ABSTRACT
The aim of the present comparative study was to compare some salivary characteristics between exclusive waterpipe smokers (EWPS) and non-smokers. 72 males (36 EWPS) were recruited. The volume of stimulated saliva was determined and divided by the duration of saliva collection. The pH was measured directly using a pH meter. The buffering capacity was determined using a quantitative method which involved the addition of 10 µl HCl. Up to a total of 160 µL was titrated up to obtain a pH titration curve. At 50 µL of titrated HCl, buffering capacity was ranked into three categories: high, medium and low. EWPS and non-smoker groups had similar flow rates (1.81 ± 0.79 and 1.78 ± 1.14 mL min-1) and similar baseline pH (6.60 ± 0.37 and 6.76 ± 0.39). Statistically significant differences in the two groups’ pH were observed from 30 to 160 µL of titrated up HCl. At 50 µL of titrated up HCl, the EWPS group compared to the non-smoker group had a significantly higher pH (4.79 ± 0.72 vs. 5.32 ± 0.79). To conclude, waterpipe tobacco smoking alters the buffering capacity but does not alter either salivary flow rates or the baseline pH and consistency.
KEYWORDS: Tobacco, narghile, shisha, hookah, oral health, hydrogen-ion concentration
1. Introduction
Saliva, a body fluid secreted by several salivary glands, contains essentially water, proteins, glycoproteins, electrolytes, small organic molecules and compounds transported from the blood.[1] Saliva plays an important role in maintaining oral health due to its biological functions such as food swallowing, protection of mucosa from bacterial attack and fungal growth, lubrication of oral tissues, and washing off of food debris and harmful agents.[2] Furthermore, it can help maintain the integrity of dental tissues and especially to prevent caries.[3] First, it protects the enamel against demineralization caused by the acids produced by microorganisms.[3] Secondly, it facilitates the remineralization of primary lesions of caries because it contains inorganic ions such as calcium and phosphorus.[3] Thirdly, it has an antimicrobial function.[2] In daily practice, saliva can be evaluated by some characteristics, such as flow rate, buffering capacity, hydrogen-ion concentration (pH) and consistency.[2,4–7] Their alteration may increase the risk of caries in individuals.[8]
Tobacco smoking increases the occurrence of dental caries.[9–12] For example, the mean number of carious lesions was significantly higher in 15 cigarette smokers than 15 non-smokers (respectively, 7.5 ± 3.0 vs. 4.9 ± 2.5).[10] However, the effects of tobacco consumption on some salivary characteristics (e.g. flow rate, buffering capacity, pH and consistency) are controversial.[2,13–17] While some studies reported a decrease in flow rate [15,17] and pH [14,17] in exclusive cigarette smokers (ECS) when compared to non-smokers, another study [2] showed similar results between the two groups. However, to the best of the authors’ knowledge, all the published studies concerned cigarette smokers [2,13–17] while no study included exclusive waterpipe smokers (EWPS). This form of tobacco use (better known as ‘narghile’ or ‘sheesha’ in the Arab world) has traditionally been associated with the Eastern Mediterranean region, Southeast Asia and Northern Africa.[18,19] However, according to the World Health Organization study group on tobacco product regulation,[20] waterpipe tobacco smoking (WTS) is increasing globally,[19–21] particularly among university students.[22–24] For example, the prevalence of WTS among students was 20, 8 and 7–11%, respectively in a poor urban community in Johannesburg,[22] in a national study in the USA [23] and in the UK.[24] Among the numerous studies analyzing the effects of WTS on oral-health, which were recently criticized [25,26] and reviewed,[27] no one investigated the saliva. Medline and Scopus searches carried out on 20 December 2016 and using a combination of the following keywords: ‘waterpipe’ or its different synonyms and ‘saliva’, found no manuscripts. Since saliva is the first biological fluid exposed to WTS, and since it has been confirmed that the latter is rich in hundreds of substances [18] potentially unsafe to health,[28] it is probable that WTS may affect some salivary variables. For example, nicotine, a toxic component of WTS (smoke from one gram of waterpipe tobacco includes 2.96 mg of nicotine [18]) may act on certain cholinergic receptors in the brain and on other organs causing neural activation leading to altered salivary secretion.[17] The hypothesis that WTS may alter saliva is therefore made and this study aimed at comparing some salivary characteristics (e.g. flow rate, pH, buffering capacity and consistency) between EWPS and non-smokers.
