Abstract
Aim
Both oxidized LDL and carbamylated LDL are considered important for initiating atherosclerosis in patients with end-stage kidney disease through vascular endothelial cell dysfunction or injury. However their effects on each other and their relationship related to pro-atherosclerotic effects on endothelial cells and macrophages have not been investigated. In this study, we analyzed the competition between LDL carbamylation and oxidation, tested biological effects of carbamylated-oxidized LDL (coxLDL) toward the endothelial cells, assessed its ability to cause foam cell development, and determined the roles of scavenger receptors in this process.
Methods
Cross-competition between carbamylation and oxidation of LDL particles was tested using cell-free fluorescent ligand-receptor assay. Pro-atherogenic properties (cell proliferation, cytotoxicity, and foam cell formation) of all LDL isoforms were tested in vitro and ex vivo using endothelial cells and peritoneal macrophages. In addition, coxLDL was assessed in human sera and in vivo atherosclerotic plaques which were developed in mouse model of uremia-induced atherosclerosis.
Results
Our data suggest that there is potential competition between carbamylation and oxidation of LDL, and that oxidation is a much stronger inhibitor of carbamylation than vice versa. coxLDL is highly cytotoxic to endothelial cells and strongly induce their proliferation measured by DNA synthesis. All three tested LDL isoforms demonstrated strong ability for transformation of primary mouse peritoneal macrophages to foam cells using predominantly CD36 scavenger receptor. coxLDL was the most potent inducer of foam cell development and macrophages/foam cell injury assessed by cell count and TUNEL, respectively. Finally, LDL particles modified by oxidation and carbamylation were detected in blood and shown to co-localize in atherosclerotic plaques in mice.
Conclusion
Our study demonstrated that LDL particles can be simultaneously carbamylated and oxidized and modifications are likely coexisting in the same LDL particle. We also demonstrated pro-atherosclerotic properties of coxLDL and proposed its role in atherosclerosis.
Keywords: Carbamylated LDL, Oxidized LDL, Carbamylated-oxidized LDL, Scavenger receptors, Atherosclerosis, Endothelial cells, Foam cells
Introduction
Chronic kidney disease (CKD) affects approximately 10% of the worldwide population and is associated with a significantly increased risk of developing cardiovascular disease due to the acceleration of atherosclerosis1). Multiple studies have shown that oxidized LDL (oxLDL) can initiate the development of atherosclerosis by inducing endothelial cell injury2). Increased levels of oxLDL have been shown in uremic patients with end-stage renal disease (ESKD)3); however, a mechanistic link between uremia and oxLDL-induced atherosclerosis has not been established.
CKD is known to induce the generation of modified LDL in blood plasma. The special type of LDL that appears as a result of apolipoprotein B (ApoB) carbamylation in CKD patients was named “uremic LDL” by Gonen et al.4) because it possesses different absorption and binding-to-receptor properties than native LDL (nLDL). Subsequently, Kraus et al.5) described cLDL generation as a product of the chemical modification of nLDL by urea-derived isocyanate. Most LDL carbamylation occurs spontaneously, and no regulators of this process have been identified. Urea dissociates to cyanate and ammonia in aqueous solutions, causing elevation of the level of cyanate in uremic patients. The active form of cyanate, isocyanic acid, reacts irreversibly with the N-terminal group of amino acids5). Irreversible carbamylation forming epsilon-amino-carbamyl-lysine occurs at multiple lysine sites within a protein, accumulating over the life span of the protein5). The resulting in vivo carbamylation changes the structure of ApoB and other proteins. An alternative pathway of protein carbamylation has recently been described by Wang and coauthors6), who showed that the oxidative enzyme mieloperoxidase contributes to the LDL carbamylation that occurs inside atherosclerotic plaques.
A pathogenetic role of carbamylated LDL (cLDL) in the development of atherosclerosis in uremic patients was first hypothesized by Horkko et al.7). Later, Roxborough and Young8) suggested that the carbamylation of LDL induced by elevated levels of urea results in the formation of cLDL and makes LDL more susceptible to oxidation. Our previously developed quantitative cLDL sandwich ELISA method showed that the concentration of cLDL in the human sera is comparable to that of oxLDL, as measured using a similar method9). Acting alone, cLDL produces a variety of biological effects in vascular cells in vitro, including endothelial cell death, induction of cell adhesion molecules, attraction of monocytes, induction of endothelial and vascular smooth muscle cell proliferation, activation of mitogen-activated protein kinases (MAPK), overexpression and binding to scavenger receptors and activation of apoptotic endonuclease G10–14). Recent studies have shown that cLDL induces oxidative stress and accelerates senescence in human endothelial progenitor cells15). cLDL has also been shown to exacerbate the development of atherosclerosis in ApoE−/− mice16). In addition, cLDL has been proposed to be a potential nontraditional marker of chronic kidney failure (CRF) and atherosclerosis17) and is colocalized in atherosclerotic lesions in both mice and humans.
Despite the similarities in the biochemical characteristics of oxLDL and cLDL, including their effects on vascular cells, sites of accumulation and common increases in uremic and cardiovascular patients, the interactions between the carbamylation and oxidation of LDL have not been evaluated. In the present study, we investigated the interactions between the oxidation and carbamylation of LDL and found that they compete with each other. This competition is partial and thus allows for the production of double-modified LDL particles. The LDL isoform that carries both modifications, carbamylated-oxidized LDL (coxLDL), was found to be more cytotoxic to endothelial cells and a more potent inducer of foam cells than cLDL or oxLDL alone.
Materials and Methods
Preparation of Modified LDLs
All reagents were purchased from Sigma-Aldrich (Milwaukee, WI), unless otherwise specified. Native human LDL (nLDL) was purchased from Intracel Resources (Frederick, MD). cLDL and oxLDL were prepared as previously described12). Briefly, cLDL was prepared by exposing nLDL to potassium cyanate (KOCN) at 20 mg/mg of LDL protein. The mixture was incubated at 35 °C for up to four hours. KOCN was removed using excessive dialysis under sterile conditions at 4 °C against 0.15 M NaCl and 0.01% EDTA with a pH of 7.0 three times for 12 hours each. The concentration of potassium cyanate after the first cycle of dialysis was 3.7 mM and could not be detected after the second or third cycles of dialysis using the Dimension RxL analyzer (Dade Behring). oxLDL was prepared by exposing nLDL to 5 mM CuSO4 for up to 24 hours at 37 °C. The reaction was stopped by adding 200 μM of sterile EDTA. coxLDL was prepared by oxidizing of cLDL, as described above. All modified LDL isoforms were kept at 4 °C away from light and used within four weeks after preparation.
Assessment of Carbamylation and Oxidation
A colorimetric diacetyl monoxime method was used to measure the degree of carbamylation in the LDL preparations, as previously described9). A standard curve was generated using homocitrulline (carbamyl lysine, 0–30 nmol) (Advanced Asymmetrics, Millstadt, IL). The results were expressed in nmol homoc-itrulline/mg of LDL protein. The oxidation of LDL was evaluated using a thiobarbituric acid reactive substances (TBARS) assay11). Freshly prepared 1,1,3,3-tet-ramethoxypropane, which yields malondialdehyde (MDA), was used as a standard. The results were expressed in nmol MDA/mg of LDL protein.
Endothelial Cell Culture
Human coronary artery endothelial cells (HCAECs) were obtained from Lonza (Walkersville, MD) and used at passages between 4 and 8. The cells were maintained in EGM-2-MV medium (Lonza) supplemented with 5% fetal bovine serum (FBS). The HCAECs were grown in a humidified incubator (5% CO2/95% air, 37°C) and treated with 25 to 400 μg/mL of LDL isoforms in serum-free EGM-2-MV medium for 24 hours.
