Abstract
The iliopsoas of the rat is composed of two muscles – the psoas major muscle and the iliacus muscle. The psoas major muscle arises from all the lumbar vertebrae and the iliacus muscle from the fifth and sixth lumbar vertebrae and ilium. Their common insertion point is the lesser trochanter of the femur, and their common action is the lateral rotation of the femur and flexion of the hip joint. Unlike humans, the rat is a quadruped and only occasionally rises up on its hind legs. Therefore, it is expected that the fibre type composition of the rat iliopsoas muscle will be different than that of humans. The iliopsoas muscle of the rat is generally considered to be a fast muscle. However, previous studies of the fibre type composition of the rat psoas muscle showed different results. Moreover, very little is known about the composition of the rat iliacus muscle. The aim of our study was to examine the fibre type composition of the rat iliopsoas muscle in order to better understand the complex function of the listed muscle. The psoas major muscle was examined segmentally at four different levels of its origin. Type I, IIA, IIB and IIX muscle fibres were typed using monoclonal antibodies for myosin heavy chain identification. The percentage of muscle fibre types and muscle fibre cross‐sectional areas were calculated. In our study we showed that in the rat iliopsoas muscle both the iliacus and the psoas major muscles had a predominance of fast muscle fibre types, with the highest percentage of the fastest IIB muscle fibres. Also, the IIB muscle fibres showed the largest cross‐sectional area (CSA) in both muscles. As well, the psoas major muscle showed segmental differences of fibre type composition. Our results showed changes in percentages, as well as the CSAs of muscle fibre types in cranio‐caudal direction. The most significant changes were visible in type IIB muscle fibres, where there was a decrease of percentages and the CSAs from the cranial towards the caudal part of the muscle. From our results it is evident that the rat iliopsoas muscle has a heterogeneous composition and is composed of all four muscle fibre types. Primarily, it is a fast, dynamic muscle with a predominance of fast type IIB muscle fibres with the largest CSAs. The composition of the rat psoas major muscles changes in a cranio‐caudal direction, thus pointing to a more postural role of the caudal part of the muscle.
Keywords: fibre types, iliopsoas muscle, immunohistochemistry, myosin heavy chain, rat
Introduction
In humans, the iliopsoas muscle has a very complex function. In particular this relates to the psoas major muscle, as, unlike iliacus muscle, beside the hip joint it crosses the sacroiliac joint as well as joints of the lumbar spine (Williams & Newel, 2005). In addition to its dynamic function, it also has a postural function due to the upright stance of humans (Walther, 1981). Such a complex function is reflected by an appropriate composition of the psoas muscle, so in humans there is remarkably high percentage of fast as well as slow muscle fibre types (Johnson et al. 1973; Havenith et al. 1990; Zheng et al. 1992; Parkkola et al. 1993; Arbanas et al. 2009). Moreover, the psoas major muscle shows segmental differences in its composition (Arbanas et al. 2009). On the other hand, unlike humans, the rat is a quadruped and only occasionally rises up on its hind legs. Therefore, it is expected that the composition of the rat iliopsoas muscle will be different when compared with that of humans.
The iliopsoas of the rat is composed of two muscles – the psoas major muscle and the iliacus muscle. The psoas major muscle arises from all the lumbar vertebrae and the iliacus muscle from the fifth and sixth lumbar vertebrae and ilium. Their common insertion point is the lesser trochanter of the femur, and their common action is the lateral rotation of the femur and flexion of the hip joint. Moreover, m. iliopsoas act as stabilizer of the lumbar spine (Hebel & Stromberg, 1986; Wingerd, 1988).
The psoas major muscle of the rat is generally considered to be a fast muscle. However, previous studies of the composition of the rat psoas muscle showed different results. Cassidy et al. (1988) studied the influence of the upright posture and bipedalism on the lumbosacral spine and paravertebral muscles of the rat. Muscle fibres were typed by staining micron sections for NADH‐tetrazolium reductase. They did not separate different type II muscle fibres. They showed a predomination of slow type I muscle fibres in the psoas major muscle of healthy rats. The ratios of type I to type II fibres in right and left muscles were 1.28 and 1.24, respectively (Cassidy et al. 1988).
