Abstract
Background
The aim of this study was to assess the clinical course and distinctive features of different white dot syndromes (WDS) in patients attending the Ophthalmology Department, Medical University of Warsaw in the years 1995–2015.
Material/Methods
Sixty-two (62) patients (43 females and 19 males), aged 18 to 77 years, referred with a WDS were included in this prospective study, with observation period ranging from 5 months to 16 years. All patients underwent a complete ophthalmological examination and multimodal imaging studies.
Results
In this cohort of 62 patients, the following WDS entities were identified: multifocal choroiditis with panuveitis (MFCPU), multifocal choroiditis (MFC), punctate inner choroidopathy (PIC), birdshot, acute posterior multifocal placoid pigment epitheliopathy (APMPPE), subretinal fibrosis and uveitis, multiple evanescent white dot syndrome (MEWDS), serpiginous choroiditis, and single cases of acute annular outer retinopathy (AAOR).
Conclusions
The study was performed at a Polish referral center and may to some extent reflect the varied geographical distribution of white dot syndromes, as none of the subjects was found to suffer from acute zonal occult outer retinopathy (AZOOR), acute macular neuroretinopathy (AMN), or diffuse unilateral subacute neuroretinitis (DUSN). Long-term follow-up is warranted by the evolution of lesions in the eye fundus, while management depends on correct diagnosis of WDS. When the posterior pole is involved in some cases of the WDS an immunosuppressive treatment, the use of the PDT or anti-VEGF injections were necessary.
MeSH Keywords: Autoimmune Diseases, Follow-Up Studies, Immunosuppressive Agents, Photography, Uveitis, Virus Diseases
Background
White dot syndromes (WDS) are a group of inflammatory chorioretinopathies distinguished by ophthalmoscopic findings, characterized by the presence of whitish or yellowish fundus lesions which vary in shape, size, and location, and at times are associated with uveitis in the anterior segment and/or the vitreous body [1–4].
The etiology of WDS is unknown and the hypothesized causes include viral infection, parasitic infestation, and autoimmune or genetic predisposition [4,5].
To date, a dozen different WDS have been described. In addition to such commonly known entities as multifocal choroiditis with panuveitis (MFCPU), multifocal choroiditis (MFC), punctate inner choroidopathy (PIC), and multiple evanescent white dot syndrome (MEWDS), there have also been reports of relentless placoid chorioretinitis (RPC), acute idiopathic exudative polymorphous vitelliform maculopathy (AIEPVM), and peripheral multifocal chorioretinitis (PMC) [6–9].
Ophthalmoscopic and angiographic findings differ between particular WDS. In the 1990s, Gass proposed found that most WDS, such as MEWDS, PIC, acute annular outer retinopathy (AAOR), acute zonal occult outer retinopathy (AZOOR), acute macular retinopathy (AMN), and MFC, are in fact variants of the same disease [10,11]. According to Essex et al. and Fung et al., MFC and PIC are the same disorder [12,13].
The aim of this study was to report the long-term clinical course and characteristic features of the fundus lesions in patients with different WDS treated at the Department of Ophthalmology, Medical University of Warsaw in the years 1995–2015.
There is a need for long-term follow-up evaluation because the fundus lesions in WDS evolve, and imaging studies, especially color photography, are used to document this evolution.
Material and Methods
We reviewed the medical records of 62 patients (43 females, 19 males) aged 18 to 77 years (mean age 41.8+16.7) who underwent diagnostic examinations for WDS at the Department of Ophthalmology, Medical University of Warsaw. The follow-up period ranged from 5 months to 16 years. During that period, the patients underwent a complete ophthalmological examination, including best-corrected visual acuity (BCVA), slit-lamp examination, and ophthalmoscopy. The other examinations included color photography of the posterior pole and peripheral retina, and fluorescein and indocyanine green angiography (VISUPAC 5.0, Carl Zeiss Meditec AG, Germany; Spectralis HRA+OCT, Heidelberg Engineering; Heidelberg, Germany). Spectralis HRA+OCT is a full name of the device, which combines confocal scanning laser ophthalmoscopy (cSLO) and spectral domain optical coherence tomography (SD-OCT), ultrasonography (AVISO, Quantel Medical, France), visual fields (Dicon Auto Perimetr, Vision System, INC, Canada; Goldmann), and optical coherence tomography (OCT) (CARL ZEISS Stratus OCT™; Cirrus HD-OCT, Carl Zeiss Meditec AG, Germany, Spectralis HRA+OCT, Heidelberg Engineering; Heidelberg, Germany).
The research adhered to the tenets of the Declaration of Helsinki.
Results
The most common diagnosis in the group of 62 patients with WDS was MFCPU (21 patients, 33.8%), followed by MCF (8 patients, 12.9%), PIC (8 patients, 12.9%), birdshot chorioretinopathy (8 patients, 12.9%), acute posterior multifocal placoid pigment epitheliopathy (APMPPE) (7 patients, 11.3%), subretinal fibrosis and uveitis (SFU) (4 patients, 6.4%), MEWDS (3 patients, 4.8%), serpiginous choroidopathy (2 patients, 3.2%), and AAOR (1 patient, 1.6%). Mean follow-up period was 3.9±2.8 years. In 40 patients (64.5%), the lesions were bilateral and in 22 patients (35.4%) they were unilateral. Myopia (−1.25 Dsph to −6.5 Dsph) was found in 20 patients (32.2%) (i.e., in all patients with MCF and PIC), 2 with APMPPE and 2 with SFU. Four patients with MFCPU (6.4%) had a coexisting systemic disease (sarcoidosis in 3 cases and multiple sclerosis in 1).
The ocular symptoms were preceded by the manifestations of a respiratory infection in 4 out of 7 patients with APMPPE and in 1 patient with AAOR.
Two patients with APMPPE who suffered from a low-grade fever and persistent cough were tested for influenza virus, and a generalized infection was confirmed in 1 patient. The PCR study of nasal swabs found the genetic material of type A influenza virus, but not of type B influenza virus.
The HAI (hemagglutinin inhibition) (PB-01-LEI/D) test confirmed in the serum the presence of anti-influenza hemagglutinin antibody, which tested positive for the following influenza strains: subtype A/H1N1 (A/California/7/2009, titre 1: 80), subtype A/H3N2 (A/Victoria/361/2011, titre 1: 80), and type B (B/Massachusetts/2/2012, titre 1: 40). The patient was not vaccinated against influenza in the 2013/14 season. Pneumonia and cardiovascular complications were excluded.
Differences in visual acuities ranging from “counting fingers” to 20/20 depended on complications of WDS such as cataract or various forms of macular changes (epiretinal membrane, cystoid macular edema, fibrosis, subretinal neovascularization, and atrophy).
