Abstract
In utero, baleen whales initiate the development of several dozens of teeth in upper and lower jaws. These tooth germs reach the bell stage and are sometimes mineralized, but toward the end of prenatal life they are resorbed and no trace remains after birth. Around the time that the germs disappear, the keratinous baleen plates start to form in the upper jaw, and these form the food‐collecting mechanism. Baleen whale ancestors had two generations of teeth and never developed baleen, and the prenatal teeth of modern fetuses are usually interpreted as an evolutionary leftover. We investigated the development of teeth and baleen in bowhead whale fetuses using histological and immunohistochemical evidence. We found that upper and lower dentition initially follow similar developmental pathways. As development proceeds, upper and lower tooth germs diverge developmentally. Lower tooth germs differ along the length of the jaw, reminiscent of a heterodont dentition of cetacean ancestors, and lingual processes of the dental lamina represent initiation of tooth bud formation of replacement teeth. Upper tooth germs remain homodont and there is no evidence of a secondary dentition. After these germs disappear, the oral epithelium thickens to form the baleen plates, and the protein FGF‐4 displays a signaling pattern reminiscent of baleen plates. In laboratory mammals, FGF‐4 is not involved in the formation of hair or palatal rugae, but it is involved in tooth development. This leads us to propose that the signaling cascade that forms teeth in most mammals has been exapted to be involved in baleen plate ontogeny in mysticetes.
Keywords: baleen, baleen whales, bowhead whale, Cetacea, embryology, FGF, keratin, mysticetes, ontogeny, tooth development
Introduction
About 34 million years ago, a macroevolutionary event took place in which the ancestors of baleen whales (Mysticeti, Cetacea, Mammalia) lost their teeth and replaced them with baleen, allowing them to feed efficiently on plankton (Deméré et al. 2008; Marx, 2011). In all modern mysticetes, the baleen rack is implanted on the left and right side of the upper jaw (Fig. 1), leaving a gap rostrally in the median plane in some (balaenids, eschrichtiids, neobalaenids), but not all species (balaenopterids). Each rack is composed of a row of several hundred baleen plates, and each of those plates consists of a flat sheet of baleen, which is mostly composed of the protein keratin (Werth, 2000; Lambertsen et al. 2005). The inside (lingual) of the plates frays into a matted mass of hair‐like filaments that are used to filter food from water taken into the mouth. Individual plates grow throughout life, and wear along their lingual border and their (ventral) tip. The rack is implanted more or less where the tooth rows would be, but there is no trace of teeth (Deméré et al. 2008).
Figure 1.
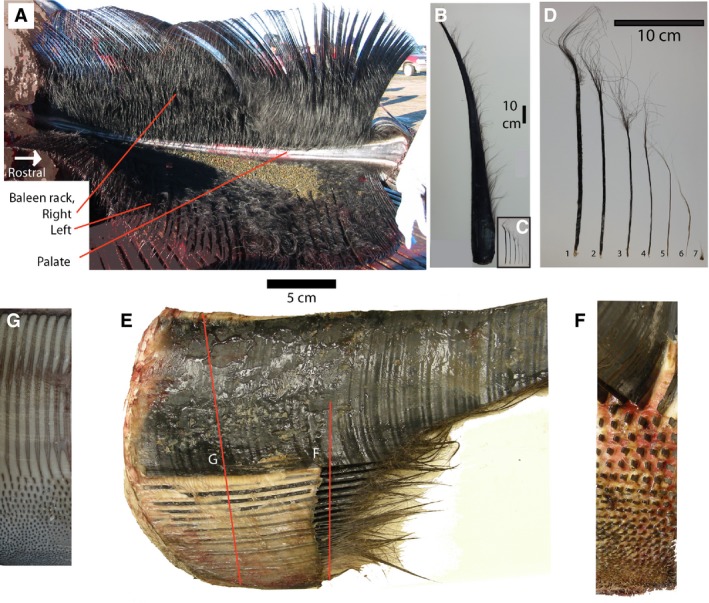
(A) Palate of bowhead whale showing left and right sides of baleen rack, each consisting of approximately 320 plates. Note the narrow palate and baleen plates flaring laterally from it. The lingual side of the plates is frayed and makes a matted surface. (B) Single baleen plate, medial to right. (C) Minor plates from the posterior side of the palate, arranged from lateral to medial on the palate, to the same scale as (B). (D) Enlarged view of (C), showing flat, bilateral symmetrical plates (1, 2, 3); radially symmetrical plates (4, 5, 6) and baleen hair (7). (E) Cranial view of baleen plate (cut off) and the minor plates medial to it (bottom of image). The oral cavity is to the right in this image, and the full depth of gingiva (gums) is seen (white material). Red lines indicate sectional planes of (F) and (G). (F) Cut section through (E), occlusal view, with minor plates cut off to show pattern of implantation, medial to bottom. (G) Section through gingiva, showing cross‐sections of plate and minor plates as embedded in palate, medial to bottom.
Interestingly, tooth development is initiated in baleen whale embryos, and proceeds through several developmental stages (Karlsen, 1962). At least in some species, some mineralization of teeth occurs (Dissel‐scherft & Vervoort, 1954), but enamel never forms (Deméré et al. 2008). Later in fetal life, all traces of teeth and tooth development disappear (Ishikawa & Amasaki, 1995; Deméré et al. 2008). Saint Hilaire (1807) was the first to describe tooth germs in a mysticete studying the bowhead whale (Balaena mysticetus L., 1758). Baleen development in this species commences late in fetal life, they are born with baleen plates 10 cm long and, after approximately 10 years, the longest plates are more than 2 m in length (Lubetkin et al. 2008). After that, as the whale keeps growing, growth of the plates exceeds their wear, and in an old, large whale, plates may be 4 m in length. Bowhead whales appear to keep growing throughout life, and may reach 200 years (George, 2009).
Baleen takes on different forms in different species of cetaceans, as well as in different parts of the mouth. Lambertsen et al. (1989, 2005) provided a full description of the baleen rack in the bowhead whale, and the histology of baleen was described in great detail for balaenopterid whales (Fudge et al. 2009). In bowhead whales, about 320 baleen plates are implanted along the length of the upper jaw (Lambertsen et al. 2005). For most of the length of the jaw, there is only a single bilateral large plate on each palatal cross‐section, although, as an anomaly, a second row of small plates may occur.
Near the posterior edge of the palate, bowheads have an array of keratinous elements medial to the large, lateral baleen plate. Collectively these smaller structures are referred to as accessory plates and bristles (Fudge et al. 2009). These elements increase in size and complexity from medial to lateral (Fig. 1), and include, respectively: baleen hairs, radially symmetrical columns with terminal hairs, and flattened platelets with terminal hairs. The last element in this series is the asymmetrical baleen plate, much larger than any of the structures medial to it. In addition to size and complexity of individual elements, the implantation of the accessory plates and bristles in the gingiva is complex. Elements are implanted in rostro‐caudal rows, but they also form rows that are oblique at approximately 45 ° and 315 ° with the sagittal plane (Fig. 1F,G). Adding further complexity, larger elements are spaced more widely than smaller elements. The implantation pattern is best seen if the protruding part of the plates and bristles is cut off, leaving only stubs protruding from the gingiva (Fig. 1F) or when the gingiva is cut off the maxilla, and that cut face is studied from dorsal (Fig. 1G).
In contrast to baleen, mammal teeth consist of inorganic hydroxyl‐apatite (mostly calcium phosphate) and do not grow after mineralization, except in special cases such as the hypsodont teeth of elephants and beavers. Mammal teeth are also limited in number (precisely 11 teeth per jaw quadrant in ancestral placentals; isodonty; Bown & Kraus, 1979), and are replaced just once (diphyodonty; Van Nievelt & Smith, 2005). The typical mammalian dentition is differentiated into tooth classes (incisors, canines, premolars and molars), each of which consists of several teeth that are similar to each other but different from teeth in other tooth classes (heterodonty), and in the caudal part of the jaw (sometimes called the distal part), the molars have a complex crown morphology with multiple cusps (elevated areas) and crests. The position of these cusps is highly stable within species. In plesiomorphic placental mammals, there are three incisors, one canine, four premolars, and three molars on each side, both in upper and lower jaw. This is often expressed as a dental formula: 3.1.4.3/3.1.4.3. The dental formula of the deciduous dentition of these placentals lacks molars: 3.1.4/3.1.4 (Bown & Kraus, 1979). Adult archaic placental mammals thus have four times 11 teeth per jaw quadrant, 44 teeth in total. This number is reduced in many placental lineages, but increases of tooth counts, polydonty, are rare among extant mammals and occur only in odontocete cetaceans (Miyazaki, 2002) and the armadillo Priodontes.
Cetacea (whales, dolphins and porpoises) are descended from terrestrial artiodactyls, with Hippopotamidae and Raoellidae, respectively, the modern and fossil family most closely related to cetaceans (Gatesy et al. 2013). Raoellids, as well as basal whales (pakicetids) are diphyodont, heterodont, have complex molar crown morphologies, and their dental formula is 3.1.4.3/3.1.4.3 (Fig. 2A). Hippopotamids and raoellids have upper molars with four elevated areas (cusps) arranged in a square (Fig. 2C), whereas pakicetids have three large cusps on their upper molars (Fig. 2D; Thewissen et al. 2001, 2007; Cooper et al. 2009). Raoellids have lower molars with a high anterior part (trigonid) and a low posterior part (talonid), each with two cusps (Fig. 2E). In pakicetids and some younger whales (protocetids), the lower molars also have a high trigonid and low talonid, but each is dominated by a single large cusp (Fig. 2F; Cooper et al. 2009; Gatesy et al. 2013). Late Eocene whales, basilosaurids, are heterodont with four tooth classes (Fig. 2B). Their lower molars do not show a distinct trigonid and talonid (Fig. 2G; Uhen, 2004), but the molars have several accessory cusps.
Figure 2.
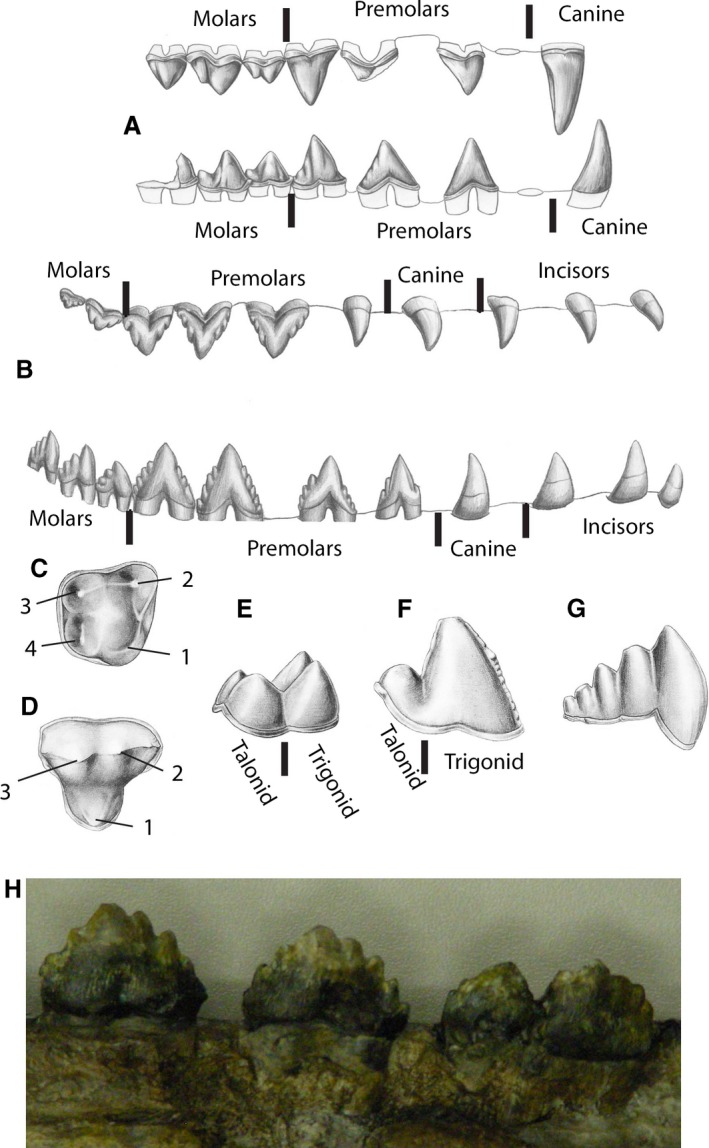
Teeth of fossil cetaceans. (A) Right upper and lower dentition of Pakicetus, the oldest known whale, in labial (lateral) view. Incisors not shown, first premolar indicated as alveolus. (B) Right upper and lower dentition of Dorudon, a late Eocene whale, in labial view. (C, D) Right upper molars of Indohyus and Pakicetus, rostral to right, with homologous cusps identified by numbers. Lower molars of Indohyus (E), a middle Eocene protocetid whale (F), and late Eocene Zygorhiza (G), in lingual view. (H) Labial view of three cheek teeth (rostral to left) of toothed mysticete (aetiocetid), notice that the last tooth appears to consist of two teeth that are fused. Drawings from Thewissen (2014).
