Abstract
The increased prevalence of HIV among adults >50 years underscores the importance of improving our understanding of mechanisms causing HIV-associated neurocognitive disorders (HAND). Identifying novel and non-invasive diagnostic predictors of HAND prior to clinical manifestation is critical to ultimately identifying means of preventing progression to symptomatic HAND. Here using a task-switching paradigm, in which subjects were cued (unpredictably) to perform a face-gender or a word-semantic task on superimposed face and word images, we examined the behavioral and neural profile of impaired cognitive control in older HIV+ adults (N=14, 9 HIV+). Functional magnetic resonance imaging (fMRI) and behavioral data were acquired while subjects were performing the face-gender or word-semantic task. We found that, despite comparable performance in standard neuropsychology tests that are designed to probe executive deficits, HIV infected participants were significantly slower than uninfected controls in adapting to change in task demand, and the behavioral impairments can be quantitatively related to difference in fMRI signal at the dorsal anterior cingulate cortex (ACC). Due to the limited sample size of this hypothesis generating study, we should take caution with these findings and future studies with a large and better matched sample size are needed. However, these rather novel findings in this study have a few important implications: first, the prevalence of cognitive impairments in HIV+ older adults might be even higher than previously proposed; secondly, ACC (in particularly its dorsal region) might be one of the key regions underlying cognitive impairments (in particularly executive functions) in HIV; and thirdly, it might be beneficial to adopt paradigms developed and validated in cognitive neuroscience to study HAND, as these techniques might be more sensitive to some aspects of HIV-associated neurocognitive impairments than standard neuropsychology tests.
Keywords: Cognitive Control, HIV-Associated Neurocognitive Disorders, FMRI, Anterior Cingulate Cortex, HAND
Introduction
In the era of combined antiretroviral therapy (cART), HIV-associated neurocognitive disorder, or HAND, remains one of most common disorders in individuals with HIV infection (Heaton et al., 2010), and executive deficit is one of the most prominent neurocognitive impairments in HIV (Heaton et al., 2011). While reduced executive function has been attributed to neuronal dysfunction in the fronto-striatal circuit (Plessis et al., 2014), the neural mechanisms underlying this dysfunction remain to be elucidated. The purpose of this study was to examine potential early and subtle impairments in executive function in HIV-infected older adults, with a focus on cognitive control (Miller, 2000). Cognitive control is essential for everyday activities which require frequent shifts between different tasks and mental sets; impairments in cognitive control can negatively impact the safety and quality of life of people with HAND (Woods, Moore, Weber, & Grant, 2009). We hypothesize that, prior to the onset of clinically diagnosable HAND, subtle deficits in cognitive control may be already present in HIV+ older adults, due to damages to certain regions in the executive circuits, such as the anterior cingulate cortex (ACC) (Shackman et al., 2011). To test this hypothesis, we adopted a task-switching functional magnetic resonance imaging (fMRI) paradigm (Yeung, Nystrom, Aronson, & Cohen, 2006) in a cross-sectional design, to examine whether impaired cognitive control is detectable in HIV-infected adults compared to HIV-uninfected controls, even when standard neuropsychological testing fail to detect deficits.
Methods
Participants
After being screened for eligibility, including MRI safety, general neurocognitive function (via Mini-Mental Status Examination) (a minimum score of 27 or higher is needed), substance abuse (based on questions adapted from National HIV Behavioral Surveillance core questionnaire, CDC), depressive symptomatology (based on the CES-D), 14 older adults from local community enrolled in the study, including 9 HIV+ subjects who are on cART and have suppressed viral load (RNA PCR <400 copies/mL, self-report). Age (52-64), education, and other factors were comparable between the HIV- and HIV+ groups (Table 1). This study was approved by local Institutional Review Boards.
Table 1. Demographic and behavioral characteristics of participants (N=14).
