Abstract
The development of the enteric nervous system (ENS) and intestinal smooth muscle occurs in a spatially and temporally correlated manner, but how they influence each other is unknown. In the developing mid‐gut of the chick embryo, we find that α‐smooth muscle actin expression, indicating early muscle differentiation, occurs after the arrival of migrating enteric neural crest‐derived cells (ENCCs). In contrast, hindgut smooth muscle develops prior to ENCC arrival. Smooth muscle development is normal in experimentally aganglionic hindguts, suggesting that proper development and patterning of the muscle layers does not rely on the ENS. However, inhibiting early smooth muscle development severely disrupts ENS patterning without affecting ENCC proliferation or apoptosis. Our results demonstrate that early intestinal smooth muscle differentiation is required for patterning the developing ENS.
Keywords: development, enteric nervous system, gut, neural crest, smooth muscle
Introduction
Normal development of the enteric nervous system (ENS) is essential in order to ensure the proper regulation of gut function, including motility, secretion, absorption and immunity (Furness, 2006). As enteric neural crest‐derived cells (ENCCs) migrate along the length of the gastrointestinal tract, they pattern into two concentric rings of ganglia that contain neurons and glial cells. These rings are referred to as the submucosal and myenteric plexuses, and are located on either side of the circular smooth muscle layer. Despite their spatial proximity and the similar timing of their development, little is known about how ENCCs and smooth muscle cells influence each other during intestinal organogenesis.
The majority of ENCCs originate from the vagal level of the neural tube and colonize the entire gastrointestinal tract (Yntema & Hammond, 1954). A second population, originating from the sacral neural crest, contributes a smaller number of ENCCs to the distal intestine (Burns & Douarin, 1998; Nagy et al. 2007; Wang et al. 2011). Vagal ENCCs arrive in the avian foregut at embryonic day 3 (E3), migrate antero‐posteriorly, and reach the end of the hindgut at E8 (Le Douarin & Teillet, 1973; Nagy et al. 2012). Mice and humans exhibit a similar pattern of ENS development that occurs at E9.5–E14 (Young et al. 1998, 1999) and at gestational weeks 4–8 (Fu et al. 2004; Wallace & Burns, 2005), respectively. In mice, Schwann cell precursors have also been shown to contribute cells to the ENS (Uesaka et al. 2015). The concentric patterning of the enteric ganglia is preserved in all species, with the two plexuses forming on either side and in close proximity to the circular muscle.
Intestinal smooth muscle arises from the splanchnic mesoderm (Roberts, 2000). Undifferentiated mesenchymal cells elongate and begin to express various smooth muscle lineage markers: α‐smooth muscle actin (SMA) appears first as a marker of early smooth muscle cell differentiation; γ‐SMA and smooth muscle protein 22 appear later; and calponin labels fully differentiated smooth muscle cells (Duband et al. 1993; McHugh, 1996; Nagy et al. 2001; Gabella, 2002; Thomason et al. 2012; McKey et al. 2016). In humans, the circular muscle layer develops first, followed by the longitudinal muscle layer, and finally the muscularis mucosae (Wallace & Burns, 2005). A similar stepwise pattern occurs in the chick (Shyer et al. 2013). Intestinal smooth muscle has been reported to develop bidirectionally, starting at the anterior and posterior ends of the gut (Duband et al. 1993; McHugh, 1996).
The close anatomic relationship between intestinal smooth muscle and the ENS during development suggests a potential link between these two cell lineages. In zebrafish, smooth muscle and enteric neuronal differentiation are concomitant, and mutations in smooth muscle development lead to ENS abnormalities (Wallace et al. 2005), suggesting that proper smooth muscle development is required for ENS patterning. Conversely, stomach smooth muscle differentiation in the chick occurs after colonization by ENCCs, and ablation of ENCCs prior to colonization impairs smooth muscle development (Faure et al. 2015). In the human intestine, smooth muscle differentiation occurs during ENCC colonization (Fu et al. 2004; Wallace & Burns, 2005), but whether a functional relationship exists is unknown.
The goal of this study was to identify and characterize the relationship between smooth muscle and ENS development in the chick embryo. Understanding the interaction between these two cell types during gut development may provide insights into the signals controlling ENS development and the pathogenesis of various congenital neuropathies and myopathies of the intestine.
