ABSTRACT
Colorectal cancer is third leading cause of cancer mortality. About 60% of patients had already developed metastasis at the time of diagnosis. Vascular endothelial growth factor (VEGF) is crucial for the development of neovascularization and hence metastasis. This study aimed at investigating the relation between the expression of VEGF in biopsies from surgically dissected colon cancer and the survival of those patients. Biopsies were collected from 86 patients with advanced colon cancer and sections were stained by immunohistochemistry for VEGF. Patients received chemotherapy after the operation and were followed up for disease progression and survival. The clinical data were statistically analyzed with respect to the immunohistochemistry results. The survival of the patients was significantly longer in the patients for whom biopsies showed negative or weak expression of VEGF in comparison to those with moderate to high expression (p-value = 0.04). The expression of VEGF was more frequent in the patients who died as a consequence of the disease in comparison to the 10-year survivors. In conclusion, VEGF could be related to the survival of the patients with colorectal carcinoma and should be considered as a predictor of the prognosis.
KEYWORDS: Vascular endothelial growth factor, colorectal cancer, survival, oncology, angiogenesis, chemoresistance
1. Introduction
Colorectal carcinoma is the third most common cause of cancer related mortality in the world.[1] Targeted biologic agents have increased the overall median survival in metastatic colorectal carcinoma (mCRC) to 23.5 months.[2] Kirsten-ras (KRAS) mutations in the epidermal growth factor receptor (EGFR) pathway have led to chemotherapy becoming a more personalized, tailored approach using EGFR monoclonal antibodies.[3] Different biomarkers were assessed in numerous studies, but a predictive biomarker for bevacizumab has not been identified.[4]
Even after radical surgery and adjuvant chemotherapy, approximately 50% of colorectal cancer (CRC) patients subsequently relapse and yield to the disease.[5] In locally advanced or mCRC, surgical resection is unlikely to be curative. The five-year survival rate of metastatic disease is less than 5%.[6] However, chemotherapy can yield improvements in survival and is the main treatment modality for the majority of these patients.[7]
Metastatic disease is the major cause of CRC-related mortality as a consequence of disease progression, tumour involvement of critical organs or adverse events of treatment. The understanding of the growth and spread of tumours as being dependent on angiogenesis has opened new avenues of research to improve knowledge of cancer biology and to facilitate the development of new therapeutic strategies.
The process of angiogenesis consists of multiple, sequential, and interdependent steps with several positive and negative regulators being involved. The survival of tumours, and thus their metastases, is dependent upon a delicate balance between endogenous angiogenic and anti-angiogenic factors, favouring increased formation of blood vessels. Neoangiogenesis, the formation of new capillaries from pre-existing blood vessels, is essential for tumour development beyond a diameter of 2–3 mm3.[8] Angiogenesis provides tumour cells the opportunity to enter the circulation and thus the ability to metastasize, in addition to providing nutrients for tumour growth. Angiogenesis is mediated by angiogenic cytokines.[9] The most potent of these cytokines is vascular endothelial growth factor (VEGF-A), a heparin-binding glycoprotein with potent angiogenic, mitogenic, and vascular permeability-enhancing activities specific for endothelial cells. Of the anti-angiogenic factors, thrombospondin is of special interest.[10,11]
Evidence from preclinical and clinical studies indicates that VEGF is the predominant angiogenic factor in human CRC and is associated with the formation of metastases and poor prognosis.[12] VEGF is expressed in approximately 50% of CRCs with minimal to no expression in normal colonic mucosa and adenomas. Increased VEGF expression significantly correlates with advanced lymph node status and distant metastasis. The survival of patients with strong VEGF expression is significantly worse than of those patients with weak or no expression.[13]
In this study, we examined the expression of VEGF-1 in CRC samples from patients with stage II, III or IV disease, to determine its association with several clinicopathological variables, response to treatment and its influence on disease survival.