Hämäläinen & Pette (1993) analysed the profiles of the fast type IIB, IID (type IID muscle fibres correspond to IIX muscle fibres), and IIA muscle fibres in the skeletal muscles of the mouse, rat and rabbit. In contrast to the aforementioned study, they described a predominance of fast muscle fibre types compared with slow ones. Using the electrophoretic separation of myosin heavy chain isoforms, the major histocompatibility complex (MHC) I isoform was present in less than 1% in the psoas muscle of rat, whereas the MHC IIa isoform was present at 8%. The most predominant were MHC IId and MHC IIb isoforms, with 45 and 46%, respectively. With regard to the cross‐sections stained for mATP‐ase, in the rat skeletal muscles, type IIB fibres had the largest CSAs, the CSAs of the type IID fibres were intermediate in size, and the IIA fibres were the smallest (Hämäläinen & Pette, 1993).
Edstrom et al. (1982) described the rat psoas muscle as composed exclusively of type IIB fibres (glycolytic fibres; Edstrom et al. 1982).
Schilling et al. (2005) analysed fibre type distribution pattern in all paravertebral muscles on laboratory rat at caudal thoracic and lumbar regions of the back. They used the alkaline combination reaction based on Ziegan's protocol for identification of type I (slow twitch, oxidative muscle fibres), type IIa (fast twitch, oxidative glycolytic muscle fibres) and type IIb (fast twitch, glycolytic muscle fibres). Psoas major muscle showed a heterogeneous distribution of fibre types with the highest percentage of glycolytic fibres. At origin the psoas major muscle was completely free of oxidative fibres and the proportion increased slightly towards the caudal and central region of the muscle belly.
Hesse et al. (2010) segmentally investigated the composition of perivertebral musculature in mice. They described larger CSAs of type IIB muscle fibres than other fibre types. Regarding the proportion of the different muscle fibre types, the psoas muscle was almost entirely composed of type IIA and IIB muscle fibres and did not show any difference in composition from the cranial to the caudal part of the muscle (Hesse et al. 2010).
The rat iliacus muscle is also considered to be a fast muscle, but very little is known about its composition.
The aim of our study was to examine immunohistochemically the fibre type composition of the rat psoas muscle at different levels of its origin, and of the rat iliacus muscle in order to better understand the complex function of the listed muscles.
Materials and methods
Materials
All work described in this study was performed according to the protocols approved by the Ethical Committee of the Faculty of Medicine, University of Rijeka, Croatia. Three‐month‐old male Wistar rats (n = 10) weighing about 250 g were used. All animals were provided with food and water ad libitum. The animals were euthanized with intraperitoneal injection of high doses of ketamine hydrochloride and xylazine hydrochloride. After the extraction of the complete left and right iliopsoas muscles, iliacus muscle was separated from psoas major muscle. M. psoas minor was excluded from this analysis. The psoas major muscle was segmentally cut on four levels at constant intervals from origin to insertion. Afterwards, the muscle samples were frozen in liquid nitrogen and samples were stored in a freezer at −80 °C. The frozen blocks were then serially cut into 5‐μm‐thick cryosections (CM1850, Leica, Vienna, Austria). Sections were cut perpendicular to the fibre direction (cross‐sections).
Methods
Immunohistochemistry
Type I, IIA, IIB and IIX muscle fibres were typed using monoclonal antibodies for myosin heavy chain identification: BA‐F8 specific to the MHC‐I isoform; SC‐71 specific to the MHC‐IIa isoform; BF‐F3 specific to the MHC‐IIb isoform; and BF‐35 specific to all MHC isoforms except the MHC‐IIx isoform (Fig. 1). These monoclonal antibodies were kindly provided by Prof. Stefano Schiaffino (University of Padua, Italy; Schiaffino et al. 1989). The slides were incubated for 1 h with primary antibodies at room temperature in a humidified environment and were washed afterwards in phosphate‐buffered saline – Tween 20 for 3 × 5 min. The secondary antibody, biotinylated anti‐mouse/anti‐rabbit IgG, was applied at room temperature (22 °C) for 30 min and the slides then incubated for 15 min with peroxidase‐labelled streptavidin (Dako, Copenhagen, Denmark). After washing the sections with phosphate‐buffered saline, the bound antigen–antibody complex was visualized with 3.3′‐diaminobenzidine in chromogen solution (Dako).
Figure 1.
