Abstract
Perfluoroalkyl and polyfluoroalkyl substances are used in commercial applications and developmental exposure has been implicated in alterations in neurobehavioral functioning. While associations between developmental perfluorooctanoic acid (PFOA) exposure and human outcomes have been inconsistent, studies in experimental animals suggest alterations in motor related behaviors. To examine a dose-response pattern of neurobehavioral effects following gestational exposure to PFOA, pregnant CD-1 mice received PFOA (0, 0.1, 0.3, 1.0 mg/kg/day) via oral gavage from gestational day 1–17 and the male offspring examined. Motor activity assessments on postnatal day (PND)18, 19, and 20 indicated a shift in the developmental pattern with an elevated activity level observed in the 1.0 mg/kg/day dose group on PND18. In the adult, no alterations were observed in body weights, activity levels, diurnal pattern of running wheel activity, startle response, or pre-pulse startle inhibition. In response to a subcutaneous injection of saline or nicotine (80 µg/kg), all animals displayed a transient increase in activity likely associated with handling with no differences observed across dose groups. Inhibition of motor activity over 18 days of 400µg/kg nicotine injection was not significantly different across dose groups. Hyperactivity induced by 2mg/kg (+)-methamphetamine hydrochloride intraperitoneal injection was significantly lower in the 1.0 mg/kg/day PFOA dose group as compared to controls. Taken together, these data suggest that the effects on motor-related behaviors with gestational PFOA exposure do not mimic those reported for acute postnatal exposure. Changes were not observed at dose level under 1.0 mg/kg/day PFOA. Further examination of pathways associated with methamphetamine-induced activity is warranted.
Keywords: development, motor activity, novel environment, neurotoxicity, nicotine, amphetamine
1. Introduction
Perfluorooctanoic acid (PFOA) is a member of the perfluoroalkyl and polyfluoroalkyl substances designated by the acronym PFAS (Buck et al., 2011). These compounds have been used in numerous industrial and commercial applications (Kissa, 2001; Lehmler, 2005) resulting in contamination of food, house dust, and water supplies (D’Hollander et al., 2010; Boiteux et al., 2012; Post et al., 2012; Perez et al., 2014; Schwanz et al., 2016). The detection of PFOA in human and laboratory animal umbilical cord blood (Llorca et al., 2012; Kato et al., 2014; Yang et al., 2016) and breast milk (So et al., 2006; Fenton et al., 2009; Fromme et al., 2010; Llorca et al., 2010; Kang et al., 2016) has raised concern for exposure to offspring during development.
Associations between developmental PFOA exposure and human birth outcomes are inconsistent (Olsen et al., 2009; Bach et al., 2015). In a hospital-based cross-sectional study of singleton deliveries in Baltimore, Maryland, Apelberg et al. (2007) reported no association between PFOA cord serum concentrations and body length or gestational age at birth; however, negative associations for birth weight and head circumference were reported. Data from the Danish National Birth Cohort (1996–2002) showed a negative association between maternal PFOA plasma concentrations in early pregnancy and birth weight (Fei et al., 2007) that persisted over the first year of life (Andersen et al., 2010). Associations were observed with smaller abdominal circumference and birth length but not with effects on developmental milestones over the first 18 months of life (Fei et al., 2008a,b). Additionally, Washino et al., (2009) reported no association between maternal serum PFOA and birth weight, while Lenters et al. (2016) reported an association with lower birth weights and Maisonet et al. (2012) a significant trend across tertiles of PFOA for lower birth weights but not birth length. Examinations of a mother-infant pair cohort from the Taiwan Birth Panel Study found no association of cord blood PFOA with gestational age, birth weight, and head circumference (Chen et al., 2012) or with performance at 2 years of age on the Comprehensive Developmental Inventory for Infants and Toddlers (Chen et al., 2013). While, individual human studies have not clearly identified an association between maternal PFOA exposure and birth weight, Lam et al (2014) reported an association with reduced birth weight following a meta-analysis of 9 human studies combined with 8 mouse studies that had been previously found to show an association with reduced birth weight (Koustas et al., 2014).
In the Danish Birth Cohort, an association of prenatal exposure to PFOA was not observed with behavioral outcome or motor coordination measures assessed during childhood (Fei and Olsen, 2011). In one recent study, Donauer et al., (2015) examined mother/infant pairs participating in the Health Outcomes and Measures of the Environment study in Cincinnati, Ohio and found no association between maternal serum PFOA and individual outcomes as measured by the Neonatal Intensive Care Unit Network Neurobehavioral Scale. However, individuals gestationally exposed to PFOA at 10x higher serum concentrations showed increased odds ratio of being categorized as hypotonic rather than social/easygoing. Studies of children from the Swedish cohort or the Dutch cohort Linking Maternal Nutrition to Child Health reported no PFOA exposure-related association with attention deficit hyperactivity disorder (Ode et al., 2014; Quaak et al., 2016). Recent studies with a Danish cohort found no evidence of exposure-related associations between maternal serum concentrations of PFOA and offspring behavioral or affective disorders, including scholastic achievement over a 20-yr follow-up period (Strom et al., 2014) or in increased risk of attention deficit/hyperactivity disorder or autism (Liew et al., 2015). In the INUENDO Polish and Ukraine cohort, there was no statistically significant association reported between exposure to PFOA and hyperactive behavior (Hoyer et al., 2015).
In experimental animal studies, the prominent neurobehavioral effects reported in adult mice following developmental PFOA exposure are increased motor activity (Johansson et al., 2008; Sobolewski et al., 2014) and changes in activity patterns in the light and dark phases (Onishchenko et al., 2011). Activity-related behaviors in adult NMRI mice were altered by a single oral dose of 8.7 mg/kg PFOA at PND10, including a diminished stimulation of activity in response to a pharmacological challenge of nicotine (80 µg s.c./kg; Johansson et al., 2008). This diminish response to nicotine was interpreted by the authors as a change in responsiveness of the cholinergic neurotransmitter system. While interesting, the previous studies on developmental effects of PFOA exposure are limited with regards to dose response, developmental allocation of animals, and evaluation of neurotransmitter systems following gestational exposure. The current study was undertaken to assess the dose-related effects of gestational PFOA exposure on activity-related development and motor activity, startle reactivity, and activity-related changes following pharmacological challenge to provide information to integrate across the different effects reported in the literature for different exposure paradigms.
2. Materials and methods
2.1. Animals and Dosing
Four cohorts of timed-pregnant CD-1 mice were obtained from Charles River Laboratory (Raleigh, NC) on gestational day (GD) 0 upon confirmation of copulatory plug. Within each cohort, dams were randomly assigned to one of four dosing groups. Pregnant mice were individually housed in Techniplast Blue Line cages (396 × 215 × 172 mm) with micro-barrier lids and autoclaved, hardwood bedding (Sani-chips, PJ Murphy, Montville, NY) within a semi-barrier room (40–60% relative humidity; 12-h light/dark cycle: 6:00 – 18:00 EST; 430–460 Lux; 20–24°C). Rodent chow (Purina 5001) and reverse osmosis water (RO-H2O) (polysulfone water bottles) were available ad libitum. All animal procedures were conducted in accordance with protocols approved by the NIEHS Animal Care and Use Committee.
Perfluorooctanoic acid (PFOA; ammonium perfluorooctanoate; > 98% pure) was obtained from Fluka Chemical (Steinheim, Switzerland) dissolved in RO-H2O and dosing solutions prepared fresh daily. On the mornings of GDs 1–17, dams received an oral gavage of 0 (RO-H2O), 0.1, 0.3, or 1 mg PFOA/kg body weight (bwt) per day, at a dosing volume of 10 mL/kg bwt. Parturition was monitored three times daily (approximately 08:00, 13:00, 18:00h). The day of birth was defined as postnatal day (PND) 0. On PND3, litters were each culled to 10. Pups were weaned on PND21 and 3 randomly selected males were individually identified by ear-punch and group housed with their littermates. From these 3 males per litter, individual mice were randomly assigned to behavioral test, maintaining criteria of only one pup per litter. Female pups were assigned to a separate study (Tucker et al., 2015). The four cohorts were generated over 18 months. Cohorts of male offspring were assigned to behavioral assessments as identified in each test description with only one male pup per litter examined in any specific behavioral test. [cohort 1 – running wheel (RW); cohort 2 – pre-pulse startle inhibition (PPI), RW; cohort 3 – PPI; methamphetamine challenge; cohort 4 – developmental motor activity; nicotine challenges. Thus, RW and PPI were assessed over 2 cohorts while all other endpoints were assessed in only 1 cohort.] With the exception of the 24-hr home cage RW activity measures, all behavioral testing was conducted between the hours of 10:00 and 15:00 within a designated behavioral testing room. For each test, the assignments of mice to one of 4 testing apparatus and time of testing were counterbalanced for dose group.
2.2. Development of Locomotor Activity
As a measure of motor ontogeny, mice from cohort 4 (n=5–7 per dose group) were repeatedly assessed on PND 18, 19, and 20, for exploratory activity in an open field chamber (42 cm × 42 cm; Columbus Instruments, Columbus, OH). Each chamber was outfitted with photocell detectors (0.32 cm diameter) spaced 2.5 cm from the chamber bottom and 1.27 cm apart linearly around the chamber. Ambulatory activity, as measured by photocell breaks, was recorded in 5-min epochs over a 20-min test session by Opto-Max version 2.27 software (Columbus Instruments).