2. Population and methods
2.1. Study design
The present comparative and cross-sectional study was carried out over the period of January and February 2016 at the Department of Oral Physiology at the Faculty of Dental Medicine, Monastir University, Tunisia. Permission from the ethical committee of Farhat HACHED University Hospital of Sousse (approval number: 17052013), was obtained, prior to the study.
This investigation was part of a project that evaluated the effects of WTS on oral health.[29] Some arguments were previously advanced to support that tobacco use, especially WTS, is a risk factor to periodontal disease.[25–27,29] After explaining the purpose of the study to the participants, an informed written consent was signed by all of them in accordance with the Declaration of Helsinki. Subjects diagnosed with any oral pathology were given treatment or were scheduled for the right specialist. This study is in compliance with the Strobe guidelines for cohort studies (http://www.strobe-statement.org).
2.2. Populations
Subjects were recruited by convenience sampling among the students of the Monastir University (Dentistry, Engineering, and Sciences). Only healthy male exclusive tabamel-smokers or non-smokers aged 20 to 29 years were included. The applied non-inclusion criteria were: tobacco use < five waterpipe-years for EWPS, Jurak and/or Tombac tobacco use, known systemic medical condition, previous head or neck radiation therapy, consumption of any drugs, wearing of intra oral appliances, food intake and smoking during the last three hours before the saliva sampling. The inability of participants to cooperate and/or to follow the instructions given regarding the saliva sampling were applied as exclusion criteria. Students were divided into two groups: EWPS and non-smokers.
2.3. Sample size
The sample size was estimated using the following formula:[30]: N = [(Zα /2)2 × P × (1 – P) × D]/E 2; where P was the proportion of the main event of interest (i.e. high buffering capacity), E was the margin of error, Zα /2 was the normal deviate for two-tailed alternative hypothesis at a level of significance, and D was the design (= 1 for simple random sampling). According to one study,[31] among a group aged 20–29 years (n = 40), 67.5% (p = 0.675) have a high buffering capacity. Assuming a confidence interval of 80% (Zα /2 = 1.28) and an E of 0.07, the total sample size was 73 subjects.
2.4. Collected data
The data included general information (age, smoking habits), clinical data (number of missing/decayed/filled teeth, plaque index) and salivary data (flow rate, pH, buffering capacity, consistency). All data were collected and/or measured by an experienced dentist (MK).
2.5. Medical questionnaire
The subjects were interviewed using a non-standardized questionnaire written in French. The questions were with closed answers and often dichotomous. The level of tobacco exposure was expressed in terms of waterpipe-years (waterpipe session per day x year duration).[18]
2.6. Clinical examination
The clinical examination was carried out to identify oral health indicators. Caries status was scored by using missing/decayed/filled teeth given by Klein et al. [32]. The Silness and Löe plaque index [33] assessed the oral hygiene. A plaque indicator was used to assist PI evaluation. The presence of visible dental plaque was recorded on four sites (vestibular, lingual, mesial and distal) of all existing teeth, except the third molars. As previously carried out by some authors,[29,34] three plaque index classes ([0–1]; [1–2]and [2–3]) were arbitrarily defined.
2.7. Saliva sampling
Stimulated saliva was collected between 10 and 12 am. The participants were allowed to sit on a chair and relax for a few minutes. Chewing-gum-stimulated saliva was collected continuously for 5 min [6] into a calibrated sterile tube. During the first 30 s of chewing, the saliva was swallowed.
2.8. Flow-rate
The flow rate, expressed in ml min–1, represents the amount of saliva produced by salivary glands.[4] The volume of stimulated saliva was determined and divided by saliva collection duration, equal to 5 min.[6] Hypo salivation, defined by a stimulated-salivary flow rate < 7 ml min– 1,[35] is an objective and measurable datum.[36]
2.9. Salivary pH and buffering capacity
Immediately after the collection, 0.5 ml of each saliva sample was placed in a tube.[6] The pH was measured directly using a pH Meter cyberscan PH510 Eutech Instruments, Singapore).[6] The accuracy of the pH meter was checked daily using pH 4, pH 7 and pH 10 standard buffers to ensure that the interpretations were correct. Salivary buffering capacity is defined as the ability of saliva to buffer acids produced by bacteria.[2,5] The latter was determined by using a quantitative method which involved the addition of 10 µl of 0.1 N hydrochloric-acid (HCl) using a micropipette.[6] The mixture was rigorously shaken, then stabilized for a few seconds and finally the pH reading was taken. HCl (160 µl) was titrated up to obtain a pH titration curve for each subject in order to determine the salivary buffering capacity. At 50 µl of titrated HCl, the salivary buffering capacity was ranked into one of the following categories: high (above pH 5.5), medium (from pH 5.5 to pH 4.4) and low (below pH 4.5).[6] A salivary pH above 5.5 after the addition of 50 µl of HCl was used as a cutoff signifying a good buffering capacity.[7] This value represented the critical pH above which the tooth is protected against demineralization.[7]
2.10. Salivary consistency
Saliva can be fluid or viscous.[2] The consistency was determined visually by the investigator.