Bromodeoxyuridine (BrdU) Assay
Following 24 hours of growth arrest in serum-free medium, the HCAECs were exposed to 25 to 200 μg/mL of LDL isoforms for eight hours. The level of cell proliferation was measured using a BrdU Cell Proliferation assay (Oncogene, Cambridge, MA), as previously described12).
LDL Labeling with AlexaFluor 594 Dye
Fluorescent labeling of nLDL and the modified LDLs was performed using AlexaFluor 594 Protein labeling kits (Invitrogen, Carlsbad, CA), as previously reported14). Briefly, 1 mg of each LDL isoform was diluted with 0.1 M sodium bicarbonate, mixed with AlexaFluor 594-carboxylic acid tetrafluorophenyl ester and exposed at 4°C for two hours. The prepared labeled LDLs were purified using Sephadex G-50 (Invitrogen). The specific fluorescence per mg of protein was equilibrated by adding appropriate amounts of unlabeled LDL isoforms.
Cytotoxicity and Apoptosis Assays
To measure the level of cytotoxicity, an LDH release assay kit (Promega, Madison, WI) was used, as previously reported 11). The level of toxicity was expressed as the ratio of LDH released in the treated cell medium to that of the maximal amount of LDH released. In some experiments, trypan blue exclusion was used as previously described11) as an additional measure of cytotoxicity.
Cell-Free Fluorescent Ligand-Receptor Assay
To study the ability of modified LDLs to bind to receptors, a fluorescent ligand-receptor assay was performed as previously described14). For this assay, 96-well plates were coated with 100 μL/well of recombinant proteins (LOX-1, CD36 or SR-A1 from R&D Systems Inc., Minneapolis, MN) at 5 μg/mL in PBS for 16 hours at 4 °C. After blocking with 2% BSA containing PBS buffer and performing several washes with PBS, AlexaFluor 594-labeled LDLs (10 μg/mL) were applied for two hours at 37 °C. The total fluorescence before and the remaining fluorescence after the washings were measured at 530/645 nm. The amount of LDL that was specifically bound to scavenger receptors was calculated based on the specificity of fluorescent labeling (fluorescence per 1 mol of protein) and measured in fluorescence numbers. The data were presented as the amount of LDL (μmol) per the amount of receptor (mol) used to capture LDL. In some experiments, the relative competition between cLDL, oxLDL and coxLDL for scavenger receptors was studied. In these cases, AlexaFluor 594-labeled LDLs (1 μg/mL) were exposed to an equal (1-fold) amount of unlabeled competitor or the same LDL isoform in the presence or absence of an excessive (30-fold) amount of an unlabeled competitor or the same LDL isoform. After measuring the fluorescence, the level of binding of LDL to the receptor observed without a competitor was accepted as 100%, and the relative level of binding was calculated and averaged from four individual measurements.
Ex vivo Foam Cell Development Assay
All animal experiments were approved by the Animal Care and Use Committee of the Central Arkansas Veterans Healthcare System. For the assay, C57BL6J mice were used as described by Zhao et al.18). Briefly, 10- to 12-week-old male mice were injected intraperitoneally with 1 mL of sterile BBL Thioglycollate Brewer Modified Medium (Becton, Dickinson and Company, Sparks, MD). Seventy-two hours later, the mice were euthanized, and peritoneal macrophages were collected via peritoneal lavage with ice-cold PBS. The macrophages were plated in 8-well slide chambers at 80% confluence and allowed to rest for 24 hours in RPMI-1640 medium/10% FBS. The cells were then exposed to LDL isoforms (200 μg/mL) in serum-free medium. The control cells were exposed to an appropriate volume of medium or vehicle (PBS, 200 μM EDTA). In some experiments, nonspecific immunoglobulins G or specific inhibiting antibodies to LOX-1 (R&D Systems), SR-A1 (R&D Systems) and CD36 (Abcam, Cambridge, MA) scavenger receptors were added to serum-free medium at a final concentration of 10 μg/mL. The inhibitory properties of the antibodies were confirmed in a cell free system using appropriate recombinant protein controls, as previously reported14). It was also shown that all three antibodies inhibited the internalization of the modified LDLs into cultured endothelial cells and interfered with the biological effects of the modified LDLs14).
In vivo Model of CRF and Atherosclerosis
A model of CRF-induced atherosclerosis was created using a previously published technique16). Eight-week-old ApoE−/− male mice were subjected to biphase surgery of electrocautery of the right kidney followed by left kidney removal. The control mice underwent sham surgery. Two weeks after the surgeries, the CRF and sham mice were transferred to a high-fat diet (adjusted to 42% calories from fat) for 12 weeks. To control the effects of diet, a separate group of mice was fed a regular chow diet.
Detection of coxLDL in Sera
All measurements of human sera were approved by the IRB Committee of the University of Arkansas for Medical Sciences. To detect coxLDL, a sandwich ELISA was used of murine and human sera (healthy individuals) at a dilution of 1:50 and 1:400, respectively, as we previously described9). Briefly, the diluted sera were applied to 96-well plates coated with anti-oxLDL antibodies, followed by hybridization with anti-cLDL antibodies directly labeled with horse radish peroxidase (HRP). After assay development with 3,3′, 5,5″-tetramethylbenzidine and termination with 2N sulfuric acid, the data were read using a Bio-Tek Synergy 4.0 plate reader at 450 nm. In vitro prepared coxLDL served as a positive control.
Immunohistochemistry, Cytochemistry and Terminal Deoxynucleotidyl Transferase dUTP Nick End Labeling (TUNEL) Assay
Immunohistochemistry was performed according to the previously described standard technique13). The tissues were fixed with 10% buffered formalin, dehydrated and embedded in paraffin. 5-μm sections were probed with the mixture of specific antibodies raised in different species, including rabbits and goats. Rabbit polyclonal antibodies to cLDL (prepared and tested as previously described9, 16)) and oxLDL (Abcam) were used for staining. Prior to use, primary antibodies to cLDL and oxLDL were directly labeled with Alexa-Fluor 488 and AlexaFluor 594, respectively. TUNEL staining was performed as previously described13). An Olympus IX81 image system with a Hamamatsu ORCA-ER camera was used for microscopy and imaging. The SlideBook 4.2 software program was used for the processing and quantitative analysis of the images.
Cytochemistry and image analyses were used to detect and quantify foam cells stained with Oil Red O, as previously described19). The cells were fixed with 4% paraformaldehyde and stained with 0.15% Oil Red O in a 55:45 (vol/vol) mixture of isopropanol and water to visualize lipids. Microscopy was performed using an Olympus IX51 image system equipped with an INFINITY2 color digital camera and the Infinity analysis software package (v. 4.6.0, Lumenera Corporation, Ottawa, ON). The ImageJ software (NIH) program was used for color image quantification. The images were split to the red, green and blue spectra, and the red spectrum was subsequently analyzed in two ways: (1) the percentage of foam cell development was counted as the relative number of cells that contained three or more fatty droplets; (2) the amount of lipids per cell was calculated as the absolute brightness of the red spectrum divided by the number of cells in each field of view. A minimum of 20 fields of view were used for each experimental point. The data of three separate experiments were averaged, and each experiment had four points.
Protein Measurement
The concentration of protein was measured using a BCA protein assay (Pierce, Rockford, IL). Bovine serum albumin was used as the standard.
Statistical Analysis
The statistical analysis was performed using the SPSS 14.0 software package for Windows (SPSS Inc. Chicago IL). Continuous variables were evaluated using the unpaired t -test, and the results were expressed as the mean±standard error of the mean (SEM). Pearson’s correlation test was used to evaluate linear correlations between two continuous variables. All statistical tests were two-sided, and a p-value of 0.05 or less was considered to be significant.