2.3. Startle Response and Prepulse Startle Inhibition (PPI)
At 2 months of age, male offspring from cohorts 2 and 3 (n=14–17 per dose group) were assessed for auditory startle response, habituation, and PPI as a measure of sensorimotor gating using a computer assisted SR-LAB startle apparatus (San Diego Instruments, San Diego, CA). Mice were allowed a 5-min habituation period in the holder placed within the apparatus. Background noise level was set at 70 dB. The session began with 6 120 dB trials (pulse-alone) followed by 2 blocks of 26 trial comprised of 2 no-stimulus trials, 6 acoustic startle stimulus (40-msec null period followed by 40-msec 120 dB pulse) trials alone, 18 pre-pulse stimulus trials of 6 for each pre-pulse trial (40-msec null period followed by 20-msec pre-pulse of 3, 6, or 12 dB above background [73, 76 and 82 dB] followed by a 100-msec null period and a 40-msec 120dB pulse; for an entire recording period of 200 msec) presented in a random order, followed by 6 trials with acoustic startle stimulus at 120dB. Trials were presented at 15 sec variable inter-trial intervals (ITI; 5–25 sec).
Startle reactivity (120 dB Vmax) over the test session, % habituation, and prepulse startle inhibition (%PPI) for the 3, 6, and 12-dB prepulse+pulse trials were determined. Startle response amplitudes were log-normally distributed across doses and pulse types (Csomor et al., 2008). The 120 dB pulse-only trials over the test session were allocated into four “blocks” of 6 trials [six trials at the beginning, 6 trials within the first 24-trial block, 6 within the 2nd 24-trial block, and 6 trials at the end of the session]. Habituation was calculated as the difference in the mean log10 of 120 dB Vmax between the 1st and the 4th "block" of trials. Differences in means of log-transformed responses are equivalent to the logarithm of the ratios of medians of untransformed values (Yee et al., 2013) and we expressed habituation as the ratio of medians. Geyer and Swerdlow (1998) calculate prepulse startle inhibition (% PPI) as a percentage of 120 dB startle response: [(mean(Vmax120 dB)− mean(Vmaxprepulse))/mean(Vmax120 dB)] × 100, or [1−mean(Vmaxprepulse))/mean(Vmax120 dB)] × 100. In this study, robust estimates of % PPI were calculated based on log10–transformed responses and expressed as 100 × [1 – median (Vmaxprepulse)]/median(Vmax120 dB)].
2.4. Running Wheel Activity
Twenty-four-hr home-cage activity levels were measured over 12 days using an automated running wheel apparatus (Novak et al., 2012). At 2 months of age, individual mice (n=12 per dose group from cohorts 1 and 2) were transferred from the home-cage room to the testing room, allowed to acclimate for 1 hr, then placed in a Techniplast Blue Line cage (396 × 215 × 172 mm) with a stainless steel running wheel (RW) attached to the top cover (Mini-Mitter®; Respironics Co., Bend, OR). Testing was conducted under conditions in an animal facility room confirmed to meet physical conditions (temperature, humidity, lighting) as that of the home-cage room. This system allowed for free access to the RW and computer-assisted recording of wheel revolutions in 1-hr epochs (Vital View Data Acquisition, Respironics Co.) during the light-phase (6:00–18:00) and the dark phase (18:00–6:00). Food and water were available ad libitum.
2.5. Nicotine-induced changes in locomotor activity
Six month-old drug naive male mice from cohort 4 (n= 4–6 per dose group) were allowed to acclimate to an open field chamber (42 cm × 42 cm; Columbus Instruments, Columbus, OH) configured as previously described. Ambulatory activity, as measured by photocell breaks, was recorded in 5-min epochs over 40 min. The mice were removed from the arena, given a single subcutaneous (sc.) injection of either 80 µg/kg nicotine base (nicotine bitartrate salt; Sigma, St Louis, MO; dissolved in saline and adjusted to 7.2 pH) or saline vehicle, at a dosing volume of 10 ml/kg bwt. Mice were immediately returned to the arena and activity measured in 5-min epochs for 50 min. According to a cross-over design, each mouse received both saline and nicotine. A 7-day drug clearance period was allowed between injections.
As a complementary assessment of nicotinic receptor function, a separate group of drug naive adult (5-month-old) mice from cohort 4 (n=5–7 per dose group) that had been previously used to assess activity development was examined for development of tolerance to activity-depressant effects of nicotine. Mice received saline (10 ml/kg bwt, sc.) and, at 5-min post-injection, were placed within the motor activity chamber for 15 min. This was repeated daily for 3 consecutive days to acclimate animals to handling, injection, and arena. The last day of acclimation was subsequently represented as baseline Day 0. This was followed on 8 consecutive days (days 1–8) by a sc. injection of 400 µg/kg nicotine base in saline (pH 7.2). Five min post-injection, mice were placed in the arena and ambulatory activity was recorded for 15 min. On days 9–17, mice received 400 µg/kg nicotine and were kept in their home cage. On day 18, mice received nicotine and, at 5 min post-injection, placed in the arena and activity was recorded for 15 min.
2.6. Methamphetamine induced hyperactivity
Drug naïve adult (6-month-old) mice from cohort 3 (n=10–12 per dose group) were examined for alterations in motor activity following methamphetamine challenge. Mice were placed in the arena and activity recorded in 5-min epochs over 50 min. Mice were removed from the chamber and injected with 2 mg/kg intraperitoneally (i.p.; 5 ml/kg bwt) (+)-methamphetamine hydrochloride (> 98% purity; CAS # 51-57-0 Sigma-Aldrich, St Louis, MO) in saline and immediately returned to the chamber. Ambulatory activity was recorded for 60 min in 5-min epochs.
2.7. Statistical Analysis
Data were initially tested for homogeneity of variance using Levene's tests and for non-normality using Shapiro-Wilk tests. Body weights collected with each pharmacological challenge were analyzed using an analysis of variance (ANOVA). Total ambulatory activity on PND 18, 19 and 20 was analyzed by repeated measures (RM) ANOVA with dose and age as factors followed Bonferroni tests. Log10–transformed startle response (Vmax) was analyzed with a RM ANOVA with block and dose as factors. One-way RM ANOVA was used to analyze Vmax response for no-stimulus trials. Percent habituation and %PPI for each pre-pulse level were modeled as a function of dose using beta regression, an extension of the generalized linear model with a beta distributed response to account for bounded percentages with skew and a logit link function. Significant dose effects were determined by Wald’s tests. Negative PPI values were set to 0.
Total daily RW rotations were modeled using linear mixed-effects models (Pinheiro and Bates, 2000) with dose and cohort as factors, time as a linear term, and a random intercept to account for random deviation. Separate models were fit for rotations captured during the light-phase and during the dark-phase. Data was analyzed for the entire 12-day test interval and additionally for days 4–12 to exclude any possible period of re-entrainment to subtle differences across the two animal rooms. Model errors followed a temporal autocorrelation structure, where correlation between two time points decreased in absolute value with distance. Daily light-phase rotations showed a skewed distribution and were log10–transformed. Due to a lack of homogeneity of variance, analysis of dose-related dark-phase rotations on day 1, day 12, differences between days 1 and 12, and total rotations during first 6 hr for days 1 and 2 were evaluated by cohort using Kruskal-Wallis tests (Hollander and Wolfe, 1999), followed by post hoc Westfall-Young permutation-based tests (Westfall et al., 1999) between dose groups and controls. Peaks of activity were defined as 200% increase within any one 1-hr epoch and evaluated over the first 2 days in an attempt to facilitate comparison to data presented in Onishchenko et al., (2011)
Square root transformed ambulatory activity data obtained prior to saline or nicotine (80 µg/kg) injection and data obtained between 15 – 45 min post-injection were analyzed with a linear mixed-effects model with PFOA dose and injection type as factors and time as a linear term, with temporally autocorrelated errors and a random intercept. Dunnett's tests were used to compare PFOA dose groups with controls. Total ambulatory activity during the 15-min period preceding the injection was analyzed by RM ANOVA with dose and injection type as factors, followed by Dunnett’s test. All animals displayed increased activity during the first 10–15 min after injection of either saline or nicotine. To compare to previous reports, total ambulatory activity during the 20-min interval immediately following injection was analyzed with a RM ANOVA with dose and injection type as factors, followed by a Dunnett’s test. To evaluate tolerance development to nicotine, a mixed linear model was fit to the log10-transformed total ambulatory activity levels on days 1–8 following nicotine (400 µg/kg) injection, with dose as a factor and day as a linear term, accounting for autocorrelated errors and individual animal variation. Post hoc comparisons were performed using Dunnett’s tests. Individual differences in activity level for day 1 and day 18 failed to meet homogeneity of variance criteria and were evaluated by Kruskal-Wallis followed by Westfall-Young permutation-based tests.