Mysticetes are generally thought to be derived from basilosaurids (Boessenecker & Fordyce, 2015; Marx & Fordyce, 2015). The oldest fossil mysticetes have teeth and are heterodont (Clementz et al. 2014). In the family Aetiocetidae, there are three incisors and one canine, and cheek teeth increase in complexity posteriorly (Barnes et al. 1994). Some archaic mysticetes are isodont (Janjucetus; Fitzgerald, 2006), but others have up to 14 teeth per half jaw, three more than the original 11 teeth (e.g. Mammalodon; Fitzgerald, 2009; aetiocetids; Barnes et al. 1994). The fossil mysticete with the most teeth is Aetiocetus polydentatus, in which tooth numbers on the left and right differ (Barnes et al. 1994). In aetiocetids (Barnes et al. 1994), cheek teeth increase in complexity caudally: they are triangular in labial view with one main cusp, but one or more small additional cusps may occur on the slopes of the large cusp (Fig. 2H). This is similar to basilosaurids (Fig. 2G).
The pattern of dental evolution in mysticetes is thus counterintuitive, first the number of teeth increases in evolution but then teeth disappear altogether suddenly. Around the time that the teeth disappeared, mysticetes developed baleen (Deméré et al. 2008). Skull anatomy of the Oligocene mysticete Waharoa indicates that it had baleen, but it also retained alveoli rostrally in the upper and lower jaw (Boessenecker & Fordyce, 2015) and was not polydont.
Because of the vast differences between them, teeth and baleen are usually considered to be independently evolved structures, and baleen is often thought to be derived from the transverse keratinous ridges on the palate, the palatal rugae (Werth, 2000; Fudge et al. 2009, a translated and annotated version of Tullberg, 1883).
Developmentally, teeth and baleen, as well as feathers, hair and palatal rugae, are epithelial organs: they form at the interface of an ectodermal placode that overlies mesenchyme, and much of the signaling involved in forming these is self‐contained. Many proteins are involved in signaling related to formation of these organs, and we will not review them here. Instead, we here discuss only those that we were able to investigate in bowhead whales.
The fibroblast growth factor (FGF) family of signaling proteins plays an important role in the formation of ectodermal organs, and different FGFs are involved in the ontogeny of different organs. While nothing is known about signaling related to the development of baleen, signaling in other keratinized tissues can involve FGF‐7 and FGF‐10 (Mitsui et al. 1997; Nakatake et al. 2001; Porntaveetus et al. 2010a,b), whereas FGF‐4 and FGF‐8 have not been shown to be involved in signaling related to the formation of keratinized structures (Rosenquist & Martin, 1996; Mitsui et al. 1997; Porntaveetus et al. 2010a).
The embryology of teeth and baleen shows limited similarities. Recent reviews of tooth development were provided by Catón & Tucker (2009) and Jernvall & Thesleff (2012). Tooth development begins early in the fetal period when an epithelial placode on the palate invaginates into the underlying mesenchyme (bud stage). Relevant to our study, FGF‐8 is expressed in what eventually will be the molar field of the jaw (Tucker & Sharpe, 2004; Mitsiadis & Smith, 2006). The epithelium then folds into shapes that foreshadow the shape of the occlusal surface of the tooth (the inner enamel epithelium in the cap and bell stages). Tips of cusps on a tooth are initiated partly as a result of sonic hedgehog (SHH) signaling deep to the inner enamel epithelium, and area called the enamel knot (Jernvall & Thesleff, 2000; Thesleff, 2000). The number of enamel knots per tooth cap determines the number of cusps on a tooth. FGF‐4 is also expressed in the enamel knot (Niswander & Martin, 1992; Kettunen & Thesleff, 1998; Tucker et al. 1998). After folding of the inner enamel epithelium, hydroxyl‐apatite is deposited on both sides of this surface in the form of enamel (on the epithelial side) and dentin (on the mesenchymal side).
In this paper, we investigate several aspects of the development and evolution of teeth and baleen in mysticetes. Very little is known about the signaling that forms teeth in cetaceans. Ishikawa et al. (1999) and Armfield et al. (2013) studied the signaling that gives rise to the loss of tooth classes in dolphins. Morphological studies of the embryology of odontocete teeth are also rare. Mišek et al. (1996) studied tooth anlagen, and Štěrba et al. (2000) described tooth formation as part of a monograph on dolphin ontogeny.
Baleen development also begins as an epithelial placode, but that placode does not invaginate into the mesenchyme, and instead evaginates directly into the oral cavity as baleen (Fudge et al. 2009). Baleen development commences long after tooth development in ontogeny, and is morphologically simpler (Ishikawa & Amasaki, 1995), although nothing is known about the genetic pathways involved.
Tooth development and signaling related to it is well studied in laboratory models, especially the mouse, although tooth development in land‐living artiodactyls is probably a better model for whales, given that cetaceans are the sister group of one of the terrestrial artiodactyls (Gatesy et al. 2013) and we use some comparative samples of developing pig teeth, mostly from the work of Armfield (2010).
First, we study the arrangement of tooth buds in bowhead whale fetuses, determining the number of teeth that are formed (isodonty or polydonty), what their shape is along the tooth row (homodonty or heterodonty), whether there is evidence of a secondary dentition (monophyodonty or diphyodonty), and how complex their crowns are. Second, we report on the ontogeny of baleen, with particular interest in signaling related to it.
Materials and methods
The acquisition of mysticete embryos is difficult. Mysticetes are protected under US laws, and no individuals are in captivity in the USA. Stranded specimens are usually in a state of decomposition that does not allow meaningful histological study of their embryos, and there are no complete ontogenetic series of mysticetes in museums. However, a small number of bowhead whales, Balaena mysticetus, are harvested annually by Alaskan natives, Iñupiat Eskimos. This harvest is permitted under the U.S. Marine Mammal Protection Act, as well as approved by the International Whaling Commission. Occasionally and accidentally, a pregnant female is harvested allowing extraction of an embryo or fetus under NOAA‐NMFS permit 17350. This hunt takes place only twice per year and, therefore, no complete ontogenetic series can be collected as females of this species tend to get pregnant in the same season and the gestation time is slightly longer than 1 year. Embryos and fetuses collected this way will be in similar stages: about 8 weeks, 7 months and full‐term (George et al. 2016). Our study is based on six prenatal specimens (Table 1), one embryo and five fetuses, four from the spring hunt and two from the fall hunt, collected between 1999 and 2015. Additional fetuses from this hunt were described by Durham (1980). Armfield et al. (2011) published several of these fetuses, and Drake et al. (2015) published a photo of the hair pattern on the face of one of them. We staged specimens following the criteria of Thewissen & Heyning (2007) for dolphins.
Table 1.
Bowhead whale prenatal specimens available
| ID number | TL (cm) | Stage | Tooth stage | Baleen stage | Characteristic |
|---|---|---|---|---|---|
| 1999B7F | 8.7 | 18 | Dental lamina | None | Tail conical |
| 1999B6F | 16.6 | 20 | Early cap | None | Tail lanceolate |
| 2000B3F | 40.3 | 21 | Bell | None | Fluke fully formed, hair on snout |
| 2007B16F | 159 | 23 | None | Placode | Full hair pattern |
| 2009KK1F | 163 | 23 | None | Placode | Full hair pattern |
| 2015B9F | 422 | 23 | None | Plates present | Full term |
For small specimens, the entire head was processed in a dehydration series embedded in paraffin, and cut on a microtome at 7 μm. For larger specimens, subsampled specimens were excised out of fetal heads. Histological stains used included hematoxylin/eosin and thionin. We also employed immunohistochemical methods with commercial antibodies. Because these antibodies were not designed for bowhead whales, caution is required in interpreting them. For each staining session, we used negative controls to make sure that no staining takes place if the primary antibody is omitted from the protocol. Specimens stained with antibodies were counterstained with thionin.
We used an FGF‐4 antibody (Santa Cruz Biotechnology, sc‐1361) at 1 : 150 dilution (except for Fig. 6J,K, where it was 1 : 100), and an FGF‐7 antibody (Santa Cruz Biotechnology, sc‐1365) at 1 : 100. The concentration for our FGF‐8 antibody (Santa Cruz Biotechnology sc‐6958) was 1 : 250. This antibody has been shown to be competent in cetacean tissues (Armfield et al. 2013). The concentration of the SHH antibody (Santa Cruz Biotechnology, sc‐9024) was 1 : 100 and this antibody was used before in cetaceans by Thewissen et al. (2006). Our BMP‐4 antibody (Santa Cruz Biotechnology, sc‐6896) was used at 1 : 100. The SP‐6 antibody (epiprofin) was used at 1 : 100 (Santa Cruz Biotechnologies, SC‐134025).
Figure 6.
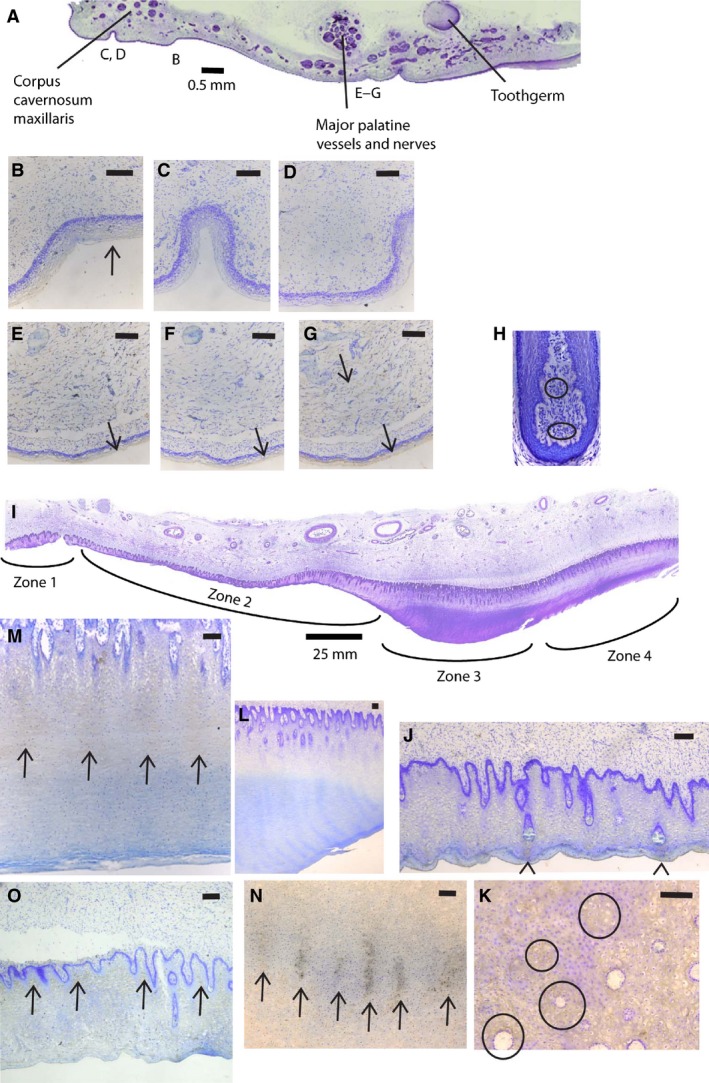
Histological sections showing baleen formation. (A) Cross‐section through half of the palate of fetus NSB‐DWM 2000B3F, showing bell‐shaped tooth germ and epithelium that will form baleen (slides P6:36). Medial to left, letters indicate approximate location of figures (B)–(G) in antibody‐stained sections of this figure. (B–D) Details of medial palatal epithelium of NSB‐DWM 2000B3F (B, slides P6:32, FGF‐4 stain; C, slides P6:33, FGF‐7 stain; D, slides P6:31, FGF‐8 stain). (E–G) Details of palatal epithelium near where baleen will form of NSB‐DWM 2000B3F (E, slides P6:32, FGF‐4 stain; F, slides P6:33, FGF‐7 stain; G, slides P6:31, FGF‐8). (H) Hair follicle of bowhead whale 2011B8 (slide 2, FGF‐7 stain). (I) Cross‐section through half of the palate of fetus NSB‐DWM 2009KK1F, same orientation as (A) (slides P12:26). (J, K) Detail of Zone 2 of the palatal epithelium of NSB‐DWM 2009KK1F (J, slides P12:24, cross‐section; K, slides P13:47, horizontal section; FGF‐4 stain). (L, M) Detail of Zone 3 of palatal epithelium of NSB‐DWM 2009KK1F with FGF‐4 stain (L, slides P12:24, cross‐section; M, slides P6:3, parasagittal section). (N) Detail of Zone 3 of palatal epithelium of NSB‐DWM 2009KK1F (slides P13:78) with FGF‐4 stain, horizontal section, labial to top. (O) Detail of Zone 2 of palatal epithelium of NSB‐DWM 2009KK1F (slides P12:29, cross‐section, FGF‐8 stain). Scale bars: 0.1 mm, unless otherwise indicated.