Results
Susceptibility of LDL to Dual Modification by Oxidation and Carbamylation
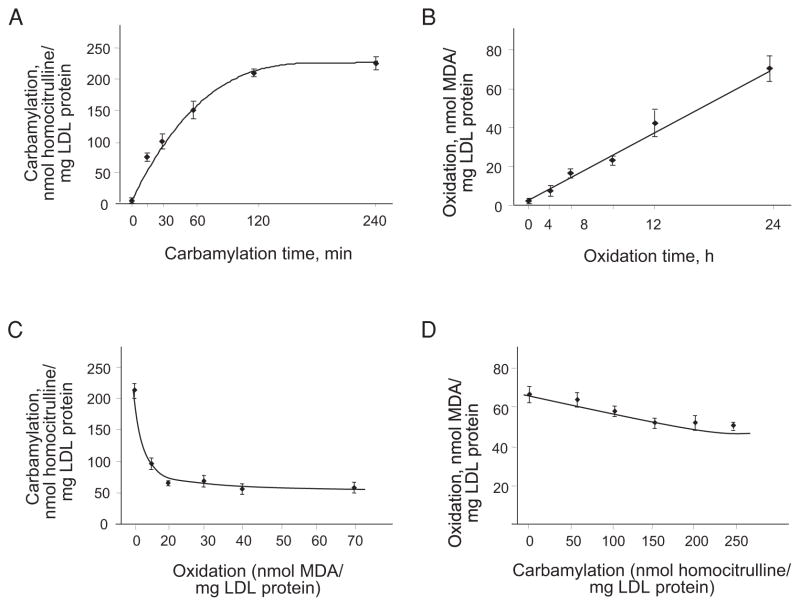
It has previously been reported that nLDL is highly susceptible to both oxidation and carbamylation, the two most abundant LDL modifications found in patients with cardiovascular disease and/or uremia20). The possibility of dual modification has not been previously explored. To investigate this issue, we first characterized the kinetics of the individual reactions. The level of in vitro LDL carbamylation was found to be significantly higher after 15 minutes of exposure to cyanate and reached a plateau within 120 minutes (Fig. 1A). At the 240-minute time point, the degree of LDL carbamylation increased from 5±1 to 221±16 nmol homocitrulline/mg of LDL protein. The level of oxidation of the in vitro prepared cLDL measured according to TBARS was not significantly different from that of nLDL (0.9±0.3 nmol MDA/mg LDL protein in nLDL and 0.7±0.3 nmol MDA/mg LDL protein in cLDL).
Fig. 1.
LDL carbamylation, oxidation and competition between the two modifications.
A. Carbamylation of nLDL occurred in a time-dependent manner and reached a plateau at 120 minutes. B. Oxidation of nLDL occurred in a time-dependent manner and reached a maximum at 24 hours. C. Carbamylation of LDLs oxidized to different degrees. Native LDL was gradually oxidized, as shown in panel C, and then carbamylated for 240 minutes. The carbamylation was inhibited with oxidation of >20 nmol MDA/mg of LDL protein. D. Oxidation of LDLs carbamylated to different degrees. Native LDL was gradually carbamylated, as shown in panel A, and then oxidized for 24 hours. The oxidation was inhibited proportionally to the degree of carbamylation. Every experiment was performed at least four times with triplication of every experimental/control point. The data are presented as the mean±SEM.
The rate of oxidation appeared to be slower than that of carbamylation. Statistically significant elevation of LDL oxidation was detected at 4-hour time points of exposure to cupric sulfate (Fig. 1B). At 24 hours, the level of LDL oxidation was increased from less than 1 nmol MDA/mg of protein to ~70 nmol MDA/mg of protein of oxLDL. There was no detectable level of carbamylation of LDL subjected to oxidation.
To investigate the possibility of dual LDL modification by carbamylation and oxidation, the competition between the two processes was studied. First, the possibility of carbamylation of LDL previously oxidized to different degrees, including 10, 20, 30, 40 and 70 (maximal) nmol MDA/mg of protein, was assessed (Fig. 1C). Two hundred and forty minutes of exposure to cyanate revealed that the smallest degree of LDL oxidation (10 nmol MDA/mg protein) inhibited LDL carbamylation by 54%. oxLDL with a higher degree of oxidation (20–60 nmol MDA/mg protein) prevented from 65% to 75% of carbamylation compared to carbamylation of non-oxidized LDL. In the second step, interference between carbamylation and oxidation was explored. cLDL with different degrees of carbamylation (50, 100, 150, 200 and 250 (maximal) nmol homocitrulline/mg of LDL) protein was oxidized using cupric sulfate for 24 hours (Fig. 1D). The data demonstrated that cLDL with 50–100 nmol homocitrulline/mg of LDL carbamylation did not significantly prevent further oxidation of LDL. cLDL with above 150 nmol homocitrulline/mg of LDL carbamylation was able to prevent ~25% of oxidation compared to nLDL.
Hence, our data suggest that there is potential competition between the carbamylation and oxidation of LDL and that oxidation is a much stronger inhibitor of carbamylation than carbamylation is of oxidation.
coxLDL Exerts Proatherogenic Effects on Endothelial Cells
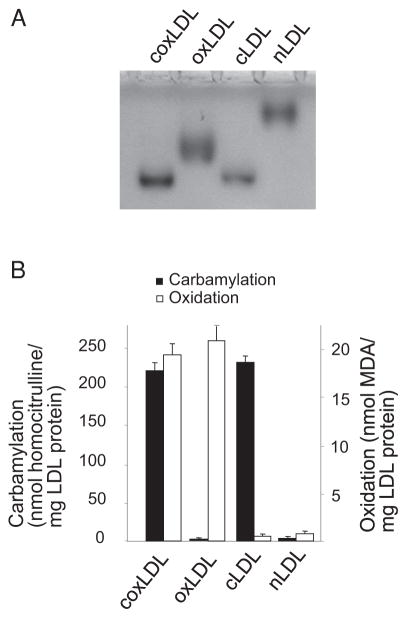
Both cLDL and oxLDL have been previously shown to induce proliferation and injury in endothelial cells11, 12). To assess the effects of coxLDL on endothelial cells in comparison to those of cLDL and oxLDL, the three LDL isoforms were prepared as described above using the latest time points. Electrophoresis in agarose gel showed that the LDLs had the following maximal relative electrophoresis mobility (REM) values: 2.7 for cLDL, 2.1 for oxLDL and 2.9 for coxLDL (Fig. 2A). coxLDL and cLDL were measured to have carbamylation degrees of nearly 220 nmol homocitrulline/mg of LDL protein, while the degree of carbamylation of nLDL and oxLDL was negligible (Fig. 2B). Oxidation of coxLDL and oxLDL was detected at nearly 20 nmol MDA/mg of LDL protein and was less than 0.5 nmol MDA/mg of LDL protein in cLDL and nLDL.
Fig. 2.
Characterization of nLDL and modified LDLs.
The relative electrophoresis mobility (REM) values were measured in 0.5% agarose gel stained with Sudan black (A). The carbamylation and oxidation assessment revealed a high degree of carbamylation in cLDL and coxLDL and a high degree of oxidation in oxLDL and coxLDL (B). Every experiment was performed at least three times with duplication of every experimental/control point. The data are presented as the mean±SEM.