The square root of ambulatory activity in the 50-min period prior to methamphetamine injection and in the 60-min interval following injection were analyzed by a linear mixed-effects model for each cohort, with PFOA dose as a factor, a linear term for time, autocorrelated errors and a random intercept. A non-parametric Jonckheere-Terpstra test (Jonckheere, 1954) was used to test for a decreasing dose-related trend in total post-injection activity, followed by a post-hoc Williams test (Williams, 1971).
2. Results
2.1. Locomotor activity
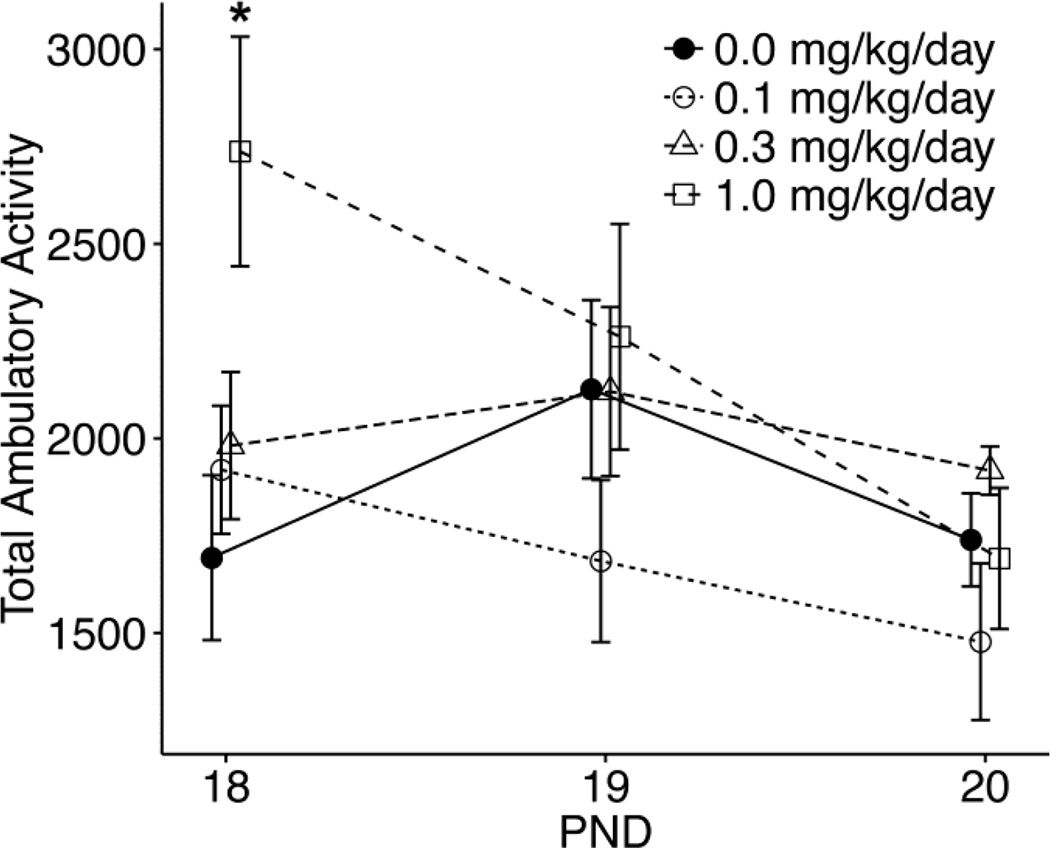
RM ANOVA of total ambulatory activity levels over the 3 pre-weaning days indicated a significant dose by day interaction (F6,38=3.36, p<0.01). Control mice showed no significant difference in activity across ages. Mice gestationally exposed to 1.0 mg/kg PFOA showed significantly higher ambulatory activity levels at PND 18 as compared to controls (p<0.002) and as compared to levels in the same dose group at PND 20 (p<0.002). No statistically significant differences were observed in the 0.1 and 0.3 mg/kg dose groups as compared to controls at any age (Fig. 1).
Figure 1.
Total ambulatory activity (counts) over 20 min at postnatal day (PND) 18, 19, and 20. Data represent means (+/− SEM) response in male mice gestationally exposed to vehicle (0.0) or PFOA (0.1, 0.3, 1.0 mg/kg/day; n=5–7 per dose group). **significantly different by Bonferroni tests as compared to controls (p <0.002) or as compared to PND 20 (p <0.002).
2.2. Startle Reactivity and PPI
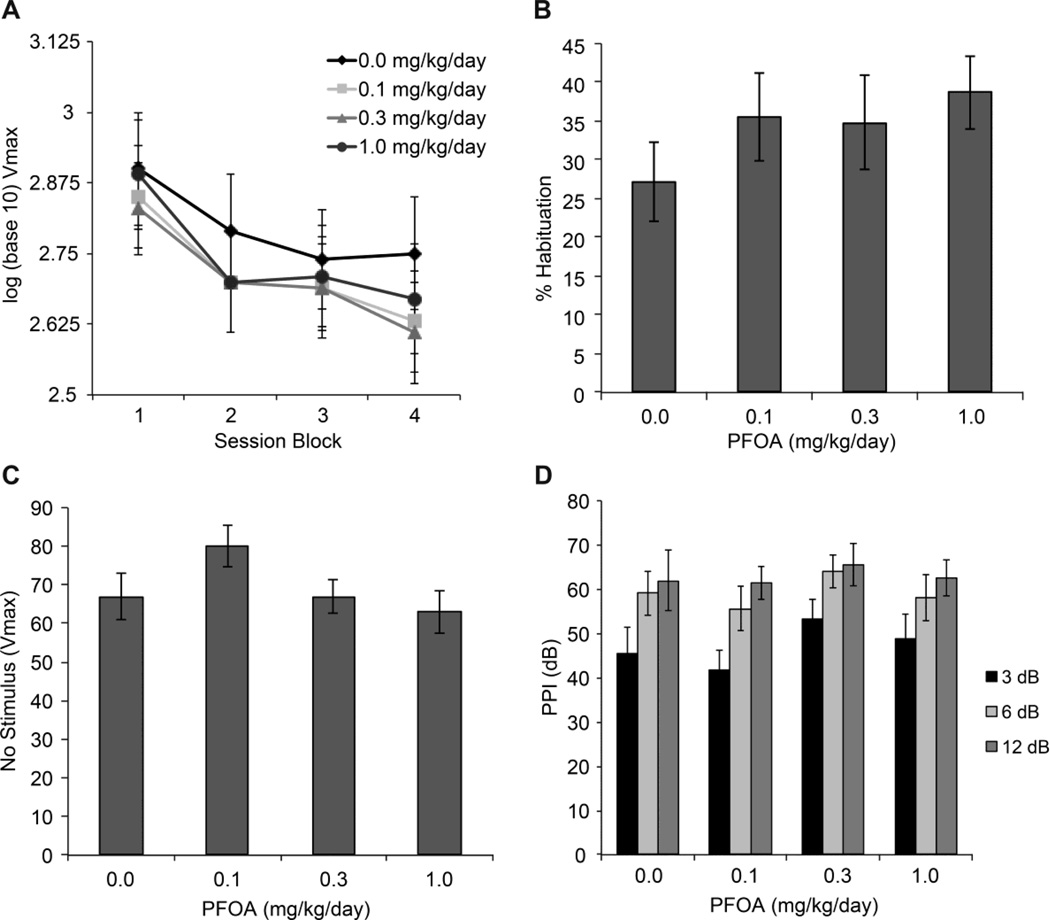
In adult mice, RM ANOVA of the log10–transformed 120 dB startle response showed a significant block effect (F3,183=42.68, p<0.001) with a decrease occurring over trials for all dose groups (Fig. 2A). No statistically significant main effect of dose or dose × block interaction were found. Relative change in startle response between first and last block (% habituation) was not significantly different across dose groups (Fig. 2B). Background Vmax levels recorded on no-stimulus trials were similar across dose groups (Fig. 2C). The level of pre-pulse startle inhibition (PPI) significantly increased with increasing prepulse intensities but there were no significant differences observed in PPI as a function of PFOA dose (Fig. 2D).
Figure 2.
