Abstract
Low fermentation temperatures are of importance to food and beverage industries working with Saccharomyces cerevisiae. Therefore, the identification of genes demonstrating a positive impact on fermentation kinetics is of significant interest. A set of 121 mapped F1 progeny, derived from a cross between haploid strains BY4716 (a derivative of the laboratory yeast S288C) and wine yeast RM11-1a, were fermented in New Zealand Sauvignon Blanc grape juice at 12.5°. Analyses of five key fermentation kinetic parameters among the F1 progeny identified a quantitative trait locus (QTL) on chromosome I with a significant degree of linkage to maximal fermentation rate (Vmax) at low temperature. Independent deletions of two candidate genes within the region, FLO1 and SWH1, were constructed in the parental strains (with S288C representing BY4716). Fermentation of wild-type and deletion strains at 12.5 and 25° confirmed that the genetic linkage to Vmax corresponds to the S288C version of the FLO1 allele, as the absence of this allele reduced Vmax by ∼50% at 12.5°, but not at 25°. Reciprocal hemizygosity analysis (RHA) between S288C and RM11-1a FLO1 alleles did not confirm the prediction that the S288C version of FLO1 was promoting more rapid fermentation in the opposing strain background, suggesting that the positive effect on Vmax derived from S288C FLO1 may only provide an advantage in haploids, or is dependent on strain-specific cis or trans effects. This research adds to the growing body of evidence demonstrating the role of FLO1 in providing stress tolerance to S. cerevisiae during fermentation.
Keywords: fermentation kinetics, genetic linkage analysis, low temperature, wine
The use of low temperatures (<18°) for many commercially important fermentative processes carried out by Saccharomyces cerevisiae, including baking, white winemaking, and rosé winemaking, is currently the industry norm. Although there is a widely held belief by winemakers and oenologists that low fermentation temperatures increase white wine quality (Uchimoto and Cruess 1952; Killian and Ough 1979; Llauradó et al. 2002; Molina et al. 2007), there is also an increase in the risk of stuck and sluggish fermentations, longer lag phase, and a decrease in the rate of yeast growth and fermentation, slowing down industrial processes and increasing financial costs (Charoenchai et al. 1998; Llauradó et al. 2002; Torija et al. 2003; Coleman et al. 2007; Chiva et al. 2012). Therefore, the identification of genes encoding proteins with the ability to confer cold tolerance during fermentation can be useful for selecting S. cerevisiae strains to be used in industries working with low fermentation temperatures, resulting in improved efficiencies and lower costs.
Low temperature environments are highly stressful for yeast, impacting on a multitude of cellular and metabolic processes: a reduction in membrane fluidity; a reduction in oxygen solubility; changes in nutrient uptake, transport and consumption; an increase in the biosynthesis of protective compounds; and a reduction in the rate of biochemical reactions (Sahara et al. 2002; Schade et al. 2004; Aguilera et al. 2007; Tai et al. 2007; Pizarro et al. 2008; Chiva et al. 2012). Environments that promote fermentation already contain many stresses that impact on yeast cells, e.g., high sugar, ethanol and toxic fatty acid concentrations, low pH, reduced concentrations of oxygen, and limited nitrogen. Therefore, the added stress of low fermentation temperatures requires an even greater response by S. cerevisiae, corresponding to altered transcription of ∼500–1000 genes depending on the strain and conditions used (Beltran et al. 2006; Deed et al. 2015). The transcriptional response to low temperature fermentation is initiated in two steps, first via the induction of cold-specific stress genes, followed by the more generalized environmental stress response and fermentation stress response (Gasch and Werner-Washburne 2002; Beltran et al. 2006; Marks et al. 2008; Deed et al. 2015). It has been well documented that different S. cerevisiae strains vary greatly in their ability to grow and ferment at lower temperatures (Charoenchai et al. 1998; Torija et al. 2003), and it has been suggested that these phenotypic differences are due to strain differences in gene expression, particularly via variation in gene promoter regions and the expression of transcription factors (Beltran et al. 2006; Chiva et al. 2012; Treu et al. 2014; Deed et al. 2015).
We have carried out genetic linkage analysis, using a set of 121 completely mapped (>99% of the genome) F1 progeny from a cross between haploid strains BY4716 and RM11-1a [denoted as BY and RM respectively in Brem et al. (2002)], to identify QTL with a positive influence on yeast fermentation kinetics at low temperature (12.5°). A region on chromosome I showed statistically significant genetic linkage to Vmax among the F1 progeny, and gene deletions and RHA were used to investigate the causative gene within this region.