In 9 cases (1 patient with MFC, 3 with PIC, 1 with serpiginous choroiditis, 3 with SFU, and 1 with AMPPE), coexisting subretinal neovascularization at different stages of activity was observed.
In 1 case of PIC, 3 injections of Macugen (pegaptanib sodium) were administered, photodynamic laser treatment (PDT) was used in 2 cases (MFC, AMPPE), and systemic corticosteroids in 3 (2 patients with SFU and 1 with serpiginous choroiditis).
In 2 patients with PIC, subretinal neovascularization resolved without treatment. In the remaining patients (PIC, SFU) no treatment was given due to either very significant scarring or pregnancy.
Coexisting secondary glaucoma was found only in patients with MFCPU and topical glaucoma, and anti-inflammatory treatment stabilized the intraocular pressures.
Among the patients included in the present study, the most severe clinical course was seen in all cases of subretinal fibrosis, with low visual acuity at presentation and poor response to systemic corticosteroids.
Epidemiological data are presented in Table 1.
Table 1.
Epidemiological data of patients with white dot syndromes.
| Disease | Number of patients, sex, age | Inititial visual acuity | Final visual acuity | Unilateral | Bilateral | CMO/ ERM/CMO+ERM | CNV/Macularscar | Fibrosis | Damage of macular RPE | Glaucoma | Cataract | Treatment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MFCPU | 21 (17W, 4M) age 24–77 | Count finger to 16/20 | Count finger to 20/20 | 3 | 18 | 2/7/3 | 0 | 0 | 0 | 3 | 6 |
|
| MFC | 8 (6W, 2M) age 25–37 | Count finger to 20/20 | Count finger to 20/20 | 4 | 4 | 0 | 1/5 | 0 | 0 | 0 | 0 |
|
| PIC | 8 (5W, 3M) age 18–43 | 1/100 To 18/20 | 1/100 to 18/20 | 2 | 6 | 0 | 3/2 | 0 | 0 | 0 | 0 |
|
| birdshot | 8 (5W, 3M) age 23–70 | Count finger to 20/20 | Count finger to 20/20 | 1 | 7 | 3/2/1 | 0 | 0 | 0 |
|
||
| AMPPE | 7 (6W, 1M) age 21–51 | 4/20 To 20/20 | 4/20 to 20/20 | 3 | 4 | 0 | 1 | 0 | 7 | 0 | 0 |
|
| SFU | 4 (2W, 2M) age 26–37 | Count finger to 20/20 | Count finger to 20/20 | 1 | 3 | 0 | 0/4 | 4 | 0 | 0 | 0 | 2 oral prednisone |
| Serpiginous | 2 M aged 24 and 44 | 4/20 to 8/20 | 4/20 to 8/20 | 0 | 2 | 0 | 0/1 | 1 | 1 | 0 | 0 | 1 oral prednisone 1 oral prednisone + cyclosporin |
| MEWDS | 2W, 1M (19–33) | 4/20 | 20/20 | 3 | 0 | 0 | 0 | 0 | 3 | 0 | 0 | Topical non-steroidal antinflammatory |
| AAOR | W 24 | 4/20 | 16/20 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | Oral prednisone + acyclovir |
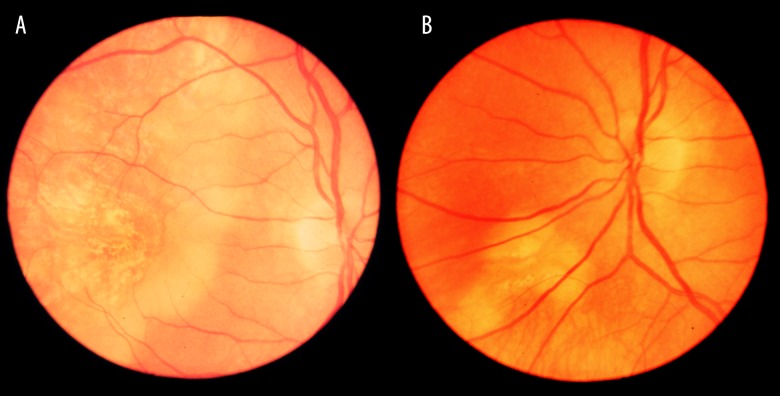
The evolution of the fundus lesions in Patient 1 initially classified as PIC is presented in Figures 1–4.
Figure 1.
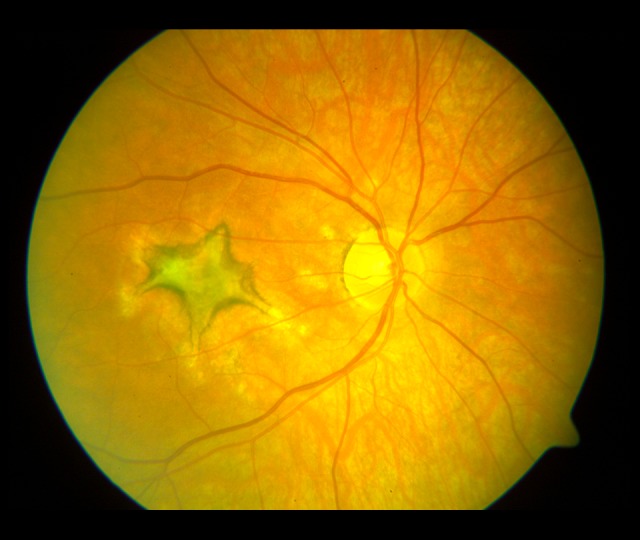
Lesions in the fundus of the right eye (2003). A star-shaped fibrotic lesion and several irregular atrophic foci in the posterior pole.
Figure 2.
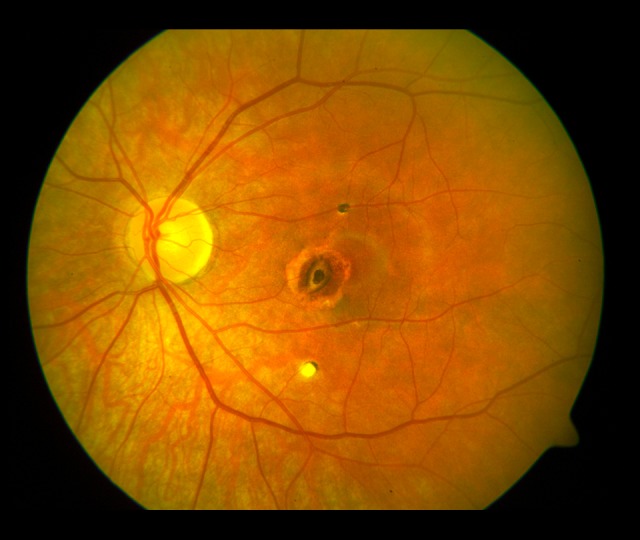
Lesions in the fundus of the left eye (2003). Foci of atrophy with pigment accumulation.