Startle reactivity and prepulse inhibition (PPI) in 2-month-old male mice gestationally exposed to vehicle (0.0) or PFOA (0.1, 0.3, 1.0 mg/kg/day; n=14–17 per dose group). (A) log10–scale plots of mean +/− SEM of startle amplitude (Vmax) in response to 120 dB pulses over four blocks of 5 trials showing a decrease over blocks (p<0.001). (B) Percent startle habituation calculated as the attenuation in startle response from the first block to the fourth block. (C) Vmax recorded in no-stimulus trials. (D) Percent inhibition of 120dB response from pre-pulse stimulus intensities of 3, 6, or 12 dB above background. Data represent means +/− SEM
2.3. Running Wheel Activity
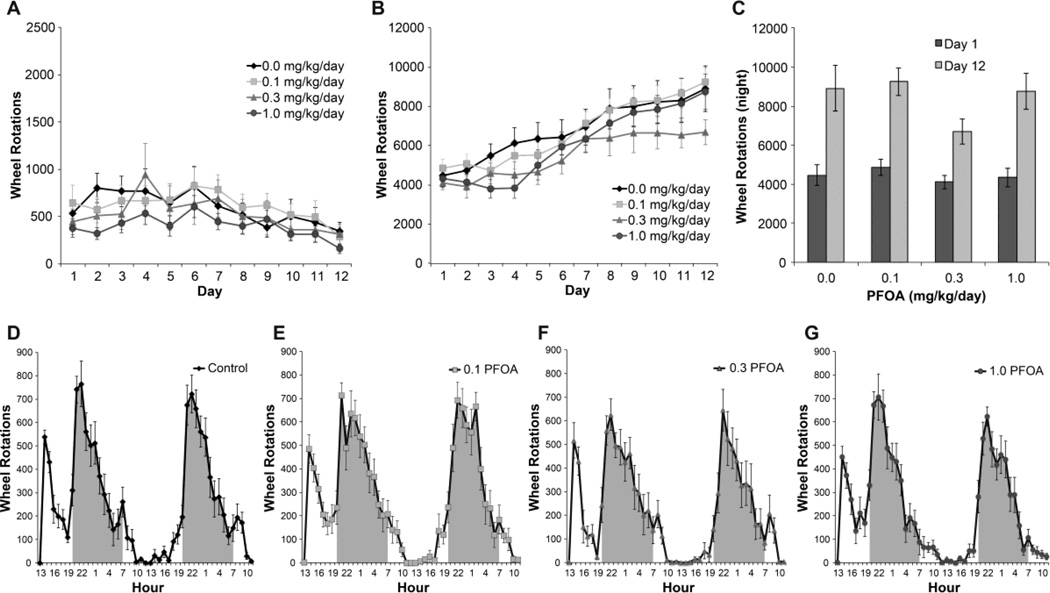
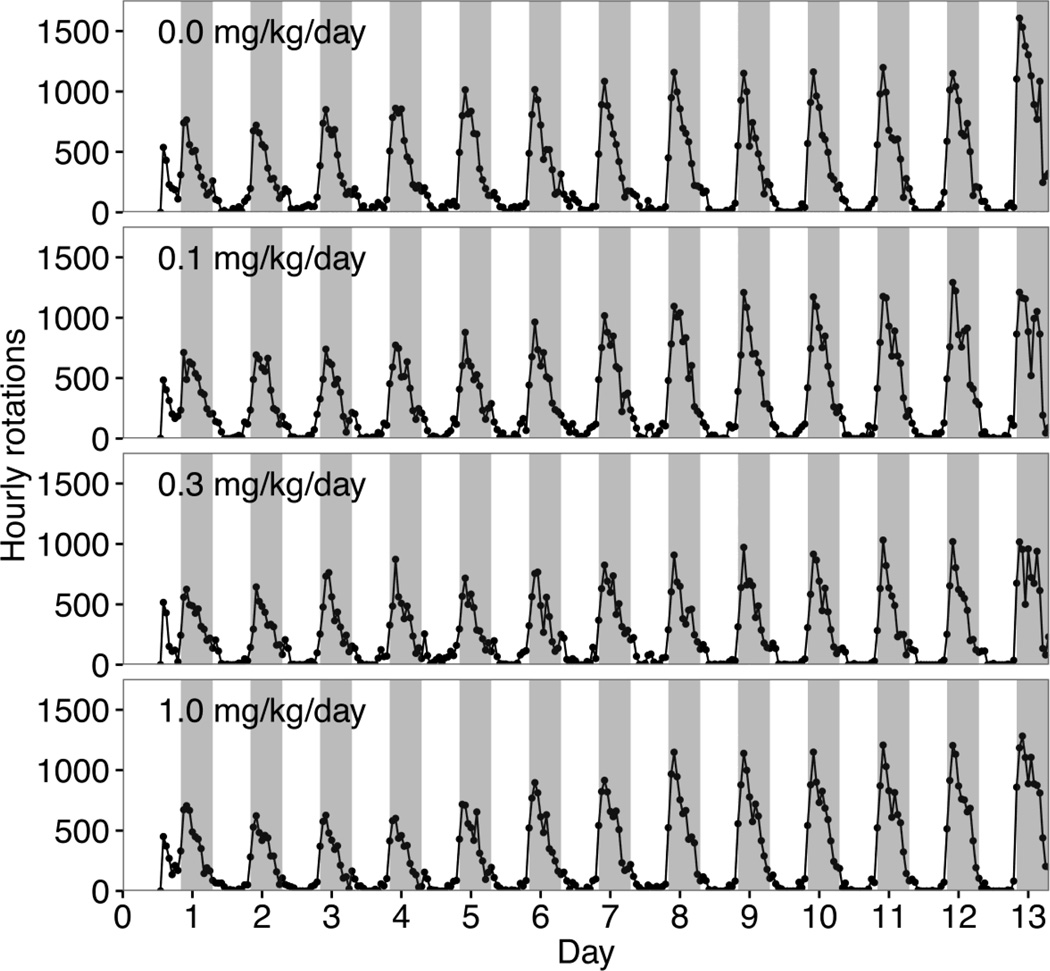
All mice displayed less activity during the light-phase (Fig. 3A; Fig. 4) and increased activity during the dark-phase (Fig. 3B; Fig 4). A RM ANOVA for each phase showed no significant main effect of PFOA exposure. A linear mixed-effects model for daily dark-phase rotations showed no significant main effect of cohort, PFOA exposure, or interactions of PFOA exposure and day or cohort. A main effect of day and a day × cohort interaction were found to be statistically significant. A significant increase in rotations over time was observed (F2,616=63.11, p<0.0001 for the combined effects of day and day by cohort). RW activity increased from day 1 to day 12 for all dose groups with no statistically significant dose-related differences observed (Fig. 3C). An analysis of RW activity between day 4 and day 12 was conducted to allow for re-entrainment and showed similar results with no statistically significant difference observed between dose groups. Peaks of activity, defined as an epoch showing a 200% change from previous epoch, were not observed over the first 48 hr (Fig. 3D–G) nor over the subsequent 10 days (Fig. 4).
Figure 3.
Running wheel activity over 12 days. Daily wheel rotations during (A) light phase and (B) dark phase in 2 month old male mice gestationally exposed to vehicle (0.0) or PFOA (0.1, 0.3, 1.0 mg/kg/day; n=12 per dose group). (C) total rotations recorded during dark phase of day 1 and day 12. (D–G) Hourly rotations recorded during day 1 and day 2 for (D) controls, and PFOA dose groups of (E) 0.1, (F) 0.3, and (G) 1.0 mg/kg/day. Shaded areas represent dark phase. Data represent means +/− SEM.
Figure 4.
Running wheel activity over 12 days. Hourly running wheel rotations recorded during day 1 to day 12 in adult CD-1 male mice exposed gestationally to vehicle (0.0) or 0.1, 0.3, and 1.0 mg PFOA/kg/day. Shaded areas represent dark phase of each day. Data represent mean total rotations occurring over a 1 hr interval showing a gradual increase over days. (n=12 per dose group).
2.4. Nicotine effects on ambulatory activity
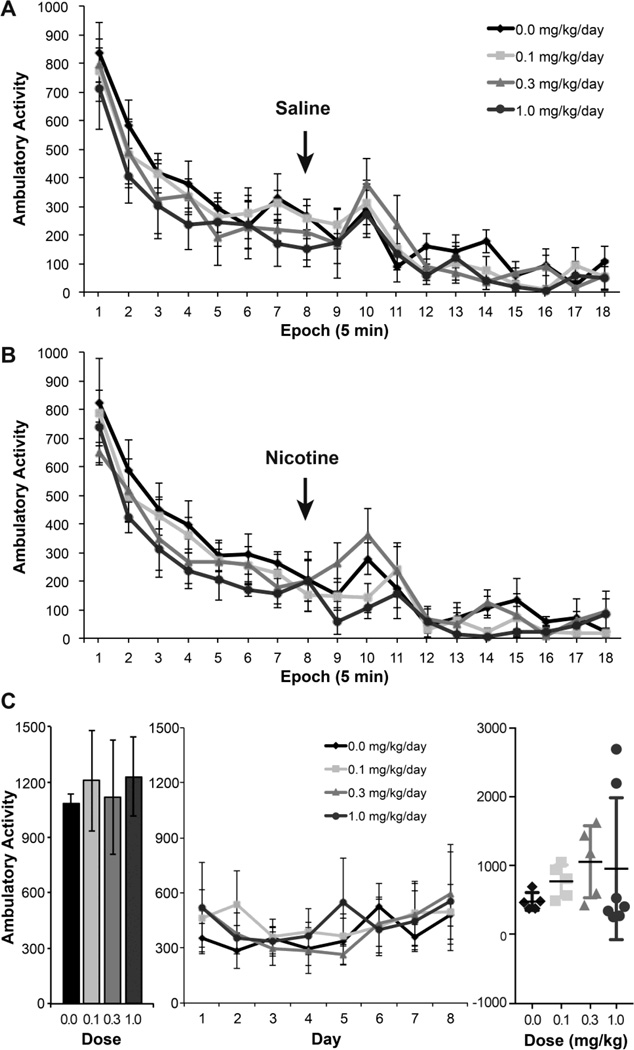
Baseline ambulatory activity levels and the normal pattern of decreasing activity over the initial 40-min session prior to injection were similar across dose groups (Fig. 5A,B) with a significant decrease observed over time (F1,298=31.14 for the linear time term, p<0.0001; Fig. 5A,B). Following disturbance of the mice with removal from the arena and injection of either saline (Fig. 5A) or nicotine (Fig. 5B), a minor stimulation of activity was observed. To compare to Johansson et al. (2008), total activity during the 20-min interval immediately following injection was analyzed. There was no evidence of a statistically significant difference in activity levels during this time as a function of PFOA dose group or in the substance injected (saline or nicotine).