Materials and Methods
S. cerevisiae strains
We utilized 121 segregant F1 progeny derived from a cross between laboratory strain BY4716 (MATα, lys2-Δ0), an isogenic derivative of laboratory strain S288C (Brachmann et al. 1998), and RM11-1a (MATa, leu2-Δ0, ura3-Δ0, HO::KanMX), a haploid derived from the wild vineyard-associated isolate Bb32 (Mortimer et al. 1994). BY4716 × RM11-1a F1 progeny were generated by Brem et al. (2002) for linkage analysis using 2957 mapped loci (kindly gifted by E. Smith and L. Kruglyak, Princeton University). S288C (MATα), representing the BY4716 parent, and the RM11-1a parent were used as reference strains to compare against fermentation phenotypes observed across the F1 progeny. Gene deletions and RHA were carried out in the S288C and RM11-1a strain backgrounds.
Growth and fermentation conditions
BY4716 × RM11-1a F1 progeny, parental strains, and diploid F1 hybrids generated for RHA were fermented at 12.5° (and 25° for the RHA strains) in Sauvignon Blanc grape juice, containing ∼22° Brix and 281 mg L−1 yeast assimilable nitrogen (Pernod Ricard, Marlborough, New Zealand). Grape juice was sterilized via overnight incubation at 25° with 200 μl L−1 dimethyl dicarbonate and supplemented with the following amino acids: 10 × leucine (300 mg L−1), 10 × lysine (300 mg L−1), and 10 × uracil (100 mg L−1). Yeast cultures were propagated in yeast-peptone-dextrose medium (YPD) and incubated overnight at 28°, with orbital shaking at 150 rpm. Grape juice was mixed well before being used to make 8 ml aliquots in 13-ml ventilation cap polypropylene tubes to ensure an even distribution of grape solids. Fermentations were inoculated with 1 × 106 cells ml−1 and a <0.5 mm2 pin-hole was punctured into each tube lid to allow for CO2 escape. Fermentations were monitored daily by measuring cumulative weight loss (g) (Bely et al. 1990). To reduce variability within triplicate fermentations, outliers were removed if they deviated from other replicates by >10% weight loss at three consecutive time points, after >50% total weight loss. Fermentations of RHA strains were performed in nonaplicates (n = 9).
Analysis of fermentation kinetic parameters
Phenotypes for five fermentation-related kinetic variables, maximal fermentation rate (Vmax) (dCO2/dt), maximal acceleration rate (Amax) (d2CO2/dt2), length of lag phase (h), final weight loss (g), and finishing time of alcoholic fermentation (AF time) (h), were determined from cumulative weight loss data, as per Marullo et al. (2006).
Linkage analysis
Quantitative phenotypic data for the five fermentation kinetic parameters were sent to J. Bloom and J. Gerke (Princeton University) for QTL mapping and identification of relevant loci. Logarithm (base 10) of odds (LOD) scores were generated for 2957 genetic markers across the 16 S. cerevisiae chromosomes using R/QTL’s scanone function, and a nonparametric model to compare the likelihood of obtaining the phenotypic data if mapped loci are linked against the likelihood of obtaining the data by chance (Broman et al. 2003). GBrowse maps of chromosomal regions with LOD scores > 3 (significant with a 5% chance of error) were obtained from the Saccharomyces Genome Database (SGD) (http://www.yeastgenome.org/) to determine candidate open reading frames (ORFs) linked to Vmax and lag phase.
Gene deletions and RHA
Deletion of candidate genes, FLO1 and SWH1, within the chromosome I QTL linked to Vmax, were constructed in S288C and RM11-1a using a modification of the Schiestl and Gietz (1989) lithium acetate yeast transformation protocol. The KanMX construct within the HO gene of RM11-1a was replaced with a hygromycin resistance (HGMR) cassette, HphMX, to allow for subsequent integration of KanMX into the two candidate genes. Transformation of haploid S288C and RM11-1a was performed independently to generate mutants with KanMX insertions in FLO1 and SWH1 using constructs amplified from the BY4743 EUROSCARF strains, FLO1ΔYAR050W::KanMX and SWH1ΔYAR042W::KanMX. Deletions of FLO1 and SWH1 were confirmed by PCR (list of oligonucleotide primers in Table 1). Crosses were made between wild-type S288C, RM11-1a, and flo1 and swh1 deletion mutants in order to construct diploid F1 hybrids for RHA (Steinmetz et al. 2002) (crosses shown in Table 2). A multiplex PCR to amplify 10 variable microsatellite markers and two mating type loci, MATa and MATα, was used to ensure that the F1 hybrids were constructed correctly (Table 3) (Richards et al. 2009).