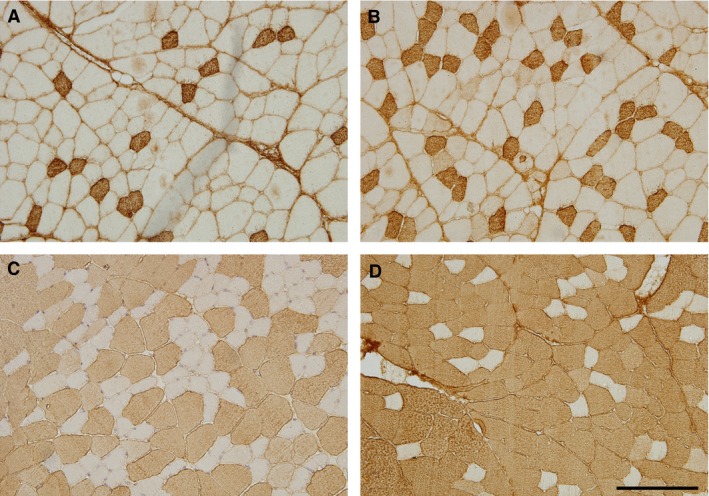
Determining the types of the muscle fibre types using myosin heavy chain identification: (A) Type I; (B) Type IIA; (C) Type IIB; and (D) Type IIX (white, not stained) muscle fibres. Scale bar: 200 μm.
Fibre typing and morphometry
Muscle sections were observed and digital images acquired using a microscope (Olympus BX51; Olympus, Tokyo, Japan) connected to a digital microscope camera (Olympus DP70; Olympus) connected to a personal computer. Images were captured at a magnification of 200× and analysed using an image analysis toolbox jmicrovision [Roduit, N. jmicrovision: Image analysis toolbox for measuring and quantifying components of high‐definition images. Version 1.2.7.6 http://www.jmicrovision.com (accessed 25 September 2012)]. The percentages of muscle fibre types and muscle fibre CSAs were calculated from the five fields of view of the muscle samples obtained from the right and left iliacus muscle. In addition, the five fields of view were obtained from each of the four different levels of origin of the right and left psoas major muscle.
Statistics
The results of the measurement are displayed as means and standard deviations. A two‐way anova was used to make comparisons between the CSAs and percentages of muscle fibre types in the psoas major and iliacus muscles (n = 10 rats). Tukey's HSD test was used to identify posthoc differences between the groups. An independent t‐test was used to test differences in fibre type composition between the psoas major and the iliacus muscle, and to test differences in fibre type composition between the different levels of the psoas major muscle. The statistical significance was set at P < 0.05.
medcalc 16.2.1. (MedCalc, Belgium) and statistica 12 (StatSoft Inc., Tulsa, OK, USA) computer software was used for statistical analysis.
Results
Composition of the rat iliacus muscle
The percentages of the iliacus muscle fibres are presented in Table 1. The CSAs of iliacus muscle fibres are presented in Table 2. There were no differences in percentages and CSAs of the same muscle fibre types between the left and right iliacus muscle (anova P < 0.001; all posthoc P > 0.05).
Table 1.
Percentages (%) of the muscle fibres in iliopsoas muscle
| Type | n | Left | Right | ||||
|---|---|---|---|---|---|---|---|
| Iliacus | Psoas major | P a | Iliacus | Psoas major | P a | ||
| I | 10 | 5.72 ± 4.30 | 6.51 ± 1.48b | 0.587 | 7.90 ± 3.36 | 7.00 ± 1.15b | 0.432 |
| IIA | 10 | 17.28 ± 9.56 | 23.10 ± 6.43 | 0.127 | 23.50 ± 10.90 | 25.45 ± 2.42 | 0.588 |
| IIB | 10 | 57.34 ± 18.40c | 48.15 ± 4.83d | 0.144 | 48.24 ± 21.95c | 47.24 ± 3.10d | 0.888 |
| IIX | 10 | 19.66 ± 8.21 | 22.23 ± 3.10 | 0.368 | 20.36 ± 9.23 | 20.31 ± 2.73 | 0.988 |
Values are mean ± SD.
P‐value stands for the comparison of iliacus to psoas major muscle.
Type I muscle fibres are present at a significantly lower percentage compared with other muscle fibre types in the left and right psoas major muscle (P < 0.001).
Type IIB fibres are present at a significantly higher percentage compared with other muscle fibre types in the left and right iliacus muscles (P < 0.001).