Figure 5.
Ambulatory activity following nicotine. (A) Ambulatory activity levels (counts) in 5-min epochs for 40 min followed by an acute injection (arrow) of (A) saline or (B) nicotine base (80 µg/kg, sc.) and activity measured over 50 min in 6-month-old male mice gestationally exposed to PFOA or vehicle (n: vehicle 0.0 = 6; 0.1 = 5; 0.3 = 4; 1.0 = 5). A transient increase in activity level was noted following injections. (C) Total ambulatory activity levels (counts) over 15 min on day 0 following a sc. injection of saline (baseline). Total ambulatory activity levels (counts over 15 min) on days 1–8 initiated 5 min following sc. injection of 400ug/kg nicotine base (pH 7.2). Once daily nicotine injections were continued on days 9 – 17. On day 18, total ambulatory activity levels were recorded over 15 min (scatter plot) initiated 5 min following sc. injection of 400µg/kg nicotine base. Data represents mean +/− SEM (n= 5–7 per dose group).
The development of tolerance to the activity-depressant effects of nicotine (400 µg/kg) was examined over 18 daily injections (Fig. 5C). Baseline activity levels (Day 0) were not significantly different between controls and PFOA dose groups. Depression of ambulatory activity following an injection of nicotine (400 µg/kg) was observed over days 1–8 that was not significantly different between PFOA dose groups and controls (Fig. 5C). After 10 additional days of nicotine injections, ambulatory activity measured on day 18 after the injection was not significantly different between dose groups and controls.
2.5. Methamphetamine-induced hyperactivity
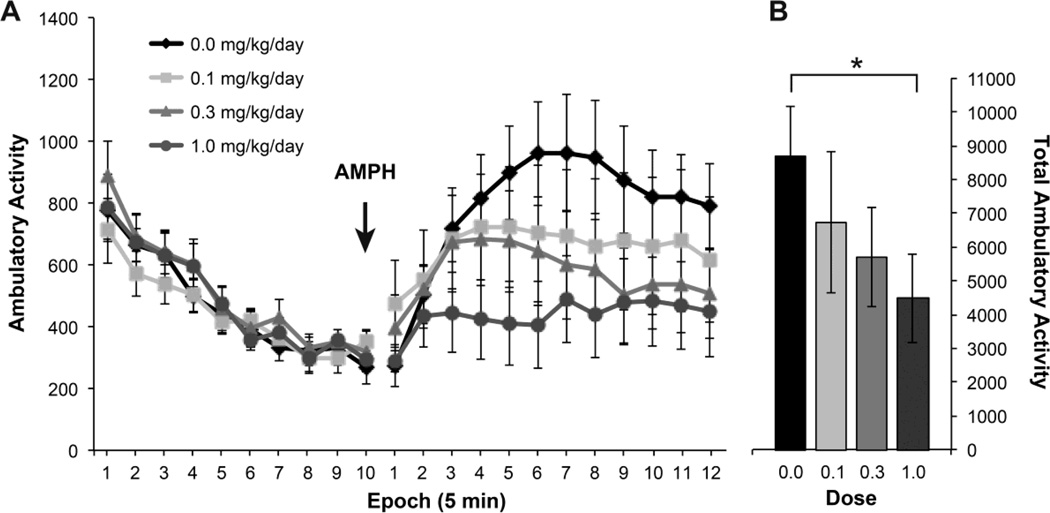
Under a similar activity-monitoring paradigm to that used for acute nicotine, changes in activity were recorded following an acute injection of methamphetamine (Fig. 6A). Ambulatory activity levels during the 50-min acclimation period in the arena were similar between dose groups and controls. After the methamphetamine injection, stimulatory actions of the drug were observed as elevations in activity. The level of stimulation was diminished with increasing doses of PFOA (Jonckheere-Terpstra test statistic = 282, p=0.047). The total post-methamphetamine injection activity of the highest PFOA dose group (1.0 mg/kg/day) was significantly less than that of controls (p=0.044, one-sided Williams test). Activity levels following methamphetamine of the 0.1 and 0.3 mg/kg/day dose groups failed to reach statistical significance (p=0.051 and p=0.058, respectively, one-sided Williams test).
Figure 6.
Ambulatory activity following methamphetamine. (A) Baseline ambulatory activity level (counts) in 5 min epochs over 50 min followed by sc injection of methamphetamine (2 mg/kg; arrow) and activity measured in 5-min epochs over 60 min in adult male mice gestational exposed to PFOA. (n = 10–12 per dose group). (B) Total ambulatory activity level following amphetamine injection. *statistically significant (p<0.05) as compared to controls as determined by Williams test.
4. Discussion
Previous work has suggested that developmental exposure to PFOA alters activity response to novel environments and responsiveness of the cholinergic system in the adult mouse (Johansson et a., 2008; Onishchenko et al., 2011; Mariussen, 2012; Sobolewski et al., 2014). In the current study, such effects were not evident in the adult CD-1 mouse following gestational exposure to PFOA. No effects were observed on adult motor activity in the open field or the running wheel and we found no evidence of a specific effect on the cholinergic system as indicated by response to nicotine administration. However, the data suggested altered responsiveness to methamphetamine in adult mice gestationally exposed to 1.0 mg/kg/day PFOA.
Motor activity is often used to assess general activity levels that can be related to lethargy and muscle strength. However, in the absence of a motor deficit, motor activity measures collected within various types of test paradigms are often considered indicative of integrated nervous system functioning including emotionality/anxiety in reaction to a novel environment, exploratory drive, and even learning (Archer 1973; Walsh and Cummins 1976; Stanford 2007). Johansson et al., (2008) reported lower ambulatory activity in 2-month-old mice that received PFOA (8.7 mg/kg) at PND10. By 4-months of age, a decrease was also observed in the lower (0.58 mg/kg) dose group. With gestational exposure (0.3 mg/kg/day) Onishchenko et al., (2011) reported no deficit in motor strength or activity in 5–8 week old mice. However, with dietary exposure from GD7 until PND 21, elevated activity levels were observed in 2-month-old male mice exposed to a lower dose of PFOA (0.1 mg/kg/day) (Sobolewski et al., 2014). Using a gestational dosing model, the current study showed no alterations in ambulatory activity levels in adult male CD-1 mice. However, in mice exposed to PFOA (1 mg/kg/day) a biological shift in motor activity development was suggested (Li et al., 2005) with higher ambulatory activity at PND18
Alterations in response to a novel environment have been suggested following developmental PFOA exposure. Using a novel object recognition task, Sobolewski et al. (2014) reported no PFOA related differences in the number of contacts made by male mice to a novel versus familiar object. Using the response to a novel home-cage environment, Onishchenko et al. (2011) reported elevated activity levels during the first hour within a group activity cage and higher activity levels during transition periods (last 2 hr of the first light phase and first hr of the subsequent 2 light phases) in PFOA exposed mice. The authors considered the early onset and delayed down-regulation of activity, as well as an alteration in circadian rhythm, as indicative of increased response to a novel environment. However, in the same publication, both control mice and mice exposed to perfluorooctanesulfonic acid (PFOS) demonstrated higher levels of activity during the first 1-hr interval of the light phases. This suggests that the activity pattern seen in the PFOA exposed mice fell within the normal range of the behavior in C57BL/6/Bkl mice and likely did not reflect a specific effect of PFOA exposure. In the current study, we found no evidence of increased activity within 1-hr epochs prior-to or following a shift in light. The increase in activity levels observed by Onishchenko et al., (2011) in PFOA exposed males was attributed by the authors to “peaks of activity”. In the present study, we measured activity of individual mice in a home-cage running wheel over the course of 12 days and found activity patterns similar across all dose groups. The differences in findings reported by Onishchenko et al., (2011) and our current study may be related to the nature of the apparatus to measure activity. While we examined individual mice, Onishchenko et al. (2011) examined activity occurring in multiple animals within a group-housing environment. It is possible that the behavior observed was related to interactions of the social group housing rather than activity or diurnal effects. In both studies, the 1-hr epochs for data capture would compromise any examination of subtle phase shifts in the diurnal onset and offset of activity.