2.11. Statistical analysis
Variable distributions were normal and results were expressed as mean ± standard deviation or ± standard error (95% confidence interval). The Student’s t and the chi-squared tests were used to compare, respectively, the two groups’ quantitative and qualitative data. All mathematical computations and statistical procedures were performed using Statistica software (Statistica Kernel version 6; Stat Software, Maisons-Alfort, France). Significance was set at the 0.05 level.
3. Results
Seventy-two subjects were included. They were divided into two groups [EWPS (n = 36) and non-smokers (n = 36)].
Table 1 shows their main characteristics. The two groups were matched to age, remaining teeth, plaque index and missing/decayed/filled teeth.
Table 1.
Characteristics of exclusive waterpipe smokers (EWPS, n = 36) and non-smokers (n = 36).
| EWPS | Non-smokers | Probability | ||
|---|---|---|---|---|
| Age (years)a | 23 ± 4 | 22 ± 3 | 0.68 | |
| [21 to 24] | [21 to 24] | |||
| Quantity of used tobacco (waterpipe-years)a | 7.92 ± 3.72 | 0 | - | |
| [6.66 to 9.18] | ||||
| Remaining teetha | 27.92 ± 0.28 | 27.83 ± 0.38 | 0.29 | |
| [27.82 to 28.01] | [27.71 to 27.96] | |||
| Plaque indexa | 1.41 ± 0.59 | 1.29 ± 0.69 | 0.42 | |
| [1.21 to 1.61] | [1.06 to 1.52] | |||
| Decayed/missing/filled teethb | 1.97 ± 0.36 | 1.81 ± 0.40 | 0.76 | |
| [1.24 to 2.70] | [0.99 to 2.63] | |||
| Classes of plaque indexc | [0–1] | 5 (13.9) | 7 (19.4) | 0.82 |
| [1–2] | 19 (52.8) | 18 (50.00) | ||
| [2–3] | 12 (33.3) | 11 (30.6) | ||
Data are: amean ± standard deviation [95% confidence-interval]; bmean ± standard error [95% confidence-interval]; cnumber (percentage).
* p < 0.05 (t-test): EWPS vs. non-smokers
† p < 0.05 (chi-squared): EWPS vs. non-smokers
Table 2 and Figure 1 display, respectively, the stimulated-salivary data (flow rate, baseline pH, buffering capacity and consistency) and the pH titration curve of the two groups. Their main results were:
The two groups have similar flow rates, baseline pH and consistency (Table 2).
The frequencies of individuals in the three buffering capacity categories were statistically different in the two groups. This indicated that WTS alters buffering capacity (Table 2).
Statistical significant differences in the two groups’ pH were observed between 30 and 160 µl of titrated HCl (Figure 1). At 50 µl of titrated HCl, the EWPS group compared to the non-smoker group had a significantly higher pH (respectively, 4.79 ± 0.72 vs. 5.32 ± 0.79; p = 0.004) (Figure 1).
Table 2.
Flow-rate, buffering capacity and consistency of exclusive waterpipe smokers (EWPS, n = 36) and non-smokers (n = 36).
| EWPS | Non-smokers | Probability | ||
|---|---|---|---|---|
| Baseline pHa | 6.60 ± 0.37 | 6.76 ± 0.39 | 0.08 | |
| [6.48 to 6.73] | [6.63 to 6.89] | |||
| Flow rate (ml min–1)a | 1.81 ± 0.79 | 1.78 ± 1.14 | 0.91 | |
| [1.54 to 2.08] | [1.40 to 2.17] | |||
| Categories of flow rateb | ≤0.7 ml min–1 | 4 (11.1) | 4 (11.1) | 0.7 |
| >0.7 ml min–1 | 32 (88.9) | 32 (88.9) | ||
| Categories of buffering capacityb | Low | 14 (38.9) | 3 (8.3) | 0.0003† |
| Medium | 18 (50.0) | 15 (41.7) | ||
| High | 4 (11.1) | 18 (50.0) | ||
| Consistencyb | Low | 28 (77.8) | 31 (86.1) | 0. 35 |
| High | 8 (22.2) | 5 (13.9) | ||
Data are: amean ± standard deviation [95% confidence-interval]; bnumber (percentage)
* p < 0.05 (t-test): EWPS vs. non-smokers
† p < 0.05 (chi-squared): EWPS vs. non-smokers
Figure 1.