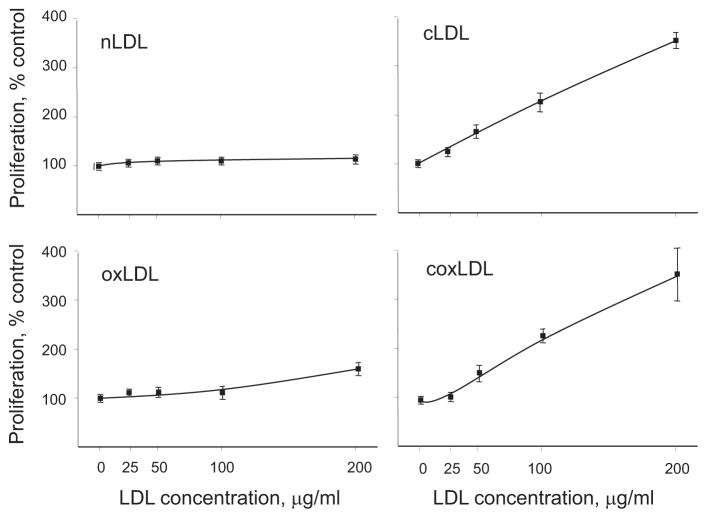
To measure the effects of the LDL isoforms on endothelial cell proliferation, cultured HCAECs were exposed to 25–200 μg/mL of the LDL isoforms for a period of 24 hours. According to the BrdU incorporation assay, at a dose of 200 μg/mL, coxLDL induced a 355±60% increase in the rate of DNA synthesis compared to the vehicle (Fig. 3). There were no significant differences in BrdU incorporation between coxLDL and cLDL; however, oxLDL exhibited a significantly milder effect on DNA synthesis.
Fig. 3.
Induction of endothelial cell proliferation by LDL isoforms.
The DNA synthesis of HCAECs treated with the LDL isoforms (200 μg/mL) for 24 hours was measured using BrdU incorporation. Both cLDL and coxLDL were found to be potent inducers of endothelial proliferation. Every experiment was performed at least four times with duplication of every experimental/control point. The data are presented as the mean±SEM.
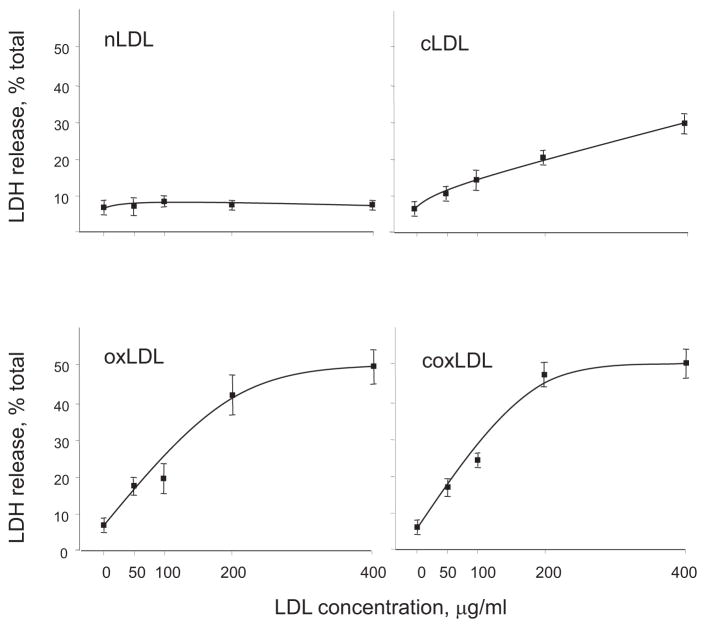
The cytotoxicity of the LDL isoforms measured according to the amount of LDH released, which demonstrated the highest effect in the HCAECs treated with 200 μg/mL of oxLDL or coxLDL and measured at 43±6% or 47±5%, respectively (Fig. 4). The cLDL- and nLDL-treated endothelial cells exhibited significantly lower levels of cytotoxicity than coxLDL and oxLDL. Trypan blue positive staining confirmed the tendency in cytotoxicity measured by LDH release and was observed in 3±2%, 9±2%, 23±9% and 27±7% of cells following exposure to 200 μg/mL of nLDL, cLDL, oxLDL and coxLDL, respectively. Therefore, coxLDL was the most cytotoxic of the studied LDLs.
Fig. 4.
Cytotoxicity of the LDL isoforms toward endothelial cells.
LDH release was induced by the exposure of all modified LDLs to HCAECs. oxLDL and coxLDL were found to be the most potent inducers of HCAEC cell death. Every experiment was performed at least three times with triplication of every experimental/control point. The data are presented as the mean±SEM.
coxLDL Induces the Most Prominent Foam Cell Production
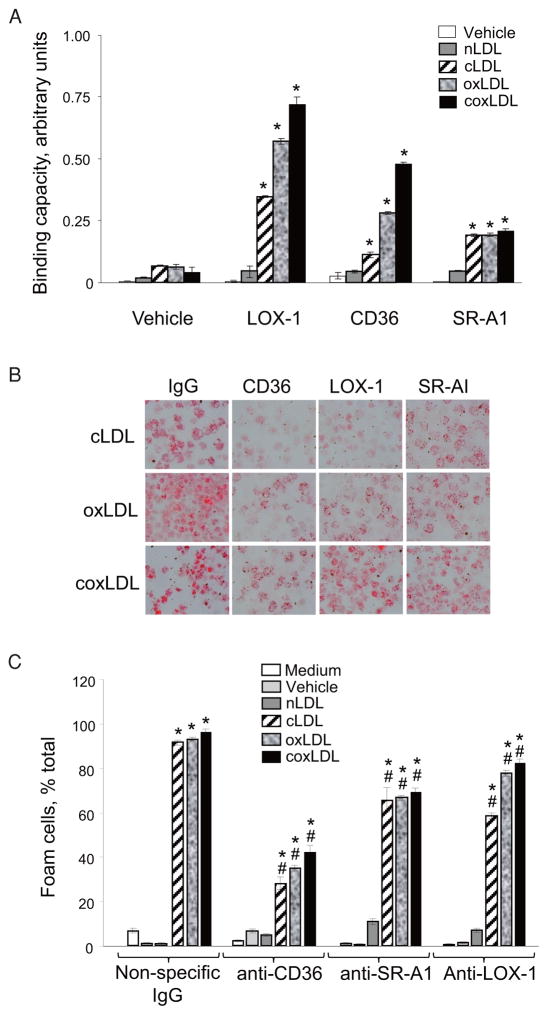
Our previous study suggested that cLDL and oxLDL induce monocyte adhesion to endothelial cells10). Subsequently, cLDL was colocalized with macrophages/foam cells in atherosclerotic plaques in a mouse model of CRF16). The modified LDLs were shown to induce extensive foam cell formation in vitro 21). To compare the ability of the LDL isoforms to induce foam cell formation, primary mouse peritoneal macrophages were isolated and treated with equal doses of the LDLs. The relative accumulation of lipids per foam cell was observed to be drastically elevated in all modified LDLs (Fig. 5A). Compared to the vehicle, all tested isoforms contributed significantly to the production of foam cells: nLDL, cLDL, oxLDL and cox-LDL converted ~8%, 62%, 82% and 90% of macrophages to foam cells, respectively (Fig. 5B and 5C). Therefore, coxLDL produced the greatest acceleration of metamorphosis of macrophages into foam cells.
Fig. 5.
Development of foam cells from murine peritoneal macrophages treated with the LDL isoforms.