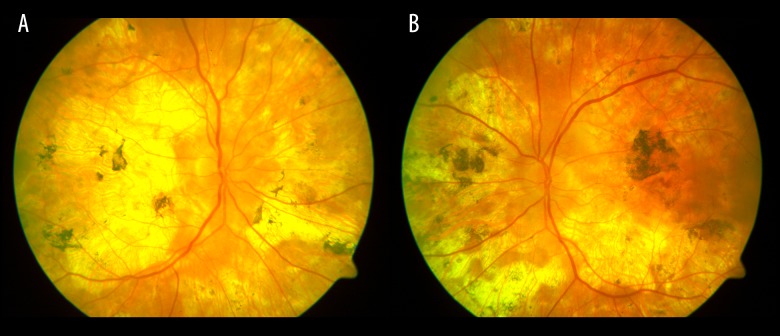
Figure 3.
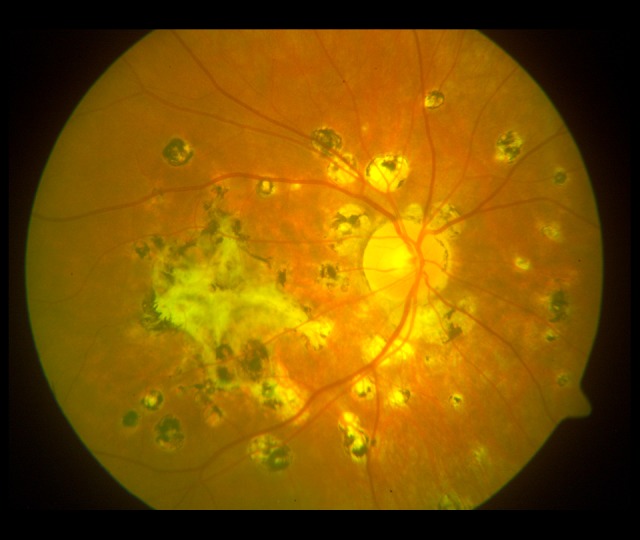
Lesions in the fundus of the right eye (2009). A large fibrotic scar in the macula and numerous various-sized pigmented atrophic foci in the posterior pole.
Figure 4.
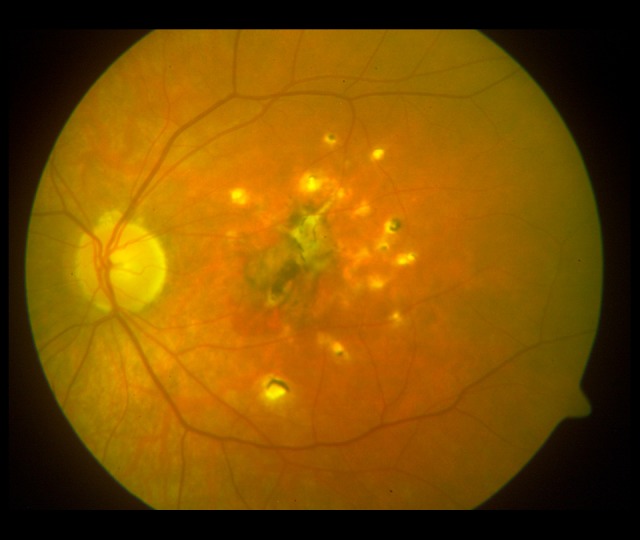
Lesions in the fundus of the left eye (2009). A fibrotic scar in the macula and numerous pigmented atrophic foci. The lesions were less advanced than in the right eye.
The evolution of the fundus lesions in Patient 2 with PIC diagnosed during 3-year follow-up is presented in Figures 5 and 6.
Figure 5.
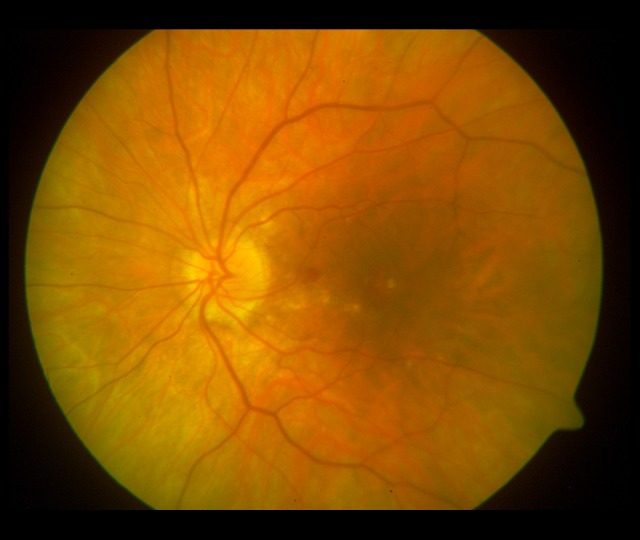
Photograph of the left eye on diagnosis: small atrophic foci in the macula and at the optic disc. The atrophic rim visible at the lower edge of the optic disc.
Figure 6.
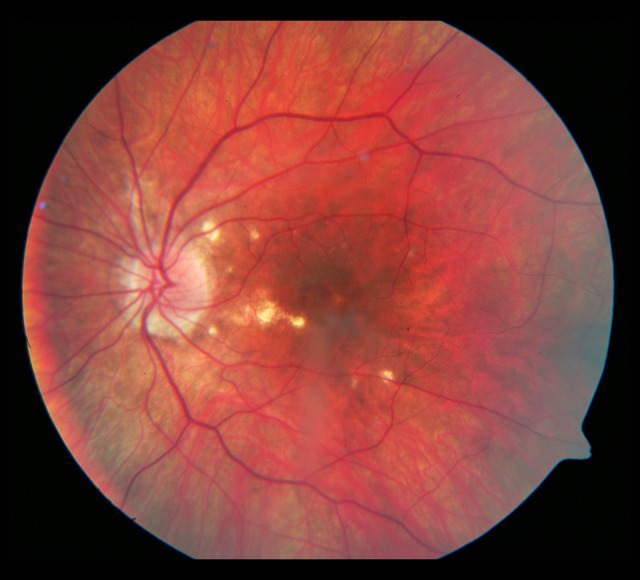
Photograph of the left eye 3 years later: widening of the atrophic rim around the optic disc. Small atrophic foci with pigment visible within some of the foci.