Materials and methods
Animals
Fertilized White Leghorn chicken (Gallus gallus) eggs were obtained from commercial breeders and incubated at 37 °C in a humidified incubator. Embryos were staged according to Hamburger and Hamilton (HH; Hamburger & Hamilton, 1992) stages or by the number of embryonic days (E). All studies were approved by the Institutional Animal Care and Use Committee at Massachusetts General Hospital.
Immunohistochemistry
Tissue was fixed in 4% paraformaldehyde. For cryosection, tissue was incubated overnight at 4 °C in 15% sucrose, for 1 h at 37 °C in 15% sucrose, 7.5% gelatin solution, and embedded in 15% sucrose, 7.5% gelatin. Gelatin blocks were rapidly frozen at −50 °C and sectioned at 12 μm thickness with a Leica CM3050 S cryostat (Leica, Buffalo Grove, IL, USA). Sections were stained with primary antibody solutions containing 1% bovine serum albumin (Sigma‐Aldrich, St Louis, MO, USA) for 1 h at room temperature. Primary antibodies included N‐cadherin (Ncad; clone: 3B6; 1 : 5; DSHB, Iowa City, IA, USA), anti‐neuronal class III β‐tubulin (clone: Tuj1; 1 : 300; Covance, Dedham, MA, USA), anti‐calponin (clone CP‐93, 1 : 5000; Sigma Aldrich) and α‐SMA (clone: 1A4; 1 : 200; Thermo Fisher, Cambridge, MA, USA). Secondary antibodies included goat anti‐mouse IgG2a, Alexa Fluor 488 and goat anti‐mouse IgG1, Alexa Fluor 594 (1 : 500; Life Technologies, Carlsbad, CA, USA). Cell nuclei were stained with DAPI (Molecular Probes, Eugene, OR, USA). Images were taken with a Nikon Eclipse 80i microscope (Nikon Instruments, Melville, NY, USA).
For analysis of cell proliferation, guts were cultured for 3 days, and 10 μm EdU was added to the media 3 h prior to fixation. EdU incorporation was detected using the Click‐iT EdU Imaging Kit (Invitrogen, Carlsbad, CA, USA). To detect apoptotic cells, serial sections were examined by double immunofluorescence using anti‐Ncad and anti‐activated caspase‐3 antibodies (1 : 50; Cell Signaling, Danvers, MA, USA).
Chorioallantoic membrane (CAM) transplants
Post‐umbilical aganglionic (n = 9) and ganglionic guts (n = 9) were removed from E5 (HH26) chick embryos and transplanted on the CAM of E9 chick as described previously (Nagy & Goldstein, 2006). Cloacas were removed from both ganglionic and aganglionic guts. Ganglionic grafts include the hindgut and post‐umbilical mid‐gut, which has ENCCs at this stage. Aganglionic guts include only the post‐cecal hindgut and therefore have no ENCCs at this stage. Guts were cultured on the CAM for 9 days, after which they were fixed and processed for immunohistochemistry.
Intestinal organ culture assay
Guts were removed from E5 (HH26) chick embryos, fixed to silicone plates with insect pins, and allowed to float in media containing 10 μm AG1295 (Merck Millipore, Billerica, MA, USA) and FK506 (Sigma‐Aldrich) or dimethylsulfoxide (DMSO; 1 : 1000; Sigma‐Aldrich). After 3 days of culture, guts (DMSO‐treated, n = 12; smooth muscle inhibitor‐treated, n = 16) were fixed and processed for immunohistochemistry.
Results
Temporal analysis of normal chick smooth muscle and ENS development
E5–E14 chick intestine was sectioned longitudinally and in cross‐section in order to examine the progression of smooth muscle and ENS development. At E5 (HH26), α‐SMA, a marker of early smooth muscle differentiation, is absent from most of the mid‐gut, from the pyloric region extending nearly to the end of the mid‐gut (Fig. 1A,B). SMA is present in the distal‐most mid‐gut and in the hindgut, but absent from the cecal buds (Fig. 1A,C). SMA is also present in the vascular smooth muscle of the mesenteric artery (Fig. 1B). While SMA is expressed in the E5 hindgut, calponin, a marker of differentiated smooth muscle, is absent from the gut at this stage (Fig. 1D). At E5, Ncad, a cell adhesion molecule that marks neural crest‐derived cells, marks the ENCC wavefront, which has migrated past the umbilicus and reached the distal mid‐gut (Fig. 1A,B).