A. Relative accumulation levels of lipids per cell. B. Relative quantification of foam cells. C. Representative images. Primary peritoneal macrophages were extracted from mice and treated with the LDL isoforms (200 μg/mL). The data demonstrate a significant increase in foam cell development following treatment with all modified LDLs. coxLDL was found to be the most potent inducer of foam cell formation. Every experiment was performed at least three times with quadruplication of every experimental/control point. The data are presented as the mean±SEM. *p < 0.05 compared to the nLDL-treated cells.
coxLDL-Induced Production of Foam Cells is Mediated by Scavenger Receptors
It has previously been reported that cLDL and oxLDL have affinity for a series of scavenger receptors involved in the development of atherosclerosis and other vascular diseases14). To test whether coxLDL is recognized by scavenger receptors and induces foam cell formation via this mechanism, we studied the binding of coxLDL to recombinant human LOX-1, CD36 and SR-A1 molecules using a fluorescent ligand-receptor assay. These receptors were chosen because they are expressed in macrophages and foam cells in atherosclerotic plaques22). Our data suggested that coxLDL exhibits the highest absolute degree of binding to CD36 and LOX-1 receptors compared to the vehicle, nLDL and the other modified LDLs (Fig. 6A). All tested modified LDLs exhibited a higher level of binding ability than nLDL to the SR-A1 receptor; however, none appeared more active than the others.
Fig. 6.
Measurement of the affinity of the LDL isoforms to CD36, LOX-1 and SR-A1 scavenger receptors using a functional ELISA (A) and the attenuation of foam cell development by the inhibition of scavenger receptors (B, C).
All modified LDLs were found to be capable of binding to LOX-1, CD36 and SR-A1 receptors; however, neutralizing CD36 prevented foam cell development to the highest degree. Every experiment was performed at least four times with triplication of every experimental/control point. The data are presented as the mean±SEM. *p<0.05 compared to the nLDL-treated cells; *p<0.05 compared to the nonspecific IgG-treated cells.
To compare the relative preferences and competition between cLDL, oxLDL and coxLDL for CD36, SR-A1 and LOX-1, the cross-competition between the LDL isoforms was measured. The data suggested that coxLDL and oxLDL are equally preferable ligands for the CD36 receptor, while cLDL is a poor competitor for CD36 compared to oxLDL and coxLDL (Table 1). Binding of coxLDL to LOX-1 and SR-A1 was significantly reduced in excess of cLDL, and vice versa, binding of cLDL to these receptors was decreased in excess of coxLDL, suggesting that both cLDL and coxLDL are close competitors for LOX-1 and SR-A1. On the other hand, oxLDL was less competitive for the LOX-1 and SR-A1 receptors compared to cLDL and coxLDL. Taken together, the data suggest that coxLDL possesses binding characteristics that are somewhat specific for both cLDL and oxLDL.
Table 1.
Cross-competition between cLDL and oxLDL or coxLDL for CD36, LOX-1 and SR-A1 receptors1
| No competitor, % | cLDL, % | oxLDL, % | coxLDL, % | ||||
|---|---|---|---|---|---|---|---|
|
|
|
|
|||||
| 1-fold | 30-fold | 1-fold | 30-fold | 1-fold | 30-fold | ||
| CD36 | |||||||
| oxLDL-AF594 | 100 | 84 | 56* | 49* | 12* | 59* | 18* |
| cLDL-594 | 100 | 51* | 15* | 64* | 28* | 52* | 21* |
| coxLDL-594 | 100 | 89 | 79 | 67* | 18* | 51* | 15* |
| SR-A1 | |||||||
| oxLDL-AF594 | 100 | 79 | 42* | 53* | 16* | 67* | 26* |
| cLDL-594 | 100 | 46* | 11* | 89 | 66* | 71 | 32* |
| coxLDL-594 | 100 | 54* | 30* | 91 | 72 | 54* | 14* |
| LOX-1 | |||||||
| oxLDL-AF594 | 100 | 75 | 46* | 53* | 14* | 63* | 29* |
| cLDL-594 | 100 | 55* | 15* | 94 | 77* | 66* | 43* |
| coxLDL-594 | 100 | 63* | 34* | 91 | 78 | 52* | 13* |
The data were calculated for every modified LDL as the level of relative binding to scavenger receptors in the presence of competitors compared to the level of binding to the scavenger receptors without competitors.
AF594, AlexaFluor 594;
p <0.05 as compared to the level of ligand binding with “no competitor.”
We then evaluated the development of foam cells in the presence of inhibitory antibodies to LOX-1, CD36 and SR-A1. The results of this experiment suggested that, compared to nonspecific immunoglobulins, antibodies to CD36 dramatically reduced the development of foam cells from peritoneal macrophages induced by all of the modified LDLs (Fig. 6B and 6C). Despite the occurrence of significant inhibition, even in the presence of anti-CD36 antibodies, coxLDL induced the development of more foam cells than any other modified LDL. Antibodies to SR-A1 and LOX-1 also significantly prevented macrophage conversion into foam cells, although less efficiently than anti-CD36 antibodies. Therefore, coxLDL appears to be recognized by all three scavenger receptors that are also recognized by cLDL and oxLDL and utilizes them to extend the development of foam cells.
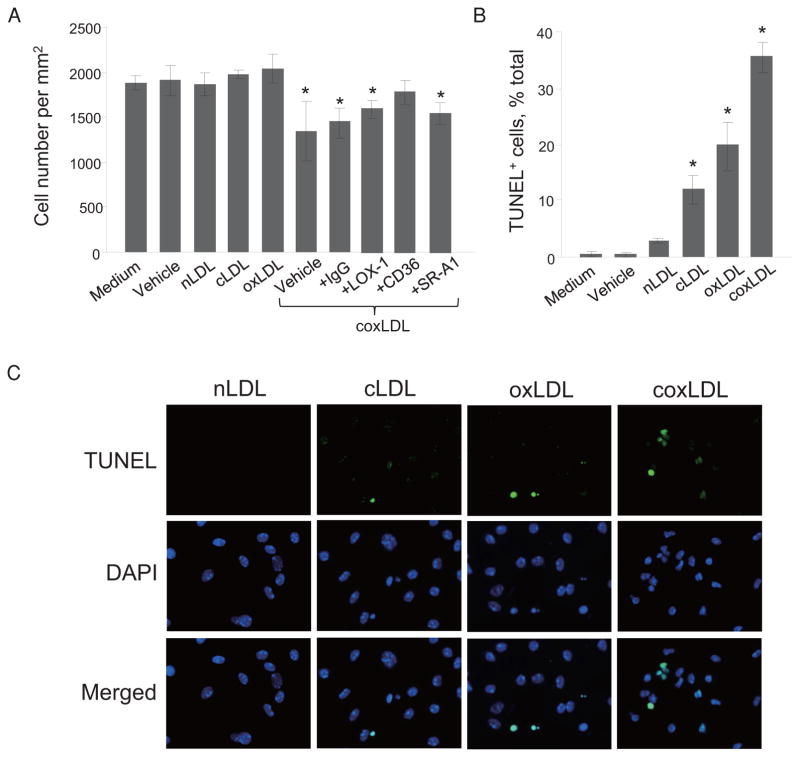
coxLDL Promotes the Degeneration and Death of Foam Cells
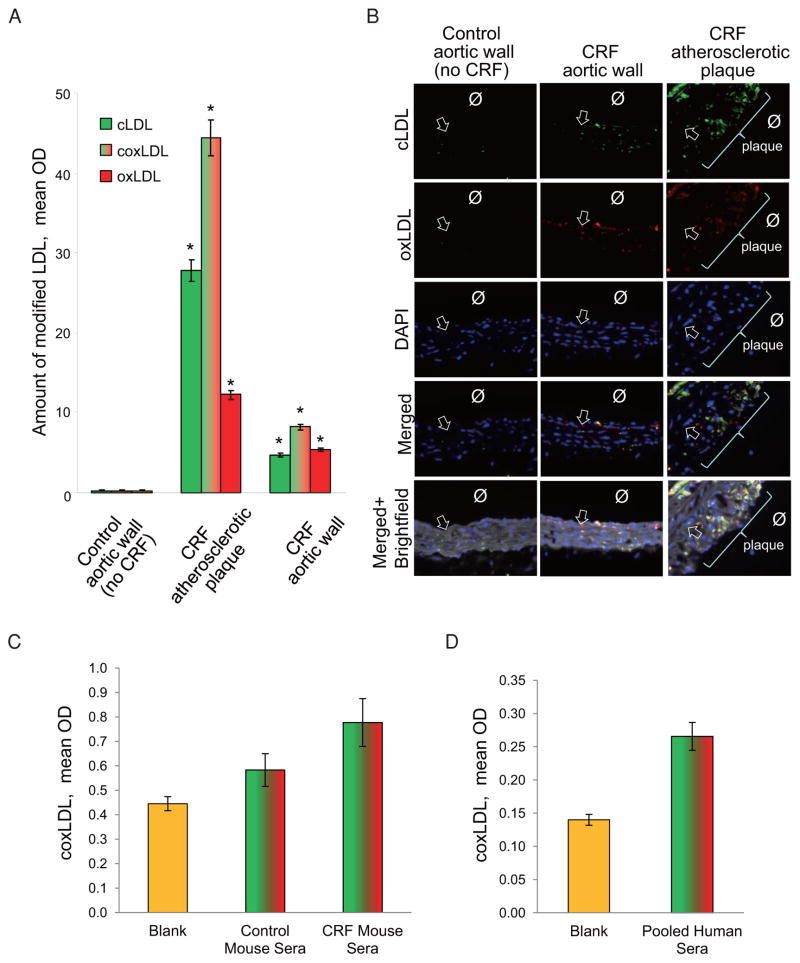