| HIV-uninfected n=5 | HIV-infected n=9 | p-value | |
|---|---|---|---|
| Demographic characteristics | n(%) | n(%) | |
| Black/African American | 0 (0) | 4 (44.4) | 0.08 |
| Age [median (IQR)] | 57 (56-58) | 55 (55-57) | 0.69 |
| Male gender*1 | 3 (60.0) | 9 (100.0) | 0.03 |
| Education less than college | 4 (80.0) | 6 (67.7) | 0.56 |
| Currently employed* | 5 (100.0) | 4 (44.4) | 0.04 |
| Current smoker | 0 (0.0) | 3 (33.3) | 0.26 |
| Currently drinks alcohol | 3 (60.0) | 6 (67.7) | 0.79 |
| Ever used illicit drugs | 5 (100.0) | 9 (100.0) | n/a |
| Ever diagnosed with a psychological/psychiatric condition* | 1 (20.0) | 7 (77.8) | 0.04 |
| CD4 [median (IQR)] | N/A | 500 (463-660) | n/a |
| Years since diagnosed [median (IQR)] | N/A | 21 (11-22) | n/a |
| MMSE [median (IQR)] 2 | 29 (28-29) | 29 (29-29) | 0.34 |
| Neurocognitive testing | Median (IQR) | Median (IQR) | |
| Wisconsin card sort (median, IQR) | |||
| Categories completed3 | 2 (1,4) | 2 (1, 4) | 0.84 |
| Categories completed percent3 | 78 (55, 81) | 62 (48, 75) | 0.31 |
| Perseverative errors4 | 5 (4,5) | 7 (1, 10) | 0.11 |
| Perseverative errors percent4 | 7.8 (6.3, 7.8) | 10.9 (1.6, 15.6) | 0.31 |
| Trail making test (TMT, median, IQR) | |||
| TMT Part A (seconds) 4 | 34.1 (29.9, 37.9) | 36.5 (34.2, 45.9) | 0.56 |
| TMT Part B (seconds) 4 | 44.9 (36.8,48.8) | 46.0 (37.1, 61.3) | 0.55 |
| Patient's assessment of own functioning inventory (PAOFI) | |||
| PAOFI subscale: higher functioning4 | 1 (1, 5) | 7 (1,9) | 0.30 |
| PAOFI subscale: memory4 | 12 (7, 16) | 10.5 (7.5, 16.0) | 0.88 |
| PAOFI subscale: language4 | 7 (7, 8) | 9.5 (4.0, 12.5) | 0.34 |
| PAOFI subscale: motor4 | 3 (2, 4) | 2 (1.5, 3.5) | 0.71 |
| PAOFI total score4 | 22 (22, 29) | 30.5 (14, 40.5) | 0.38 |
| FMRI | |||
| FMRI cost of task switch (median, IQR)*** 2# | 0.125 (0.079,0.157) | -0.076 (-0.082,-0.049) | <0.001 |
p<0.05
p<0.01
p<0.001
(p-values are provided as a means of guiding interpretation of the findings of this hypothesis generating study only. Fisher exact test was used for categorical variables, two-sample t-test was used for continuous variables, and non-parametric test (Kruskal-Wallis) was used for non-continuous numeric variables).
HIV status was associated with having lower signal change scores, with none of the HIV-uninfected persons having a score lower than the median, and 7 infected persons having a compromised score (0% vs. 77.8%, p=0.005). Similarly, older age was associated with having lower scores independent of HIV status with 1 person under the median age of 57 having a compromised score compared with 6 over that cutpoint (16.7% vs. 75.0%, p=0.031).
One HIV-negative male at birth who currently identified as transgender classified as male for this analysis.
Having MMSE 27 or higher was required as an eligibility criterion to ensure ability to consent for the study.
Higher score indicates less favorable cognitive functioning
MRI Acquisition Parameters
MRI data were acquired at local imaging center, using an echo-planar sequence on a 3T Siemens Trio scanner with a 12-channel head coil (flip angle=90°, TR=2.04s, TE=29ms, FOV=205°, 64×64 matrix). 35 interleaved axial slices (4mm thick, no gap; 3.2×3.2mm2 in-plane resolution) were acquired. One structural scan (MPRAGE, 1×1×1 mm3 voxels) was collected.
Event-Related (ER) Scans
MRI images from two ER scans were collected for each subject using a task switch paradigm (Yeung et al., 2006). Each run had two 10.2s fixation periods, one at the beginning and the other at the end. Between the two fixation periods, a total of 100 trials were presented to participants at a rate of one every 4.08s. During each trial, overlapped face and word images were displayed for 2000ms (Fig. 1A) followed by a 2080ms blank screen, and subjects were cued (unpredictably with a written cue) to perform a task on the face (to judge the gender of face) or the word (to judge whether the word is an animate (e.g., tiger) or inanimate (e.g., table) word). The cue was an English word “Face” or “Word” that was presented on-screen for 4.08s. The first trials after the cue were referred as the switch trials, and the other face/word task trials as the non-switch trials. Trial order was pseudo-randomized and counterbalanced.
Figure 1.