Figure 1.
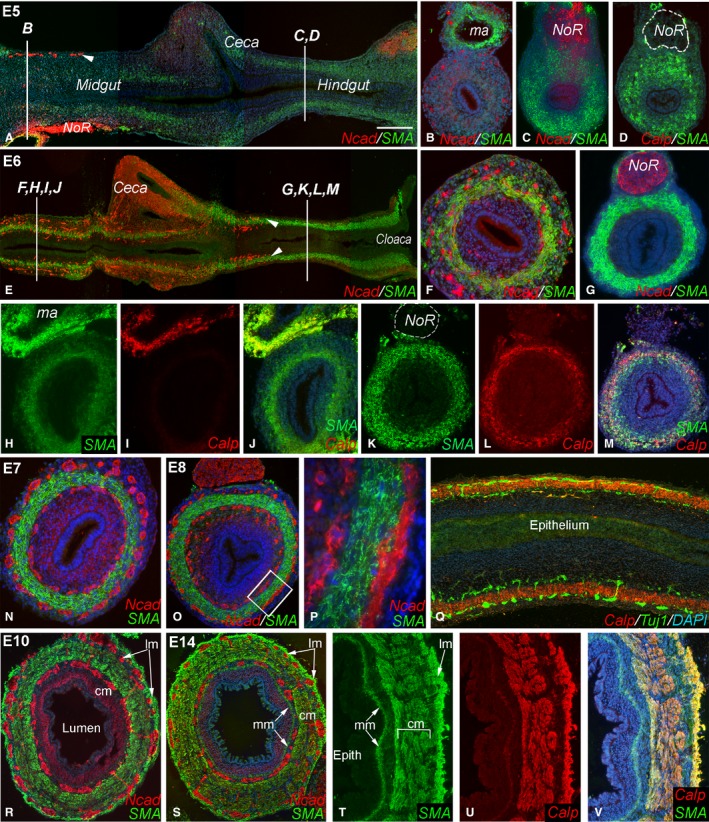
Coordinated development of smooth muscle and enteric nervous system (ENS) during chick gut development. At E5, smooth muscle actin (SMA) expression is very low in the mid‐gut and ceca (A,B). While SMA is present in the hindgut at E5, calponin is not (C,D). At E5, the enteric neural crest‐derived cell (ENCC) wavefront is proximal to the cecal region (A, arrowhead; B). By E6, ENCCs have reached the proximal hindgut (E, arrowheads) and SMA is expressed in both mid‐gut (F) and hindgut (G). E6 mid‐gut expresses SMA but not calponin (H–J), although calponin expression in vascular smooth muscle in a mesenteric artery is seen (I). E6 hindgut expresses both muscle proteins (K–M). Circular smooth muscle and both plexuses are present in E7 mid‐gut (N) and E8 hindgut (O, boxed area magnified in P). E8 longitudinal section shows Tuj1+ enteric neurons on either side of the calponin‐expressing circular muscle layer (Q). By E10, longitudinal muscle layer has formed in the hindgut (R). At E14, the muscularis mucosa is visible (S) and all muscle layers express SMA and calponin (T–V). DAPI staining (when present) is blue. Scale bar: 200 μm (A); 140 μm (B–D); 260 μm (E); 120 μm (F); 100 μm (G); 125 μm (H–Q); 120 μm (45 μ; T–V); 370 µm (P); 120 μm (R). Calp, calponin; cm, circular muscle; epith, epithelium; lm, longitudinal muscle; ma, mesenteric artery; mm, muscularis mucosae; Ncad, N‐cadherin; NoR, nerve of Remak.
At late E6 (HH29), SMA is expressed in the mid‐gut and hindgut, but still absent in the cecal buds (Fig. 1E,F). The ENCC wavefront at this stage has progressed into the proximal hindgut (Fig. 1E). Background Ncad staining is commonly observed in the ceca (Nagy et al. 2012). The remainder of the hindgut is still pre‐ganglionic, but already has well‐developed circular muscle (Fig. 1G). In the E6 distal mid‐gut, SMA is expressed, but calponin is not (Fig. 1H–J), while in the hindgut both proteins are present (Fig. 1K–M).