All specimens are part of the collection of the North‐Slope Borough, Department of Wildlife Management in Barrow, Alaska (NSB‐DWM). We used the computer program AMIRA to make three‐dimensional reconstructions based on photographs of histological sections.
Results
Dental lamina stage
Stratified cuboidal epithelium lines the oral cavity of this embryo (NSB‐DWM 1999B7F). For much of its extent, the oral epithelium is two‐layered, but the dental placode is thicker, consisting of multiple layers. The dental lamina projects into the mesenchyme and its aboral edges (Fig. 3A,B) are thickened, but there is no morphological sign of individual tooth buds (Fig. 4A). In the upper jaw, the dental lamina extends caudally much farther than in the lower jaw (approximately as far as slide 140 in the upper jaw, vs. slide 115 in the lower jaw). The cranioventral extent of the dental lamina is greater in the upper than in the lower jaw (compare Fig. 3A,D).
Figure 3.
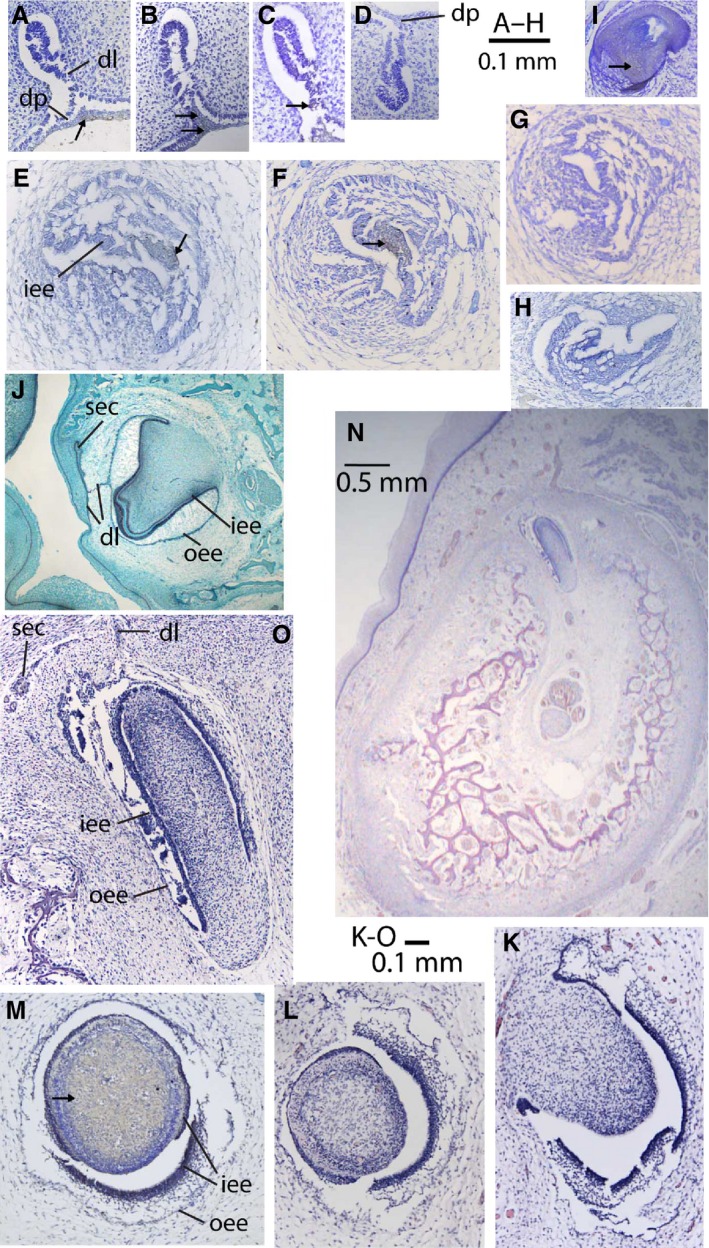
Histological sections through tooth germs and hair follicle of bowhead whale (A–I, K–O) and pig (only J). (A–D) Dental lamina stage (NSB‐DWM 1999B7F); (E–H) cap stage (NSB‐DWM 1999B6F); (K–O) bell stage (NSB‐DWM 2000B3F); staining indicated by arrows, absence of arrows indicates absence of staining. (A) Rostral part of upper dental lamina (slide 83, FGF‐8 stain). (B) Caudal part of upper dental lamina (slide 125, FGF‐8 stain). (C) Intermediate area of upper dental lamina (slide 103, SP‐6 stain for epiprofin). (D) Rostral part of lower dental lamina (slide 81, FGF‐8 stain). (E) Upper tooth cap (slide 111, SHH stain). (F) Upper tooth cap (slide 149, SHH stain). (G) Upper tooth cap (slide 195, SP‐6 stain). (H) Lower tooth cap (slide 111, SHH stain). (I) Hair follicle of NSB‐DWM 2003B3F, showing competency of antibody in bowheads (slide 29, SP‐6 stain). (J) Bell stage upper tooth germ of pig, showing complete dental lamina and germ for permanent tooth. (K, L) Bell stage upper tooth germ 4 (slide 49 and 77, no antibody stain). (M) Bell stage upper tooth germ 5 (slide 22, SHH stain). (N) Mandible with tooth germ of lower tooth 15 (slide c.30, no antibody stain), lingual to left. (O) Bell stage lower tooth germ, enlarged from (N). dl, dental lamina; dp, dental placode; iee, inner enamel epithelium; oee, outer enamel epithelium; sec, secondary tooth germ for permanent tooth.
Figure 4.
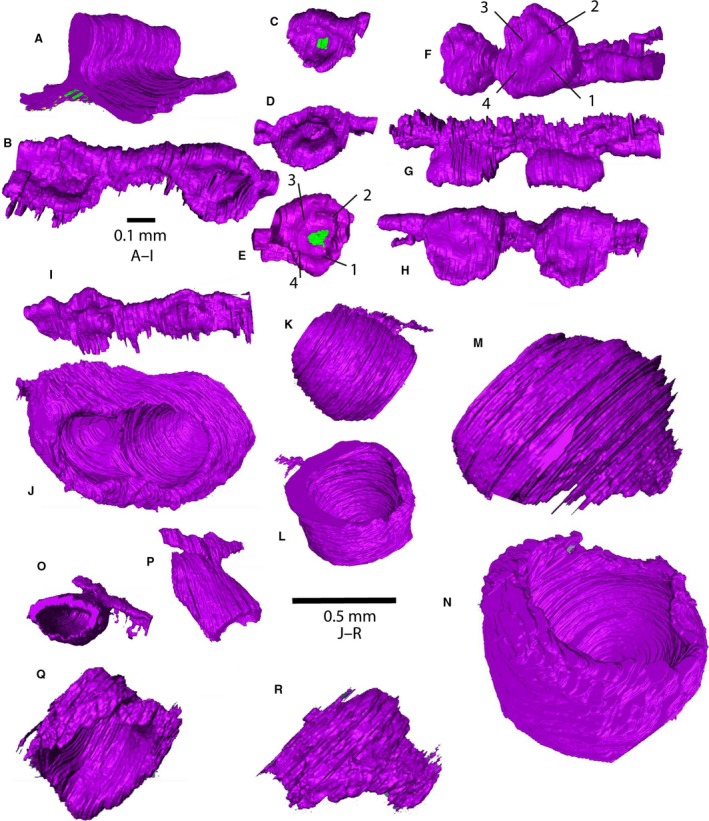
Three‐dimensional reconstructions of tooth germs of bowhead whale, made using AMIRA, based on histological sections. The green areas indicate the presence of antibody stain. (A) Dental lamina stage in bowhead whale (NSB‐DWM 1999B7F); (B–I) cap stage (NSB‐DWM 1999B6F); (J–R) bell stage (NDB‐DWM 2000B3F). Scale bars are approximate because of foreshortening of images, and magnification of images of the same specimen are similar. (A) Rostral part of upper dental lamina, caudolingual view (slides 73–93). (B) Cap stage upper tooth germs 4 and 5, dorsolingual view, rostral to right (slides 64–81). (C–E) Cap stage upper tooth germs 9 (C, dorsolingual view, slides 104–114), 10 (D, dorsolabial view, slides 115–124) and 14 (E, dorsal view, slides 146–153); the green areas in (C) and (E) indicate antibody stain for SHH. (F–H) Cap stage upper tooth germs 23 and 24 in dorsorostral, lateral and dorsal views, respectively, showing topography of inner enamel epithelium; the numbers identify folds in the inner enamel epithelium as discussed in the text (slides 249–274). (I) Cap stage lower tooth germs 19 and 20 in ventrolingual view (slides 241–259). (J) Bell stage upper tooth germ 4 (slides P1:41–83) in dorsocaudal view. (K, L) Bell stage upper tooth germ 5 in lingual and dorsal view (slides P1:1–27). (M, N) Bell stage upper tooth germ 24 (slides P4:4–42) in lingual and dorsolabial view. (O, P) Bell stage lower tooth germ 15 (slides C:13–40) in ventral and lingual view. (Q, R) Bell stage lower tooth germ 40 (slides G:24–45) in labial and lingual view.
We investigated the dental lamina for expression of shh using an antibody competent in this species, but this experiment failed to show presence of the protein. An antibody for FGF‐8 indicates that this protein is not present in the dental lamina or the dental placode of rostral slides (slide 33, not shown), although it does occur in oral epithelium elsewhere in the mouth. Farther caudally (Fig. 3A,B), FGF‐8 can be detected in the upper and lower dental placode, and occasionally in the dental lamina of the upper teeth. In general, its expression is weaker in the lower jaw. Staining for epiprofin (SP‐6) occurs in upper and lower dental lamina (Fig. 3C).
Cap stage
Developing teeth in this bowhead fetus (NSB‐DWM 1999B6F) are mostly in the early cap stage: they show a cap shape, but lack stellate reticulum. There are 41 upper and 35 lower caps. Upper tooth caps are in general larger than lower tooth caps (Fig. 3E–H), and the upper dental lamina extends farther caudal then the lower dental lamina: the last tooth bud occurs in slide 455 in the upper, and in slide 425 in the lower jaw. In most mammals the dental lamina has the shape of a sheet that invaginates from the oral epithelium into the underlying mesenchyme and remains there until long after the cap stage. For instance, the dental lamina of the pig is connected to the oral epithelium in the early cap stage and remains attached in some regions until the bell stage (Fig. 3J; Armfield, 2010). In contrast, the bowhead dental lamina is an irregular bar that extends between the tooth caps without being clearly distinct from them (Fig. 4F) and is not connected to the oral epithelium. The dental lamina does connect all tooth caps, and extends well beyond the last tooth.
Three‐dimensional reconstruction shows that the topography of the inner enamel epithelium of upper and lower tooth caps differs (Fig. 4B–H). In the upper jaw, tooth caps 4, 9, 10, 14, 23 and 24 (counting from rostral to caudal) were reconstructed. The inner enamel epithelium of all of these caps is similar: they display a more or less circular outline in dorsal view (Fig. 4H), with a raised central area that is surrounded by three or four depressed areas that are connected, arranged in a square around the central area. This is best seen in oblique views, such as Fig. 4C–E. In the lower jaw, the outline of the tooth cap, in ventral view, is elongate, and there are two depressions, a deeper one rostrally, and a shallower one caudally (Fig. 4I).
We stained some of these sections with an antibody for SHH. SHH is present in the upper tooth caps (Figs 3E,F and 4C,E), but not in the lower tooth caps (Fig. 3H). In the upper teeth, only the central depressed area of each germ shows SHH signaling (Fig. 4C,E). Staining with an antibody for epiprofin indicated that this protein is not present in the bowhead dental caps at this age (Fig. 3G), whereas the ability of this antibody to recognize the protein is shown by staining in the dental lamina (Fig. 3C) and hair follicles (Fig. 3I) of bowhead whales.