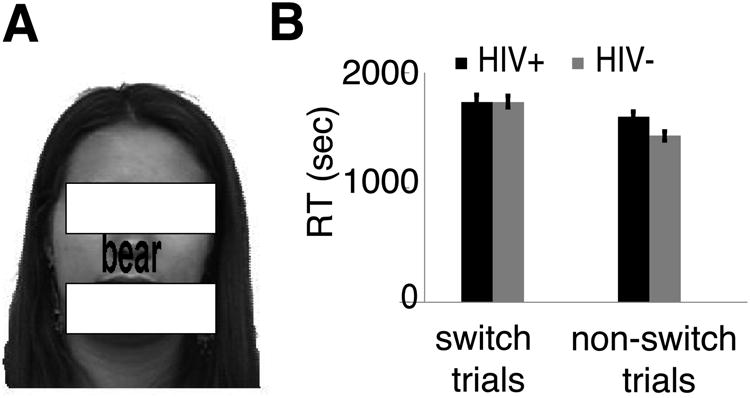
(A) Sample stimuli and the task-switching experimental paradigm. The stimuli were always a superimposed face and word image, and subjects were cued (Face vs. Word) to perform a gender identification task on the face while ignoring the word, or a word-category task (animate (e.g., tiger) vs. inanimate (e.g., table)) on the word while ignoring the face. The cue was a word “Face” or “Word” displayed for 4.08s (in the absence of task trials). The eye and mouth regions of the face image was masked here to protect the identity of this person. (B) Reaction time (RT) of task-switch (the first trials after switching task) and non-switch (other trials) trials. Both groups of subjects were highly accurate in tasks (93.2%±1.3%) and there was no difference in accuracy between groups. Error bars represent SEM.
fMRI Data Analyses
SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) was used for MRI data preprocessing and analysis. MRI preprocessing followed the steps detailed elsewhere [Citation to be added], including temporal correction, spatial realignment, normalization, and smooth (6mm Gaussian kernel). We then modeled fMRI responses with a design matrix comprising the onset of six conditions – the cue to switch to face task, the cue to switch to word task, the first face-task trial after the face-cue, the first word-task trial after the word-cue, the other face-task trials after the face-cue, and the other word-task trials after the word-cue – and movement parameters as regressors using a standard canonical hemodynamic response function (HRF). Additional analyses revealed no significant effects of age, race, and gender on fMRI results. Other independent variables (e.g., substance abuse, depression, co-morbidities) did not provide sufficient sample size to allow in depth exploration.
Results
Neuropsychological Scores
As shown in Table 1, HIV-infected participants performed more poorly than uninfected controls on all standard tests, but none statistically so, even after adjusting for the effect of age and gender.
Behavioral and fMRI Evidence for Cognitive Control Deficits in HIV+ Adults
A mixed ANOVA design of reaction time (RT) with a within-subject factor (switch vs non-switch) and a between-subject factor (HIV+ vs HIV-) revealed a significant effect of task-switching (F(1,12) = 40.944, p<0.001), but not between the two groups (p=0.307), and critically, a significant interaction between the two factors (F(1,12) = 6.565, p=0.025) (Fig. 1B). Post-hoc t-tests (paired or two-sample, two-tailed) revealed that the RT of switch-trials was compatible between the two groups (p>0.9), but was significantly higher than that of non-switch trials in both groups (at least p<0.033), suggesting a strong task switch cost in both groups of subjects. In contrast, the HIV-infected subjects were significantly slower than HIV-uninfected controls on non-switch trials (p<0.029), suggesting that HIV-infected subjects might be less efficient to adapt to current task demands than HIV-uninfected controls, as HIV-infected adults usually performed well under a choice task (in the absence of task switch) (Hardy & Hinkin, 2002).
Whole brain analysis via the contrast of (task-switch > non-switch) × (HIV+ > HIV-) revealed a single large cluster (6360mm3, threshold: pFWE <0.05, Bonferroni corrected for multiple comparison at cluster-level) in bilateral dorsal ACC (Fig. 1C). We then extracted the fMRI response in this cluster for both switch and non-switch trials, which revealed an ACC dysfunction in HIV+ older adults (Fig. 1D), and a significant correlation between the RT cost of task-switching (RTcost = RTtask-switch – RTnon-switch) and the fMRI cost of task-switching in the ACC ROI (fMRIcost = fMRItask-switch – fMRInon-switch), r=0.603, p<0.023 (Fig. 1E), supporting a direct link between the neuronal dysfunction in the ACC and the behavioral deficit, in line with literature that posits ACC as the center of cognitive control, especially its role in detecting and resolving conflict (Shenhav, Botvinick, & Cohen, 2013). Furthermore, while pointing to the right direction, there were no significant correlations between the fMRIcost and standard neuropsychology test scores (|r|≈0.38, p≈0.18 for all analyses), suggesting that the methodology employed in the present study is more sensitive than some of standard neuropsychology tests (like WCST and TMTB) in detecting early impairments in executive function.