By E7, SMA is present in the circular muscle layer of the distal mid‐gut, and the submucosal and myenteric plexuses are on either side (Fig. 1N). Similarly, at E8 (HH34), the two plexuses are in close proximity to the circular muscle in the hindgut (Fig. 1,P). In the E8 hindgut, a close physical relationship between Tuj1+ neurons and calponin‐immunoreactive smooth muscle is evident (Fig. 1Q). By E10 (HH36), the longitudinal muscle has formed on the outside of the myenteric plexus, and connecting fibers between the two plexuses are visible (Fig. 1R). At E14 (HH40), the muscularis mucosae has developed internal to the submucosal plexus (Fig. 1S). All three muscle layers co‐express SMA and calponin at this stage (Fig. 1T–V). These results indicate that the intestinal smooth muscle and ENS follow distinct temporal patterns of development in the distal gut. Notably, ENCCs arrive in the mid‐gut prior to smooth muscle specification, whereas in the hindgut they arrive after specification (SMA present) but prior to terminal differentiation (calponin not expressed).
Hindgut smooth muscle develops independently from the ENS
To determine whether ENS development is required for smooth muscle formation, intestine was explanted at E5, when the hindgut is still aneural, and grafted onto the CAM of an E9 (HH35) host embryo for 9 days. The cloaca and nerve of Remak were removed to prevent ENCC contribution by the sacral neural crest‐derived cells present in these structures. Experimental grafts only included hindgut, whereas control grafts included post‐umbilical mid‐gut, and thus contained ENCCs that were able to colonize the distal intestine during the grafting period.
As expected, by E14 (E5 grafts + 9 days of CAM culture), control grafts formed three normal muscle layers expressing SMA (Fig. 2A) and calponin (Fig. 2C). Two ENS plexuses are also present, containing Ncad + ENCCs (Fig. 2A) and Tuj1+ enteric neurons (Fig. 2C). The patterning is similar to that seen in normal E14 hindgut (Fig. 1S). In the experimental grafts, no ENS formed, as expected (Fig. 2B,D). However, normal SMA (Fig. 2B) and calponin (Fig. 2D) expression was present despite the absence of the ENS (Fig. 2B), indicating that smooth muscle development in the hindgut does not rely on the presence of an ENS.
Figure 2.
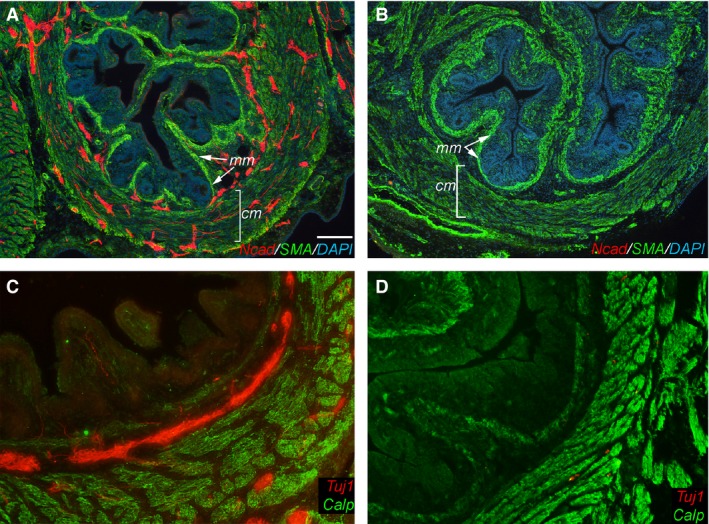
Intestinal smooth muscle differentiation is independent of enteric nervous system (ENS) formation. Ganglionic (A,C) and aganglionic (B,D) E5 chick hindgut was grafted onto the choriallantoic membranes (CAM) and assessed for smooth muscle actin (SMA) (A,B) and calponin (C,D) immunoreactivity 9 days later. N‐Cadherin (Ncad) (A,B) and Tuj1 (C,D) were used to detect enteric neural crest‐derived cells (ENCCs) and differentiated neurons, respectively. Smooth muscle development in the aganglionic hindgut progressed normally in the absence of the ENS (B,D) and was comparable to that of normally ganglionated intestine (A,C). Scale bar in (A) corresponds to 120 μm (A,B) and 60 μm (C,D). Calp, calponin; cm, circular muscle; mm, muscularis mucosae; Ncad, N‐cadherin.