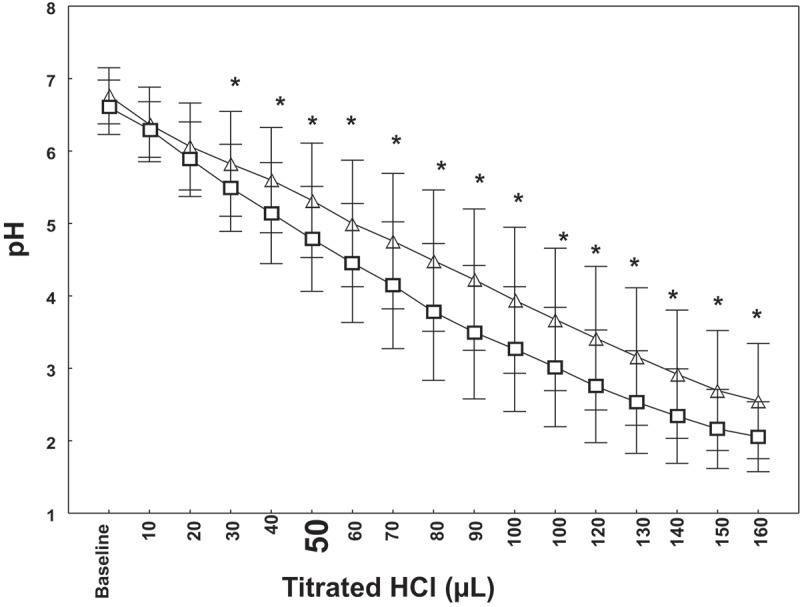
pH titration curve of exclusive waterpipe smokers (EWPS, n = 36) and non-smokers (n = 36). Data are mean (□ for non-smokers and ∆ for EWPS) and standard deviation ( ). *Student’s t-test p < 0.05: non-smokers vs. EWPS for the same volume of HCl.
). *Student’s t-test p < 0.05: non-smokers vs. EWPS for the same volume of HCl.
4. Discussion
Four salivary characteristics of two groups were compared, matched to age, remaining teeth, plaque index and missing/decayed/filled teeth [36 EWPS and 36 non-smokers]. The main result was that the WTS alters the buffering capacity as shown by a higher pH at 50 µl of titrated HCl. However, WTS does not alter either salivary flow rates or the baseline pH and consistency.
Studies analyzing the effects of WTS on oral health are scarce and their methodologies and results have recently been reported and criticized.[25–27] However, to the best of the authors’ knowledge, this is the first study that evaluates the effects of WTS on some salivary characteristics. Moreover, the effects of cigarette smoking on the above data have also been rarely evaluated in the literature.[2,13–17]
Since old age and female sex have been reported to correspond to lower the flow rates,[37,38] only local university male students under 30 years were included. Moreover, the age of the 72 recruited students [mean ± SD (minimum–maximum) = 23 ± 4 (20–29) years] was similar to the previous reported of a local study analyzing smoking behavior among Tunisian students [n = 378, age mean ± SD (minimum–maximum) = 22 ± 2 (19–29) years].[39] Almost all students have similar socioeconomic status. This reduced the variability between groups since the salivary flow rate, the pH and the buffering capacity are influenced by the socioeconomic status.[2] Moreover, some special precautions were considered, such as the inclusion of only healthy participants without any medication-use and that didn’t eat/drink anything three hours prior to saliva collection. It is known that flow rate and pH are affected by systemic diseases, oral cancer, radiation treatment, drugs and nutrition.[40,41]
The calculated sample size seems to be satisfactory. In some related studies aiming at analyzing the effects of WTS on oral health,[25,27] the sample sizes of waterpipe smokers varied from 11 to 228.[25,27]
This study has opted for mechanically stimulated saliva.[42] The latter does not vary greatly daily.[43] However, the stimulation of saliva may affect the salivary pH and the concentrations of some constituents.[44] In some studies with different aims, unstimulated saliva method was used [1,7,14,15,37,45,46] in spite of the fact that it is difficult to standardize them.[7] In order to reduce diurnal variations,[47] saliva sampling was collected in the morning.