Discussion
While HIV-infected older adults performed similarly to their uninfected counterparts when examined with standard neuropsychological tests, significant behavioral and fMRI impairments were observed using a task-switching paradigm (Yeung et al., 2006). That is, compared to negative controls, HIV+ participants adapted less quickly to changing task demands, and the behavioral deficits strongly correlated with disrupted brain activations in the bilateral dorsal ACC – a central region in executive function and the regulation of emotional processing (Shackman et al., 2011; Shenhav et al., 2013). Intriguingly, apathy has been widely reported in people with HIV-disease (Paul et al., 2005) and found to be associated with white matter atrophy that links ACC with other brain regions (Hoare et al., 2010), and neuropsychology studies have suggested a direct link between apathy and lesions to the ACC (Cummings, 1993). Here we propose that the ACC might be one of the key and early regions affected by HIV-disease, as suggested by a recent study (Garvey et al., 2014), and the manifestation of neuronal dysfunction in the ACC at a later stage may lead to a widespread cognitive decline, especially in the executive domain. Indeed, previous studies have suggested a significant correlation between apathy and cognitive performance in HIV+ individuals (Paul et al., 2005; Shapiro, Mahoney, Zingman, Pogge, & Verghese, 2013).
A few recent studies have questioned the high prevalence of HAND and suggested the existence of significant false alarms, especially in the diagnosis of the mild forms of HAND (Torti, Focà, Cesana, & Lescure, 2011). However, here we provided preliminary evidence suggesting the opposite, that is, cognitive impairments in those HIV-infected 50 years or older individuals might have been under-reported rather than over-reported in previous studies, in line with the general agreement that many neuropsychology tests might be insensitive to HAND (Kamminga, Cysique, Lu, Batchelor, & Brew, 2013), supporting an urgent need to develop neuropsychological tests that are more sensitive and specific to HAND for early diagnosis, which could lead to early and more effective interventional treatments (Ellis et al., 1997; Grant et al., 2014).
However, this conclusion needs to be taken with caution as this study has several limitations. As a pilot study of a complex novel technology, the goal was hypothesis generating not hypothesis testing; necessarily limited sample size decreases our statistical power and differences between groups may have been missed; some HIV+ older adults (i.e., those tested in the present study) might be at greater risk for HAND (Hardy & Vance, 2009). Viral load and CD4 measurements were based on self-report which may have introduced misclassification on these measures; future research should explore not only obtaining viral load measurements but other biomarker correlates of CNS saturation of HIV. In addition, the status of Hepatitis C infection and vascular disease were missing in the present study, both could affect neurocognitive functions. However, the results we have reported here clearly demonstrate the sensitivity and applicability of this technique in detecting reduced executive function in HIV, and uncovering its neural basis, with a strong implication for follow-up studies with a large and well-matched cohort.
Figure 2.
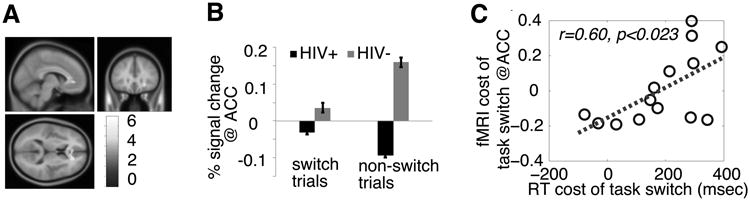
fMRI results. (A) Anterior cingulate cortex (ACC) was revealed with the contrast of (HIV+ > HIV-) × (task-switch > non-switch trials) (p<0.001, uncorrected, and p<0.05, Bonferroni corrected at cluster-level). (B) Percent signal change in the ACC ROI. Error bars represent within-subject SEM. (C) The correlation between the cost of RT and % signal change at the ACC cluster. RT cost of task switch = RTtask-switch – RTnon-switch, and fMRI cost of task-switching in the ACC ROI = fMRItask-switch – fMRInon-switch.
Acknowledgments
Funding: This project was supported by Award Number UL1RR031988 from the National Center for Research Resources and the District of Columbia Developmental Center for AIDS Research Award Number P30AI087714. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Center for Research Resources or the National Institutes of Health. Travel to CROI was generously supported by the District of Columbia Developmental Center for AIDS Research Award Number P30AI087714. We also appreciate the support of the Intellectual and Development Disorders Research Center (IDDRC) [5P30HD040677-13].