Bell stage
This fetus (NSB‐DWM 2000B3F) has a row of bell stage tooth germs in the upper jaw (Fig. 5A), evenly spaced in a more or less straight line. Macroscopically, 40 germs can be distinguished on the right side and 32 on the left side.
Figure 5.
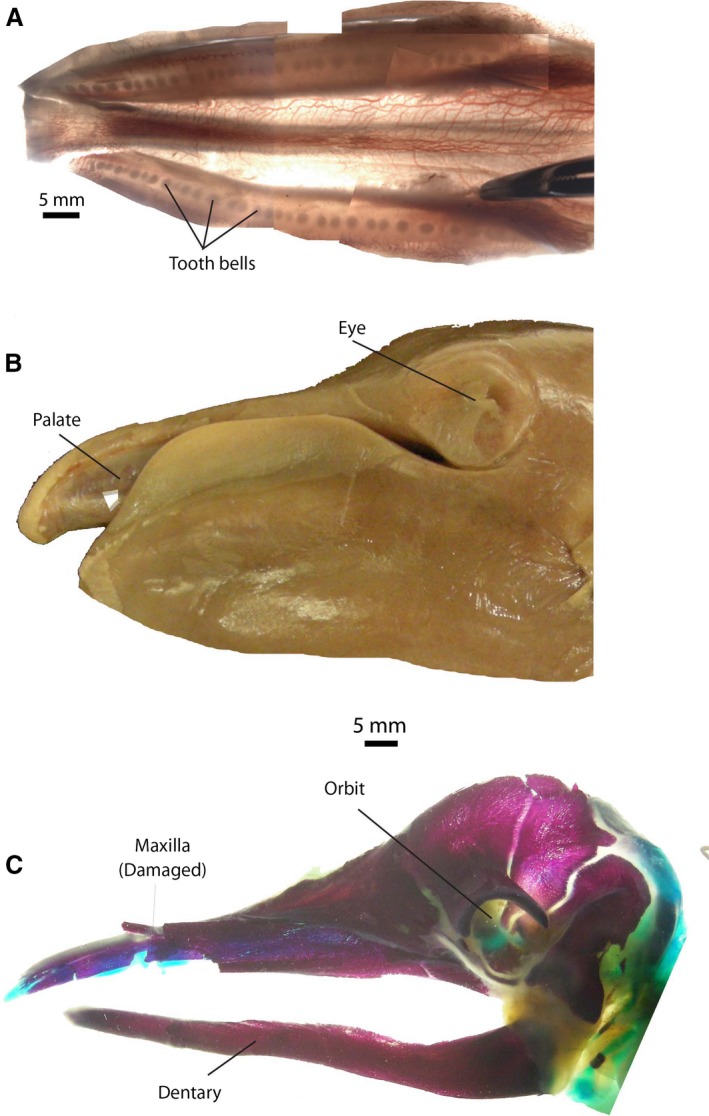
Palate and head of bowhead fetus (NSB‐DWM 2000B3F). (A) Palatal epithelium, as removed from skull, backlit showing transmitted light to show developing tooth germs (in the bell stage). (B) Head of fetus showing great extent of the lower lip. (C) Clear/stain preparation of head of the same fetus, showing the narrow dentary that does not match the outline of the lower lip. Cartilage is blue and bone is red in this preparation.
Most of the upper tooth germs are simple bowl‐ or bell‐shaped structures (Fig. 4K–N), separated from their neighbors by undifferentiated mesenchyme. However, a few germs are in contact with the neighboring germ and such a pair could be interpreted as a single, elongate, bicuspid germ (Fig. 4J). Macroscopically, recognizable joined pairs are numbers 26–27, 28–29, 30–31, 32–33 and 34–35 on the right side, and 22–23, 24–25, 26–27 and 29–30 on the left side. Microscopic examination after sectioning reveals that some additional pairs of germs that look unpaired macroscopically are in reality also paired. For instance, germ 4 on the right side appears externally to be single, and was counted as such, but is in fact paired (Fig. 4J). The individual cusps in this germ are lined up craniocaudally, with the anterior cusp being lower in elevation than the posterior cusp. On the left side, the most rostral bells occur well caudal to the most rostral bells in the right side (Fig. 5A).
In the lower jaw, tooth germs are embedded in the dorsolingual part of the dentary (Fig. 3N), which is ossifying at this stage. On the left side, 28 lower tooth bells are present, and the most rostral of these is located well caudal to the tip of the dentary. The most caudal germs are located well rostral to the coronoid process. Lower tooth germs thus have a relation to bone, whereas upper tooth germs do not. As a result, lower tooth germs are not close to the oral epithelium, as the edge of the lower lip at this stage flares dorsally, foreshadowing the shape of the lower jaw after birth. This is best appreciated when comparing a lateral view of a fetal head with a cleared/stained fetal head (Fig. 5B,C) of the same age. Unlike the lower jaw, upper tooth bells are close to the oral epithelium, and are not in close contact to the bones of the upper jaw.
Histologically, upper and lower tooth germs are in the early bell stage in which the inner enamel epithelium consists of columnar epithelium. In lower tooth bells (Fig. 3N,O), the inner enamel epithelium consists of cuboidal cells, whereas the outer enamel epithelium consists of squamous cells. The cells that form the tip of the dental papilla are elongating, forming odontoblasts. In the upper jaw, there is no remnant of the dental lamina, whereas in the lower jaw the dental lamina is weak, connects subsequent tooth germs, but is not connected to the oral epithelium (Fig. 3O). There is no trace of secondary tooth buds in the upper dentition, but in the lower dentition a flange of the dental lamina projects lingual to the primary tooth bud, and may represent a secondary bud (Fig. 3O). In the pig, the dental lamina at this stage shows clear lingual invaginations that will give rise to the teeth of the permanent dentition (Fig. 3J).
Investigated upper teeth (Fig. 3L–O) are similar in size and shape along the tooth row (except for those that are paired). Lower tooth germs are smaller and of more diverse shape (Fig. 3O–R). More anterior teeth are elongate (Figs 3O and 4O,P), whereas more posterior teeth are more globular but less regular and smaller than upper bells (Fig. 4Q,R).
At this stage, there are no morphological signs of baleen formation in the epithelium (Fig. 6A). Many small blood vessels occur in the region medial to the tooth bells, and deep to it are the major palatine artery, vein and nerve. The area immediately adjacent to the median plane is the area where, in adult bowhead whales, a large subcutaneous plexus of blood vessels occurs that will develop into the corpus cavernosum maxillaris of Ford et al. (2013). SHH signaling occurs in the stellate reticulum of the upper and lower teeth (Fig. 4M; lower jaw, slide C‐57, not shown). Lateral to this region, immunohistochemistry indicates that FGF‐4, FGF‐7 and FGF‐8 signaling occurs in the epithelium, but there is no iterative pattern of intensity fluctuations that could be associated with initiation of individual baleen plates (Fig. 6B,E–G), although all three proteins are absent near the median plane (Fig. 6C,D).
Baleen placode stage
The palate of these fetuses (NSB‐DWM 2007B16F and 2009KK1F) is narrow, and displays no remnants of teeth. The palatal surface consists of four gross morphologically distinct zones. From medial to lateral these are: a median vascularized area that is convex and narrows caudally, the corpus cavernosum maxillaris (Zone 1; Figs 6I and 7A); a band of undifferentiated epithelium that narrows rostrally (Zone 2); a white placode that also narrows rostrally (Zone 3); and a second band of undifferentiated epithelium (Zone 4). The white placode is the area from which the baleen develops. Near the caudal edge of the palate, the Zone 2 broadens.
Figure 7.
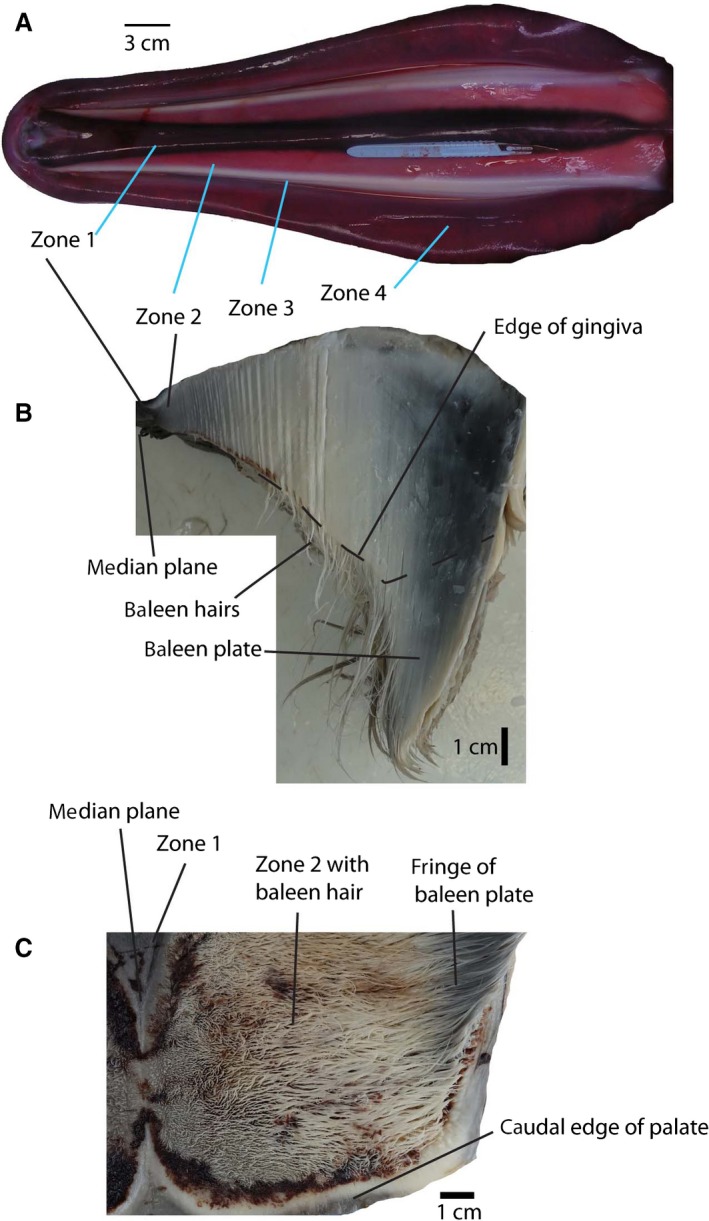
Palate and baleen in fetus. (A) Palate of fetus NSB‐DWM 2007B16F, rostral to left. (B) Rostral cross‐section through the posterior part of the unilateral palate of NSB‐DWM 2015B9F. (C) Ventral view of the posterior palate (same individual as B), showing baleen hairs and their root in the gingiva of the palate. Zones identify homologous areas in Figs 6 and 7.
FGF‐4 signaling can be demonstrated in much of the palatal epithelium. In Zone 2, such signaling occurs mostly superficial to vessels that are located in dermal papillae (Fig. 6J,K). In Zone 3, FGF‐4 signaling shows an iterative pattern: signaling occurs in dorsoventral bands that span most of the depth of the epithelium and is visible in cross‐ and parasagittal sections (Fig. 6L,M), with bands separated by areas lacking staining. In horizontal sections of some areas of Zone 3, these bands fuse into linear features that extend mediolaterally, and are lined up craniocaudally, an orientation similar to postnatal baleen plates (Fig. 6N).
Staining for FGF‐8 occurs in the area medial to Zone 3, mostly in epithelial areas away from dermal papillae (Fig. 6O), and is very faint to absent in the placode. We did not detect FGF‐7 in the palatal sections, including the baleen‐forming placode, whereas the competency of this antibody in this species is shown by staining in the hair follicle of a bowhead whale (Fig. 6H).
Full term
The four zones distinguished on the palate can still be recognized in this fetus (Fig. 7B,C). The median vascular area has not changed, but the undifferentiated epithelium is now a narrow strip. Lateral to this zone extends a wide field of thin baleen hairs (Fudge et al. 2009) that are anchored into the gingiva by small accessory columns and platelets that are topographically in the same area as the undifferentiated epithelium of the previous stage. These accessory plates (Fudge et al. 2009) are not keratinized and increase in cross‐sectional area and length from medial to lateral. Further lateral yet, accessory plates are subsumed in and constitute a single large sheet of barely keratinized tissue that does not project into the oral cavity and is totally embedded into the gingiva (Zone 3). This area grades laterally into the baleen plate, which projects approximately 6 cm into the oral cavity in the middle of the baleen rack. In palatal view (Fig. 7C), the baleen hairs and frayed inner surface of the baleen plate give the appearance of a matted field of hair.