Salivary pH was determined by an electrode pH meter, which has the advantage of measuring the pH value of a single drop of saliva (0.5 ml). The salivary buffering capacity was identified using a quantitative method.[6] First, the latter can be used as a chair-side test.[1] Second, it was preferred to the colorimetric methods known to be inconclusive and subjective.[48]
Since the accuracy of pH measurements depends on the time interval between the collection and the analysis (attributed to the continuous loss of carbon dioxide from saliva being exposed to air),[45] the saliva was tested immediately after collection.
This study has four limitations to the study design, the convenience sampling, the buffering capacity and the consistency determination. The present cross-sectional comparative study measures only associations and reflects the reality of the study population.[49] Therefore, a causal relationship could not be established.[49] In the future, a longitudinal investigation would be welcome. The disadvantages of the convenience sampling consist in the risk that the sample might not represent the population as a whole, and that it might be biased by volunteers.[25] In this study, the buffering capacity and consistency data were classified into categories using thresholds.[6] It is better to opt for a direct method,[2,45,47] or at least, for example, to add a third control group of ECS, in order to determine which form of tobacco, among cigarette and waterpipe, more affects the saliva. However, taking three groups in a single study seems to have limited precedence in the literature and raises some substantive questions such as whether the prevalence of some risk factors in the three groups is comparable.
According to Ericsson and Harwick,[50] a flow rate of stimulated saliva greater than 1 ml min–1 is considered normal. This is the case for the flow rate of EWPS and non-smoker groups (Table 2). Moreover, the proportions of subjects with hyposaliva were similar among non-smokers and EWPS (Table 2). The chronic effects of cigarette use on flow rate are still unclear. While some studies [15,17] reported a decrease of ECS flow rate when compared to non-smokers, another study [2] showed similar results between the two groups. This study supports the absence of effect of WTS on flow rate.
In the literature, salivary pH has been quoted to range from 5.3 to 7.8 depending on the stimulation state.[40] In this study, the two groups of EWPS and non-smokers have similar mean pH close to 7 (Table 2). On the one hand, this is similar to results observed in elderly ECS and non-smokers.[2] However, this is contrary to other results showing a significant difference in mean pH between ECS and non-smokers.[14,17]
Evaluating buffering capacity is very important because it is one of the key factors that may affect individual caries risk.[8] WTS is responsible for a lower salivary buffering capacity (Table 2 and Figure 1). This complies with results concerning cigarette consumption.[2,13,16] The buffering capacity, which is essential to maintain pH value in oral cavity above the hydroxyapatite critical level,[7] depends on the phosphate and especially on the bicarbonate concentrations.[51] These two ions are found in higher concentrations in parotid saliva [51] and at high salivary flow rate.[46] In fact, chewing paraffin stimulated the parasympathetic response [47] which increases saliva output from the parotid gland.[47] Therefore, stimulated saliva has a high buffering capacity.
Finally, similar higher frequencies of low consistency were observed in the EWPS and the non-smoker groups (Table 2). The consistency of saliva was due to the concentrations of mucins.[52] Since a high flow rate leads to an increased aqueous content, a lower concentration of mucins and therefore a lower consistency was observed in stimulated saliva.[52]
Why does WTS influence only buffering capacity? The decrease of buffering capacity among EWPS could be explained by the fact that WTS may influence the composition of oral microflora and favor the colonization of cariogenic bacteria. The last are known to produce acid especially when pH tends toward acidity.[1,53] However, since contradictory results were observed for buffering capacity and pH, further investigations exploring the role of oral cariogenic microflora would be interesting.
To conclude, the main result of this study was that the buffering capacity varied according to the WTS status. As saliva plays an important role in protecting teeth against caries, dentists are urged to inform their patients about the health risks of WTS on oral health.
Acknowledgments
The authors wish to thank Professor Béchir Saadaoui for his invaluable contribution in the improvement of the quality of the writing in the present paper.