References
- Cummings JL. Frontal-subcortical circuits and human behavior. Archives of Neurology. 1993;50(8):873–880. doi: 10.1001/archneur.1993.00540080076020. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/8352676. [DOI] [PubMed] [Google Scholar]
- Ellis RJ, Deutsch R, RK H, al E, Heaton RK, Marcotte TD, et al. NEurocognitive impairment is an independent risk factor for death in hiv infection. Archives of Neurology. 1997;54(4):416–424. doi: 10.1001/archneur.1997.00550160054016. [DOI] [PubMed] [Google Scholar]
- Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, Winston A. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS (London, England) 2014;28(1):67–72. doi: 10.1097/01.aids.0000432467.54003.f7. [DOI] [PubMed] [Google Scholar]
- Grant I, Franklin DR, Jr, Deutsch R, Woods SP, Vaida F, Ellis RJ, et al. Group, F. the C. Asymptomatic HIV-associated neurocognitive impairment increases risk for symptomatic decline. Neurology. 2014 doi: 10.1212/WNL.0000000000000492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy DJ, Hinkin CH. Reaction time slowing in adults with HIV: results of a meta-analysis using brinley plots. Brain and Cognition. 2002;50(1):25–34. doi: 10.1016/s0278-2626(02)00007-6. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/12372349. [DOI] [PubMed] [Google Scholar]
- Hardy DJ, Vance DE. The neuropsychology of HIV/AIDS in older adults. Neuropsychology Review. 2009;19(2):263–272. doi: 10.1007/s11065-009-9087-0. [DOI] [PubMed] [Google Scholar]
- Heaton RK, Franklin DR, Ellis RJ, McCutchan JA, Letendre SL, LeBlanc S, et al. Groups, for the C. and H. HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. Journal of NeuroVirology. 2011;17(1):3–16. doi: 10.1007/s13365-010-0006-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaton RKK, Clifford DBB, Franklin DRRJ, Woods SPP, Ake C, Vaida F, et al. Group, C. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75(23):2087–2096. doi: 10.1212/WNL.0b013e318200d727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoare J, Fouche JP, Spottiswoode B, Joska JA, Schoeman R, Stein DJ, Carey PD. White matter correlates of apathy in HIV-positive subjects: a diffusion tensor imaging study. The Journal of Neuropsychiatry and Clinical Neurosciences. 2010;22(3):313–320. doi: 10.1176/appi.neuropsych.22.3.313. [DOI] [PubMed] [Google Scholar]
- Kamminga J, Cysique LA, Lu G, Batchelor J, Brew BJ. Validity of cognitive screens for HIV-associated neurocognitive disorder: a systematic review and an informed screen selection guide. Current HIV/AIDS Reports. 2013;10(4):342–55. doi: 10.1007/s11904-013-0176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nature Reviews Neuroscience. 2000;1(1):59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Paul R, Flanigan TP, Tashima K, Cohen R, Lawrence J, Alt E, et al. Hinkin C. Apathy correlates with cognitive function but not CD4 status in patients with human immunodeficiency virus. The Journal of Neuropsychiatry and Clinical Neurosciences. 2005;17(1):114–118. doi: 10.1176/appi.neuropsych.17.1.114. [DOI] [PubMed] [Google Scholar]
- Plessis S, Du Vink M, Joska JA, Koutsilieri E, Stein DJ, Emsley R. HIV infection and the fronto-striatal system: a systematic review and meta-analysis of fMRI studies. AIDS (London, England) 2014;28(6):803–811. doi: 10.1097/QAD.0000000000000151. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience. 2011;12(3):154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro ME, Mahoney JR, Zingman BS, Pogge DL, Verghese J. Apathy correlates with cognitive performance, functional disability, and HIV RNA plasma levels in HIV-positive individuals. Journal of Clinical and Experimental Neuropsychology. 2013;35(9):934–945. doi: 10.1080/13803395.2013.838941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenhav A, Botvinick MM, Cohen JD. The expected value of control: an integrative theory of anterior cingulate cortex function. Neuron. 2013;79(2):217–240. doi: 10.1016/j.neuron.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torti C, Focà E, Cesana BM, Lescure FX. Asymptomatic neurocognitive disorders in patients infected by HIV: fact or fiction? BMC Medicine. 2011;9(1):138. doi: 10.1186/1741-7015-9-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods SP, Moore DJ, Weber E, Grant I. Cognitive neuropsychology of HIV-associated neurocognitive disorders. Neuropsychology Review. 2009;19(2):152–168. doi: 10.1007/s11065-009-9102-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung N, Nystrom LE, Aronson JA, Cohen JD. Between-task competition and cognitive control in task switching. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience. 2006;26(5):1429–1438. doi: 10.1523/JNEUROSCI.3109-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]