We noted that the coxLDL-treated peritoneal macrophages also developed signs of degeneration, such as cytoplasmic granulation and vacuolization. By the end of 72 hours of exposure, coxLDL induced cell injury in macrophages/foam cells that led to a significant reduction in the number of cells (Fig. 7A). Neutralizing of CD36 scavenger receptors by inhibitory antibodies prevented the cell toxicity induced by cox-LDL. To assess the extent of cell death in the coxLDL-treated macrophages/foam cells, the cells were stained for TUNEL. This experiment confirmed the highest rate of cell injury after treatment with coxLDL compared to the vehicle, nLDL and other modified LDLs (Fig. 7B and 7C). To extend this observation to an in vivo model and explore whether coxLDL is present in atherosclerotic lesions, immunohistochemical staining was performed in ApoE−/− mouse aortas with atherosclerotic plaque produced as previously described16). We observed that the atherosclerotic lesions contained both cLDL and oxLDL, which colocalized with each other (Fig. 8A and 8B). A pixel-to-pixel correlation analysis revealed that 45–53% of the detected cLDL and oxLDL overlapped, suggesting the possible presence of coxLDL in sites of uremia-induced atherosclerotic plaque and atherosclerosis in the aortic wall in mice with CRF. Subsequently, modification of LDL by sole oxidation or carbamylation was observed in 14–29% and 26–33% of the mice, respectively. Furthermore, the coxLDL concentration was assessed in pooled murine sera and appeared to be at a detectable level above the “blank” control in both the control and CRF mice (Fig. 8C). The level of coxLDL was found to be elevated in the mice with CRF compared to that observed in the control mice (p = 0.073). Finally, pooled sera obtained from healthy individuals were also found to be positive for coxLDL (Fig. 8D). These data suggest that coxLDL is likely to be present in atherosclerotic plaque and blood in vivo and is associated with a higher incidence of vascular cell injury.
Fig. 7.
Cytotoxicity of the modified LDLs (200 μg/mL) toward foam cells and macrophages in vitro.
Cell count following treatment with medium, vehicle, nLDL, cLDL, oxLDL or coxLDL (in the presence or absence of antibodies to scavenger receptors) (A). TUNEL staining: quantification (B) and representative images (C). coxLDL induced significant toxicity toward macrophages/foam cells; however, this phenomenon was partially preventable with the inhibition of CD36 antibodies. Every experiment was performed at least three times with triplication of every experimental/control point. The data are presented as the mean±SEM. *p <0.05 compared to the nLDL-treated cells.
Fig. 8.
Immunohistochemical colocalization of cLDL and oxLDL in the aortic wall and atherosclerotic plaques in ApoE−/− mice subjected to CRF.
Quantification of cLDL and oxLDL in the aortic wall was performed using an analysis of pixel-to-pixel overlap. A. The atherosclerotic plaques obtained from mice with atherosclerosis contained significantly more cLDL, oxLDL and coxLDL (cLDL overlapped with oxLDL) compared to that observed in the aortic walls of the control mice. Each point indicates the mean±SEM for n = 4. *p <0.05 compared to the aortic wall in the control (no CRF) ApoE−/− mice. B. Representative images of cLDL/oxLDL colocalization (Ø - lumen; arrow - internal elastic lamina). C. Results of ELISA of coxLDL in pooled sera (n = 5) obtained from control and CRF mice. D. Results of ELISA of coxLDL in pooled sera (n = 10) obtained from healthy individuals.
Discussion
This study described a new form of dual modified LDL, coxLDL, which results from two most common modifications coexisting on a single LDL particle. The pixel-to-pixel colocalization suggested that at least 45–53% of modified LDL in atherosclerotic plaque is modified by both carbamylation and oxidation. We showed that oxidation of LDL inhibits carbamylation more strongly than carbamylation inhibits oxidation. coxLDL was found to induce the proliferation of endothelial cells and formation of foam cells and to be highly cytotoxic toward endothelial and macrophages/foam cells. The data showed that, during the formation of foam cells, coxLDL utilizes LOX-1, CD36 and SR-A1 scavenger receptors. Overall, the effects of coxLDL were more prominent than the effects of LDLs with single modifications.
coxLDL may be the result of spontaneous chemical modifications of LDL particles occurring in either a simultaneous or consequent fashion. For the first time, we have shown that oxidation of LDL does not allow for further LDL carbamylation, while further oxidation of cLDL is permitted. Based on this competition pattern, LDL carbamylation is more likely to occur prior to oxidation and may therefore play a role as a driving force in the formation of coxLDL. In addition to conventional nonenzymatic mechanisms, this process is also likely to involve myeloid peroxidase, which has been shown to catalyze both the oxidation and carbamylation of proteins6).
Our study does not confirm the mechanism proposed by Roxborough and Young8) that carbamylation promotes the oxidation of LDL particles. In this regard, coxLDL formation differs from that of another double-modified LDL, glycoxidized LDL (goxLDL)23). Oxidation inhibits the subsequent glycation of LDL, while glycation promotes the subsequent oxidation of the LDL particle23). The mechanisms underlying the LDL oxidation-induced inhibition of LDL carbamylation remain unknown. It can be speculated that this phenomenon occurs because oxLDL has a significantly higher molecular charge than cLDL or gLDL24). It is likely that this charge and the subsequent change in lipoprotein conformation are responsible for repealing other polar and nonpolar molecules that can further modify LDL particles. Although amino groups are largely involved in both cLDL and oxLDL modification, it is likely that the sites of oxidation and carbamylation partially overlap. There are more sites for oxidation than for carbamylation in LDL particles because LDL can be oxidized in both the lipid and protein domains, while other modified LDLs, such as cLDL and gLDL, are primarily produced through covalent modification of the amino groups of the protein.