2. Subjects and methods
2.1. Patients, treatment and follow-up
The study was approved by the National Authority for Medico-Legal Affairs Committee and was conducted in accordance with the declaration of Helsinki. Series of consecutive histological sections were obtained from surgically removed biopsies tumours from advanced CRC patients attending the Department of Oncology and Radiotherapy, Turku University Hospital, Finland between August 1998 and August 2003.
The samples were biopsies removed during surgery from 86 patients with advanced CRC, of whom 55 had metastases at diagnosis (stage IV disease). The remainder, 31, had stage II or III disease at diagnosis. Patients started treatment at the Department of Oncology and Radiotherapy, Turku University Hospital between August 1998 and August 2003. Patient characteristics are presented in Table 1. An experienced pathologist confirmed all histological diagnoses.
Table 1.
Description of the patients.
| Age, mean ± standard deviation (years) | 58.6 ± 10 |
|---|---|
| Gender, no. (%) | |
| Female | 31 (36%) |
| Male | 55 (64%) |
| TNM classification, no. (%) | |
| Tumour status | |
| T1 | |
| T2 | 1 (1.2%) |
| T3 | 6 (7.0%) |
| T4 | 57 (66.3%) |
| Unknown | 15 (17.4%) |
| 7 (8.1%) | |
| Nodal status | |
| N0 | |
| N1 | 23 (26.7%) |
| Unknown | 38 (44.2%) |
| 25 (29.1%) | |
| Histological grade | |
| Grade I | 11 (12.8%) |
| Grade II | 57 (66.3%) |
| Grade III | 15 (17.4%) |
| Unknown | 3 (3.5) |
| Dukes’ stage at the time of diagnosis | |
| B | 15 (17.4%) |
| C | 16 (18.6%) |
| D | 55 (64.0%) |
| Location of the primary tumour | |
| Ascending colon | 23 (26.7%) |
| Transverse colon | 7 (8.1%) |
| Descending colon | 35 (40.7%) |
| Rectum | 21 (24.4%) |
| Location of metastases upon starting chemotherapy | |
| Local | 4 (4.7%) |
| Liver | 36 (41.9%) |
| Lung | 5 (5.8%) |
| Multiple sites | 41 (47.7%) |
| Disease-specific survival, mean ± standard deviation (months) | 33.0 ± 24.3 |
| Disease-specific outcome | |
| Alive | 12 (14.0%) |
| Died of disease | 73 (84.8%) |
| Unknown | 1 (1.2%) |
The patients received a combination of irinotecan (180–210 mg m– 2, administered as a 60–90 min intravenous infusion) and 5-fluorouracil (5-FU) (500 mg m– 2, iv bolus), modulated by folinic acid (FA) (60 mg m– 2, iv bolus). The 5-FU/FA administrations were repeated again on the following day. This treatment combination was repeated every two weeks until disease progression or the occurrence of unacceptable toxicity (Group 2).
Tumour response was assessed every eight weeks according to WHO criteria, assigned as complete response, partial response, stable disease and progressive disease. The study was approved by the National Authority for Medico-Legal Affairs Committee and was conducted in accordance with the declaration of Helsinki.