Inhibition of smooth muscle differentiation disrupts ENS patterning
E5 (HH26) intestine was grown for 3 days in a catenary organ culture with and without inhibitors of visceral smooth muscle development, FK506 and AG1295, in order to assess the effect of smooth muscle on ENS development and patterning. Culturing E5 intestine in the presence of both drugs led to complete inhibition of smooth muscle development in the distal mid‐gut, confirmed by the absence of both SMA (Fig. 3A,B) and calponin (Fig. 3A,B′). In the hindgut, the inhibitors did not prevent SMA expression (Fig. 3A,D), but did inhibit calponin (Fig. 3A,D′).
Figure 3.
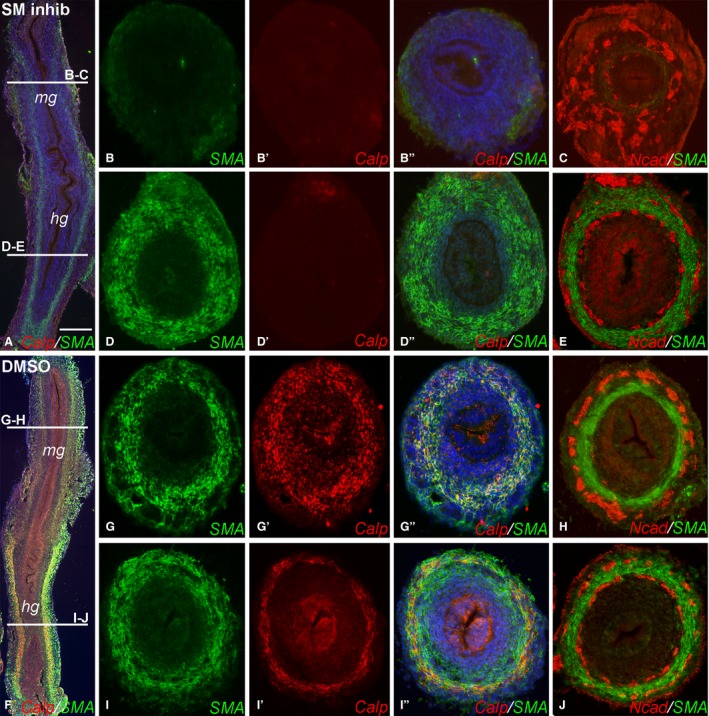
Inhibition of smooth muscle formation leads to disruption of enteric nervous system (ENS) patterning. E5 guts were placed in catenary culture for 3 days in the presence of smooth muscle inhibitors FK‐506 and AG‐1295 (A–E) or the solvent dimethylsulfoxide (DMSO) as control (F–J). Expression of smooth muscle actin (SMA) and calponin were inhibited in the mid‐gut (B–B′′), while only calponin was inhibited in the hindgut (D–D′′). ENS patterning was severely disrupted in the mid‐gut (C), but normal in the hindgut (E). Control guts showed normal SMA, calponin and ENS development in the mid‐gut (G–H) and hindgut (I–K). Scale bar in (A) corresponds to 300 μm (A,F) and 120 μm (B–E, G–J). Calp, calponin; hg, hindgut; mg, mid‐gut; Ncad, N‐cadherin.
Inhibiting smooth muscle development in the mid‐gut resulted in severe disruption of ENS organization (Fig. 3C), with loss of radial patterning and no discernable plexus formation. In contrast, ENS formation in the hindgut, where SMA expression was not inhibited, was unperturbed (Fig. 3E). As a control, E5 guts were cultured in the presence of the solvent DMSO, and formation of both smooth muscle and ENS in the mid‐gut and hindgut were unaffected (Fig. 3F–J). These findings indicate that early, but not late, smooth muscle differentiation is necessary for normal ENS patterning during gut development.
The effect of the smooth muscle inhibitors, FK506 and AG1295, on ENCC proliferation was analyzed using EdU incorporation and compared with untreated controls (Fig. 4A,B). The rate of ENCC proliferation in E5 guts treated for 3 days was the same as the proliferation rate in control guts (28.2 ± 6.1% vs. 27.9 ± 6.0%; P = NS using Student's t‐test). Cell apoptosis was examined using caspase‐3 immunoreactivity, and no significant difference in the rate of cell death was observed (Fig. 4C,D), suggesting that the inhibitors do not cause diffuse mesenchymal injury.