Type IIB muscle fibres are present at a significantly higher percentage compared with other muscle fibre types in the left and right psoas major muscle (P < 0.001).
Table 2.
Cross‐sectional areas (μm2) of the muscle fibres in the iliopsoas muscle
| Type | n | Left | Right | ||||
|---|---|---|---|---|---|---|---|
| Iliacus | Psoas major | P a | Iliacus | Psoas major | P a | ||
| I | 10 | 1950.28 ± 429.77 | 1361.38 ± 293.14 | < 0.05 | 2089.77 ± 543.08 | 1332.08 ± 228.05 | < 0.001 |
| IIA | 10 | 1710.69 ± 362.68 | 1297.98 ± 318.35 | < 0.05 | 1721.01 ± 425.92 | 1273.05 ± 186.91 | < 0.05 |
| IIB | 10 | 4476.09 ± 827.71b | 3752.27 ± 780.66c | 0.059 | 4089.42 ± 656.30b | 3850.66 ± 519.93c | 0.379 |
| IIX | 10 | 2010.47 ± 536.33 | 1761.27 ± 302.03 | 0.217 | 2060.43 ± 586.03 | 1724.59 ± 298.49 | 0.123 |
Values are mean ± SD.
P‐value stands for the comparison of iliacus to psoas major muscle.
Type II B muscle fibres have a significantly larger CSAs compared with other muscle fibre types in the left and right iliacus muscle (P < 0.001).
Type II B muscle fibres have significantly larger CSAs compared with other muscle fibre types in the left and right psoas major muscle (P < 0.001).
There was a significantly higher percentage of Type IIB fibres than other muscle fibre types (anova P < 0.001; all posthoc P < 0.001). There were no significant differences in the percentages of type I, IIA and IIX muscle fibres (anova P < 0.001; all posthoc P > 0.05; Table 1).
Type II B muscle fibres were significantly larger than other muscle fibre types (anova P < 0.001; all posthoc P < 0.001). There were no differences in the CSAs between the type I, IIA and IIX muscle fibres (anova P < 0.001; all posthoc P > 0.05; Table 2).
Composition of the rat psoas major muscle
The percentages of psoas major muscle fibres are presented in Table 1 and the CSAs in Table 2. There were no differences in the percentages and the CSAs of the same muscle fibre types between the left and right psoas major muscle (anova P < 0.001; all posthoc > 0.05).
There was a significantly higher percentage of Type IIB fibres than other muscle fibre types (anova P < 0.001; all posthoc P < 0.001). Moreover, there was a significantly lower percentage of Type I muscle fibres than other muscle fibre types (anova P < 0.001; all posthoc P < 0.001). There were no significant differences in the percentages of type IIA and IIX muscle fibres (anova P < 0.001; all posthoc P > 0.05; Table 1).
Type II B muscle fibres were significantly larger than other muscle fibre types (anova P < 0.001; all posthoc P < 0.001). There were no differences in the CSAs between the type I, IIA and IIX muscle fibres (anova P < 0.001; all posthoc P > 0.05; Table 2).
Composition of the rat psoas major muscle at the different levels
The percentages of the psoas major muscle fibres at the different levels are presented in Fig. 2 and the CSAs of the psoas major muscle fibres at the different levels are presented in Fig. 3. There were no differences in the percentages and CSAs of the same muscle fibre types between the left and right psoas major muscle at all analysed levels.
Figure 2.