2.2. Immunohistochemical detection of VEGF-1 expression
Formalin-fixed, paraffin-embedded primary tumours were obtained from 86 patients. The sections were cut serially at 5 µm for routine haematoxylin and eosin staining and for immunohistochemical (IHC) analysis. IHC staining was completed using automated staining system (DAKO Autostainer, Dako, Copenhagen, Denmark). Purified anti-human VEGF (121, 165, and 189 isoforms), clone VG-1 (Biosite company, Biosite, San Diego, CA, USA) (1:150), was used. After staining, the sections were dehydrated in ethanol, cleared in xylene and covered with Mountex and coverslips. The expression of VEGF-1 in the tumour tissue was assessed blinded to the clinical data, and weighted according to the expression in the total tumour area. Tumour tissue showed only cytoplasmic staining. The cytoplasmic staining was graded into four categories: (0) negative, no detectable staining; (1) weak but detectable staining; (2) moderate, clearly positive staining; and (3) strong staining, intense throughout the tumour.[14]
2.3. Statistical analysis
Statistical analyses were performed using the SPSS (SPSS, Inc., Chicago, IL, USA) and STATA (Stata Corp., College Station, TX, USA) software packages (SPSS for Windows, version 18.0.1 and STATA/SE 11.1). Differences in the means of continuous variables were analysed using non-parametric tests (Mann–Whitney or Kruskal–Wallis) for two and multiple independent samples, respectively. Univariate survival analysis for the outcome measure (disease-specific survival [DSS] or disease-free survival [DFS]) was based on the Kaplan–Meier method, with log-rank (Mantel–Cox) comparison test. DSS and DFS were calculated, based on the time from diagnosis to death (due to disease), and on the time from diagnosis to the appearance of metastatic disease, respectively. In all tests, the values p < 0.05 were regarded statistically significant.
3. Results
VEGF expressions was assessed in all tumours and the cases were classified as moderate/strong- and negative/weak expression groups (Figure 1).
Figure 1.
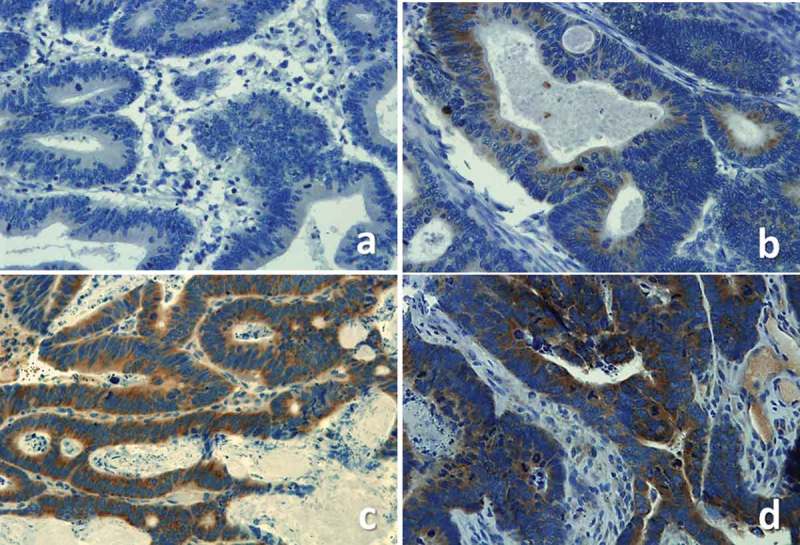
Examples of VEGF negative (a), weak (b), moderate (c) and strong (d) staining.
The cumulative survival was significantly correlated with the VEGF expression. Our results showed that patients with negative to weak VEGF expression had longer survival with the metastasis in comparison to the patients with moderate to strong expression (p-value = 0.04), which could indicate the role of VEGF in chemoresistance (Figure 2). VEGF expression was more frequent (61%) among patients who died of their disease than among the 10-year survivors (49%).
Figure 2.
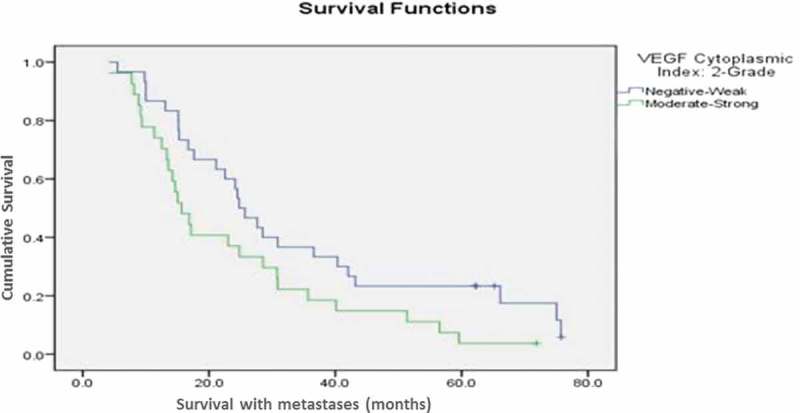
The cumulative survival of patients with combination therapy group compared according to the expression of VEGF. (p-value = 0.04).