Figure 4.

Smooth muscle inhibitors do not affect enteric neural crest‐derived cell (ENCC) proliferation or survival. E5 guts were cultured for 3 days in the presence of FK‐506 and AG‐1295 (A,C) or dimethylsulfoxide (DMSO) as control (B,D). ENCC proliferation was detected by EdU incorporation in Ncad+ cells (A,B). Cell apoptosis was detected as immunoreactivity to activated caspase‐3 (C,D). Scale bar in (A) corresponds to 60 μm.
Discussion
We find that development of the intestinal smooth muscle and the ENS follow discrete time courses in the mid‐gut and hindgut. In the mid‐gut, ENCC arrival precedes smooth muscle differentiation, with no intestinal SMA expressed at that time. Interestingly, this temporal relationship is reversed in the hindgut, where the smooth muscle develops first. Both SMA and calponin, markers of early and late muscle differentiation, respectively, are expressed before the arrival of ENCCs. As a result, at E5, migrating ENCCs encounter a mid‐gut mesenchymal environment devoid of smooth muscle, whereas 2 days later the wavefront cells are migrating adjacent to differentiated smooth muscle. This would suggest that the presence of ENCCs is not required for smooth muscle to develop in the hindgut, and our CAM grafts with aganglionic intestine confirm this. Similarly, because the ENS migratory wavefront proceeds along the mid‐gut despite the absence of smooth muscle, one can conclude that smooth muscle cells are not required for initial ENS colonization of the small intestine.
Our results reveal a novel, essential role for intestinal smooth muscle in patterning the ENS. Radial patterning of the ENS is characterized by positioning of the two major ganglionated plexuses, submucosal and myenteric, on either side of the circular muscle layer. This suggests the possibility that smooth muscle is required for establishing this concentric radial pattern. In this study, two soluble factors were used to inhibit smooth muscle differentiation. FK506 is an immunosuppressive drug that has been shown to inhibit the differentiation of smooth muscle in the developing chick gizzard (Fukuda et al. 1998). AG1295 is a platelet‐derived growth factor receptor antagonist that inhibits smooth muscle differentiation in the embryonic mouse gut (Kurahashi et al. 2008). Both drugs have been used to inhibit smooth muscle formation in E6 chick mid‐gut without influencing cell proliferation or survival (Shyer et al. 2013). We found that smooth muscle development was most effectively inhibited using a combination of both drugs. Importantly, these inhibitors do not appear to have a direct effect on ENCCs. Shyer et al. (2013) reported that they did not observe any effects on non‐smooth muscle cells in their avian gut study. We find that the ENS in the colon develops normally in the presence of these inhibitors, and also observe no effect on the rate of ENCC proliferation of survival, confirming the muscle specificity of these inhibitors.
Using catenary cultures, we show that inhibition of smooth muscle development leads to severe disruption of ENS patterning and failure of plexus development in the mid‐gut. Interestingly, the inhibitors prevented SMA expression in the mid‐gut, indicating failure of smooth muscle specification in that part of the intestine. In contrast, in the hindgut, where SMA is already expressed when the culture period begins at E5, SMA was not inhibited. However, the inhibitors prevented calponin expression, indicating inhibition of late smooth muscle differentiation in the hindgut. Despite the absence of calponin expression, ENCC migration and patterning proceeded normally in the hindgut. These results suggest that early muscle specification, marked by SMA expression, is essential for ENS patterning, whereas later muscle differentiation is not.
In the developing chick stomach (Faure et al. 2015) and human intestine (Fu et al. 2004; Wallace & Burns, 2005), smooth muscle differentiation has been suggested to occur after ENCC colonization. Lack of ENCC in the chick stomach prevents proper patterning of the smooth muscle (Faure et al. 2015), indicating an important role for neural crest‐derived cells in development of gastric smooth muscle. In contrast, we do not find this to be the case in the hindgut, where smooth muscle develops normally in the absence of ENCCs, suggesting fundamental differences in the development of these regions of the gastrointestinal tract. Our results are consistent with previous studies showing that the muscle layers form normally in cultured aneural hindgut (Smith et al. 1977; Newgreen et al. 1980).