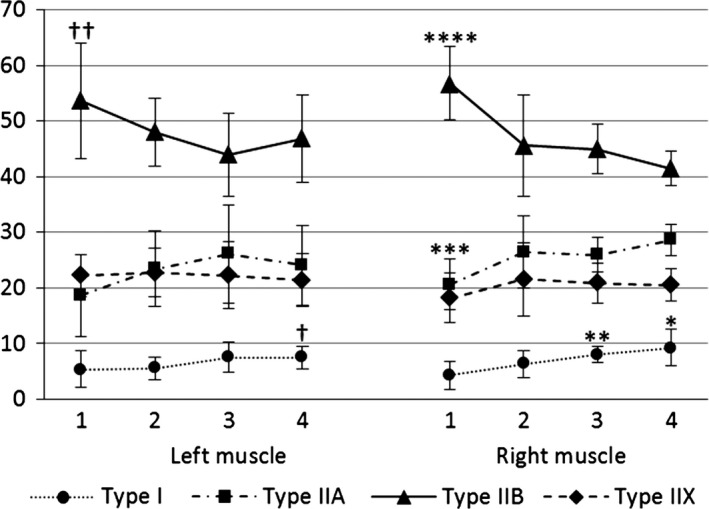
Changes of the percentages (%) of the psoas major muscle fibres between the four different levels analysed. †The percentage of the Type I muscle fibres is significantly higher at the fourth than the second analysed level on the left side (P < 0.05). ††The percentage of Type IIB muscle fibres is significantly higher at the first compared with the third level analysed on the left side (P < 0.05). *The percentage of the Type I muscle fibres is significantly higher at the fourth than at the second and first levels analysed on the right side (both P < 0.05). **The percentage of Type I muscle fibres is significantly higher at the third than at the first level analysed on the right side (P < 0.001). ***The percentage of Type IIA muscle fibres is significantly lower at the first than at the second and third (both P < 0.05) and the fourth (P < 0.001) levels analysed on the right side. ****The percentage of Type IIB muscle fibres is significantly higher at the first than at the second (P < 0.05) and the third and fourth (both P < 0.001) levels analysed on the right side.
Figure 3.
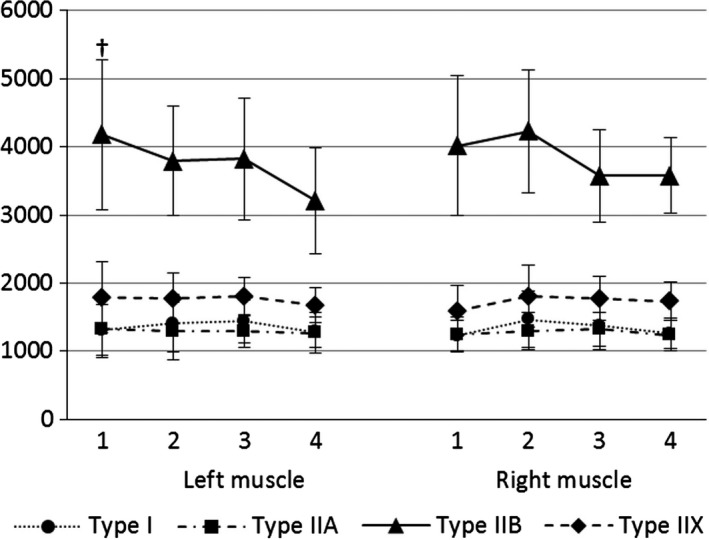
Changes of the cross‐sectional areas (μm2) of the psoas major muscle fibres between the four different levels analysed. †Type II B muscle fibres have significantly larger CSAs at level 1 compared with Type II B muscle fibres at level 4 in the left psoas major muscle (P < 0.05).
There was a significantly lower percentage of type I muscle fibres than other muscle fibre types (all P < 0.001), and a significantly higher percentage of type IIB muscle fibres than other muscle fibre types (all P < 0.001) at all levels analysed levels. There were no differences in percentages between the type IIA and IIX muscle fibres at all levels analysed. The results showed a decrease in the percentages of the type IIB muscle fibres, and an increase in the percentages of the type I and IIA muscle fibres from the first to fourth levels analysed. The percentages of the type IIX muscle fibres remained unchanged between levels. The percentage of the type I muscle fibres was significantly higher at the third than the first level analysed (P < 0.001), and significantly higher at the fourth than the second and first levels analysed on the right side (P < 0.05). Moreover, on the left side, the percentage of type I muscle fibres was significantly higher at the fourth compared with the second level analysed (P < 0.05). The percentage of the type IIA muscle fibres was significantly lower at the first compared with the second and third (both P < 0.05) and the fourth (P < 0.001) levels analysed on the right side. The percentage of the type IIB muscle fibres was significantly higher at the first compared with the third level analysed on the left side (P < 0.05). It was also significantly higher at the first than the second (P < 0.05), and third and fourth (both P < 0.001) levels analysed on the right side (Fig. 2).
Type IIB muscle fibres were significantly larger than the other muscle fibre types at all levels analysed (all P < 0.001). There were no differences in CSAs between type I, IIA and IIX muscle fibres at all levels analysed. The results showed a decrease in the CSA of type IIB muscle fibres from the first to fourth analysed levels. Furthermore, on the left side the type IIB muscle fibres were significantly larger at the first than at the fourth level (P < 0.05). The type I, IIA and IIX muscle fibres showed no differences in the CSAs between the different levels analysed (Fig. 3).