Responsible Editor Dr Omran Bakoush, University of Lund, Sweden
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Pandey P, Reddy NV, Rao VA. Estimation of salivary flow rate, pH, buffer capacity, calcium, total protein content and total antioxidant capacity in relation to dental caries severity, age and gender. Contemp Clin Dent. 2015;6(Suppl:1):S65–7. doi: 10.4103/0976-237X.152943. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islas-Granillo H, Borges-Yanez SA, Medina-Solis CE. Salivary parameters (salivary flow, pH and buffering capacity) in stimulated saliva of Mexican elders 60 years old and older. West Indian Med J. 2014;63(7):758–765. doi: 10.7727/wimj.2014.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh S, Sharma A, Sood PB. Saliva as a prediction tool for dental caries: an in vivo study. J Oral Biol Craniofac Res. 2015;5(2):59–64. doi: 10.1016/j.jobcr.2015.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar WM. Saliva: its secretion, composition and functions. Br Dent J. 1992;172(8):305–312. doi: 10.1038/sj.bdj.4807861. [DOI] [PubMed] [Google Scholar]
- Kitasako Y, Burrow MF, Stacey M. Comparative analysis of three commercial saliva testing kits with a standard saliva buffering test. Aust Dent J. 2008;53(2):140–144. doi: 10.1111/j.1834-7819.2008.00023.x. [DOI] [PubMed] [Google Scholar]
- Moritsuka M, Kitasako Y, Burrow MF. The pH change after HCl titration into resting and stimulated saliva for a buffering capacity test. Aust Dent J. 2006;51(2):170–174. doi: 10.1111/j.1834-7819.2006.tb00422.x. [DOI] [PubMed] [Google Scholar]
- Larsen MJ, Jensen AF, Madsen DM. Individual variations of pH, buffer capacity, and concentrations of calcium and phosphate in unstimulated whole saliva. Arch Oral Biol. 1999;44(2):111–117. doi: 10.1016/s0003-9969(98)00108-3. [DOI] [PubMed] [Google Scholar]
- Messer LB. Assessing caries risk in children. Aust Dent J. 2000;45(1):10–16. doi: 10.1111/j.1834-7819.2000.tb00235.x. [DOI] [PubMed] [Google Scholar]
- Seichter U. Root surface caries: a critical literature review. J Am Dent Assoc. 1987;115(2):305–310. doi: 10.14219/jada.archive.1987.0236. [DOI] [PubMed] [Google Scholar]
- Golpasand Hagh L, Zakavi F, Ansarifar S. Association of dental caries and salivary sIgA with tobacco smoking. Aust Dent J. 2013;58(2):219–223. doi: 10.1111/adj.12059. [DOI] [PubMed] [Google Scholar]
- Schenkein HA, Gunsolley JC, Koertge TE. Smoking and its effects on early-onset periodontitis. J Am Dent Assoc. 1995;126(8):1107–1113. doi: 10.14219/jada.archive.1995.0327. [DOI] [PubMed] [Google Scholar]
- Edman K, Öhrn K, Nordström B. Prevalence of dental caries and influencing factors, time trends over a 30-year period in an adult population. Epidemiological studies between 1983 and 2013 in the county of Dalarna, Sweden. Acta Odontol Scand. 2016;74(5):385–392. doi: 10.3109/00016357.2016.1163733. [DOI] [PubMed] [Google Scholar]
- Nakonieczna-Rudnicka M, Bachanek T. Selected risk factors for diseases of hard tooth tissues in tobacco smokers-preliminary study. Przegl Lek. 2012;69(10):756–759. [PubMed] [Google Scholar]
- Grover N, Sharma J, Sengupta S. Long-term effect of tobacco on unstimulated salivary pH. J Oral Maxillofac Pathol. 2016;20(1):16–19. doi: 10.4103/0973-029X.180907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rad M, Kakoie S, Niliye Brojeni F. Effect of long-term smoking on whole-mouth salivary flow rate and oral health. J Dent Res Dent Clin Dent Prospects. 2010;4(4):110–114. doi: 10.5681/joddd.2010.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wikner S, Soder PO. Factors associated with salivary buffering capacity in young adults in Stockholm, Sweden. Scand J Dent Res. 1994;102(1):50–53. doi: 10.1111/j.1600-0722.1994.tb01152.x. [DOI] [PubMed] [Google Scholar]
- Sah K, Grover N, Chandra S. Long-term effect of tobacco on resting whole mouth salivary flow rate and pH: an institutional based comparative study. European J Gen Dent. 2013;2(3):296. [Google Scholar]