VEGF expression was not statistically associated with other clinico-pathological variables such as age, sex, tumor-node-metastasis (TNM) status, grade, Duke’s stage or carcinoembryonic antigen levels (data not shown).
4. Discussion
Angiogenesis is a key player in the development of tumours as well as in metastasis. VEGF enhances the production of new capillaries that supply oxygen and nutrients to the tumour and provide channels for the transfer of cancerous cells to distant locations. Such findings were confirmed in colon cancer. Kondo et al. [15] showed that VEGF-expressing CRC had increased vascularity and metastatic potential in comparison to tumours with baseline VEGF. In addition, accumulating evidence suggests a prognostic role for VEGF in CRC, associated with poor prognosis with overexpression.[13,16–18]
Similarly, a recent meta-analysis by Des Guetz and colleagues [19] confirms that VEGF expression is associated with poor overall survival in CRC. This meta-analysis included 27 studies specifically investigating VEGF in CRC, and VEGF expression was shown to be significantly correlated with poor overall survival and was a stronger predictor of overall survival than microvessel density.[19] In the present study, a series of 360 CRCs comprising all stages of disease (and with prolonged post-treatment follow up) were analysed for VEGF expression using IHC approach. Several intriguing observations were made, as discussed below.
VEGF seems to be an indicator of poor prognosis in breast cancer as well and was shown to be correlated with tumour stage.[20] Similarly, serum VEGF-A levels have been shown to correlate with disease stage in CRC, with increasing levels being associated with more advanced disease.[21] Preoperative serum VEGF levels have also been shown to correlate with advanced tumour stage at the time of surgery.[22] When measured prospectively in a group of patients undergoing curative resection for CRC, serum VEGF levels were significantly higher in patients who subsequently developed metastases than in those who did not.[23,24] Our present observations are fully consistent with these previous reports, confirming that 66.7% of the patients with metastatic disease (stage IV) have VEGF-positive tumours. Furthermore, VEGF expression in stage IV tumours was significantly more intense than in stage II and III disease (p = 0.005), implicating a direct relationship between VEGF expression and the stage of the disease; intense VEGF expression is associated with more advanced stage and propensity to develop metastatic disease.
Another important observation in the present study was the close correlation of VEGF expression with the treatment response; a lower proportion of patients who clinically benefited from treatment had VEGF-expressing tumours as compared those who had progressive disease (49% vs. 61%, respectively, p = 0.04). This suggests that VEGF expression in the tumours bears some relationship with the response to treatment in that VEGF-expressing tumours are less likely to respond to therapy. Finally, VEGF expression seems to be of some prognostic value in CRC as suggested by the present results and some previous data.[4,13,18,19,25] Ogata and colleagues [26] studied a series of 342 patients with resected stage II or III CRC, of whom 225 received adjuvant oral fluoropyrimidines and 117 received no further treatment after surgery, reporting that VEGF overexpression had a significantly deleterious effect on DFS.[26] This is similar to the results of another study showing that an increase in blood vessel count and VEGF concentration correlated with progression and metastases of CRC.[19] In yet another study, both overall and DFS were found to be significantly lower in patients with VEGF-positive tumours.[25] In the present study, we are reporting a significant correlation between VEGF expression in colorectal tumour biopsies and patient survival, which can open the door for the use of VEGF in clinical perspective.