The induction of DNA synthesis and endothelial cell proliferation stimulated by coxLDL is similar to the previously demonstrated effects of cLDL and oxLDL12). cLDL-induced endothelial injury is markedly dependent on the induced DNA synthesis; therefore, the latter is a key factor in the mechanism of cLDL-induced cytotoxicity12). On the other hand, the level of oxLDL-induced cytotoxicity is significantly higher than the level of cLDL-induced cytotoxicity and is not related to the induction of scheduled DNA synthesis12), which can be explained by the existence of two separate mechanisms of endothelial cell injury induced by cLDL and oxLDL. It is likely that coxLDL inherits its ability to activate both mechanisms; however, because the level of cytotoxicity of coxLDL is not significantly different from that of oxLDL, the results suggest that additive or potentiating effects are absent. At this point, it can be speculated that despite differences in the mechanisms of cytotoxicity of cLDL and oxLDL, some steps are likely to be shared between the two mechanisms and thus can be saturated and limit the biological effects.
LOX-1, CD36 and SR-A1 scavenger receptors can bind to either cLDL or oxLDL14). All three scavenger receptors are considered to be important for the various cellular effects of oxLDL25). On the other hand, cLDL mostly utilizes LOX-1 and CD3614). It has been shown that cLDL has a higher affinity for LOX-1 and SR-A1 than oxLDL and is not easily displaced by oxLDL, while oxLDL has higher affinity than cLDL to CD36 and can easily displace cLDL from the recep-tor14). The current report confirmed previous observations that oxLDL has overall higher efficacy than cLDL for binding to LOX-1 and CD36 and equal binding efficacy with cLDL to SR-A114). We also demonstrated that coxLDL has higher efficacy than either cLDL or oxLDL for binding to LOX-1 and CD36 and is not different from cLDL or oxLDL with respect to efficacy for binding to the SR-A1 receptor. Likewise, gox-LDL has a significantly higher capability to bind to scavenger receptors than either gLDL or oxLDL21). Therefore, it appears to be a common trend that double modified LDLs maintain a higher capacity to bind to scavenger receptors than single modified LDLs by virtue of the presence of more binding sites and a higher charge26).
Recruiting of scavenger receptors has been proven to play a key role in the endothelial toxicity induced by cLDL and oxLDL14, 25). However, it remains an open question as to whether these receptors play an active role in the atherogenic effects on vascular endothelial cells induced by coxLDL or other double-modified LDLs. Assessments of the latter phenomenon and the potential competition between coxLDL and other known modified LDLs are pending based on the measurement of physiologically relevant coxLDL concentrations in patient sera and should be conducted in the future.
Foam cells are considered to be key cells in sites of atherosclerotic lesions, with a variety of pathophysiological properties, such as local inflammatory responses, activation of vascular smooth muscle cell proliferation and LDL modification27). oxLDL is a known stimulant of foam cell development27); however, the current study is the first to demonstrate both cLDL and cox-LDL being highly involved in foam cell development. Among the LDL isoforms, coxLDL was found to act most aggressively by accumulating the highest amount of lipids per cell. This is similar to the previously described effects of goxLDL, which demonstrated a higher ability than oxLDL or gLDL to be internalized to macrophages21).
This study showed that CD36 strongly mediates the foam cell development induced by all modified LDLs, while SR-A1 and LOX-1 receptors have a rather modest capacity. These data are in good agreement with those of previous reports suggesting a primary role of CD36 in foam cell development22, 28). CD36 is also frequently considered to be a marker of foam cells in vascular wall or atherosclerotic lesions and has been proposed to be involved in the primary mechanism of goxLDL internalization by macrophages21). Previously, Wang and coauthors6) suggested that SR-A1, but not CD36, is a preferential scavenger receptor for cLDL in macrophages. Our previous studies suggested that cLDL has a strong preference for several scavenger receptors, with LOX-1 and CD36 receptors being the most functionally important14). oxLDL is known to have the highest preference for CD36 and SR-A1 receptors in macrophages25, 29). Although the underlying mechanisms of this phenomenon remain unknown, it can be speculated that the simultaneous modification of LDL by carbamylation and oxidation changes LDL particles, leading to the high preference of cox-LDL for CD36 receptors.
Unlike cLDL or oxLDL, coxLDL exhibits marked cytotoxicity to macrophages/foam cells. On this point, our data are in contradiction with the previously reported strong proapoptotic properties of oxLDL toward foam cells and macrophages30). In contrast to the findings of our study showing the controlled presence of both cLDL and oxLDL modification, previous studies do not account for the possible presence of other modified LDLs in tested oxLDL preparations, which may be one reason for the contradiction. Interestingly, among all studied scavenger receptors, inhibition of the CD36 receptor was the only effective measure observed to significantly prevent the death of macrophages/foams cells. Therefore, CD36 should be considered a mechanistic molecule that participates in both foam cell development and the death of macrophages/foam cells in the arterial wall. The high level of cytotoxicity of coxLDL suggests that it has the potential to play an important role in exacerbating the inflammatory response in addition to the development of lipid cores of atherosclerotic plaque and the promotion of plaque degeneration and instability31).
In summary, the current report suggests that the simultaneous carbamylation and oxidation of LDL particles may lead to the production of coxLDL, a new modified LDL particle that is likely to have an aggressive atherogenic potential. Future studies are needed to determine the quantity of coxLDL in the circulation and tissues using direct proteomic methods and to uncover the mechanisms underlying the cox-LDL cytotoxicity toward foam cells and identify the significance of this LDL isoform in cardiovascular disease.
Acknowledgments
Financial support: grant from Satellite Health-care, two VA Merit Review grants (A.G.B., S.V.S.), grants from the National Center for Research Resources (5P20RR016460-11) and the National Institute of General Medical Sciences (8 P20 GM103429-11) from the National Institutes of Health (A.G.B.), fellowships from the Turkish Nephrology Association and the International Society of Nephrology (E.O.) and an American Heart Association Grant (E.O.A.).
Footnotes
Conflicts of Interest
None.