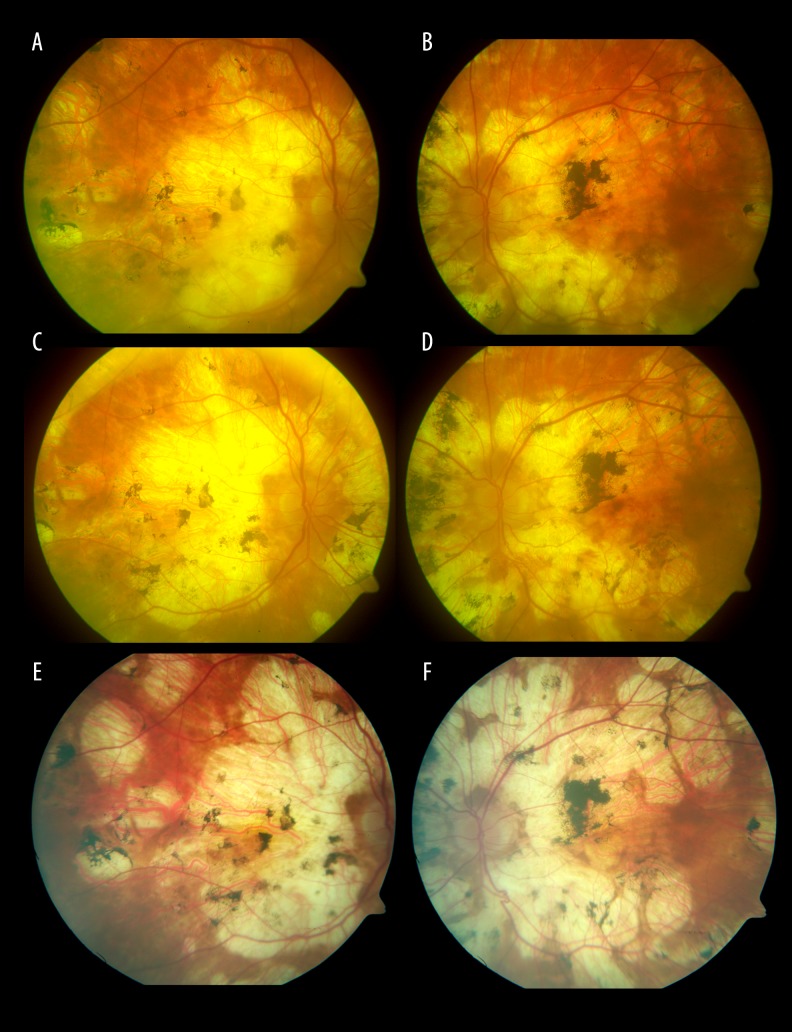
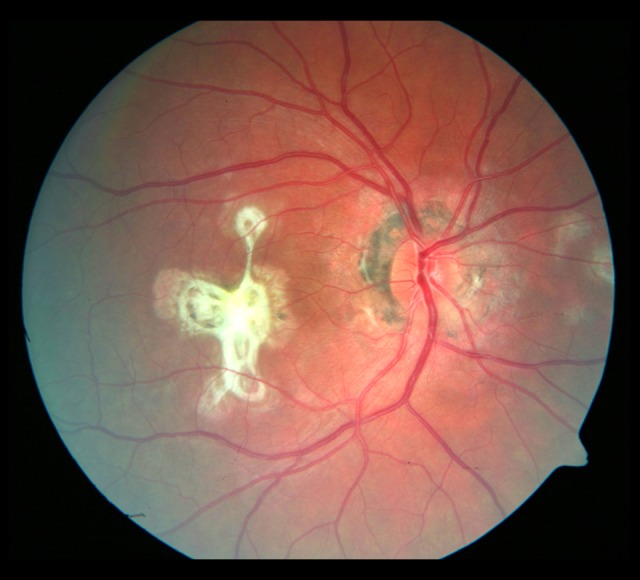
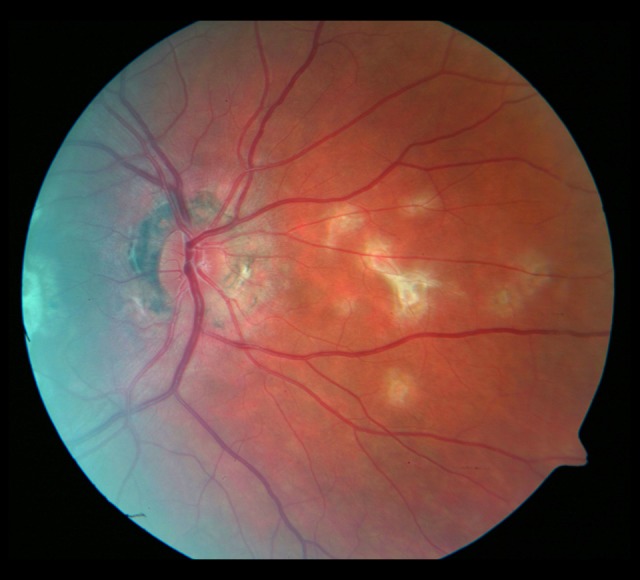
Evolution of the fundus lesions in Patient 3 with SFU diagnosed during 18-month follow-up is presented in Figures 7–14.
Figure 7.
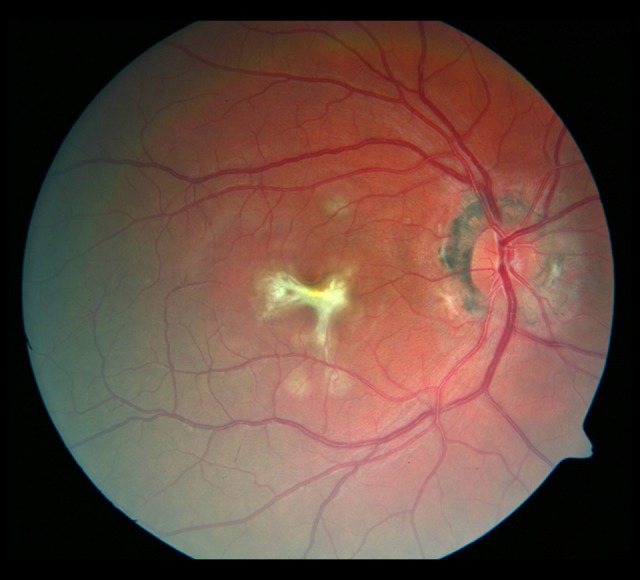
Photograph of the fundus of the right eye with the atrophic rim around the optic disc. A fibrotic scar and whitish, washed-out, irregularly shaped foci in the macula.
Figure 8.