Discussion
Tooth counts
Our bowhead specimens display polydonty, and this was also documented in other mysticete fetuses (Julin, 1880; Kükenthal, 1891; Dissel‐scherft & Vervoort, 1954; Karlsen, 1962). Embryologists have speculated on the origin of polydonty in cetaceans. Kükenthal (1893) proposed that polydonty was the result of splitting of multicuspid teeth, whereas Julin (1880) suggested the opposite: that individual, conical tooth germs fuse in development, implying that earlier embryos would have even more tooth germs than later ones. Karlsen (1962) reanalyzed Kükenthal's data and based his analysis on a collection that also included younger embryos than were available to Kükenthal. Karlsen (1962) concluded that some tooth germs fuse occasionally in ontogeny, but that the fusion pattern is consistent neither along the tooth row, nor between left and right tooth row, nor between different individuals. Karlsen (1962) also observed that crown complexity does not increase toward the caudal part of the tooth row as is common in heterodont dentitions of other mammals. We concur that fusion observed in tooth crowns of our specimens is not related to the patterns that determine tooth counts and crown shape in heterodont mammals. Consistent with the view of Karlsen (1962), we consider the high tooth number in fetal bowhead whales to be the result of dental patterning earlier in ontogeny, not of divisions in already formed dental germs long after they form.
One of the geologically youngest toothed mysticetes is Aetiocetus polydentatus. This species has the highest numbers of excess teeth of any fossil mysticete (Barnes et al. 1994), and has different numbers of teeth in the left and right jaw. Cobourne & Sharpe (2010) discussed differences between left and right jaws as symptoms of a release of stabilizing selection, and Salazar‐Ciudad & Jernvall (2010) indicated that the lack of strict occlusion in seals was correlated to higher dental variation. The same may be true for toothed mysticetes. Indeed, fused crowns that complicate precise occlusion do occur in some fossil cetaceans postnatally (Fig. 2H), and it is possible that these were formed by the same process as is documented in the mysticete fetuses we studied.
Karlsen (1962) pointed out that, in Balaenoptera, the dental lamina continues to produce new germs caudal to existing teeth for longer than in other mammals. The extension of the bowhead dental lamina caudally, long after it has disappeared in other mammals, would lengthen the period of tooth formation and thus create more tooth germs. In terrestrial mammals (Yamanaka et al. 2007) the dental lamina develops buds soon after its formation, and this appears to be the case in dolphins too (Mišek et al. 1996). It is not the case in bowhead whales, where formation of tooth buds is delayed. It has been shown experimentally that a persistent dental lamina can result in polydonty in lab animals (Rodrigues et al. 2011). Fetal embryonic polydonty in bowhead whales may be the result of the early, strong and extended development of the dental lamina. This type of heterochronic development of the dental lamina is taken to an extreme in the manatee Trichechus, where the dental lamina persists throughout life, and new molar‐shaped teeth are formed nearly indefinitely long after birth.
Nakamura et al. (2006, 2008) found that a loss‐of‐function mutation of the transcription factor epiprofin causes polydonty and simple crown morphologies in mice. In mice with functional copies of the gene (+/+), this protein is extensively expressed through the enamel epithelium from the bud through the enamel formation stage (Nakamura et al. 2006). Epiprofin development in bowhead whales is truncated, our specimens showed evidence in the dental lamina (Fig. 3C), but not at the cap stage, consistent with polydonty and simple crown morphologies of our later fetuses. It is possible that changes in epiprofin signaling underlie the morphological changes in the dental lamina.
Our working hypothesis is that the increased variability in tooth numbers as well as polydonty in early mysticetes is the result of a release of the selection pressures related to accurate occlusion as occlusion was not critical to food acquisition. The ontogenetic fingerprints thereof are still present in mysticete embryos. Reduced expression, in time or concentration, of epiprofin may be part of the mechanism by which this was accomplished.
Molar crown morphology
At the cap stage, bowhead whales display folds of the inner enamel epithelium that resemble those that are associated with secondary enamel knots in land mammals (Jernvall & Thesleff, 2000). In land mammals, these folds eventually give rise to the complex molar crown morphology of these taxa (Fig. 2C). Lower bowhead tooth germs resemble the pattern of the lower teeth of the Eocene whale Pakicetus with a high part rostrally (trigonid) and low part caudally (talonid; Fig. 2A,E). The folding pattern of the inner enamel epithelium in upper teeth resembles the cusp pattern of the artiodactyl relatives of cetaceans, hippopotamids and raoellids (Fig. 2C; Gatesy et al. 2013), with four folds that match the arrangement of the original four cusps (Fig. 4F,H).
Dissel‐scherft & Vervoort (1954) studied the mineralized fetal teeth of a Balaenoptera fetus of 102 cm, a stage for which we do not have bowhead fetuses. They found that posterior lower teeth have a complex three‐cusped crown, similar to the trigonid of raoellid relatives of cetaceans, whereas anterior teeth have single‐cusped crowns similar to incisors and canines of heterodont mammals. These teeth were composed of dentin and lacked enamel, consistent with the study of Deméré et al. (2008) and Meredith et al. (2009) that detected nonsense mutations in the genes involved with amelogenesis of mysticetes. It would be remarkable if the embryonic germs resembled molar crowns of species that lived more than 40 million years ago.
To investigate this, we used the signaling protein SHH to further explore the folds of the inner enamel epithelium. SHH is a marker for the enamel knot, the signaling center for cusp tips (Jernvall & Thesleff, 2000; Thesleff, 2000). Our experiments show that the protein is not expressed in the tips of the folds but instead in the valley between them in the upper teeth. This pattern of expression foreshadows the single‐cusped germs of the bowhead bell stage, but does not support the interpretation of the enamel folds as original cusps. With our embryos, we could not document any SHH in the lower teeth at the cap stage. Furthermore, in our specimens, even anterior upper tooth germs (germs 1–8) have complex morphologies of the inner enamel epithelium at the cap stage, while the artiodactyl relatives of cetaceans have simple crowns for these teeth (Thewissen et al. 2007).
At least two interpretations of these data are possible. The first is that the folding patterns of the inner enamel epithelium of bowhead whales are induced by secondary enamel knots (and are thus cusp homologs) at some stage but that our collection does not include specimens of that stage. The heterodonty of mineralized teeth described by Dissel‐scherft & Vervoort (1954) is consistent with this interpretation, and so is the heterodonty of lower tooth crowns in our bell stage fetus. The homodonty of the upper tooth bells then provides a discordant datum.
The second possible explanation is that these folds are not related to secondary enamel knots at all. Nakamura et al. (2008) found that the inner enamel epithelium displays incipient folding that is not related to enamel knot formation in mice that do not express epiprofin. These mice also show a deficiency in shh expression. This is consistent with the disruption of epiprofin signaling in the bowhead whale. In fact, it is possible that the small accessory cusps that occur on the anterior and posterior slope of the main cusp in fossil mysticetes and their archaeocete ancestors (Fig. 2B,G,H) are caused by truncated expression of epiprofin. We observed inner enamel epithelium folds even in anterior teeth at the cap stage, suggesting that they are not related to the original heterodonty of the mysticete ancestors. However, this does not explain the discrepancy between upper and lower tooth bells. At present, it is not possible to test these two evolutionary scenarios as specimens of the relevant developmental stages are not available.
Tooth shape along the tooth row
Signaling that is important in defining mammalian tooth classes occurs at the dental lamina stage, when morphogenetic fields are created in the jaw (Catón & Tucker, 2009; Jernvall & Thesleff, 2012). These guide the formation of different tooth classes. Such signaling occurs long before the signaling that determines the size, spacing and number of teeth, suggesting that these processes are independent to some degree. In investigated mammals (mice, shrews and minks), BMP‐4 signaling sets up the incisor field of the jaw, whereas FGF‐8 sets up the molar field. The canine and premolar fields may result, in part, on the interaction of these signaling proteins along a gradient in the jaw. Armfield et al. (2013) studied the dolphin Stenella, and showed that the signaling field of BMP‐4 has expanded caudally and overlaps with the FGF‐8 field. Both Stenella and bowhead whales retain the FGF‐8 field in the caudal part of the jaw. In bowhead whales the signaling is more distinct in the upper than in the lower jaw. All teeth in Stenella are similar, and have incisor‐like morphologies. Experimentally, it has been determined that, in mice, BMP‐4 forces posterior teeth into incisor‐like morphologies (Murashima‐Suginami et al. 2008; Munne et al. 2010), a mechanism that is consistent with the Stenella data. Unfortunately, our BMP‐4 antibody appears to be incompetent in bowhead whales, and we cannot determine the protein's signaling traces in this species.
In our specimens, there is clear morphological variation of teeth along a cline in the lower jaw, but not in the upper jaw. Lower crowns of rostral teeth are longer and more pointed, whereas those of caudal teeth are more globular in the 40.3‐cm fetus. Karlsen (1962) also found this in Balaenoptera. Julin (1880) studied the lower jaw of a Balaenoptera fetus with 41 tooth germs, where teeth 1–9 had simple, one‐cuspid tooth bells, whereas teeth 10–41 had two or three cusps per tooth, sometimes of the same height, sometimes of different heights. In the Balaenoptera mandible described by Dissel‐scherft & Vervoort (1954), the anterior 36 right and 38 left teeth were simple conical teeth, and teeth more posterior showed molar trigonid morphologies. The lower jaw of our 40.3‐cm specimen displays heterodonty too, although it is less pronounced, and clearer in the lower than in the upper jaw. Karlsen (1962) indicated that Julin's fetus may have been 45 cm in length, indicating that it was at a later ontogenetic time than our bowhead fetus.
Heterodonty occurs in all extinct toothed mysticetes, with distinct incisors and canine, and a gradual transition of simple one‐cusped teeth to more complex teeth in premolars and molars (Barnes et al. 1994; Fitzgerald, 2006, 2009). The most consistent interpretation of this pattern is that the polydont and heterodont fetal lower dentition of mysticete fetuses is a hold‐over of their Oligocene ancestors, whereas morphological remnants of the fetal upper dentition have been erased.
Tooth replacement
Julin (1880) did not find evidence of a deciduous dentition in the (single) early fetus of Balaenoptera that he studied. Kükenthal (1893) interpreted patterns observed in his collection of prenatal Balaenoptera to indicate that there were three waves of dentition development. In his view, the second of these waves proceeds to develop furthest and is homologous to the deciduous dentition of other mammals. He identified remnants of a predeciduous dentition lateral to, and germs of the adult dentition medial to, the deciduous dentition. Karlsen (1962) studied a Balaenoptera embryo with early dental caps and disputed the presence of a predeciduous dentition, instead interpreting the lateral clusters of epithelial cells as remnants of the lateral dental lamina. The dental lamina occurs in many groups of mammals, and disappears before the bell stage is reached. Karlsen (1962) interpreted the lingual clusters of cells as emanating from the dental lamina, not from other tooth buds, and part of the same tooth generation as the larger buds. In Karlsen's material (Karlsen, 1962), these clusters occasionally developed into tooth caps. It is possible that such a process in related taxa with teeth actually results in the formation of erupted tooth crowns. Fordyce (1982) reported supernumery single‐rooted teeth in a fossil odontocete that may be the result of such a process.
Karlsen (1962) noted the presence of conspicuous folds in the outer enamel epithelium in a 56‐cm fetus, and that smaller germs in the cap stage are located somewhat lingually from larger more developed germs in the lower, but not in the upper jaw.
Dissel‐scherft & Vervoort (1954) described in some detail how, in the lower jaw of Balaenoptera, tooth bells of multiple sizes and shapes seem to co‐occur in a cranio‐caudal pattern that shows no discernable regularity or trend. These authors did not go so far as to interpret these as different tooth generations, taking them instead for evidence of a gradually disappearing heterodont dentition. Our evidence is consistent with the view of Karlsen (1962) and Dissel‐scherft & Vervoort (1954): the tooth germs that reach the bell stage in modern bowhead fetuses all arise from a single tooth generation. There is evidence of anlagen for teeth of a second tooth generation in the form of lingual buds protruding from the lower, but not the upper tooth bells (Fig. 3O). Dosedělová et al. (2015) found that a weak crest that projects lingually from the monophyodontic teeth of terrestrial mammals (mouse, hedgehog, pig) is a likely remnant of a developing permanent tooth. This is similar to the crest we observed in the lower dentition of bowhead whales, and we interpret the bowhead tooth bells as part of the primary (deciduous) dentition, and hypothesize that these tooth germs represent the single tooth generation of fossil toothed mysticetes such as aetiocetids. The lingual buds would then be remnants of the permanent dentition.