The developing cholinergic system has been speculated to be sensitive to PFOA exposure (Johansson et al., 2008). This conclusion was based upon the observation that mice exposed to PFOA on PND10 displayed an altered activity response to the pharmacological effects of nicotine. Nicotine mimics the action of acetylcholine at nicotinic acetylcholine receptors (nAChRs) and stimulation releases a variety of other neurotransmitters. Changes in the response to nicotine might indicate a persistent cellular change in the nervous system following a perturbation during development. The paradigm used by Johansson et al. (2008) was based upon work showing that a single dose of nicotine increased motor activity in NMRI mice over the first 20 min following injection (Nordberg et al., 1991; Eriksson et al., 2000; Viberg et al., 2002). In 4-month-old mice exposed to PFOA at PND10, nicotine-stimulated motor activity was diminished during the first 20 min epoch, with no differences observed over the subsequent 2 epochs (Johansson et al., 2008). Nicotine-induced stimulation in activity was not observed in the current study. Rather, activity levels in the first 10–15 min were elevated after saline or nicotine injection. The absence of a split-plot design for the nicotine challenge, to include a saline injection, in the Johansson et al., (2008) paper makes it difficult to make a clear comparison between the studies. Since mice were immediately placed within the arena following injection, it is likely that increases in activity were the result of handlingFurther examination in the current study of a potential alteration in nicotine receptors showed no significant differences across dose groups in activity-depressant effects of a high dose of nicotine. The genetic background, the PFOA dose level, the age of exposure, and age of assessment also differed between the current study and the Johansson study. The age of assessment was slightly older, 6 months versus 4 months, in the current study however, it is not expected that this would have diminished the sensitivity of the nicotine receptor. While a direct comparison across NMRI and CD-1 mouse strains has not been reported, the available literature suggests that CD-1 mice are sensitive to developmental PFOA exposure and thus, the absence of an effect is likely not due to strain. The most obvious difference is age of exposure. The rodent brain approximately doubles in size with each week of life between birth and weaning (Calabrese et al., 2013) and one could consider that an acute exposure on PND 10 disrupts homeostasis at this dynamic stage of brain development. It is also possible that, with continuous gestational exposure, the developing brain adapts to a different “exposure environment” allowing for compensatory process to be initiated and thus, deficits seen with acute postnatal exposure may not manifest under a prolonged exposure period. Many of the neurobehavioral effects of nicotine are related to activation of nicotinic cholinergic receptors (nAChRs) and the subsequent influence of the GABA-ergic system (Damaj et al., 1999; Dobelis et al., 2003; Ortells and Arias, 2010). Given the absence of a response to 80 µg/kg nicotine, changes in the cholinergic system were examined further by using an 18-day regimen to examine tolerance to inhibitory effects of a higher nicotine dose on activity levels (Ksir, 1993). With repeated exposure to nicotine, an increase occurs in the density of α4β2 nAChRs resulting in the development of tolerance to the activity suppressive effects (Naylor et al., 2005). Under the testing conditions of the current study, all animals showed an inhibition of activity by nicotine injection on days 1–8 that was maintained on day 18. The few mice in the 1.0 mg/kg/day PFOA dose group showing a higher activity level on day 18 suggest that further exploration of a tolerance effect to nicotine would require a longer injection period.
The response of mice to an acute administration of methamphetamine was evaluated to further examine potential alterations in dopamine-dependent motor behaviors. Methamphetamine can act as a nicotinic receptor agonist (Liu et al., 2003) but also exerts effects on activity through a dopaminergic mechanism (Thornburg and Moore, 1973) that can be modulated by serotonin (Costall et al., 1979). Methamphetamine stimulates dopamine elevations within striatal pathways to increase activity (Fornai et al., 2009; Zombeck et al., 2009). In the current study, methamphetamine-induced hyperactivity was observed in all dose groups. The lower level of methamphetamine-induced activity in mice receiving 1 mg/kg PFOA suggested a decrease in activation of the dopaminergic system. Several studies have demonstrated that intrinsic 5-HT2 receptors can modify dopaminergic function (Lucas et al., 2000; Porras et al., 2002) with 5-HT2A antagonists reducing hyperactivity induced by amphetamine (O'Neill et al., 1999). In addition, striatal noradrenaline release can modulate the motor effects of amphetamine (Carli et al., 2015). Thus, the observed effects may be related to alterations in the serotonin system, which would require further focused experimental examination. While not observed in the current study, alterations in these neurotransmitter systems may occur in a more subtle fashion at lower PFOA dose levels and would require examination using a more specific and targeted experimental paradigm and endpoints.
In conclusion, gestational exposure to 1 mg/kg/day PFOA was shown to have an effect on components of the motor system at PND18, with no effects observed at the lower doses. In adults, there was no evidence of altered ambulatory activity in the open-field and no specific effects on activity. A decreased level of nicotine-stimulated motor activity as reported by Johansson et al. (2008) was not observed in the current study. The differential response to methamphetamine, in the absence of a response to nicotine in mice exposed to PFOA suggests the need for further examination of these pharmacological pathways and the dopaminergic system to characterize the underlying effects of PFOA gestational exposure.
Highlights.
Neurotoxicity of gestational PFOA exposure was not evident at dose levels of 0.1 and 0.3 mg/kg/day
Nicotine (80 mg/kg) did not stimulate motor activity, rather physical handling and injection procedure stimulated motor activity in first 10–15 min
Gestational exposure to PFOA 1mg PFOA/kg/day diminished methamphetamine-induced activity.
Acknowledgments
This research was supported by the Division of Intramural Research and the Division of the National Toxicology Program, National Institute of Environmental Health Sciences, National Institutes of Health, Department of Health and Human Services #1Z01ES101623; ES021164; ES102785. The study was initiated by S. White and S. Fenton, who were responsible for dosing and selection of animals; S. White and D. Goulding conducted the behavioral tests; S. McBride performed statistical analysis; and G. Harry was responsible for study design and data interpretation. All authors contributed to writing of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors declare no conflicts of interest.
References
- Andersen CS, Fei C, Gamborg M, Nohr EA, Sorensen TI, Olsen J. Prenatal exposures to perfluorinated chemicals and anthropometric measures in infancy. Am. J. Epidemiol. 2010;172:1230–1237. doi: 10.1093/aje/kwq289. [DOI] [PubMed] [Google Scholar]
- Apelberg BJ, Witter FR, Herbstman JB, Calafat AM, Halden RU, Needham LL, Goldman LR. Cord serum concentrations of perfluorooctane sulfonate (PFOS) and perfluorooctanoate (PFOA) in relation to weight and size at birth. Environ. Hlth. Perspect. 2007;115:1670–1676. doi: 10.1289/ehp.10334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer J. Test for emotionality in rats and mice: a review. Anim. Behav. 1973;21:205–235. doi: 10.1016/s0003-3472(73)80065-x. [DOI] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Brix N, Nohr EA, Bonde JP, Henriksen TB. Perfluoroalkyl and polyfluoroalkyl substances and human fetal growth: a systematic review. Crit. Rev. Toxicol. 2015;45:53–67. doi: 10.3109/10408444.2014.952400. [DOI] [PubMed] [Google Scholar]
- Boiteux V, Dauchy X, Rosin C, Munoz JF. National screening study on 10 perfluorinated compounds in raw and treated tap water in France. Arch. Environ. Contam. Toxicol. 2012;63:1–12. doi: 10.1007/s00244-012-9754-7. [DOI] [PubMed] [Google Scholar]