5. Conclusion
VEGF expression was clearly accentuated in advanced disease stages, implicating an effect on the propensity to develop a metastatic phenotype. Furthermore, VEGF expression was shown to be rarer among patients who showed clinical benefit of treatment as compared to those who had progressive disease, suggesting that VEGF expression might make the tumour cells more resistant to therapy. Finally, VEGF expression in the primary tumours seems to be associated with less favourable long-term (10-year) survival as compared with VEGF-negative tumours, possibly implicating some differences in the inherent malignancy of CRC that only become manifest after prolonged follow-up.
Numerous retrospective studies showed the prognostic value of VEGF expression in CRC. High VEGF expression in tumour tissue indicated a shorter relapse free survival and overall survival.[19] Further studies are still needed to verify the role of VEGF as a predictive biomarker for the efficacy of antiangiogenic therapy.[4] Jalbă et al. [27] showed that VEGF-A expression was a poor prognostic marker for CRC. In another study; Pohl et al. [28] found no correlation between treatment response and VEGF expression within the tumour tissue. In our study we found longer overall survival rates with negative VEGF expression, which may indicate a role for VEGF in CRC from a clinical perspective. Further studies may lead to consideration of VEGF status for CRC patients as a part of their management plan, aiming at personalized treatment as well as prediction of progress.
RESPONSIBLE EDITOR - Omran Bakoush, University of Lund, Sweden
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jemal A, Siegel R, Xu J. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–5. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- Van CE, Lambrechts D, Prenen H. Lessons from the adjuvant bevacizumab trial on colon cancer: what next? J Clin Oncol. 2011;29:1–4. doi: 10.1200/JCO.2010.32.2701. [DOI] [PubMed] [Google Scholar]
- Wong R, Cunningham D. Using predictive biomarkers to select patients with advanced colorectal cancer for treatment with epidermal growth factor receptor antibodies. J Clin Oncol. 2008;26:5668–5670. doi: 10.1200/JCO.2008.19.5024. [DOI] [PubMed] [Google Scholar]
- Asghar U, Hawkes E, Cunningham D. Predictive and prognostic biomarkers for targeted therapy in metastatic colorectal cancer. Clin Colorectal Cancer. 2010;9:274–281. doi: 10.3816/CCC.2010.n.040. [DOI] [PubMed] [Google Scholar]
- Staib L, Link KH, Blatz A. Surgery of colorectal cancer: surgical morbidity and five- and ten-year results in 2400 patients–monoinstitutional experience. World J Surg. 2002;26:59–66. doi: 10.1007/s00268-001-0182-5. [DOI] [PubMed] [Google Scholar]
- Young A, Rea D. ABC of colorectal cancer: treatment of advanced disease. Bmj. 2000;321:1278–1281. doi: 10.1136/bmj.321.7271.1278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christopoulou A. Chemotherapy in metastatic colorectal cancer. Tech Coloproctol. 2004;8(Suppl S1):s43–s46. doi: 10.1007/s10151-004-0108-7. [DOI] [PubMed] [Google Scholar]
- Folkman J. Endothelial cells and angiogenic growth factors in cancer growth and metastasis. Introduction. Cancer Metastasis Rev. 1990;9:171–174. doi: 10.1007/BF00046358. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Folkman J. Patterns and emerging mechanisms of the angiogenic switch during tumorigenesis. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- Reinmuth N, Parikh AA, Ahmad SA. Biology of angiogenesis in tumors of the gastrointestinal tract. Microsc Res Tech. 2003;60:199–207. doi: 10.1002/jemt.10258. [DOI] [PubMed] [Google Scholar]
- Ellis LM. A targeted approach for antiangiogenic therapy of metastatic human colon cancer. Am Surg. 2003;69:3–10. [PubMed] [Google Scholar]