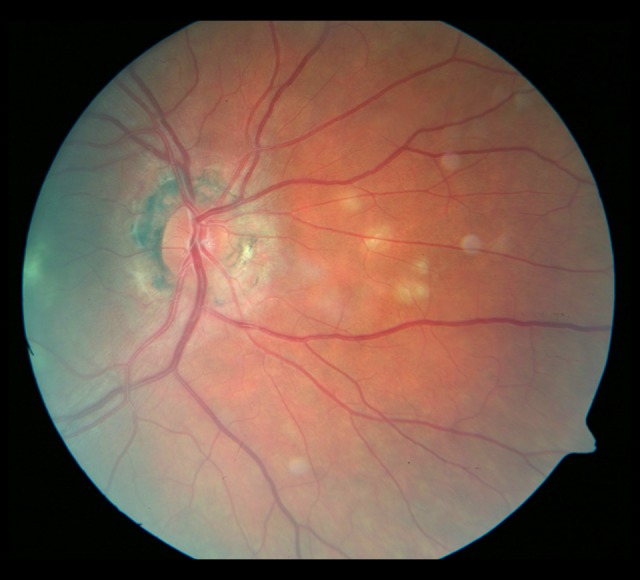
Whitish, irregularly shaped foci on the nasal side of the optic disc.
Figure 9.
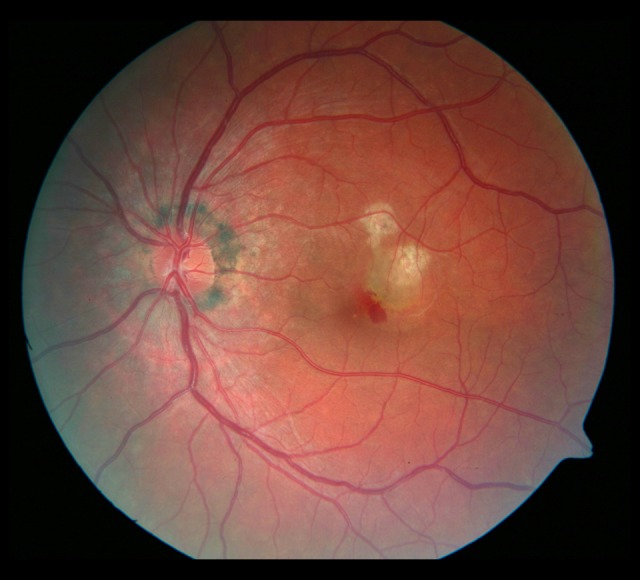
Photograph of the fundus of the left eye with the atrophic rim around the optic disc. A hemorrhage spot and whitish irregularly shaped foci in the macula.
Figure 10.
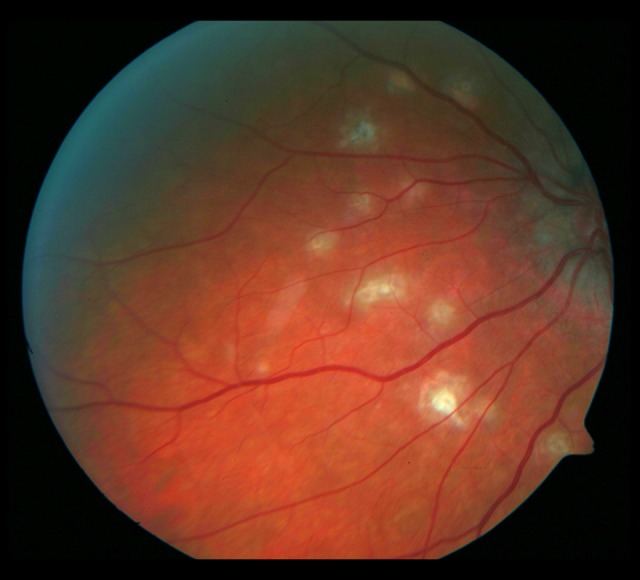
Whitish, irregularly shaped foci visible on the nasal side of the optic disc. The fundus of the left eye 18 months later. Progression of the macular fibrosis and the foci on the nasal side of the optic disc.
Figure 11.
Photograph of the fundus of the right eye 18 months later. Progression of the macular fibrosis.
Figure 12.
Photograph of the fundus of the right eye 18 months later. Progression of the focal fibrosis on the nasal side of the optic disc.
Figure 13.
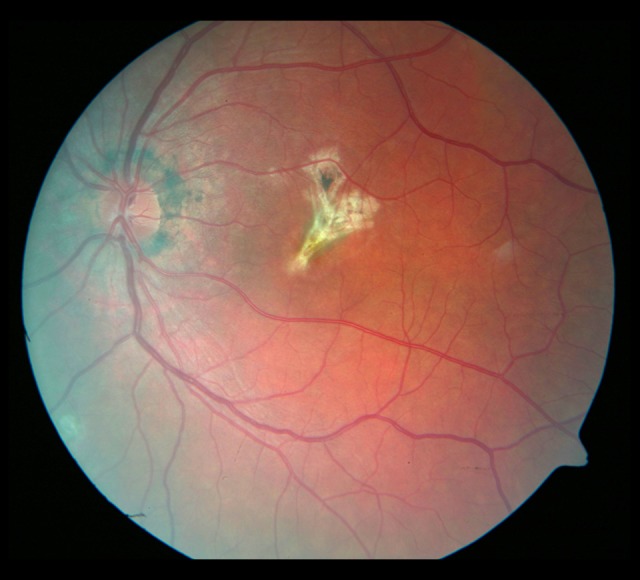
The fundus of the left eye 18 months later. Progression of the macular fibrosis.
Figure 14.
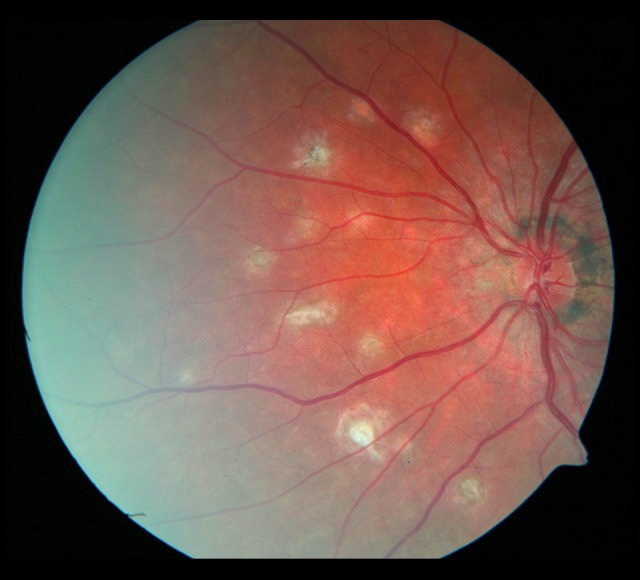
The fundus of the left eye 18 months later. Progression of the focal fibrosis and on the nasal side of the optic disc.
The evolution of the fundus lesions in a patient with serpiginous choroiditis during 16-year follow-up is presented in Figures 15–17.
Figures 15.
(A, B) Washed-out, creamy foci of inflammation in the macular region of the right eye and below the optic disc in the left eye (1999).
Figures 16.