Comparison of the rat iliacus and the psoas major muscles
There were no differences in the percentages of the same muscle fibre types in the psoas and the iliacus muscle (Table 1).
The type I and IIA muscle fibres of the iliacus muscle were significantly larger than the psoas major muscle fibres (all P < 0.05 except type I muscle fibres on the right side with P < 0.001). The type IIB and IIX muscle fibres showed no differences in CSA between the iliacus and the psoas major muscle (Table 2).
Discussion
The composition of the skeletal muscle is directly related to its function. Muscles with a primarily postural function show a predominance of slow type I muscle fibres, whereas muscles with a primarily dynamic function show a higher percentage of fast type II muscle fibres (Gibbons & Comerford, 2001). In humans, the postural muscles play a major role in maintaining an upright stance.
Humans are bipedal creatures, whereas rats are quadruped and only rise on their hind legs occasionally. Therefore, the composition of the rat iliopsoas muscle differs in relation to that of humans, which is composed in accordance with its locomotor and postural function. The human psoas muscle shows a considerably higher percentage of slow type muscle fibres compared with the psoas muscle of the rat.
A number of studies reported a very high percentage (more than 40%) or even a predominance of slow type I muscle fibres in the human psoas muscle (Johnson et al. 1973; Havenith et al. 1990; Zheng et al. 1992; Parkkola et al. 1993; Arbanas et al. 2009).
On the other hand, the psoas muscle of the rat is generally considered to be a fast muscle. Although Cassidy et al. (1988) described a predominance of slow type I muscle fibres, Hämäläinen & Pette (1993) reported less than 1% of MHC I isoform in the rat psoas muscle. However, Cassidy et al. (1988) only used NADH staining and therefore IIA fibres could possibly be among the type I fibres, as they have a high oxidative capacity. Moreover, type IIX muscle fibres, which are generally considered fast twitch fibers with low oxidative activity, in the rat have moderate to strong SDH activity (Sciaffino & Reggiani, 2011) and higher NADH activity (Termin et al. 1989). Therefore, there could also be some type IIX fibres among type I fibres. Edstrom et al. (1982) used SDH staining and described the rat psoas muscle to be composed exclusively of type IIB fibres (glycolytic fibres), which were mostly of low stainability for SDH. Also, there was a minority of small fibres characterized by high or intermediate stainability for SDH which could possibly be type I or type IIA fibres. Schilling et al. (2005) showed a heterogeneous distribution of fibre types in psoas major muscle with the highest percentage of glycolytic fibres (type IIB fibres) using the alkaline combination reaction based on Ziegan's protocol for identification of different fibre types.
Our results correspond to those of Schilling et al. (2005), Hämäläinen & Pette (1993) and Edstrom et al. (1982), and show a low percentage of slow type I muscle fibres (Table 1). Type I muscle fibres were present at a significantly lower percentage compared with other muscle fibre types in the psoas major muscle. This result confirms previous views of the rat psoas major muscle being a fast muscle. However, it should be noted that rats are considerably smaller than humans. Therefore, consideration should be given to the fact that the influence of gravity is different for smaller animals than for humans, which also affects the properties of skeletal muscles. At a small size, gravitational constraint is less important (Gasc, 2001) so in smaller animals we can expect a lower percentage of slow, type I muscle fibres.
In addition, Hämäläinen & Pette (1993) outlined rat skeletal type IIB muscle fibres with the largest CSA and type IIA muscle fibres with the smallest CSA. Our results correspond to those results (Table 2). Type II B muscle fibres had a significantly larger CSAs compared with other muscle fibre types in the psoas major muscle.