- Ben Saad H. The narghile and its effects on health. Part I: the narghile, general description and properties. Rev Pneumol Clin. 2009;65(6):369–375. doi: 10.1016/j.pneumo.2009.08.010. [DOI] [PubMed] [Google Scholar]
- Akl EA, Gunukula SK, Aleem S. The prevalence of waterpipe tobacco smoking among the general and specific populations: a systematic review. BMC Public Health. 2011;11:244. doi: 10.1186/1471-2458-11-244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Advisory note: waterpipe tobacco smoking: health effects, research needs and recommended actions for regulators - 2nd edition 2016 Dec 20; doi: 10.1186/1477-5751-5-17. http://apps.who.int/iris/bitstream/10665/161991/1/9789241508469_eng.pdf?ua=1&ua=1 cited. Available from. [DOI] [PMC free article] [PubMed]
- Morton J, Song Y, Fouad H. Cross country comparison of waterpipe use: nationally representative data from 13 low and middle-income countries from the Global Adult Tobacco Survey (GATS) Tob Control. 2014;23:419–427. doi: 10.1136/tobaccocontrol-2012-050841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkubuge F, Ayo-Yusuf OA, Louwagie GM. Water pipe and smokeless tobacco use among medical students in South Africa. Nicotine Tob Res. 2012;14:755–760. doi: 10.1093/ntr/ntr211. [DOI] [PubMed] [Google Scholar]
- Amrock SM, Gordon T, Zelikoff JT. Hookah use among adolescents in the United States: results of a national survey. Nicotine Tob Res. 2014;16:231–237. doi: 10.1093/ntr/ntt160. [DOI] [PubMed] [Google Scholar]
- Jawad M, Abass J, Hariri A. Waterpipe smoking: prevalence and attitudes among medical students in London. Int J Tuberc Lung Dis. 2013;17:137–140. doi: 10.5588/ijtld.12.0175. [DOI] [PubMed] [Google Scholar]
- Khemiss M, Rouatbi S, Berrezouga L. Critical analysis of the published literature about the effects of narghile use on oral health. Libyan J Med. 2015;10:30001. doi: 10.3402/ljm.v10.30001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khemiss M, Ben Saad H. Clinical and radiographic periodontal status of exclusive narghile smokers: some sources of confusion. J Periodontol. 2016;87:11. doi: 10.1902/jop.2016.160209. [DOI] [PubMed] [Google Scholar]
- Khemiss M, Rouatbi S, Berrezouga L. Oral health effects associated with narghile use. Tunis Med. 2016;94(7):401–411. [PubMed] [Google Scholar]
- Ben Saad H. The narghile and its effects on health. Part II: the effects of the narghile on health. Rev Pneumol Clin. 2010;66(2):132–144. doi: 10.1016/j.pneumo.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Khemiss M, Ben Khelifa M, Ben Rejeb M. Periodontal bone height of exclusive narghile smokers compared with exclusive cigarette smokers. Libyan J Med. 2016;11:31689. doi: 10.3402/ljm.v11.31689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang M, Ragan BG, Park JH. Issues in outcomes research: an overview of randomization techniques for clinical trials. J Athl Train. 2008;43(2):215–221. doi: 10.4085/1062-6050-43.2.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moritsuka M, Kitasako Y, Burrow MF. Quantitative assessment for stimulated saliva flow rate and buffering capacity in relation to different ages. J Dent. 2006;34(9):716–720. doi: 10.1016/j.jdent.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Klein H, Palmer CE, Knutson JW. Studies on dental caries: I. Dental status and dental needs of elementary school children. Public Health Reports (1896-1970) 1938;53(19):751. [Google Scholar]
- Silness J, Loe H. Periodontal disease in pregnancy. Ii. Correlation between oral hygiene and periodontal condition. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- Bibars AR, Obeidat SR, Khader Y. The effect of waterpipe smoking on periodontal health. Oral Health Prev Dent. 2015;13(3):253–259. doi: 10.3290/j.ohpd.a32671. [DOI] [PubMed] [Google Scholar]