(A, B) Diffuse foci of chorioretinal atrophy and accumulations of pigment (2002).
Figures 17.
(A, B) (2006), (C, D) (2009), (E, F) (2015). Increases in the areas of chorioretinal atrophy and the extent of pigment dispersion in long-term observation.
Discussion
White dot syndromes are a group of ocular disorders, distinguished from one another by ophthalmoscopic and angiographic findings, whose clinical course is characterized by lesions in the retina, choroid, and pigment epithelium, and some WDS may be described as inflammatory choriocapillaropathies [14].
The incidence of WDS in adults in not known, but Spital et al. estimate the rate in pediatric patients at 1% to 5% [15].
The literature search revealed single reports of cumulative incidence rates for different WDS in particular populations [2,15].
To the best of our knowledge, it is the first report emphasizing the need of a long-term follow-up of WDS and documenting the evolution of lesions by means of color photography.
In our analysis of the clinical findings pictures of patients with WDS, we identified 9 entities out of a dozen described WDS, but found no cases of AZOOR, DUNS, or AMN [3,14,16–18]. The lesions were bilateral in 64.5% of patients and unilateral in 35.4%. Fung et al. found bilateral lesions during follow-up of over 4 years in patients with MFC [13]. In our study, no fellow eye involvement was observed in patients with initially unilateral lesions.
Myopia may accompany WDS, especially MFC, PIC, and MEWDS [13,14,19]; we found it in 21 patients (33.8%), in all patients with MFC and PIC, 2 patients with AMPPE, and 3 with SFU.
Coexisting systemic disease occurred in 4 patients, all of them with MFCPU (sarcoidosis in 3 cases and multiple sclerosis in 1 case). Perlman et al., in their follow-up study of 114 patients with WDS, found autoimmune disease in one-fourth of patients, mostly disorders of the thyroid gland (Hashimoto or Graves disease) or skin (psoriasis, alopecia areata, atopic dermatitis) [5].
MFCPU was the most common WDS (33.8%) and typical complications such as cataract, glaucoma, cystoid macular edema, and epiretinal membrane were found in 21 patients, which is in agreement with reports by other authors [17,20–22].
In contrast to the studies by Wachtlin et al. and by Gerth et al., Perlman el al did not observe any development of choroidal neovascularization [5,23,24], nor did they find optic neuropathy, which was described by Thorne et al. [25].
In 2005, we published a report of 14 cases of changes described as multifocal choroiditis and panuveitis (MCP), but at present the term multifocal choroiditis with panuveitis (MFCPU) is preferred [26].
In the present follow-up study, we originally assumed that the size of the lesion was an important feature which distinguished MFC from PIC, but we now realize that such a final differentiation may be arbitrary [27]. The evolution of lesions in Patient 1 seems very interesting in this respect.
In 2003, in our assessment, the clinical picture appeared to be characteristic of PIC, and 6 years later, the appearance of lesions suggested MFC, although we are aware that some other ophthalmologists might diagnose POCHS in the right eye and PIC in the left eye.
There have been reports of coexisting choroidal neovascularization in patients with WDS, especially MFC and PIC, and much less frequently in the course of APMPPE, serpiginous choroiditis, SFU, MEWDS, or birdshot chorioretinopathy [13,20,23,27–34]. In our study group, choroidal neovascularization occurred in 9 patients (14.5%), mostly in the course of PIC and SFU and in single cases of serpiginous choroiditis, MFC, and APMPPE.
Depending on the activity of the lesions, most promising treatment options available at the time were used, ranging from immunosuppressive agents to photodynamic laser therapy to intravitreal injections of Macugen (pegaptanib sodium).
In 1 case, choroidal neovascularization occurred in early pregnancy (7 weeks) in a 31-year-old woman with PIC. The lesions healed spontaneously within 5 weeks. There have been single reports of choroidal neovascularization in pregnant women, observed in the course of presumed ocular histoplasmosis syndrome, inner punctate choroidopathy, or idiopathic disease [35–38].
According to Vuorela et al., from the onset of pregnancy, vascular endothelial growth factor (VEGF) and placental growth factor (PIGF) are highly expressed not only in the placenta, but also in the maternal blood, which can be the cause of choroidal neovascularization [39].
In patients with birdshot chorioretinopathy, we observed its typical complications, but not subretinal neovascularization as reported by Nguyen et al. and Oueghlani et al. [30,40].
Out of 7 patients with APMPPE, the visual acuity returned with general treatment in 6 after treatment with prednisone or acyclovir or both [41]. Similar findings were reported by Gass et al., but Fiore reported that nearly half of the patients did not recover full visual acuity [42,43].
In our study, we proved the association of flu infection with the development of APMPPE, a finding that to date has not been reported by other authors. In 1 case, APMPPE was considered to be recurrent since active and healed lesions in the fundus were accompanied by pigmented scarring and choroidal neovascularization subsequently healed with photodynamic therapy. Choroidal neovascularization secondary to APMPPE is rare [31].
In all cases of subretinal fibrosis, poor response to systemic corticosteroids was observed.
The reports by Amaro el al and by Adnan et al. are similar in this respect [44,45]. Adnan et al. and Cornish et al. found that treatment with infliximab or rituximab was effective in diffuse subretinal fibrosis [45,46].
In our study, the clinical course of MEWDS and AAOR was typical and in agreement with the observations of other authors [3,18]. The diagnosis of WDS may pose a problem, because although the fundus lesions meet the diagnostic criteria of WDS, the appearance of the foci or their location may make the identification of a specific WDS difficult. Occasionally, the WDS may overlap, and in one patient, lesions characteristic of 2 different WDS may be found in one or both eyes [47–50].
Our group of 62 cases of different WDS in patients attending the Ophthalmology Department, Medical University of Warsaw in the years 1995–2015 may seem small, but, like Kedhar, we think that it is due to the fact that as a referral center, we admit mostly selected patients requiring rapid diagnosis and treatment for macular lesions [27].
Conclusions
In our opinion, it is extremely important for clinicians to learn more about WDS, because some patients with features of WDS are still referred to infectious diseases hospitals with the diagnosis of infectious chorioretinitis, especially suspected ocular toxoplasmosis. Monitoring of the lesions by means of color photography and angiography is also very important, and allow objective assessment of the evolution. The wide variety of lesions observed in the eye fundus suggests different entities, but in its final stage WDS may present with a similar appearance.
Footnotes
Source of support: Departmental sources
Declaration of interest
All the authors declare no conflicts of interest.