The muscles of the rat may show variations in the intramuscular distribution of different fibre types. The regionalization in rat hindlimb muscles has been described. Differences can be found with regard to the deeper or superficial parts, as well as with regard to the proximal and distal parts of the muscle (Wang & Kernell, 2000). Moreover, in rabbits, differences in the expression of MHC can be found over the length of the muscle fibres (Korfage et al. 2016). The results of our study showed changes in the composition of the rat psoas muscle with regard to its segmental origin. The rat psoas major muscle arises from all the lumbar vertebrae and inserts into the lesser trochanter of the femur (Hebel & Stromberg, 1986; Wingerd, 1988). In our study, we demonstrated a decrease of percentages of the type IIB muscle fibres. Furthermore, our results showed an increase of the percentages of the type IIA and slow type I muscle fibres in the psoas major muscle from the cranial toward its caudal part (Fig. 2). Schilling et al. (2005) showed similar results. In its origin the psoas major muscle was completely free of oxidative fibres, but their proportion increased slightly towards the caudal and central region of the muscle belly (Schilling et al. 2005). Moreover, the results of our study showed a decrease of the CSAs of the type IIB muscle fibres in the psoas major muscle from the cranial toward its caudal part. The CSAs of type I, IIA and IIX muscle fibres remained unchanged (Fig. 3). These results suggest differences in the function of the muscle in such a way that function could change from a locomotory to a postural role in the cranio‐caudal direction. Wang & Kernell (2000) described a decrease of density of type I muscle fibres in rat hindlimb muscles in the proximo‐distal direction. In comparison, our results showed the opposite pattern in the distribution of fast and slow muscle fibres in the rat psoas major muscle, which might suggest different functional demands of the psoas muscle compared with hindlimb muscles. Unlike hindlimb muscles, the linear origin of the psoas major muscle spans multiple separate segments of lumbar vertebrae (Hebel & Stromberg, 1986; Wingerd, 1988). Moreover, the lumbar spine in rats plays a huge role in hindlimb protraction during fast gait (Gasc, 2001). Therefore, differences in the composition of the psoas major muscle might be related to the direct contribution of the lumbar spine to hindlimb movements in rats.
Hesse et al. (2010) also segmentally investigated the composition of perivertebral musculature, including the psoas major muscle in rodents. In contrast to our research, they investigated the musculature in mice but not in rats. They analysed the muscles from the intervertebral joint between the 11th and 12th thoracic to the joint between the sixth lumbar and first sacral vertebrae. However, it should be emphasized that mice have an even smaller body size than rats and therefore an even smaller postural problem. Hesse et al. (2010) showed a larger CSA of the type IIB muscle fibres than other fibre types (1.5–2 times larger). Regarding the proportion of the different muscle fibre types, the psoas muscle was almost entirely composed of type IIA and IIB muscle fibres and did not show any differences in composition from the cranial to the caudal part of the muscle. Nevertheless, they did show differences in other perivertebral muscles in the cranio‐caudal direction (Hesse et al. 2010). However, those authors did not investigate the most caudal part of the m. psoas major muscle, which could be why they did not observe an increase of type I and IIA fibres.
Previous studies have not provided a detailed composition of the rat iliacus muscle. Our results showed a similar composition of the iliacus muscle and the psoas muscle, at least regarding the percentages of muscle fibres. No significant differences in the percentages of the same muscle fibre types between the psoas and the iliacus muscle were found (Table 1). However, differences in the CSAs of the muscle fibres. Our results showed larger type I and IIA muscle fibres of the iliacus muscle compared with the psoas major muscle, but the CSAs of the type IIB and IIX muscle fibres did not differ (Table 2).
A similar composition of the iliacus muscle compared with the psoas muscle can be attributed to its anatomical position. The rat iliacus muscle arises from the ilium and also from the lumbar vertebrae, similar to the psoas muscle. Therefore, although we distinguish between two separate muscles, anatomically and functionally they are analogous.
However, the CSAs of type I and type IIA muscle fibres are significantly larger in the iliacus muscle (Table 2), which indicates a shift toward the static role of the muscle. Furthermore, as mentioned before, if we look at the psoas major muscle, its caudal part, which is anatomically most similar to the iliacus muscle, shows more static composition compared with its cranial part.
Conclusion
The rat iliopsoas muscle has a heterogeneous composition and is composed of all four muscle fibre types. Primarily, it is a fast, dynamic muscle with a predominance of fast type IIB muscle fibres with the largest CSAs. The composition of the rat psoas major muscles changes in a cranio‐caudal direction, thus pointing to the more postural role of the caudal part of the muscle.
Conflict of interest
The authors declare that they have no conflict of interest.
Author contributions
J.A. and D.M. contributed to study design, acquisition of data and data analysis. H.V. and V.M. contributed to acquisition of data. T.S.V. contributed to data interpretation. K.B. performed statistical analysis. All authors contributed to drafting of the manuscript, critical revision of the manuscript and approval of the article.