The mechanisms that regulate ENCC development are incompletely understood, although a growing body of evidence supports the importance of interactions between ENCCs and the gut microenvironment. These include interactions between ENCC surface receptors and ligands present in the mesenchyme (Goldstein et al. 2013) and extracellular matrix (ECM) proteins (Akbareian et al. 2013; Nagy et al. 2016). Like the smooth muscle, blood vessels share a close anatomic relationship with the ENS, and endothelial cells may act as a substrate for guiding and promoting ENCC migration and proliferation (Nagy et al. 2009). This guided migration is mediated through interactions between β1 integrins on ENCCs and ECM proteins expressed by endothelial cells. Smooth muscle may act in a similar manner to regulate radial patterning of the ENS, which is essential so that appropriate connections can be made between neurons and glial cells and their intended targets. Our results support a role for the intestinal smooth muscle in directing patterning of the ENS during gut development. The circular muscle layer ensures that the enteric plexuses are positioned properly in concentric rings within the gut wall. The underlying mechanisms are unknown, but could rely on signals from smooth muscle cells or associated ECM proteins that direct ENCC positioning. Further studies are needed to identify the nature of these regulatory signals.
Conflict of interest
The authors declare that there is no conflict of interest regarding the publication of this paper.
Acknowledgements
AMG is supported by NIH‐NIDDK R01DK080914. NN is supported by a Bolyai Fellowship of the Hungarian Academy of Sciences.
References
- Akbareian SE, Nagy N, Steiger CE, et al. (2013) Enteric neural crest‐derived cells promote their migration by modifying their microenvironment through tenascin‐C production. Dev Biol 382, 446–456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns AJ, Douarin NM (1998) The sacral neural crest contributes neurons and glia to the post‐umbilical gut: spatiotemporal analysis of the development of the enteric nervous system. Development 125, 4335–4347. [DOI] [PubMed] [Google Scholar]
- Duband JL, Gimona M, Scatena M, et al. (1993) Calponin and SM 22 as differentiation markers of smooth muscle: spatiotemporal distribution during avian embryonic development. Differentiation 55, 1–11. [DOI] [PubMed] [Google Scholar]
- Faure S, McKey J, Sagnol S, de Santa Barbara P (2015) Enteric neural crest cells regulate vertebrate stomach patterning and differentiation. Development 142, 331–342. [DOI] [PubMed] [Google Scholar]
- Fu M, Tam PK, Sham MH, Lui VC (2004) Embryonic development of the ganglion plexuses and the concentric layer structure of human gut: a topographical study. Anat Embryol 208, 33–41. [DOI] [PubMed] [Google Scholar]
- Fukuda K, Tanigawa Y, Fujii G, et al. (1998) cFKBP/SMAP; a novel molecule involved in the regulation of smooth muscle differentiation. Development 125, 3535–3542. [DOI] [PubMed] [Google Scholar]
- Furness JB (2006) The organisation of the autonomic nervous system: peripheral connections. Auton Neurosci 130, 1–5. [DOI] [PubMed] [Google Scholar]
- Gabella G (2002) Development of visceral smooth muscle. Results Probl Cell Differ 38, 1–37. [DOI] [PubMed] [Google Scholar]
- Goldstein AM, Hofstra RM, Burns AJ. (2013) Building a brain in the gut: development of the enteric nervous system. Clin Genet 83, 307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, Hamilton HL (1992) A series of normal stages in the development of the chick embryo. 1951. Dev Dyn. 195, 231‐72. [DOI] [PubMed] [Google Scholar]
- Kurahashi M, Niwa Y, Cheng J, et al. (2008) Platelet‐derived growth factor signals play critical roles in differentiation of longitudinal smooth muscle cells in mouse embryonic gut. Neurogastroenterol Motil 20, 521–531. [DOI] [PubMed] [Google Scholar]
- Le Douarin NM, Teillet MA (1973) The migration of neural crest cells to the wall of the digestive tract in avian embryo. J Embryol Exp Morphol 30, 31–48. [PubMed] [Google Scholar]
- McHugh KM (1996) Molecular analysis of gastrointestinal smooth muscle development. J Pediatr Gastroenterol Nutr 23, 379–394. [DOI] [PubMed] [Google Scholar]
