem. 2007;387(2):497–511. doi: 10.1007/s00216-006-0710-0. [Epub 2006/09/13] [DOI] [PubMed] [Google Scholar]
- 28.Pavord I.D., Pizzichini M.M., Pizzichini E., Hargreave F.E. The use of induced sputum to investigate airway inflammation. Thorax. 1997;52(6):498–501. doi: 10.1136/thx.52.6.498. [Epub 1997/06/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cystic Fibrosis Trust. Laboratory standards for processing microbioloigcal samples from people with cystic fibrosis2010. https://http://www.cysticfibrosis.org.uk/media/82034/CD_Laboratory_Standards_Sep_10.pdf Available from:
- 30.Lee T.W., Brownlee K.G., Conway S.P., Denton M., Littlewood J.M. Evaluation of a new definition for chronic Pseudomonas Aeruginosa infection in cystic fibrosis patients. J Cyst Fibros. 2003;2(1):29–34. doi: 10.1016/S1569-1993(02)00141-8. [Epub 2004/10/07] [DOI] [PubMed] [Google Scholar]
- 31.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [Epub 1988/09/01] [PubMed] [Google Scholar]
- 32.Equi A.C., Pike S.E., Davies J., Bush A. Use of cough swabs in a cystic fibrosis clinic. Arch Dis Child. 2001;85(5):438–439. doi: 10.1136/adc.85.5.438. [Epub 2001/10/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bala A., Kumar R., Harjai K. Inhibition of quorum sensing in Pseudomonas aeruginosa by azithromycin and its effectiveness in urinary tract infections. J Med Microbiol. 2011;60(Pt 3):300–306. doi: 10.1099/jmm.0.025387-0. [Epub 2010/12/04] [DOI] [PubMed] [Google Scholar]
- 34.Fletcher M.P., Diggle S.P., Crusz S.A., Chhabra S.R., Camara M., Williams P. A dual biosensor for 2-alkyl-4-quinolone quorum-sensing signal molecules. Environ Microbiol. 2007;9(11):2683–2693. doi: 10.1111/j.1462-2920.2007.01380.x. [Epub 2007/10/10] [DOI] [PubMed] [Google Scholar]
- 35.Brenner H., Gefeller O. Variation of sensitivity, specificity, likelihood ratios and predictive values with disease prevalence. Stat Med. 1997;16(9):981–991. doi: 10.1002/(sici)1097-0258(19970515)16:9<981::aid-sim510>3.0.co;2-n. [Epub 1997/05/15] [DOI] [PubMed] [Google Scholar]
- 36.Donnelly L.F., MacFall J.R., McAdams H.P., Majure J.M., Smith J., Frush D.P. Cystic fibrosis: combined hyperpolarized 3He-enhanced and conventional proton MR imaging in the lung--preliminary observations. Radiology. 1999;212(3):885–889. doi: 10.1148/radiology.212.3.r99se20885. [Epub 1999/09/09] [DOI] [PubMed] [Google Scholar]
- 37.Granstrom M., Ericsson A., Strandvik B., Wretlind B., Pavlovskis O.R., Berka R. Relation between antibody response to Pseudomonas aeruginosa exoproteins and colonization/infection in patients with cystic fibrosis. Acta Paediatr Scand. 1984;73(6):772–777. doi: 10.1111/j.1651-2227.1984.tb17774.x. [Epub 1984/11/01] [DOI] [PubMed] [Google Scholar]
- 38.Brett M.M., Ghoneim A.T., Littlewood J.M. Serum IgG antibodies in patients with cystic fibrosis with early Pseudomonas aeruginosa infection. Arch Dis Child. 1987;62(4):357–361. doi: 10.1136/adc.62.4.357. [Epub 1987/04/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brett M.M., Ghoneim A.T., Littlewood J.M. Prediction and diagnosis of early Pseudomonas aeruginosa infection in cystic fibrosis: a follow-up study. J Clin Microbiol. 1988;26(8):1565–1570. doi: 10.1128/jcm.26.8.1565-1570.1988. [Epub 1988/08/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guina T., Purvine S.O., Yi E.C., Eng J., Goodlett D.R., Aebersold R. Quantitative proteomic analysis indicates increased synthesis of a quinolone by Pseudomonas aeruginosa isolates from cystic fibrosis airways. Proc Natl Acad Sci U S A. 2003;100(5):2771–2776. doi: 10.1073/pnas.0435846100. [Epub 2003/02/26] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Collier D.N., Anderson L., McKnight S.L., Noah T.L., Knowles M., Boucher R. A bacterial cell to cell signal in the lungs of cystic fibrosis patients. FEMS Microbiol Lett. 2002;215(1):41–46. doi: 10.1111/j.1574-6968.2002.tb11367.x. [Epub 2002/10/24] [DOI] [PubMed] [Google Scholar]
- 42.Fletcher M.P., Diggle S.P., Camara M., Williams P. Biosensor-based assays for PQS, HHQ and related 2-alkyl-4-quinolone quorum sensing signal molecules. Nat Protoc. 2007;2(5):1254–1262. doi: 10.1038/nprot.2007.158. [Epub 2007/06/05] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material