- Gomez-Moreno G, Guardia J, Aguilar-Salvatierra A. Effectiveness of malic acid 1% in patients with xerostomia induced by antihypertensive drugs. Med Oral Patol Oral Cir Bucal. 2013;18(1):e49–55. doi: 10.4317/medoral.18206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazesh M, Christensen C, Brightman V. Clinical criteria for the diagnosis of salivary gland hypofunction. J Dent Res. 1992;71(7):1363–1369. doi: 10.1177/00220345920710070301. [DOI] [PubMed] [Google Scholar]
- Fenoll-Palomares C, Munoz Montagud JV, Sanchiz V. Unstimulated salivary flow rate, pH and buffer capacity of saliva in healthy volunteers. Rev Esp Enferm Dig. 2004;96(11):773–783. doi: 10.4321/s1130-01082004001100005. [DOI] [PubMed] [Google Scholar]
- Narhi TO, Kurki N, Saliva AA. salivary micro-organisms, and oral health in the home-dwelling old elderly-a five-year longitudinal study. J Dent Res. 1999;78(10):1640–1646. doi: 10.1177/00220345990780100901. [DOI] [PubMed] [Google Scholar]
- Ben Rejeb M, Abroug H, Khefacha-Aissa S. Smoking behavior, knowledge, and attitudes towards anti-smoking regulations of nursing students in Sousse, Tunisia. Rev Epidemiol Sante Publique. 2016;64(2):121–127. doi: 10.1016/j.respe.2015.12.014. [DOI] [PubMed] [Google Scholar]
- Kazakov VN, Udod AA, Zinkovych II. Dynamic surface tension of saliva: general relationships and application in medical diagnostics. Colloids Surf B Biointerfaces. 2009;74(2):457–461. doi: 10.1016/j.colsurfb.2009.06.010. [DOI] [PubMed] [Google Scholar]
- Chitra S, Shyamala Devi CS. Effects of radiation and alpha-tocopherol on saliva flow rate, amylase activity, total protein and electrolyte levels in oral cavity cancer. Indian J Dent Res. 2008;19(3):213–218. doi: 10.4103/0970-9290.42953. [DOI] [PubMed] [Google Scholar]
- Humphrey SP, Williamson RT. A review of saliva: normal composition, flow, and function. J Prosthet Dent. 2001;85(2):162–169. doi: 10.1067/mpr.2001.113778. [DOI] [PubMed] [Google Scholar]
- Ben-Aryeh H, Fisher M, Szargel R. Composition of whole unstimulated saliva of healthy children: changes with age. Arch Oral Biol. 1990;35(11):929–931. doi: 10.1016/0003-9969(90)90075-l. [DOI] [PubMed] [Google Scholar]
- Liu J, Saliva DY. a potential media for disease diagnostics and monitoring. Oral Oncol. 2012;48(7):569–577. doi: 10.1016/j.oraloncology.2012.01.021. [DOI] [PubMed] [Google Scholar]
- Foglio-Bonda A, Pattarino F, Foglio-Bonda PL. Kinematic viscosity of unstimulated whole saliva in healthy young adults. Eur Rev Med Pharmacol Sci. 2014;18(20):2988–2994. [PubMed] [Google Scholar]
- Wang P, Zhou Y, Zhu YH. Unstimulated and stimulated salivary characteristics of 12-13-year-old schoolchildren with and without dental erosion. Arch Oral Biol. 2011;56(11):1328–1332. doi: 10.1016/j.archoralbio.2011.04.013. [DOI] [PubMed] [Google Scholar]
- Gittings S, Turnbull N, Henry B. Characterisation of human saliva as a platform for oral dissolution medium development. Eur J Pharm Biopharm. 2015;91:16–24. doi: 10.1016/j.ejpb.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Ericson D, Bratthall D. Simplified method to estimate salivary buffer capacity. Scand J Dent Res. 1989;97(5):405–407. doi: 10.1111/j.1600-0722.1989.tb01453.x. [DOI] [PubMed] [Google Scholar]
- Ferreira-Nobilo Nde P, Tabchoury CP, Sousa Mda L. Knowledge of dental caries and salivary factors related to the disease: influence of the teaching-learning process. Braz Oral Res. 2015:7. doi: 10.1590/1807-3107BOR-2015.vol29.0061. [DOI] [PubMed] [Google Scholar]
- Ericsson Y, Hardwick L. Individual diagnosis, prognosis and counselling for caries prevention. Caries Res. 1978;12(Suppl:1):94–102. doi: 10.1159/000260369. [DOI] [PubMed] [Google Scholar]
- Mandel ID, Wotman S. The salivary secretions in health and disease. Oral Sci Rev. 1976;8:25–47. [PubMed] [Google Scholar]
- Haward SJ, Odell JA, Berry M. Extensional rheology of human saliva. Rheologica Acta. 2011;50(11–12):869–879. [Google Scholar]
- Frenkel ES, Ribbeck K. Salivary mucins protect surfaces from colonization by cariogenic bacteria. Appl Environ Microbiol. 2015;81(1):332–338. doi: 10.1128/AEM.02573-14. [DOI] [PMC free article] [PubMed] [Google Scholar]