- Buck RC, Franklin J, Berger U, Conder JM, Cousins IT, de Voogt P, Jensen AA, Kannan K, Mabury SA, van Leeuwen SPJ. Perfluoroalkyl and polyfluoroalkyl substances in the environment: terminology, classification, and origins. Integr. Environ. Assess. Manag. 2011;7:513–541. doi: 10.1002/ieam.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E, Badea A, Watson C, Johnson GA. A quantitative magnetic resonance histology atlas of postnatal rat brain development with regional estimates of growth and variability. NeuroImage. 2013;71:196–206. doi: 10.1016/j.neuroimage.2013.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carli M, Kostoula C, Sacchetti G, Mainolfi P, Anastasia A, Villani C, Invernizzi RW. Tph2 gene deletion enhances amphetamine-induced hypermotility: effect of 5-HT restoration and role of striatal noradrenaline release. J. Neurochem. 2015;135:674–685. doi: 10.1111/jnc.13280. [DOI] [PubMed] [Google Scholar]
- Chen MH, Ha EH, Wen TW, Su YN, Lien GW, Chen CY, Chen PC, Hsieh WS. Perfluorinated compounds in umbilical cord blood and adverse birth outcomes. PLoS One. 2012;7(8):e42474. doi: 10.1371/journal.pone.0042474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MH, Ha EH, Liao HF, Jeng SF, Su YN, Wen TW, Lien GW, Chen CY, Hsieh WS, Chen PC. Perfluorinated compound levels in cord blood and neurodevelopment at 2 years of age. Epidemiology. 2013;24:800–808. doi: 10.1097/EDE.0b013e3182a6dd46. [DOI] [PubMed] [Google Scholar]
- Costall B, Hui SC, Naylor RJ. The importance of serotonergic mechanisms for the induction of hyperactivity by amphetamine and its antagonism by intra-accumbens (3,4-dihydroxy-phenylamino)-2-imidazoline (DPI) Neuropharmacol. 1979;18:605–609. doi: 10.1016/0028-3908(79)90112-6. [DOI] [PubMed] [Google Scholar]
- Csomor PA, Yee BK, Vollenweider FX, Feldon J, Nicolet T, Quednow BB. On the influence of baseline startle reactivity on the indexation of prepulse inhibition. Behav. Neurosci. 2008;122:885–900. doi: 10.1037/0735-7044.122.4.885. [DOI] [PubMed] [Google Scholar]
- D'Hollander W, de Voogt P, De Coen W, Bervoets L. Perfluorinated substances in human food and other sources of human exposure. Rev. Environ, Contam, Toxicol. 2010;208:179–215. doi: 10.1007/978-1-4419-6880-7_4. [DOI] [PubMed] [Google Scholar]
- Damaj MI, Glassco W, Dukat M, Martin BR. Pharmacological characterization of nicotine-induced seizures in mice. J. Pharmacol. Exp. Ther. 1999;291:1284–1291. [PubMed] [Google Scholar]
- Dobelis P, Hutton S, Lu Y, Collins AC. GABAergic systems modulate nicotinic receptor-mediated seizures in mice. J. Pharmacol. Exp. Ther. 2003;306:1159–1166. doi: 10.1124/jpet.103.053066. [DOI] [PubMed] [Google Scholar]
- Donauer S, Chen A, Xu Y, Calafat AM, Sjodin A, Yolton K. Prenatal exposure to polyborminated diphenyl ethers and polyfluoroalkyl chemicals and infant neurobehavior. J. Pediat. 2015;166:736–742. doi: 10.1016/j.jpeds.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson P, Ankarberg E, Fredriksson A. Exposure to nicotine during a defined period in neonatal life induces permanent changes in brain nicotinic receptors and in behaviour of adult mice. Brain Res. 2000;853:41–48. doi: 10.1016/s0006-8993(99)02231-3. [DOI] [PubMed] [Google Scholar]
- Fei C, Olsen J. Prenatal exposure to perfluorinated chemicals and behavioral or coordination problems at age 7 years. Environ. Health Perspect. 2011;119:573–578. doi: 10.1289/ehp.1002026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. Perfluorinated chemicals and fetal growth: a study within the Danish National Birth Cohort. Environ. Health Perspect. 2007;115:1677–1682. doi: 10.1289/ehp.10506. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Prenatal exposure to perfluorooctanoate (PFOA) and perfluorooctanesulfonate (PFOS) and maternally reported developmental milestones in infancy. Environ. Health Perspect. 2008a;116:1391–1395. doi: 10.1289/ehp.11277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Tarone RE, Olsen J. Fetal growth indicators and perfluorinated chemicals: a study in the Danish National Birth Cohort. Am J Epidemiol. 2008b;168:66–72. doi: 10.1093/aje/kwn095. [DOI] [PubMed] [Google Scholar]
- Fenton S, Reiner J, Nakayama S, Delinsky A, Stanko J, Hines E, White SS, Lindstron AB, Strynar MJ, Petropoulou SS. Analysis of PFOA in dosed CD-1 mice. Part 2. Disposition of PFOA in tissues and fluids from pregnant and lactating mice and their pups. Reprod. Toxicol. 2009;27:365–372. doi: 10.1016/j.reprotox.2009.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fornai F, Biagioni F, Fulceri F, Murri L, Ruggieri S, Paparelli A. Intermittent dopaminergic stimulation causes behavioral sensitization in the addicted brain and parkinsonism. Int. Rev. Neurobiol. 2009;88:371–398. doi: 10.1016/S0074-7742(09)88013-6. [DOI] [PubMed] [Google Scholar]
- Fromme H, Mosch C, Morovitz M, Alba-Alejandre I, Boehmer S, Kiranoglu M, Faber F, Hannibal I, Genzel-Boroviczény O, Koletzko B, Völkel W. Pre- and postnatal exposure to perfluorinated compounds (PFCs) Environ. Sci. Technol. 2010;44:7123–7129. doi: 10.1021/es101184f. [DOI] [PubMed] [Google Scholar]
- Geyer MA, Swerdlow NR. Measurement of startle response, prepulse inhibition, and habituation. Curr. Protocol. Neurosci. 1998;(Suppl. 3):8.7.1–8.7.15. doi: 10.1002/0471142301.ns0807s03. [DOI] [PubMed] [Google Scholar]
- Hollander M, Wolfe DA. Nonparametric Statistical Methods. 3rd. New York, NY: Wiley; 2013. [Google Scholar]
- Høyer BB, Ramlau-Hansen CH, Obel C, Pedersen HS, Hernik A, Ogniev V, Jönsson BA, Lindh CH, Rylander L, Rignell-Hydbom A, Bonde JP, Toft G. Pregnancy serum concentrations of perfluorinated alkyl substances and offspring behavior and motor development at age 5–9 years--a prospective study. Environ. Health. 2015;14:2. doi: 10.1186/1476-069X-14-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson N, Fredriksson A, Eriksson P. Neonatal exposure to perfluorooctane sulfonate (PFOS) and perfluorooctanoic acid (PFOA) causes neurobehavioural defects in adult mice. Neurotoxicology. 2008;29:160–169. doi: 10.1016/j.neuro.2007.10.008. [DOI] [PubMed] [Google Scholar]
- Jonckheere AR. A distribution-free k-sample test against ordered alternatives. Biometrika. 1954;41:133–145. [Google Scholar]
- Kang H, Choi K, Lee HS, Kim DH, Park NY, Kim S, Kho Y. Elevated levels of short carbon-chain PFCAs in breast milk among Korean women: Current status and potential challenges. Environ. Res. 2016;148:351–359. doi: 10.1016/j.envres.2016.04.017. [DOI] [PubMed] [Google Scholar]
- Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, Calafat AM. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003–2006. Environ. Sci. Technol. 2014;48:9600–9608. doi: 10.1021/es501811k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koustas E, Lam J, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. The Navigation Guide-Evidence-Based Medicine Meets Environmental Health: Systematic Review of Nonhuman Evidence for PFOA Effects on Fetal Growth. Environ. Hlth. Perspect. 2014;122:1015–10127. doi: 10.1289/ehp.1307177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ksir C. Acute and chronic nicotine effects on measures of activity in rats; a multivariate analysis. Psychopharm. 1994;115:105–109. doi: 10.1007/BF02244758. [DOI] [PubMed] [Google Scholar]
- Lam J, Koustas E, Sutton P, Johnson PI, Atchley DS, Sen S, Robinson KA, Axelrad DA, Woodruff TJ. The Navigation Guide-Evidence-Based Medicine Meets Environmental Health: Integration of Animal and Human Evidence for PFOA Effects on Fetal Growth. Environ. Hlth. Perspect. 2014;122:1040–1051. doi: 10.1289/ehp.1307923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmler HJ. Synthesis of environmentally relevant fluorinated surfactants—a review. Chemosphere. 2005;58:1471–1496. doi: 10.1016/j.chemosphere.2004.11.078. [DOI] [PubMed] [Google Scholar]
- Lenters V, Portengen L, Rignell-Hydbom A, Jonsson BA, Lindh CH, Piersma AH, Toft G, Bonde JP, Heederik D, Ruylander L, Vermeulen R. Prenatal phthalate, perfluoroalkyl acid, and organochlorine exposure and term birth weight in three birth cohorts: Multi-pollutant models based on elastic net regression. Environ. Health Perspect. 2016;124:365–372. doi: 10.1289/ehp.1408933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AA. Regulatory developmental neurotoxicology testing: data evaluation for risk assessment purposes. Environ. Toxicol. Pharmacol. 2005;19:727–733. doi: 10.1016/j.etap.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Liew Z, Ritz B, von Ehrenstein OS, Bech BH, Nohr EA, Fei C, Bossi R, Henriksen TB, Bonefeld-Jørgensen EC, Olsen J. Attention deficit/hyperactivity disorder and childhood autism in association with prenatal exposure to perfluoroalkyl substances: a nested case-control study in the Danish National Birth Cohort. Environ. Hlth. Perspect. 2015;123:367–373. doi: 10.1289/ehp.1408412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu PS, Liaw CT, Lin MK, Shin SH, Kao LS, Lin LF. Amphetamine enhances Ca2+ entry and entry and catecholamine release via nicotinic receptor activation in bovine adrenal chromaffin cells. Eur. J. Pharmacol. 2003;460:9–17. doi: 10.1016/s0014-2999(02)02870-4. [DOI] [PubMed] [Google Scholar]