References
- 1.Jampol LM, Becker KG. White spot syndromes of the retina: A hypothesis based on the common genetic hypothesis of autoimmune/inflammatory disease. Am J Ophthalmol. 2003;135(3):376–79. doi: 10.1016/s0002-9394(02)02088-3. [DOI] [PubMed] [Google Scholar]
- 2.Yaghi NE, Hartono SP, Hodge DO, et al. White dot syndromes: A 20-year study of incidence, clinical features, and outcomes. Ocul Immunol Inflamm. 2011;19(6):426–30. doi: 10.3109/09273948.2011.624287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Crawford CM, Igboeli O. A review of the inflammatory chorioretinopathies: The white dot syndromes. ISRN Inflamm. 2013;2013:783190. doi: 10.1155/2013/783190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quillen DA, Davis JB, Gottlieb JL, et al. The white dot syndromes. Am Journal Ophthalmol. 2004;137(3):538–50. doi: 10.1016/j.ajo.2004.01.053. [DOI] [PubMed] [Google Scholar]
- 5.Pearlman RB, Golchet PR, Feldmann MG, et al. Increased prevalence of autoimmunity in patients with white spot syndromes and their family members. Arch Ophtalmol. 2009;127(7):869–74. doi: 10.1001/archophthalmol.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jones BE, Jampol LM, Yannuzzi LA, et al. Relentless placoid chorioretinitis: A new entity or an unusual variant of serpiginous chorioretinitis? Arch Ophthalmol. 2000;118:931–38. [PubMed] [Google Scholar]
- 7.Vianna RN, Socci D, Nehemy MB, et al. The white dot syndromes. Arq Bras Oftalmol. 2007;70(3):554–62. doi: 10.1590/s0004-27492007000300031. Review. [DOI] [PubMed] [Google Scholar]
- 8.Vianna RN, Muralha A, Muralha L. Indocyanine green angiography in acute idiopathic exudative polymorphous vitelliform maculopathy. Retina. 2003;23(4):539–41. doi: 10.1097/00006982-200308000-00016. [DOI] [PubMed] [Google Scholar]
- 9.Koop A, Ossewaarde A, Rothova A. Peripheral multifocal chorioretinitis: Complications, prognosis and relation with sarcoidosis. Acta Ophthalmol. 2013;91(6):492–97. doi: 10.1111/j.1755-3768.2012.02483.x. [DOI] [PubMed] [Google Scholar]
- 10.Gass JDM. Acute zonal occult outer retinopathy. J Clin Neuroophthalmol. 1993;13:79–97. [PubMed] [Google Scholar]
- 11.Gass JD. The acute zonal outer retinopathies. Am J Ophthalmol. 2000;130(5):655–57. doi: 10.1016/s0002-9394(00)00738-8. [DOI] [PubMed] [Google Scholar]
- 12.Essex RW, Wong J, Jampol LM, et al. Idiopathic multifocal choroiditis: A comment on present and past nomenclature. Retina. 2013;33(1):1–4. doi: 10.1097/IAE.0b013e3182641860. [DOI] [PubMed] [Google Scholar]
- 13.Fung AT, Pal S, Yannuzzi NA, et al. Multifocal choroiditis without panuveitis: Clinical characteristics and progression. Retina. 2014;34(1):98–107. doi: 10.1097/IAE.0b013e31829234cb. [DOI] [PubMed] [Google Scholar]
- 14.Cimino L, Mantovani A, Herbort CP. Primary inflammatory choriocapillaropathies. In: Pleyer U, Mondino B, editors. Essentials in Ophthalmology: Uveitis and Immunological Disorders. Berlin, Heidelberg, New York: Springer; 2004. pp. 209–31. [Google Scholar]
- 15.Spital G, Heiligenhaus A, Scheider A, et al. „White dot syndromes” in childhood. Klin Monbl Augenheilkd. 2007;224(6):500–6. doi: 10.1055/s-2007-963179. [DOI] [PubMed] [Google Scholar]
- 16.Essex RW, Wong J, Fraser-Bell S, et al. Punctate inner choroidopathy: Clinical features and outcomes. Arch Ophtalmol. 2010;128(8):982–87. doi: 10.1001/archophthalmol.2010.157. [DOI] [PubMed] [Google Scholar]
- 17.Vianna RN, Ozdal PC, Filho JP, et al. Longterm follow-up of patients with multifocal choroiditis and panuveitis. Acta Ophtalmol Scand. 2004;82(6):748–53. doi: 10.1111/j.1600-0420.2004.00343.x. [DOI] [PubMed] [Google Scholar]
- 18.Fekrat S, Wilkinson CP, Chang B, et al. Acute annular outer retinopathy: Report of four cases. Am J Ophthalmol. 2000;130(5):636–44. doi: 10.1016/s0002-9394(00)00560-2. [DOI] [PubMed] [Google Scholar]
- 19.Herbort CP, Papadia M, Neri P. Myopia and inflammation. J Ophthalmic Vis Res. 2011;6(4):270–83. [PMC free article] [PubMed] [Google Scholar]
- 20.Kedhar SR, Thorne JE, Wittenberg S, et al. Multifocal choroiditis with panuveitis and punctate inner choroidopathy: Comparison of clinical characteristics at presentation. Retina. 2007;27(9):1174–79. doi: 10.1097/IAE.0b013e318068de72. [DOI] [PubMed] [Google Scholar]
- 21.Nölle B, Faul S, Jenisch S, Westphal E. Peripheralmultifocal chorioretinitis with panuveitis clinical and immunogenetic characterization in older patients. Graefes Arch Clin Exp Ophthalmol. 1998;236(6):451–60. doi: 10.1007/s004170050105. [DOI] [PubMed] [Google Scholar]
- 22.Thorne JE, Wittenberg S, Jabs DA, et al. Multifocal choroiditis with panuveitis incidence of ocular complications and of loss of visual acuity. Ophthalmology. 2006;113(12):2310–16. doi: 10.1016/j.ophtha.2006.05.067. [DOI] [PubMed] [Google Scholar]
- 23.Wachtlin J, Heimann H, Behme T, Foerster MH. Long-term results after Figuredynamic therapy with verteporfin for choroidal neovascularizations secondary to inflammatory chorioretinal diseases. Graefes Arch Clin Exp Ophtalmol. 2003;241(11):899–906. doi: 10.1007/s00417-003-0734-5. [DOI] [PubMed] [Google Scholar]
- 24.Gerth C, Spital G, Lommatzsch A. Figuredynamic therapy for choroidal neovascularization in patients with multifocal choroiditis and panuveitis. Eur J Ophthalmol. 2006;16(1):111–18. doi: 10.1177/112067210601600118. [DOI] [PubMed] [Google Scholar]