Acknowledgements
This work was supported by the University of Rijeka, Croatia, grant number 841.10.1241.
References
- Arbanas J, Starcevic Klasna G, Nikolic M, et al. (2009) Fibre type composition of the human psoas major muscle with regard to the level of its origin. J Anat 215, 636–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy JD, Yong‐Hing K, Kirkaldy‐Willis WH, et al. (1988) A study of the effects of bipedism and upright posture on the lumbosacral spine and paravertebral muscles of the Wistar rat. Spine 13, 301–308. [DOI] [PubMed] [Google Scholar]
- Edstrom L, Hultman E, Sahlin K, et al. (1982) The contents of high‐energy phosphates in different fibre types in skeletal muscles from rat, guinea‐pig and man. J Physiol 332, 47–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasc JP (2001) Comparative aspects of gait, scaling and mechanics in mammals. Comp Biochem Physiol A Mol Integr Physiol 131, 121–133. [DOI] [PubMed] [Google Scholar]
- Gibbons STG, Comerford MJ (2001) Strength versus stability: Part 1: concept and terms. Orthop Div Rev 12, 21–27. [Google Scholar]
- Hämäläinen N, Pette D (1993) The histochemical profiles of fast fiber types IIB, IID, and IIA in skeletal muscles of mouse, rat, and rabbit. J Histochem Cytochem 41, 733–743. [DOI] [PubMed] [Google Scholar]
- Havenith MG, Visser R, Schrijvers‐van Schendel JM, et al. (1990) Muscle fiber typing in routinely processed skeletal muscle with monoclonal antibodies. Histochemistry 93, 497–499. [DOI] [PubMed] [Google Scholar]
- Hebel R, Stromberg MW (1986) Anatomy and Embriology of the Laboratory Rat, pp. 25–45. Wörthsee: BioMed Verlag. [Google Scholar]
- Hesse B, Fischer MS, Schilling N (2010) Distribution pattern of muscle fiber types in the perivertebral musculature of two different sized species of mice. Anat Rec 293, 446–463. [DOI] [PubMed] [Google Scholar]
- Johnson MA, Polgar J, Weightman D, et al. (1973) Data on the distribution of fibre types in thirty‐six human muscles. An autopsy study. J Neurol Sci 18, 111–129. [DOI] [PubMed] [Google Scholar]
- Korfage JA, Kwee KE, Everts V, et al. (2016) Myosin heavy chain expression can vary over the length of jaw and leg muscles. Cells Tissues Organs 201, 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkkola R, Alanen A, Kalimo H, et al. (1993) MR relaxation times and fiber type predominance of the psoas and multifidus muscle. An autopsy study. Acta Radiol 34, 16–19. [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, et al. (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10, 197–205. [DOI] [PubMed] [Google Scholar]
- Schilling N, Arnold D, Wagner H, et al. (2005) Evolutionary aspects and muscular properties of the trunk – implications for human low back pain. Pathophysiology 12, 233–242. [DOI] [PubMed] [Google Scholar]
- Sciaffino S, Reggiani C (2011) Fiber types in mammalian skeletal muscles. Physiol Rev 91, 1447–1531. [DOI] [PubMed] [Google Scholar]
- Termin A, Staron RS, Pette D (1989) Myosin heavy chain isoforms in histochemically defined fiber types of rat muscle. Histochemistry 92, 453–457. [DOI] [PubMed] [Google Scholar]
- Walther DS (1981) Applied Kinesiology, Vol. I, pp. 302–305. Pueblo: System DC. [Google Scholar]
- Wang LC, Kernell D (2000) Proximo‐distal organization and fibre type regionalization in rat hindlimb muscles. J Muscle Res Cell Motil 21, 587–598. [DOI] [PubMed] [Google Scholar]
- Williams A, Newel RLM (2005) Pelvic girdle, gluteal region and hip joint In: Gray's Anatomy, 39th edn (ed. Standring S.), pp. 1444–1446 . Edinburgh: Churchill Livingstone. [Google Scholar]
- Wingerd BD (1988) Rat Dissection Manual, pp. 28 Baltimore: The Johns Hopkins University Press. [Google Scholar]
- Zheng A, Rahkila P, Vuori J, et al. (1992) Quantification of carbonic anhydrase III and myoglobin in different fiber types of human psoas muscle. Histochemistry 97, 77–81. [DOI] [PubMed] [Google Scholar]