- McKey J, Martire D, de Santa Barbara P, Faure S (2016) LIX1 regulates YAP1 activity and controls the proliferation and differentiation of stomach mesenchymal progenitors. BMC Biol 14, 34. doi: 10.1186/s12915‐016‐0257‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, Goldstein AM (2006) Endothelin‐3 regulates neural crest cell proliferation and differentiation in the hindgut enteric nervous system. Dev Biol 293, 203–217. [DOI] [PubMed] [Google Scholar]
- Nagy N, Magyar A, Oláh I (2001) A novel monoclonal antibody identifies all avian embryonic myogenic cells and adult smooth muscle cells. Anat Embryol (Berl) 204, 123–134. [DOI] [PubMed] [Google Scholar]
- Nagy N, Brewer KC, Mwizerwa O, Goldstein AM. (2007) Pelvic plexus contributes ganglion cells to the hindgut enteric nervous system. Dev Dyn 236, 73–83. [DOI] [PubMed] [Google Scholar]
- Nagy N, Mwizerwa O, Yaniv K, et al. (2009) Endothelial cells promote migration and proliferation of enteric neural crest cells via beta1 integrin signaling. Dev Biol 330, 263–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, Burns AJ, Goldstein AM (2012) Immunophenotypic characterization of enteric neural crest cells in the developing avian colorectum. Dev Dyn 241, 842–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy N, Barad C, Graham HK, et al. (2016) Sonic hedgehog controls enteric nervous system development by patterning the extracellular matrix. Development 143, 264–275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newgreen DF, Jahnke I, Allan IJ, Gibbins IL (1980) Differentiation of sympathetic and enteric neurons of the fowl embryo in grafts to the chorio‐allantoic membrane. Cell Tissue Res 208, 1–19. [DOI] [PubMed] [Google Scholar]
- Roberts DJ (2000) Molecular mechanisms of development of the gastrointestinal tract. Dev Dyn 219, 109–120. [DOI] [PubMed] [Google Scholar]
- Shyer AE, Tallinen T, Nerurkar NL, et al. (2013) Villification: how the gut gets its villi. Science 342, 212–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J, Cochard P, Le Douarin NM (1977) Development of choline acetyltransferase and cholinesterase activities in enteric ganglia derives from presumptive adrenergic and cholinergic levels of the neural crest. Cell Differ 6, 199–216. [DOI] [PubMed] [Google Scholar]
- Thomason RT, Bader DM, Winters NI (2012) Comprehensive timeline of mesodermal development in the quail small intestine. Dev Dyn 241, 1678–1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uesaka T, Nagashimada M, Enomoto H (2015) Neuronal Differentiation in Schwann Cell Lineage Underlies Postnatal Neurogenesis in the Enteric Nervous System. J Neurosci. 35, 9879‐88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace A, Burns A (2005) Development of the enteric nervous system, smooth muscle and interstitial cells of Cajal in the human gastrointestinal tract. Cell Tissue Res 319, 367–382. [DOI] [PubMed] [Google Scholar]
- Wallace KN, Akhter S, Smith EM, et al. (2005) Intestinal growth and differentiation in zebrafish. Mech Dev 122, 157–173. [DOI] [PubMed] [Google Scholar]
- Wang X, Chan AK, Sham MH, et al. (2011) Analysis of the sacral neural crest cell contribution to the hindgut enteric nervous system in the mouse embryo. Gastroenterology 141, 992–1002, e1–6. [DOI] [PubMed] [Google Scholar]
- Yntema CL, Hammond WS (1954) The origin of intrinsic ganglia of trunk vicera from vagal neural crest in the chick embryo. J Comp Neurol 101, 515–541. [DOI] [PubMed] [Google Scholar]
- Young HM, Hearn CJ, Ciampoli D, et al. (1998) A single rostrocaudal colonization of the rodent intestine by enteric neuron precursors is revealed by the expression of Phox2b, Ret, and p75 and by explants grown under the kidney capsule or in organ culture. Dev Biol 202, 67–84. [DOI] [PubMed] [Google Scholar]
- Young HM, Ciampoli D, Hsuan J, Canty AJ (1999) Expression of Ret‐, p75(NTR)‐, Phox2a‐, Phox2b‐, and tyrosine hydroxylase‐immunoreactivity by undifferentiated neural crest‐derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev Dyn 216, 137–152. [DOI] [PubMed] [Google Scholar]