References
- 1.Nissenson AR, Pereira BJ, Collins AJ, Steinberg EP. Prevalence and characteristics of individuals with chronic kidney disease in a large health maintenance organization. Am J Kidney Dis. 2001;37:1177–1183. doi: 10.1053/ajkd.2001.24520. [DOI] [PubMed] [Google Scholar]
- 2.Galle J, Hansen-Hagge T, Wanner C, Seibold S. Impact of oxidized low density lipoprotein on vascular cells. Atherosclerosis. 2005 doi: 10.1016/j.atherosclerosis.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Van Tits L, De Graaf J, Hak-Lemmers H, Bredie S, Demacker P, Holvoet P, Stalenhoef A. Increased levels of low-density lipoprotein oxidation in patients with familial hypercholesterolemia and in end-stage renal disease patients on hemodialysis. Lab Invest. 2003;83:13–21. doi: 10.1097/01.lab.0000048633.76607.e0. [DOI] [PubMed] [Google Scholar]
- 4.Gonen B, Goldberg AP, Harter HR, Schonfeld G. Abnormal cell-interactive properties of low-density lipoproteins isolated from patients with chronic renal failure. Metabolism. 1985;34:10–14. doi: 10.1016/0026-0495(85)90052-6. [DOI] [PubMed] [Google Scholar]
- 5.Kraus LM, Kraus AP., Jr Carbamoylation of amino acids and proteins in uremia. Kidney Int Suppl. 2001;78:S102–107. doi: 10.1046/j.1523-1755.2001.59780102.x. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z, Nicholls SJ, Rodriguez ER, Kummu O, Horkko S, Barnard J, Reynolds WF, Topol EJ, DiDonato JA, Hazen SL. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat Med. 2007;13:1176–1184. doi: 10.1038/nm1637. [DOI] [PubMed] [Google Scholar]
- 7.Horkko S, Savolainen MJ, Kervinen K, Kesaniemi YA. Carbamylation-induced alterations in low-density lipo-protein metabolism. Kidney Int. 1992;41:1175–1181. doi: 10.1038/ki.1992.179. [DOI] [PubMed] [Google Scholar]
- 8.Roxborough HE, Young IS. Carbamylation of proteins and atherogenesis in renal failure. Med Hypotheses. 1995;45:125–128. doi: 10.1016/0306-9877(95)90059-4. [DOI] [PubMed] [Google Scholar]
- 9.Apostolov EO, Shah SV, Ok E, Basnakian AG. Quantification of carbamylated LDL in human sera by a new sandwich ELISA. Clin Chem. 2005;51:719–728. doi: 10.1373/clinchem.2004.044032. [DOI] [PubMed] [Google Scholar]
- 10.Apostolov EO, Shah SV, Ok E, Basnakian AG. Carbamylated low-density lipoprotein induces monocyte adhesion to endothelial cells through intercellular adhesion molecule-1 and vascular cell adhesion molecule-1. Arterioscler Thromb Vasc Biol. 2007;27:826–832. doi: 10.1161/01.ATV.0000258795.75121.8a. [DOI] [PubMed] [Google Scholar]
- 11.Ok E, Basnakian AG, Apostolov EO, Barri YM, Shah SV. Carbamylated low-density lipoprotein induces death of endothelial cells: a link to atherosclerosis in patients with kidney disease. Kidney Int. 2005;68:173–178. doi: 10.1111/j.1523-1755.2005.00391.x. [DOI] [PubMed] [Google Scholar]
- 12.Apostolov EO, Basnakian AG, Yin X, Ok E, Shah SV. Modified LDLs induce proliferation-mediated death of human vascular endothelial cells through MAPK pathway. Am J Physiol Heart Circ Physiol. 2007;292:H1836–1846. doi: 10.1152/ajpheart.01079.2006. [DOI] [PubMed] [Google Scholar]
- 13.Apostolov EO, Ray D, Alobuia WM, Mikhailova MV, Wang X, Basnakian AG, Shah SV. Endonuclease G mediates endothelial cell death induced by carbamylated LDL. Am J Physiol Heart Circ Physiol. 2011;300:H1997–2004. doi: 10.1152/ajpheart.01311.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apostolov EO, Shah SV, Ray D, Basnakian AG. Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL. Arterioscler Thromb Vasc Biol. 2009;29:1622–1630. doi: 10.1161/ATVBAHA.109.189795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carracedo J, Merino A, Briceno C, Soriano S, Buendia P, Calleros L, Rodriguez M, Martin-Malo A, Aljama P, Ramirez R. Carbamylated low-density lipoprotein induces oxidative stress and accelerated senescence in human endothelial progenitor cells. Faseb J. 2011;25:1314–1322. doi: 10.1096/fj.10-173377. [DOI] [PubMed] [Google Scholar]
- 16.Apostolov EO, Ray D, Savenka AV, Shah SV, Basnakian AG. Chronic Uremia Stimulates LDL Carbamylation and Atherosclerosis. J Am Soc Nephrol. 2010;21:1852–1857. doi: 10.1681/ASN.2010040365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaisson S, Pietrement C, Gillery P. Carbamylation-derived products: bioactive compounds and potential biomarkers in chronic renal failure and atherosclerosis. Clin Chem. 2011;57:1499–1505. doi: 10.1373/clinchem.2011.163188. [DOI] [PubMed] [Google Scholar]
- 18.Zhao W, Varghese M, Yemul S, Pan Y, Cheng A, Marano P, Hassan S, Vempati P, Chen F, Qian X, Pasinetti GM. Peroxisome proliferator activator receptor gamma coacti-vator-1alpha (PGC-1alpha) improves motor performance and survival in a mouse model of amyotrophic lateral sclerosis. Mol Neurodegener. 2011;6:51. doi: 10.1186/1750-1326-6-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lazarenko OP, Rzonca SO, Suva LJ, Lecka-Czernik B. Netoglitazone is a PPAR-gamma ligand with selective effects on bone and fat. Bone. 2006;38:74–84. doi: 10.1016/j.bone.2005.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basnakian AG, Shah SV, Ok E, Altunel E, Apostolov EO. Carbamylated LDL. Adv Clin Chem. 2010;51:25–52. doi: 10.1016/s0065-2423(10)51002-3. [DOI] [PubMed] [Google Scholar]
- 21.Lam MC, Tan KC, Lam KS. Glycoxidized low-density lipoprotein regulates the expression of scavenger receptors in THP-1 macrophages. Atherosclerosis. 2004;177:313–320. doi: 10.1016/j.atherosclerosis.2004.08.003. [DOI] [PubMed] [Google Scholar]
- 22.de Villiers WJ, Smart EJ. Macrophage scavenger receptors and foam cell formation. J Leukoc Biol. 1999;66:740–746. doi: 10.1002/jlb.66.5.740. [DOI] [PubMed] [Google Scholar]
- 23.Sakata N, Uesugi N, Takebayashi S, Nagai R, Jono T, Horiuchi S, Takeya M, Itabe H, Takano T, Myint T, Taniguchi N. Glycoxidation and lipid peroxidation of low-density lipoprotein can synergistically enhance atherogenesis. Cardiovasc Res. 2001;49:466–475. doi: 10.1016/s0008-6363(00)00262-5. [DOI] [PubMed] [Google Scholar]
- 24.Chiu JH, Peng YN, Yang YL, Tsai MH, Ho YL, Wu CY, Liu MY. In vitro oxidized and glycated human low-density lipoprotein particles characterized by capillary zone electrophoresis. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;875:383–391. doi: 10.1016/j.jchromb.2008.09.014. [DOI] [PubMed] [Google Scholar]
- 25.Boullier A, Bird DA, Chang MK, Dennis EA, Friedman P, Gillotre-Taylor K, Horkko S, Palinski W, Quehenberger O, Shaw P, Steinberg D, Terpstra V, Witztum JL. Scavenger receptors, oxidized LDL, and atherosclerosis. Ann N Y Acad Sci. 2001;947:214–222. doi: 10.1111/j.1749-6632.2001.tb03943.x. discussion 222–213. [DOI] [PubMed] [Google Scholar]
- 26.Avogaro P, Bon GB, Cazzolato G. Presence of a modified low density lipoprotein in humans. Arteriosclerosis. 1988;8:79–87. [PubMed] [Google Scholar]
- 27.Orso E, Grandl M, Schmitz G. Oxidized LDL-induced endolysosomal phospholipidosis and enzymatically modified LDL-induced foam cell formation determine specific lipid species modulation in human macrophages. Chem Phys Lipids. 2011;164:479–487. doi: 10.1016/j.chemphyslip.2011.06.001. [DOI] [PubMed] [Google Scholar]
- 28.Shashkin P, Dragulev B, Ley K. Macrophage differentiation to foam cells. Curr Pharm Des. 2005;11:3061–3072. doi: 10.2174/1381612054865064. [DOI] [PubMed] [Google Scholar]
- 29.Nicholson AC, Febbraio M, Han J, Silverstein RL, Hajjar DP. CD36 in atherosclerosis. The role of a class B macrophage scavenger receptor. Ann N Y Acad Sci. 2000;902:128–131. discussion 131–123. [PubMed] [Google Scholar]
- 30.Hegyi L, Skepper JN, Cary NR, Mitchinson MJ. Foam cell apoptosis and the development of the lipid core of human atherosclerosis. J Pathol. 1996;180:423–429. doi: 10.1002/(SICI)1096-9896(199612)180:4<423::AID-PATH677>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 31.Martinet W, Kockx MM. Apoptosis in atherosclerosis: focus on oxidized lipids and inflammation. Curr Opin Lipidol. 2001;12:535–541. doi: 10.1097/00041433-200110000-00009. [DOI] [PubMed] [Google Scholar]