Whitlock & Richman (2013) reviewed tooth replacement mechanisms. The dental lamina plays a key role in this process (Järvinen et al. 2009). In mammals, the loss of a connection between dental lamina and oral cavity stops new tooth generations from forming (Buchtová et al. 2012). In humans, the dental lamina begins to break up at the bell stage by detaching from the oral epithelium (Bhussry, 1980), whereas in bowheads this connection is lost by the cap stage.
We hypothesize that the early loss of a connection between dental lamina and oral epithelium in bowhead prenatal development plays a role in the loss of diphyodonty. Traces of a second tooth generation can still be found in the lower, but not in the upper dentition.
Developmental tooth loss
Complete tooth loss in bowheads takes place between the stages of fetuses of a total length 40.3 and 159 cm, and is not documented by our specimens. Several aspects of the process of tooth loss are known for mysticetes. Karlsen (1962) found evidence of dentin formation in Balaenoptera, but not enamel formation, consistent with the absence of enamel formation gene expression shown by Deméré et al. (2008). Dissel‐scherft & Vervoort (1954) describe mineralized crowns in detail, noting several histological features that are indicative of resorption. Davit‐Béal et al. (2009) reviewed the little that is known about tooth loss in tetrapods in a developmental context. Comparing ontogenetic data for different taxa, it is clear that different developmental processes underlie tooth loss in different taxa (Louchart & Viriot, 2011; Tokita et al. 2013). It is also clear that the developmental processes that arrest tooth development are unrelated to and overprint the processes related to tooth formation. This implies that, in cladistics analyses, the absence of teeth in a clade should be considered a character independent from that related to the precise number of teeth in its toothed relatives.
Developmental origin of baleen
The first morphological signs of baleen formation occur when the teeth are at the bell stage. When the palate is backlit, a dense area is visible medial to the tooth germs (Fig. 5A). This area is underlain by a neurovascular bundle (Fig. 6A) and develops into the baleen placode later in ontogeny (Fig. 6I), when all traces of teeth disappear. FGF‐4 signaling occurs in an iterative pattern in the placode and could be related to eventual iterative pattern of the baleen plates. In mammals with teeth, FGF‐4 is expressed in the enamel knot (Niswander & Martin, 1992; Kettunen & Thesleff, 1998; Tucker et al. 1998), but the protein is not involved in hair formation (Mitsui et al. 1997) or nail formation.
FGF‐8 plays an important role in the initiation of tooth development in mammals (Niswander & Martin, 1992; Kettunen & Thesleff, 1998; Nadiri et al. 2004), including cetaceans (Armfield et al. 2013), but plays no role in the development of hair (Rosenquist & Martin, 1996) or palatal rugae (Porntaveetus et al. 2010a). By contrast, FGF‐7 is involved in signaling in the mammalian hair follicle (Mitsui et al. 1997; Nakatake et al. 2001), and FGF‐10 in palatal rugae development (Porntaveetus et al. 2010a), and these are not involved in tooth signaling (Porntaveetus et al. 2010b). We could not detect signaling of FGF‐7 and FGF‐10 related to baleen formation.
Involvement of FGF‐4 in baleen development instead suggests that some of the original signaling related to tooth formation now partakes in the formation of baleen. There are other indications that this may be the case. In most mammals, upper and lower teeth follow similar ontogenetic trajectories, but in bowheads they deviate early in ontogeny. The upper dental lamina differs morphologically from the lower dental lamina: it is longer (rostrocaudally) and higher (dorsoventrally) and creates more tooth germs. Those tooth germs are more homodont than those in the lower jaw. In some sense then, the tooth germs of the upper jaw appear to foreshadow baleen development, where many, near‐identical plates form. Lower tooth germs develop close to the dentary, and they are far from the lower lip because the lip bulges dorsally (Fig. 5B,C). Thus, the distance between the lower mandibular epithelium and the developing tooth germs is great. This epithelium is known to play a role in tooth development (Järvinen et al. 2009), and that topography may affect lower tooth development. While this may explain the differences in upper and lower dentition development in bowhead whales, the lower lip of other fossil and modern mysticetes (e.g. balaenopterids) is closer to the mandible with its tooth germs. A different mechanism may underlie the loss of tooth germs in these taxa.
Ishikawa et al. (1999) noted similarities in extracellular matrix proteins involved in baleen development to those involved in tooth development, and Davit‐Béal et al. (2009) speculated that baleen development was functionally related to tooth loss based on developmental timing. These observations support our interpretation of the signaling evidence. Our working hypothesis is that development of the upper dentition in the bowheads is a necessary step in the development of baleen, and that differences between upper and lower dentition are related to the lack of this function in the lower teeth.
The fossil record is also consistent with this hypothesis. There is a trend in the earliest fossil mysticetes toward homodonty of the molars and premolars, but polydonty was limited, with no more than a single additional tooth (beyond the original 11 per quadrant) in most clades and four additional teeth in one taxon (Aetiocetus polydentatus). It is puzzling that modern mysticete embryos have more than 40 tooth germs if known members related to their ancestors never had more than 15 teeth, unless those germs serve a function beyond that of teeth. This situation differs from odontocetes, where polydonty is widespread across fossil and modern clades. We hypothesize that mysticetes that were highly polydont as adults never existed, and that the iterative signaling that underlies the formation of so many tooth germs is related to the signaling that is important in baleen formation later in ontogeny, but was not engaged until later in evolution, when postnatal teeth had been lost already.
The pattern of signaling in the palate coincides with results presented by Fudge et al. (2009) regarding baleen development: initially undifferentiated epithelium is induced to form accessory plates and, as the fetus grows, these structures are incorporated into the main baleen plate. The pattern of implantation of baleen plates, accessory plates and bristles on the back of the palate has no match in mammals, but is reminiscent of other ectodermal structures in the mouth of other vertebrates. Generations of replacement teeth in reptiles, for instance, are arranged in patterns that show a maturation gradient from medial to lateral, but implantation of individual elements in rows that are oblique to the mediolateral plane (Westergaard & Ferguson, 1990; Whitlock & Richman, 2013).
It might seem strange that two structures that are very different in composition, teeth and baleen, are formed by similar signaling pathways. However, it should be remembered that enamel and keratin have shared the oral cavity since the earliest vertebrates, as evidenced by the keratinous teeth of cyclostomes (Trott & Lucow, 1964), although the formation of these has no relation to tooth origins (Smith & Coates, 2000; Huysseune et al. 2009). The link between keratin and the oral cavity also virtually characterizes birds. Louchart & Viriot (2011) discussed the evolution of tooth loss and the formation of a rhamphoteca (keratin beak) in birds, noting that local keratinization may be a cause for arrest of tooth formation. Wu et al. (2004) noted the developmental similarity in signaling among morphologically very different keratinized epithelial appendages, and teeth too share some of these pathways. Clearly, there is great plasticity in epithelial appendages, both developmentally and evolutionarily (Chuong, 1998; Dhouailly, 2009), and common developmental pathways underlie all of them.
Some of the significant differences in developmental processes forming teeth and baleen may also be bridged by minor changes in patterning. For instance, mammalian teeth form by invagination of epithelium into underlying mesenchyme, whereas baleen forms by evagination into the oral cavity. The protein IKKα is involved with the development of hair and whiskers, and, possibly, in the regulation of other ectodermal–mesenchymal interactions (Karin & Delhase, 2000; Ohazama et al. 2010). In Ikkα mutant mice, incisors and whiskers form by evagination, not invagination. Furthermore, in the talpid2 mutant of chicks, tooth germs form by evagination, not invagination (Harris et al. 2006). There are many other genes that play a role both in the development of teeth and keratin structures. For instance, fgf‐20 is involved in tooth and hair development, and its subtle effects on the morphology of teeth could be a potent agent in evolutionary changes in tooth morphology (Häärä et al. 2012). Obviously, shifts in single genes are insufficient to turn teeth into baleen in ontogeny, but it does challenge the assumption that the morphological difference between these processes is unbridgeable.
Our evidence suggests a greater level of homology between teeth and baleen than their homology as epithelial organs (sensu Chuong, 1998), namely that the particular gene expression pathway that leads to two generations of teeth in most mammals has been co‐opted to be involved in baleen formation in cetaceans. Several approaches can be used to investigate this in greater depth. First, signaling that underlies the number of palatal rugae that forms and the spacing between them is suggestive (Pantalacci et al. 2008) and may elucidate the developmental basis for baleen plate spacing. Second, a detailed understanding of the development of the compounds that compose baleen could help to understand selective powers at work (Szewciw et al. 2010). These compounds may include a large diversity of keratins (Bragulla & Homberger, 2009), and their developmental pathways may be quite different from each other.
Conclusion
We integrate our data and discussion into a working hypothesis that comprises the development of teeth and baleen in bowhead whales. Until the bell stage, bowhead dental ontogeny is easily recognizable as a variation on the dental ontogeny of generalized mammals. We interpret differences that do exist as precursors of events that happen after the bell stage: the complete loss of teeth and the development of baleen. Here, we summarize that process and our interpretation of it and provide an evolutionary context (Fig. 8).
Figure 8.
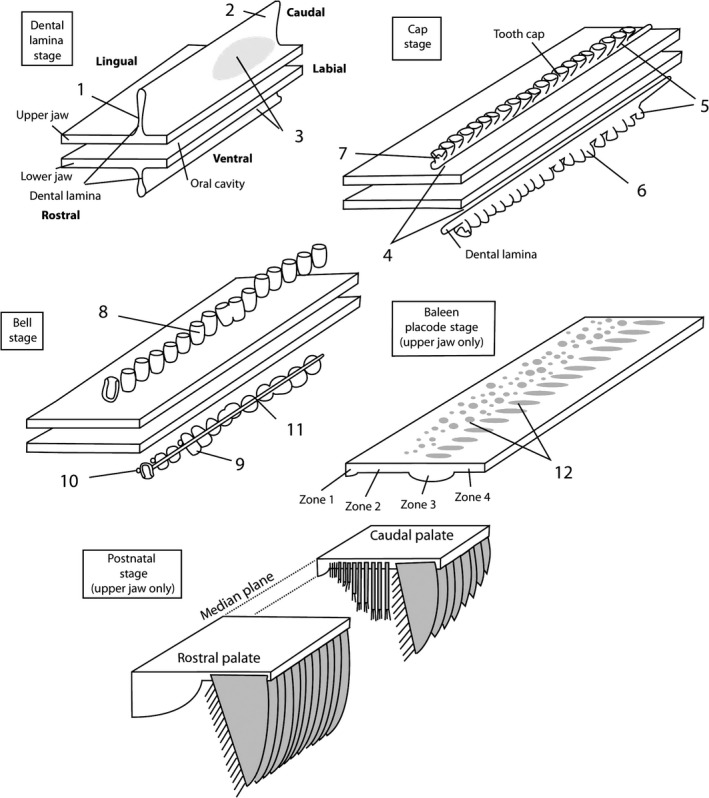
Diagrams showing tooth and baleen development in bowhead whale. Upper and lower oral epithelium and structures derived from it are shown, mesenchyme is not shown. For baleen placode and postnatal stage, the lower jaw is not shown. Numbers refer to important features discussed in the text. (1) Upper dental lamina is higher than lower dental lamina. (2) Upper dental lamina extends further caudal than lower dental lamina. (3) FGF‐8 expression in caudal (molar) region of dental lamina. (4) Dental lamina lacks contact with oral epithelium. (5) More tooth caps occur in the upper than in the lower jaw. (6) Lower tooth caps are more variable in shape and size than upper tooth caps. (7–8) Inner enamel epithelium of tooth caps shows complex folds, whereas that of upper tooth bells (8) shows single fold that corresponds to single cusp of tooth. (9) Lower tooth bells are heterodont. (10) Secondary tooth buds develop in lower, but not upper jaw. (11) Dental lamina persists longer in lower than upper jaw. (12) FGF‐4 expression in upper jaw epithelium. The pattern of expression of fgf‐4 foreshadows the footprint of baleen plates.