- Llorca M, Farré M, Picó Y, Teijón ML, Alvarez JG, Barceló D. Infant exposure of perfluorinated compounds: levels in breast milk and commercial baby food. Environ. Int. 2010;36:584–592. doi: 10.1016/j.envint.2010.04.016. [DOI] [PubMed] [Google Scholar]
- Llorca M, Pérez F, Farré M, Agramunt S, Kogevinas M, Barceló D. Analysis of perfluoroalkyl substances in cord blood by turbulent flow chromatography coupled to tandem mass spectrometry. Sci. Total Environ. 2012;433:151–160. doi: 10.1016/j.scitotenv.2012.05.080. [DOI] [PubMed] [Google Scholar]
- Lucas G, Spampinato U. Role of striatal serotonin2A and serotonin2C receptor subtypes in the control of in vivo dopamine outflow in the rat striatum. J. Neurochem. 2000;74:693–701. doi: 10.1046/j.1471-4159.2000.740693.x. [DOI] [PubMed] [Google Scholar]
- Maisonet M, Terrell ML, McGeehin MA, Christensen KY, Holmes A, Calafat AM, Marcus M. Maternal concentrations of polyfluoroalkyl compounds during pregnancy and fetal and postnatal growth in British girls. Environ. Hlth. Perspect. 2012;120:1432–1437. doi: 10.1289/ehp.1003096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mariussen E. Neurotoxic effects of perfluoroalkylated compounds: mechanisms of action and environmental relevance. Arch. Toxicol. 2012;86:1349–1367. doi: 10.1007/s00204-012-0822-6. [DOI] [PubMed] [Google Scholar]
- Naylor C, Quarta D, Fernandes C, Stolerman IP. Tolerance to nicotine in mice lacking α7 nicotinic receptors. Psychopharm. 2005;180:558–563. doi: 10.1007/s00213-005-2187-5. [DOI] [PubMed] [Google Scholar]
- Nordberg A, Zhang XA, Fredriksson A, Eriksson P. Neonatal nicotine exposure induces permanent changes in brain nicotinic receptors and behaviour in adult mice. Brain Res. Dev. Brain Res. 1991;63:201–207. doi: 10.1016/0165-3806(91)90079-x. [DOI] [PubMed] [Google Scholar]
- Novak CM, Burghardt PR, Levine JA. The use of a running wheel to measure activity in rodents: Relationship to energy balance, general activity, and reward. Neurosci. Biobehavioral Rev. 2012;36:1001–1014. doi: 10.1016/j.neubiorev.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ode A, Källén K, Gustafsson P, Rylander L, Jönsson BA, Olofsson P, Ivarsson SA, Lindh CH, Rignell-Hydbom A. Fetal exposure to perfluorinated compounds and attention deficit hyperactivity disorder in childhood. PLoS One. 2014;9(4):e95891. doi: 10.1371/journal.pone.0095891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill MF, Heron-Maxwell CL, Shaw G. 5-HT2 receptor antagonism reduces hyperactivity induced by amphetamine, cocaine, and MK-801 but not D1 agonist C-APB. Pharmacol. Biochem. Behav. 1999;63:237–243. doi: 10.1016/s0091-3057(98)00240-8. [DOI] [PubMed] [Google Scholar]
- Olsen GW, Butenhoff JL, Zobel LR. Perfluoroalkyl chemicals and human fetal development: an epidemiologic review with clinical and toxicological perspectives. Reprod. Toxicol. 2009;27:212–230. doi: 10.1016/j.reprotox.2009.02.001. [DOI] [PubMed] [Google Scholar]
- Onishchenko N, Fischer C, Norhamidah W, Ibrahim W, Negri S, Spulber S, Cottica D, Ceccatelli S. Prenatal exposure to PFOS or PFOA alters motor function in mice in a sex-related manner. Neurotox. Res. 2011;19:452–461. doi: 10.1007/s12640-010-9200-4. [DOI] [PubMed] [Google Scholar]
- Ortells MO, Arias HR. Neuronal networks of nicotine addiction. Inter. J. Biochem. Cell Biol. 2010;42:1931–1935. doi: 10.1016/j.biocel.2010.08.019. [DOI] [PubMed] [Google Scholar]
- Perez F, Llorca M, Kock-Schulmeyer M, Skrbic B, Oliveira LS, da Boit Martinello K, Al-Dhabi NA, Antic I, Farre M, Barcelo D. Assessment of perfluoroalkyl substances in food items at global scale. Environ. Res. 2014;135:181–189. doi: 10.1016/j.envres.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed-effects models in S and S-PLUS. 2000th. New York, NY: Springer; 2000. ISBN: 978-1441903174. [Google Scholar]
- Porras G, Di Matteo V, Fracasso C, Lucas G, De Deurwaerdère P, Caccia S, Esposito E, Spampinato U. 5-HT2A and G-HT2C/2B receptor subtypes modulate dopamine release induced in vivo by amphetamine and morphine in both the rat nucleus accumbens and striatum. Neuropsychopharm. 2002;26:311–324. doi: 10.1016/S0893-133X(01)00333-5. [DOI] [PubMed] [Google Scholar]
- Post GG, Cohn PD, Cooper KR. Perfluorooctanoic acid (PFOA), an emerging drinking water contaminant: a critical review of recent literature. Environ. Res. 2012;116:93–117. doi: 10.1016/j.envres.2012.03.007. [DOI] [PubMed] [Google Scholar]
- Quaak I, Decock M, de Boer M, Lamoree M, Leonards P, van de Bor M. Prenatal exposure to perfluoroalkyl substances and behavioral development in children. Int. J. Environ. Res. Public Health. 2016;13:511. doi: 10.3390/ijerph13050511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quiñones O, Snyder SA. Occurrence of perfluoroalkyl carboxylates and sulfonates in drinking water utilities and related waters from the United States. Environ. Sci. Technol. 2009;43:9089–9095. doi: 10.1021/es9024707. [DOI] [PubMed] [Google Scholar]
- Schwanz TG, Llorca M, Farre M, Barcelo D. Perfluoroalkyl substances assessment in drinking waters from Brazil, France and Spain. Sci. Total Environ. 2016;539:143–152. doi: 10.1016/j.scitotenv.2015.08.034. [DOI] [PubMed] [Google Scholar]
- So MK, Yamashita N, Taniyasu S, Jiang Q, Giesy JP, Chen K, Lam PK. Health risks in infants associated with exposure to perfluorinated compounds in human breast milk from Zhoushan, China. Environ. Sci. Technol. 2006;40:2924–2929. doi: 10.1021/es060031f. [DOI] [PubMed] [Google Scholar]
- Sobolewski M, Conrad K, Allen JL, Weston H, Martin K, Lawrence BP, Cory-Slechta DA. Sex-specific enhanced behavioral toxicity induced by maternal exposure to a mixture of low dose endocrine-disrupting chemicals. Neurotoxicology. 2014;45:121–130. doi: 10.1016/j.neuro.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford SC. The open field test: reinventing the wheel. J. Psychopharm. 2007;21:134–135. doi: 10.1177/0269881107073199. [DOI] [PubMed] [Google Scholar]
- Strøm M, Hansen S, Olsen SF, Haug LS, Rantakokko P, Kiviranta H, Halldorsson TI. Persistent organic pollutants measured in maternal serum and offspring neurodevelopmental outcomes--a prospective study with long-term follow-up. Environ. Int. 2014;68:41–48. doi: 10.1016/j.envint.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Thornburg JE, Moore KE. The relative importance of dopaminergic and noradrenergic neuronal systems for the stimulation of locomotor activity induced by amphetamine and other drugs. Neuropharm. 1973;12:853–866. doi: 10.1016/0028-3908(73)90038-5. [DOI] [PubMed] [Google Scholar]
- Tucker DK, Macon MB, Strynar MJ, Dagnino S, Andersen E, Fenton SE. The mammary gland is a sensitive pubertal target in CD-1 and C57Bl/6 mice following perinatal perfluorooctanoic acid (PFOA) exposure. Reprod. Toxicol. 2015;54:26–36. doi: 10.1016/j.reprotox.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viberg H, Fredriksson A, Eriksson P. Neonatal exposure to the brominated flame retardant 2,2',4,4',5-pentabromodiphenyl ether causes altered susceptibility in the cholinergic transmitter system in the adult mouse. Toxicol. Sci. 2002;67:104–107. doi: 10.1093/toxsci/67.1.104. [DOI] [PubMed] [Google Scholar]
- Walsh RN, Cummins RA. The open-field test: a critical review. Psychol. Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- Washino N, Saijo Y, Sasaki S, Kato S, Ban S, Konishi K, Ito R, Nakata A, Iwasaki Y, Saito K, Nakazawa H, Kishi R. Correlations between prenatal exposure to perfluorinated chemicals and reduced fetal growth. Environ. Hlth. Persp. 2009;117:660–667. doi: 10.1289/ehp.11681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westfall P, Hochberg Y, Rom D, Wolfinger R, Tobias R. Multiple Comparisons and Multiple Tests Using the SAS System. Cary, NC: SAS Institute; 1999. [Google Scholar]
- Williams DA. A test for differences between treatment means when several dose levels are compared with a zero dose control. Biometrics. 1971;27:103–117. [PubMed] [Google Scholar]
- Yang L, Li J, Lai J, Luan H, Cai Z, Wang Y, Zhao Y, Wu Y. Placental transfer of perfluoroalkyl substances and associations with thyroid hormones: Beijing prenatal exposure study. Sci. Rep. 2016;22(6):21699. doi: 10.1038/srep21699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee BK, Singer P. A conceptual and practical guide to the behavioral evaluation of animal models of the symptomatology and therapy of schizophrenia. Cell Tissue Res. 2013;354:221–246. doi: 10.1007/s00441-013-1611-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zombeck JA, Gupta T, Rhodes JS. Evaluation of a pharmacokinetic hypothesis for reduced locomotor stimulation from methamphetamine and cocaine in adolescent versus adult male C57BL/6J mice. Psychopharmacology. 2009;201:589–599. doi: 10.1007/s00213-008-1327-0. [DOI] [PubMed] [Google Scholar]