- 25.Thorne JE, Wittenberg S, Kedhar SR, et al. Optic neuropathy complicating multifocal choroiditis and panuveitis. Am J Ophthalmol. 2007;143(4):721–23. doi: 10.1016/j.ajo.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brydak-Godowska J, Swituła M, Dróbecka-Brydak E, et al. [Multifocal choroiditis and panuveitis (MCP)-diagnosis, ocular symptoms and treatment]. Klin Oczna. 2005;107(10–12):665–67. [in Polish] [PubMed] [Google Scholar]
- 27.Kedhar SR, Thorne JE, Wittenberg S, et al. Multifocal choroiditis with panuveitis and punctate inner choroidopathy: Comparison of clinical characteristics at presentation. Retina. 2007;27(9):1174–79. doi: 10.1097/IAE.0b013e318068de72. [DOI] [PubMed] [Google Scholar]
- 28.Chang LK, Spaide RF, Brue C, et al. Bevacizumab treatment for subfoveal choroidal neovascularization from causes other than age-related macular degeneration. Arch Ophthalmol. 2008;126:941–45. doi: 10.1001/archopht.126.7.941. [DOI] [PubMed] [Google Scholar]
- 29.Leung TG, Moradi A, Liu D, et al. Clinical features and incidence rate of ocular complications in punctate inner choroidopathy. Retina. 2014;34(8):1666–74. doi: 10.1097/IAE.0000000000000125. [DOI] [PubMed] [Google Scholar]
- 30.Nguyen QD, Shah SM, Hafiz G, et al. Intravenous bevacizumab causes regression of choroidal neovascularization secondary to diseases other than agerelated macular degeneration. Am J Ophthalmol. 2008;145:257–66. doi: 10.1016/j.ajo.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bowie EM, Sletten KR, Kayser DL, Folk JC. Acute posterior multifocal placoid pigment epitheliopathy and choroidal neovascularization. Retina. 2005;25:362–64. doi: 10.1097/00006982-200504000-00018. [DOI] [PubMed] [Google Scholar]
- 32.Christmas NJ, Oh KT, Oh DM, Folk JC. Long-term follow-up of patients with serpinginous choroiditis. Retina. 2002;22(5):550–56. doi: 10.1097/00006982-200210000-00004. [DOI] [PubMed] [Google Scholar]
- 33.Gandorfer A, Ulbig MW, Kampik A. Diffuse subretinal fibrosis syndrome. Retina. 2000;20(5):561–63. doi: 10.1097/00006982-200005000-00028. [DOI] [PubMed] [Google Scholar]
- 34.Shen Z, Zhang L, Li Z, et al. Comparison on clinical characteristics of multifocal choroiditis and punctate inner choriodopathy. Eye Sci. 2011;26(3):161–65. doi: 10.3969/j.issn.1000-4432.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Rhee P, Dev S, Mieler WF. The development of choroidal neovascularization in pregnancy. Retina. 1999;19:520–24. doi: 10.1097/00006982-199911000-00007. [DOI] [PubMed] [Google Scholar]
- 36.Sim DA, Sheth HG, Kaines A, Tufail A. Punctate inner chorioretinopathy-associated choroidal neovascular membranes during pregnancy. Eye. 2008;22:725–27. doi: 10.1038/eye.2008.93. [DOI] [PubMed] [Google Scholar]
- 37.Tarantola RM, Folk JC, Boldt HC, Mahajan VB. Intravitreal bevacizumab during pregnancy. Retina. 2010;30(9):1405–11. doi: 10.1097/IAE.0b013e3181f57d58. [DOI] [PubMed] [Google Scholar]
- 38.Anastasilakis K, Symeonidis C, Kaprinis K, et al. Peripapillary neovascular membrane in a young pregnant woman and prompt response to ranibizumab injections following uneventful delivery. Case Rep Ophthalmol. 2011;2(1):129–33. doi: 10.1159/000328385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vuorela P, Hatva E, Lymboussaki A, et al. Expression of vascular endothelial growth factor and placenta growth factor in human placenta. Biol Reprod. 1997;56:489–94. doi: 10.1095/biolreprod56.2.489. [DOI] [PubMed] [Google Scholar]
- 40.Oueghlani E, Westcott M, Pavésio CE. Anti-VEGF therapy for choroidal neovascularisation secondary to Birdshot chorioretinopathy. Klin Monbl Augenheilkd. 2010;227(4):340–41. doi: 10.1055/s-0029-1245246. [DOI] [PubMed] [Google Scholar]
- 41.Dróbecka-Brydak E, Skórska I, Swituła M, et al. [Acute posterior multifocal placoid epitheliopathy]. Klin Oczna. 1996;98(4):319–21. [in Polish] [PubMed] [Google Scholar]
- 42.Gass JDM. Acute posterior multifocal placoid pigment epitheliopathy: A long-term follow-up study. In: Fine SL, Owens SL, editors. Management of retinal vascular and macular disorders. Baltimore: Williams & Wilkins; 1983. pp. 176–81. [Google Scholar]
- 43.Fiore T, Iaccheri B, Androudi S, et al. Acute posterior multifocal placoid pigment epitheliopathy: outcome and visual prognosis. Retina. 2009;29(7):994–1001. doi: 10.1097/IAE.0b013e3181a0bd15. [DOI] [PubMed] [Google Scholar]
- 44.Amaro MH, Muccioli C, Motta MM. Progressive subretinal fibrosis and multifocal granulomatous chorioretinitis. Arq Bras Oftalmol. 2006;3:413–15. doi: 10.1590/s0004-27492006000300024. [DOI] [PubMed] [Google Scholar]
- 45.Adan A, Sanmarti R, Bures A, et al. Successful treatment with infliximab in a patient with diffuse subretinal fibrosis syndrome. Am J Ophthalmol. 2007;143:533–34. doi: 10.1016/j.ajo.2006.10.047. [DOI] [PubMed] [Google Scholar]
- 46.Cornish KS, Kuffova L, Forrester JV. Treatment of diffuse subretinal fibrosis uveitis with rituximab. Br J Ophthalmol. 2015;99(2):153–54. doi: 10.1136/bjophthalmol-2013-304686. [DOI] [PubMed] [Google Scholar]
- 48.Gass JDM, Hamed LM. Acute macular neuroretinopathy and multiple evanescent white dot syndrome in the same patient. Arch Ophthalmol. 1989;107:189–93. doi: 10.1001/archopht.1989.01070010195021. [DOI] [PubMed] [Google Scholar]
- 49.Holz FG, Kim RY, Schwartz SD, et al. Acute zonal occult outer retinopathy (AZOOR) associated with multifocal choroidopathy. Eye. 1994;8:77–83. doi: 10.1038/eye.1994.15. [DOI] [PubMed] [Google Scholar]
- 50.Bryan RG, Freund KB, Yannuzzi LA, et al. Multiple evanescent white dot syndrome in patients with multifocal choroiditis. Retina. 2002;22:317–22. doi: 10.1097/00006982-200206000-00010. [DOI] [PubMed] [Google Scholar]