The first differences between dental development of bowheads and generalized mammal development already occur at the dental lamina stage, and were observed before by Karlsen (1962) in Balaenoptera: the dental lamina of the upper jaw is higher dorsoventrally (Fig. 8.1) and longer cranio‐caudally (Fig. 8.2) than the dental lamina of the lower jaw. FGF‐8 signaling occurs in the posterior regions of both, but is stronger in the upper jaw than in the lower jaw (Fig. 8.3). Such signaling is the basis for molar morphologies of posterior teeth in generalized mammals, and is also found in homodont dolphins. In dolphins, FGF‐8 signaling in the posterior jaw is overprinted by BMP‐4 signaling, which may induce the incisor‐like morphologies of the cheek teeth (Armfield et al. 2013), as it is known to do in mutant mice (Munne et al. 2010). It is likely that the FGF‐8 signaling causes the heterodontic features of the lower jaw of bowhead fetuses, as well as the limited degree of heterodonty in extinct toothed mysticetes (e.g. Janjucetus, Mammalodon and aetiocetids; Barnes et al. 1994; Fitzgerald, 2006, 2009).
At the dental cap stage, the dental lamina has lost contact with the oral epithelium (Fig. 8.4), an event that takes place much later in generalized mammals. The dental lamina is necessary for the formation of subsequent tooth generations, and we propose that its loss underlies the absence of a second tooth generation even in fossil, toothed mysticetes.
At the bell stage, upper and lower dentitions vary significantly. The upper tooth germs are homodont (Fig. 8.8), there is no evidence of primordia for a second tooth generation, and individual bells are not connected by the dental lamina. In the lower jaw, there are fewer, more variably developed germs (Fig. 8.5 and 8.6) and they are smaller than the upper teeth, there is a gradient of heterodonty along the jaw (Fig. 8.9), while the dental lamina remains. This is consistent with the findings of Karlsen (1962). A lingual crest protrudes from the tooth bells that may be the remnant of the secondary dental lamina that is involved in the formation of permanent teeth. We interpret these differences in the context of the imposition of selection forces on the upper dentition, leading to greater polydonty, homodonty and monophyodonty. In contrast, the lower teeth maintain some of the ancestral patterns of heterodonty and diphyodonty, while they are underdeveloped in general.
Individual tooth caps show patterns of folds in the inner enamel epithelium that resemble cusps of the Eocene ancestors of whales in the upper molars, and lower molar morphology of the earliest whales in the lower teeth (Fig. 8.7). Although this pattern is suggestive, there is only a single area of SHH signaling in these teeth, and this area probably is related to the single cusps that occur in the bell stage of these teeth. Folding of the inner enamel epithelium could also be caused by absence of epiprofin signaling and, indeed, epiprofin signaling is reduced in Balaena. Mice with disrupted epiprofin signaling display polydonty and simple crown morphologies along the tooth row (Nakamura et al. 2008). The dental patterns of teeth in −/− epiprofin mice suggest that if enamel formation were to take place in these tooth caps, small cuspules would develop on these teeth, a feature that is characteristic for the posterior cheek teeth of toothed mysticetes such as Mammalodon, Janjucetus and Aetiocetus. Järvinen et al. (2006) showed that simple crowns and polydonty are not independent developmentally. It is possible that lack of epiprofin contributes to both effects in bowheads. Many other genes are involved in the determination of cusp patterns (e.g. Eda, Spry, Sostdc; Jernvall & Thesleff, 2012), and further investigations are needed to test these hypotheses.
The first signs of homodonty, monophyodonty, polydonty and simple molar crown patterns all appear in the fossil record closely spaced, near the origin of mysticetes. Evidence presented by us suggests that multiple factors may play a role in the evolution of these features. These include the disruption of epiprofin signaling and the lengthening of the dental lamina and its early detachment from the oral epithelium. Selection for precise occlusion may have been released at this time, similar to the mechanism proposed for phocid seal cheek tooth evolution proposed by Salazar‐Ciudad & Jernvall (2010) and for odontocete tooth crown morphology by Armfield et al. (2013). In contrast, Tsai & Fordyce (2014) reached the opposite conclusion for the evolution of mysticete cranial morphology; they studied heterochrony in late‐term fetal and postnatal mysticete skulls and showed highly constrained heterochonic evolution in some clades.
In the ontogeny of one tooth germ, several developmental genes are used multiple times during development (Tucker & Sharpe, 2004). That same developmental cassette is also replayed for topographically sequential teeth in the tooth row, as well as for chronologically subsequent teeth such as the deciduous and permanent dentitions of mammals, or the polyphyodont dentitions of other vertebrates. One of the proteins involved in this cassette is FGF‐4, a member of its family that does not play a great role in other tissues of the head.
Based on these findings, we formulate a working hypothesis that the tooth development cascade was adapted and plays a role in baleen development in mysticetes. This is an example of exaptation in baleen whale evolution, and implies that teeth and baleen are homologous to some degree. Obviously, there are many differences, including developmental ones. Homology is not an absolute concept, baleen is homologous with teeth as an epithelial organ, and as an organ formed by the iterative signaling sequence that initiates teeth. However, this level of homology is less deep than the homology of the first molar of a pakicetid to that of a dorudontid cetacean.
Author's contributions
Thewissen, George, Suydam and Stimmelmayr collected the samples; Hieronymus and McBurney prepared the samples and did the experiments; and Thewissen, Hieronymus and George did the writing.
Acknowledgements
The authors thank the captains of Barrow, Alaska, and the Alaska Eskimo Whaling Commission for allowing for examination of their whales and collection of samples. We thank all of those involved, especially Director Taqulik Hepa and Deputy Director Harry Brower Jr. Funding was provided by the North Slope Borough and NEOMED.
All authors declare that there is no conflict of interest that might affect their objectivity in preparing this paper.
References
- Armfield BA (2010) The evolution and development of mammalian tooth class. PhD dissertation, Kent State University: Kent, Ohio, USA. [Google Scholar]
- Armfield BA, George JC, Vinyard CJ, et al. (2011) Allometric patterns of fetal head growth in mysticetes and odontocetes: comparison of Balaena mysticetus and Stenella attenuata . Mar Mam Sci 27, 819–827. [Google Scholar]
- Armfield BA, Zheng Z, Bajpai S, et al. (2013) Development and evolution of the unique cetacean dentition. PeerJ 1, e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LG, Kimura M, Furusawa H, et al. (1994) Classification and distribution of Oligocene Aetiocetidae (Mammalia, Cetacea, Mysticeti) from western North American and Japan. Island Arc 3, 392–431. [Google Scholar]
- Bhussry BR (1980) Development and growth of teeth In: Orban's Histology and Embryology, 9th edn (ed. Bhaskar SN.), pp. 24–45. St. Louis, Missouri: CV Mosby. [Google Scholar]
- Boessenecker RW, Fordyce RE (2015) Anatomy, feeding ecology, and ontogeny of a transitional baleen whale: a new genus and species of Eomysticetidae (Mammalia, Cetacea) from the Oligocene of New Zealand. PeerJ 3, e1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bown TM, Kraus MJ (1979) Origin of the tribosphenic molar and metatherian and eutherian dental formulae In: Mesozoic Mammals, The First Two‐thirds of Mammalian History. (eds Lillegraven JA, Kielan‐Jaworowska Z, Clemens WA.), pp. 172–181. Berkeley: Univ California Press. [Google Scholar]
- Bragulla HH, Homberger DG (2009) Structure and functions of keratin proteins in simple, stratified, keratinized, and cornified epithelia. J Anat 214, 516–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchtová M, Stembírek J, Glocová K, et al. (2012) Early regression of the dental lamina underlies the development of diphyodont dentitions. J Dent Res 91, 491–498. [DOI] [PubMed] [Google Scholar]
- Catón J, Tucker AS (2009) Current knowledge of tooth development: patterning and mineralization of the murine dentition. J Anat 214, 502–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuong C‐M (1998) Morphogenesis of epithelial appendages: variations on top of a common theme and implications for regeneration In: Molecular Basis of Epithelial Appendage Morphogenesis. (ed. Chuong C‐M.), pp. 3–13. Austin, Texas: R. G. Landes. [Google Scholar]
- Clementz MT, Fordyce RE, Peek SL, et al. (2014) Ancient marine isoscapes and isotopic evidence of bulk‐feeding by Oligocene cetaceans. Palaeogeog Palaeoclim Palaeoec 400, 28–40. [Google Scholar]
- Cobourne MT, Sharpe PT (2010) Making up the numbers: the molecular control of mammalian dental formula. Semin Cell Dev Biol 21, 314–324. [DOI] [PubMed] [Google Scholar]
- Cooper LN, Thewissen JGM, Hussain ST (2009) New middle Eocene archaeocetes (Cetacea, Mammalia) from the Kuldana formation of Northern Pakistan. J Vertebr Pal 29, 1289–1299. [Google Scholar]
- Davit‐Béal T, Tucker AS, Sire J‐Y (2009) Loss of teeth and enamel in tetrapods: fossil record, genetic data and morphological adaptations. J Anat 214, 477–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deméré TA, McGowen MR, Berta A, et al. (2008) Morphological and molecular evidence for a stepwise evolutionary transition from teeth to baleen in mysticete whales. Syst Biol 57, 15–37. [DOI] [PubMed] [Google Scholar]
- Dhouailly D (2009) A new scenario for the evolutionary origin of hair, feather, and avian scales. J Anat 214, 587–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissel‐scherft MCV, Vervoort W (1954) Development of the teeth in fetal Balaeonoptera physalus (L.) (Cetacea, Mystacoceti). Proc Nederl Akad Wetensch Amsterdam C 57, 196–210. [Google Scholar]
- Dosedělová H, Dumková J, Lesot H, et al. (2015) Fate of the molar dental lamina in the monophyodont mouse. PLoS ONE 10, e0127543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake SE, Crish SD, George JC, et al. (2015) Sensory hairs in the bowhead whale, Balaena mysticetus (Cetacea, Mammalia). Anat Rec Adv Integr Anat Evol Bio 298, 1327–1335. [DOI] [PubMed] [Google Scholar]
- Durham FE (1980) External morphology of bowhead fetuses and calves. Mar Fish Rev 42, 74–80. [Google Scholar]
- Fitzgerald EMG (2006) A bizarre new toothed mysticete (Cetacea) from Australia and the early evolution of baleen whales. Proc Roy Soc B 273, 2955–2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald EM (2009) The morphology and systematics of Mammalodon colliveri (Cetacea: Mysticeti), a toothed mysticete from the Oligocene of Australia. Zool J Linn Soc 158, 367–476. [Google Scholar]
- Ford TJ Jr, Werth AJ, George JC (2013) An intraoral thermoregulatory organ in the bowhead whale (Balaena mysticetus) the corpus cavernosum maxillaris. Anat Rec 296, 701–708. [DOI] [PubMed] [Google Scholar]
- Fordyce RE (1982) Dental anomaly in a fossil squalodont dolphin from New Zealand, and the evolution of polydonty in whales. New Zealand J Zool 9, 419–426. [Google Scholar]
- Fudge DS, Szewciw LJ, Schwalb AN (2009) Morphology and development of blue whale baleen: an annotated translation of Tsycho Tullberg's classic 1883 paper. Aq Mamm 35, 226–252. [Google Scholar]
- Gatesy J, Geisler JH, Chang J, et al. (2013) A phylogenetic blueprint for a modern whale. Mol Phyl Evol 66, 479–506. [DOI] [PubMed] [Google Scholar]
- George JC (2009) Growth, morphology, and energetics of bowhead whales (Balaena mysticetus). PhD Dissertation, University of Alaska: Fairbanks. [Google Scholar]
- George JC, Stimmelmayr R, Suydam R, et al. (2016) Severe bone loss as part of the life history strategy of bowhead whales. PLoS ONE 11, e0156753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Häärä O, Harjunmaa E, Lindfors PH, et al. (2012) Ectodysplasin regulates activator‐inhibitor balance in murine tooth development through Fgf20 signaling. Development 139, 3189–3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris MP, Hasso SM, Ferguson MW, et al. (2006) The development of archosaurian first‐generation teeth in a chicken mutant. Curr Biol 16, 371–377. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire J‐Y, Witten PE (2009) Evolutionary and developmental origins of the vertebrate dentition. J Anat 214, 465–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Amasaki H (1995) Development and physiological degradation of tooth buds and development of rudiment of baleen plate in southern minke whale Balaenoptera acutorostrata . J Vet Med Sci 57, 665–670. [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Amasaki H, Dohguchi H, et al. (1999) Immunohistological distributions of fibronectin, tenascin, Type I, III, and IV collagens, and laminin during tooth development and degeneration in fetuses of minke whale, Balaenoptera acutorostrata . J Vet Med Sci 61, 227–232. [DOI] [PubMed] [Google Scholar]
- Järvinen E, Salazar‐Ciudad I, Birchmeier W, et al. (2006) Continuous tooth generation in mouse is induced by activated epithelial Wnt/beta‐catenin signaling. Proc Natl Acad Sci USA 103, 18 627–18 632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Järvinen E, Tummers M, Thesleff I (2009) The role of the dental lamina in mammalian tooth replacement. J Exp Zool B Mol Dev Evol 312B, 281–291. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I (2000) Reiterative signaling and patterning during mammalian tooth morphogenesis. Mech Dev 15, 19–29. [DOI] [PubMed] [Google Scholar]
- Jernvall J, Thesleff I (2012) Tooth shape formation and tooth renewal: evolving with the same signals. Development 139, 3487–3497. [DOI] [PubMed] [Google Scholar]
- Julin C (1880) Recherches sur l'ossification du maxillaire inférieur et sur la constitution du système dentaire chez le foetus de la Balaenoptera rostrata . Arch Biol 1, 75–136. [Google Scholar]
- Karin M, Delhase M (2000) The I kappa B kinase (IKK) and NF‐kappa B: Key elements of proinflammatory signalling. Semin Immunol 12, 85–98. [DOI] [PubMed] [Google Scholar]
- Karlsen K (1962) Development of the tooth germs and adjacent structures in the whalebone whale (Balaenoptera physalus (L.)) with a contribution to the theories of the mammalian dentition. Hvalrådets Skrifter 45, 1–56. [Google Scholar]
- Kettunen P, Thesleff I (1998) Expression and function of FGFs‐4, ‐8, and ‐9 suggest functional redundancy and repetitive use as epithelial signals during tooth morphogenesis. Dev Dyn 211, 256–268. [DOI] [PubMed] [Google Scholar]
- Kükenthal W (1891) Űber den Ursprung und die Entwicklungsgeschichte der Saügetiere. Gegenb Morph Jb 1891, 469–489. [Google Scholar]
- Kükenthal W (1893) Vergleichend anatomische und entwicklungsgeschichtliche Untersuchungen an Waltieren. Denkschr Med‐Naturwis Ges Jena, I‐III, 1–448. [Google Scholar]
- Lambertsen RH, Hintz RJ, Lancaster WC, et al. (1989) Characterization of the functional morphology of the mouth of the bowhead whale, Balaena mysticetus, with special emphasis on feeding and filtration mechanisms. Report for the period 1 July 1987 through 30 June 1989. Department of Wildlife Management, North Slope Borough, Barrow, Alaska, pp. 1–134.