- Guba M, Seeliger H, Kleespies A. Vascular endothelial growth factor in colorectal cancer. Int J Colorectal Dis. 2004;19:510–517. doi: 10.1007/s00384-003-0576-y. [DOI] [PubMed] [Google Scholar]
- Lee JC, Chow NH, Wang ST. Prognostic value of vascular endothelial growth factor expression in colorectal cancer patients. Eur J Cancer. 2000;36:748–753. doi: 10.1016/s0959-8049(00)00003-4. [DOI] [PubMed] [Google Scholar]
- Bendardaf R, Lamlum H, Ristamäki R. Mismatch repair status is a predictive factor of tumour response to 5-fluorouracil and irinotecan chemotherapy in patients with advanced colorectal cancer. Tumour Biol. 2007;28:212–220. doi: 10.1159/000107417. [DOI] [PubMed] [Google Scholar]
- Kondo Y, Arii S, Furutani M. Implication of vascular endothelial growth factor and p53 status for angiogenesis in noninvasive colorectal carcinoma. Cancer. 2000;88:1820–1827. [PubMed] [Google Scholar]
- Tokunaga T, Oshika Y, Abe Y. Vascular endothelial growth factor (VEGF) mRNA isoform expression pattern is correlated with liver metastasis and poor prognosis in colon cancer. Br J Cancer. 1998;77:998–1002. doi: 10.1038/bjc.1998.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Duan Y, Cheng X. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. Biochem Biophys Res Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- Martins SF, Garcia EA, Luz MA. Clinicopathological correlation and prognostic significance of VEGF-A, VEGF-C, VEGFR-2 and VEGFR-3 expression in colorectal cancer. Cancer Genomics Proteomics. 2013;10:55–67. [PubMed] [Google Scholar]
- Des GG, Uzzan B, Nicolas P. Microvessel density and VEGF expression are prognostic factors in colorectal cancer. Meta-analysis of the literature. Br J Cancer. 2006;94:1823–1832. doi: 10.1038/sj.bjc.6603176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparini G. Prognostic value of vascular endothelial growth factor in breast cancer. Oncologist. 2000;5(Suppl 1):37–44. doi: 10.1634/theoncologist.5-suppl_1-37. [DOI] [PubMed] [Google Scholar]
- Bayhan Z, Simşek T, Ergül E. Serum cytokine levels in patients with colorectal cancers according to tumor stages and VEGF gene polymorphism. Hepatogastroenterology. 2014;61:1889–1894. [PubMed] [Google Scholar]
- Kwon KA, Kim SH, Oh SY. Clinical significance of preoperative serum vascular endothelial growth factor, interleukin-6, and C-reactive protein level in colorectal cancer. BMC Cancer. 2010;10:203. doi: 10.1186/1471-2407-10-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishigami SI, Arii S, Furutani M. Predictive value of vascular endothelial growth factor (VEGF) in metastasis and prognosis of human colorectal cancer. Br J Cancer. 1998;78:1379–1384. doi: 10.1038/bjc.1998.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akagi K, Ikeda Y, Miyazaki M. Vascular endothelial growth factor-C (VEGF-C) expression in human colorectal cancer tissues. Br J Cancer. 2000;83:887–891. doi: 10.1054/bjoc.2000.1396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White JD, Hewett PW, Kosuge D. Vascular endothelial growth factor-D expression is an independent prognostic marker for survival in colorectal carcinoma. Cancer Res. 2002;62:1669–1675. [PubMed] [Google Scholar]
- Ogata Y, Matono K, Mizobe T. The expression of vascular endothelial growth factor determines the efficacy of post-operative adjuvant chemotherapy using oral fluoropyrimidines in stage II or III colorectal cancer. Oncol Rep. 2006;15:1111–1116. [PubMed] [Google Scholar]
- Jalbă CS, Jalbă BA, Nicula C. Clinical relevance of vascular endothelial growth factor-A in colorectal cancer. Rom J Morphol Embryol. 2011;52:775–781. [PubMed] [Google Scholar]
- Pohl M, Werner N, Munding J. Biomarkers of anti-angiogenic therapy in metastatic colorectal cancer (mCRC): original data and review of the literature. Z Gastroenterol. 2011;49:1398–1406. doi: 10.1055/s-0031-1281752. [DOI] [PubMed] [Google Scholar]