- Lambertsen RH, Rasmussen KJ, Lancaster WC, et al. (2005) Functional morphology of the mouth of the bowhead whale and its implications for conservation. J Mamm 86, 342–352. [Google Scholar]
- Louchart A, Viriot L (2011) From snout to beak: the loss of teeth in birds. Tr Ecol Evol 26, 663–673. [DOI] [PubMed] [Google Scholar]
- Lubetkin SC, Zeh JE, Rosa C, et al. (2008) Age estimates for young bowhead whales (Balaena mysticetus) using annual baleen growth increments. Can J Zool 86, 525–538. [Google Scholar]
- Marx F (2011) The more the merrier? A large cladistics analysis of mysticetes, and comments on the transition from teeth to baleen. J Mamm Evol 18, 77–100. [Google Scholar]
- Marx FG, Fordyce RE (2015) Baleen boom and bust: a synthesis of mysticete phylogeny, diversity, and disparity. R Soc Open Sci 2, 140 434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith W, Gatesy J, Murphy WJ, et al. (2009) Molecular decay of the tooth gene Enamelin (ENAM) mirrors the loss of enamel in the fossil record of placental mammals. PLoS Genet 5, e1000634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mišek I, Witter K, Peterka M, et al. (1996) Initial period of tooth development in dolphins (Stenella attenuata, Cetacea) – a pilot study. Acta Veter Brno 65, 277–284. [Google Scholar]
- Mitsiadis TA, Smith MM (2006) How do genes make teeth to order through development? J Exp Zool 306B, 177–182. [DOI] [PubMed] [Google Scholar]
- Mitsui S, Ohuchi A, Hotta M, et al. (1997) Genes for a range of growth factors and cyclin‐dependent kinase inhibitors are expressed by isolated human hair follicles. Br J Dermatol 137, 693–698. [PubMed] [Google Scholar]
- Miyazaki N (2002) Teeth In: Encyclopedia of Marine Mammals, 1st edn (eds Perrin WF, Würsig B, Thewissen JGM.), pp. 1227–1235. San Diego: Academic Press. [Google Scholar]
- Munne PM, Felszeghy S, Jussila M, et al. (2010) Splitting placodes: effects of bone morphogenetic protein and activing on the patterning and identity of mouse incisors. Evol Devel 12, 383–392. [DOI] [PubMed] [Google Scholar]
- Murashima‐Suginami A, Takahashi K, Sakata T, et al. (2008) Enhanced BMP signaling results in supernumerary tooth formation in USAG‐1 deficient mice. Biochem Biophys Res Comm 16, 1012–1016. [DOI] [PubMed] [Google Scholar]
- Nadiri A, Kuchler‐Bopp S, Lesot H (2004) Immunolocalization of BMP‐2/‐4, FGF‐4, and WNT10b in the developing mouse lower first molar. J Histochem Cytochem 52, 103–112. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Unda F, de‐Vega S, et al. (2006) The krüppel‐like factor epiprofin is expressed by epithelium of developing teeth, hair hollicles, and limb buds and promotes cell proliferation. J Biol Chem 279, 626–634. [DOI] [PubMed] [Google Scholar]
- Nakamura T, de Vega S, Fukumoto S, et al. (2008) Transcription factor epiprofin is essential for tooth morphogenesis by regulating epithelial cell fate and tooth number. J Biol Chem 283, 4825–4833. [DOI] [PubMed] [Google Scholar]
- Nakatake Y, Hoshikawa M, Asaki T, et al. (2001) Identification of a novel fibroblast growth factor, FGF‐22, preferentially expressed in the inner root sheath of the hair follicle. Biochim Biophys Acta 1517, 460–463. [DOI] [PubMed] [Google Scholar]
- Niswander L, Martin GR (1992) Fgf‐4 expression during gastrulation, myogenesis, limb and tooth development in the mouse. Development 114, 755–768. [DOI] [PubMed] [Google Scholar]
- Ohazama A, Haworth KE, Ota MA, et al. (2010) Ectoderm, endoderm, and the evolution of heterodont dentitions. Genesis 48, 382–389. [DOI] [PubMed] [Google Scholar]
- Pantalacci S, Prochazka J, Martin A, et al. (2008) Patterning of palatal rugae through sequential addition reveals an anterior/posterior boundary in palatal development. BMC Dev Biol 8, 116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porntaveetus T, Oommen S, Sharpe PT, et al. (2010a) Expression of Fgf signalling pathway related genes during palatal rugae development in the mouse. Gene Expr Patt 10, 193–198. [DOI] [PubMed] [Google Scholar]
- Porntaveetus T, Otsuka‐Tanaka Y, Basson MA, et al. (2010b) Expression of fibroblast growth factors (Fgfs) in murine tooth development. J Anat 218, 534–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues HG, Marangoni P, Šumbera R, et al. (2011) Continuous dental replacement in a hyper‐chisel tooth digging rodent. Proc Natl Acad Sci USA 108, 17 355–17 359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenquist TA, Martin GR (1996) Fibroblast growth factor signaling in the hair growth cycle: expression of the fibroblast growth factor receptor and ligand genes in the murine hair follicle. Dev Dyn 205, 379–386. [DOI] [PubMed] [Google Scholar]
- Saint Hilaire G (1807) Considerations sur pieces de la tête osseuse des animaux vertébrés et particulairement sur celles du crâne des oiseaux. Ann Mus Hist Nat 10, 342–365. [Google Scholar]
- Salazar‐Ciudad I, Jernvall J (2010) A computational model of teeth and the developmental origins of morphological variation. Nature 464, 583–586. [DOI] [PubMed] [Google Scholar]
- Smith MM, Coates MI (2000) Evolutionary origins of teeth and jaws: developmental models and phylogenetic patterns In: Development, Function and Evolution of Teeth. (eds Teaford M, Smith M, Ferguson M.), pp. 133–151. Cambridge: Cambridge University Press. [Google Scholar]
- Štěrba O, Klima M, Schildger B (2000) Embryology of dolphins, staging and ageing of embryos and fetuses of some cetaceans. Adv Anat Emb Cell Bio 157, 1–133. [PubMed] [Google Scholar]
- Szewciw LJ, de Kerckhove DG, Grime GW, et al. (2010) Calcification provides mechanical reinforcement to whale baleen alpha‐keratin. Proc Biol Sci 277, 2597–2605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thesleff I (2000) Genetic basis of tooth development and dental defects. Acta Odont Scand 58, 191–194. [DOI] [PubMed] [Google Scholar]
- Thewissen JGM (2014) The Walking Whales, from Land to Water in Eight Million Years. Berkeley: University of California Press. [Google Scholar]
- Thewissen JGM, Heyning J (2007) Chapter 15, embryogenesis and development in Stenella attenuata and other cetaceans In: Reproductive Biology and Phylogeny of Cetacea. (ed. Miller D.), pp. 307–329. USA, Enfield New Hampshire: Science Publishers. [Google Scholar]
- Thewissen JGM, Williams EM, Roe LJ, et al. (2001) Skeletons of terrestrial cetaceans and the relationship of whales to artiodactyls. Nature 413, 277–281. [DOI] [PubMed] [Google Scholar]
- Thewissen JGM, Cohn MJ, Stevens LS, et al. (2006) Developmental basis for hind limb loss in dolphins and the origin of the cetacean bodyplan. Proc Natl Acad Sci USA 103, 8414–8418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thewissen JGM, Cooper LN, Clementz MT, et al. (2007) Whales originated from aquatic artiodactyls in the Eocene epoch of India. Nature 450, 1190–1194. [DOI] [PubMed] [Google Scholar]
- Tokita M, Chaeychomsri W, Siruntawineti J (2013) Developmental basis of toothlessness in turtles: insight into convergent evolution of vertebrate morphology. Evolution 67, 260–273. [DOI] [PubMed] [Google Scholar]
- Trott JR, Lucow R (1964) A macroscopic and microscopic investigation of the teeth of two cyclostomes – Petromyzon marinus and Polistotema stoutii . Act Anat 6, 201–215. [DOI] [PubMed] [Google Scholar]
- Tsai C‐H, Fordyce RE (2014) Disparate heterochronic processes in baleen whale evolution. Evol Biol 41, 299–307. [Google Scholar]
- Tucker A, Sharpe P (2004) The cutting‐edge of mammalian development; how the embryo makes teeth. Nat Rev Genet 5, 499–508. [DOI] [PubMed] [Google Scholar]
- Tucker AS, Markham HJ, Green P, et al. (1998) A novel approach for inhibiting growth factor signaling in murine tooth development, inhibition of FGF's. Eur J Oral Sci 106(Suppl. 1), 122–125. [DOI] [PubMed] [Google Scholar]
- Uhen MD (2004) Form, function, and anatomy of Dorudon atrox (Mammalia, Cetacea): an archaeocete from the middle to late Eocene of Egypt. Univ Michigan Pap Paleont 34, 1–222. [Google Scholar]
- Van Nievelt AFH, Smith KK (2005) To replace or not to replace: the significance of reduced tooth replacement in marsupial and placental mammals. Paleobiology 31, 324–346. [Google Scholar]
- Werth A (2000) Feeding in marine mammals In: Feeding, Form, Function, and Evolution in Tetrapod Vertebrates. (ed. Schwenk K.), pp. 487–526. San Diego, California: Academic Press. [Google Scholar]
- Westergaard B, Ferguson MW (1990) Development of the dentition in Alligator mississippiensis: upper jaw dental and craniofacial development in embryos, hatchlings, and young juveniles, with a comparison to lower jaw development. Am J Anat 187, 393–421. [DOI] [PubMed] [Google Scholar]
- Whitlock JA, Richman JM (2013) Biology of tooth replacement in amniotes. Int J Oral Sci 5, 66–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P, Ng CS, Yan J, et al. (2004) Topographical mapping of α‐ and β‐keratins on developing chicken skin integuments: functional interaction and evolutionary perspectives. Proc Natl Acad Sci USA 112, E6770–E6779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka A, Yasui K, Sonomura T, et al. (2007) Development of heterodont dentition in house shrew (Suncus murinus). Eur J Oral Sci 115, 433–440. [DOI] [PubMed] [Google Scholar]