Table 1. Oligonucleotide primers used for gene deletions and RHA.
| Primer Name | Sequence (5′–3′) | Purpose |
|---|---|---|
| 3′kanI-F | GGTCGCTATACTGCTGTC | Confirm integration of KanMX constructs |
| HOF2 | TGCAGAAGCTTGTTGAAGCA | Amplify HphMX insertion within HO |
| HOR2 | GCCGGTAACGCTTTTTGTAT | Amplify HphMX insertion within HO |
| MATa | ACTCCACTTCAAGTAAGAGTTTG | Amplify the MATa locus |
| Matα | GCACGGAATATGGGACTACTTCG | Amplify the MATα locus |
| MatR | AGTCACATCAAGATCGTTTATGG | Amplify the MATa/α locus |
| FLO1intL-F | CGGCACAGTTGAAAGAGTCA | Amplify KanMX from BY4743 flo1 deletion strain with flanking regions of homology |
| FLO1intR-R | GGCGATGGTTCATTAATTGC | Amplify KanMX from BY4743 flo1 deletion strain with flanking regions of homology |
| FLO1testL-F | GCCCTCACAAGAATTTGGAA | Flanking test primer used to confirm integration of KanMX into the FLO1 locus of transformants |
| FLO1testR-R | TTCCTGGGAACGAAAAGCTA | Flanking test primer used to confirm integration of KanMX into the FLO1 locus of transformants |
| SWH1intL-F | GCGTGTCCGGTTGAGTTTAT | Amplify KanMX from BY4743 swh1 deletion strain with flanking regions of homology |
| SWH1intR2-R | TTGCAGCAATTCGTTCAAAG | Amplify KanMX from BY4743 swh1 deletion strain with flanking regions of homology |
| SWH1testL2-F | GCCAGGACCGTCACTTGTAT | Flanking test primer used to confirm integration of KanMX into the SWH1 locus of transformants |
Table 2. Strains used to make crosses for RHA between S288C and RM11-1a for the FLO1 and SWH1 loci.
| Cross | Parent #1 | Parent #2 | F1 Hybrid Selection |
|---|---|---|---|
| R-FS × S-FS | RM11-1a (HO::HphMX; MATa) | S288C (MATα) | *HGMR |
| R-FS × S-fS | RM11-1a (HO::HphMX; MATa) | S288C (FLO1::KanMX; MATα) | HGMR; KanR |
| R-FS × S-Fs | RM11-1a (HO::HphMX; MATa) | S288C (SWH1::KanMX; MATα) | HGMR; KanR |
| R-fS × S-FS | RM11-1a (HO::HphMX; FLO1::KanMX; MATa) | S288C (MATα) | *HGMR; KanR |
| R-Fs × S-FS | RM11-1a (HO::HphMX; SWH1::KanMX; MATa) | S288C (MATα) | *HGMR; KanR |
The S288C parent strain in bold were added in 100 × excess of the RM11-1a parent, as S288C did not have any selectable markers that differed from RM11-1a. The F1 hybrid selections marked with * could result in the presence of the RM11-1a parent, as well as the F1 hybrid. The R-FS × S-FS cross was included as a control. HGMR, hygromycin resistance; KanR, kanamycin resistance.
Table 3. Microsatellite confirmation of F1 hybrid strains between S288C and RM11-1a for RHA.
| Strain | C3 | C5 | C8 | C4 | 091c | AT4 | AT2 | Scaat3 | 009c | 267c | α | a |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S288C | 120 | 174 | 130 | 240 | 302 | 296 | 357 | 407 | 443 | 415 | 468 | — |
| RM11-1a | 121 | 139 | 146 | 259 | 260 | 296 | 364 | 381 | 419 | 427 | — | 492 |
| R-Fs × S-FS | 120 | 139 | 130 | 240 | 260 | 296 | 358 | 381 | 419 | 415 | 468 | 492 |
| 121 | 174 | 146 | 259 | 303 | 296 | 364 | 407 | 443 | 427 | |||
| R-fS × S-FS | 120 | 139 | 130 | 240 | 260 | 296 | 358 | 381 | 419 | 415 | 468 | 492 |
| 121 | 174 | 146 | 259 | 303 | 296 | 364 | 407 | 443 | 427 | |||
| R-FS × SFs | 120 | 138 | 130 | 240 | 260 | 296 | 358 | 381 | 419 | 415 | 468 | 492 |
| 121 | 174 | 146 | 259 | 303 | 296 | 363 | 413 | 443 | 427 | |||
| R-FS × SfS | 120 | 138 | 130 | 240 | 260 | 296 | 358 | 381 | 419 | 415 | 468 | 492 |
| 121 | 174 | 146 | 259 | 302 | 296 | 363 | 407 | 443 | 427 | |||
| R-FS × S-FS | 120 | 139 | 130 | 240 | 260 | 296 | 358 | 381 | 419 | 415 | 468 | 492 |
| 121 | 174 | 146 | 259 | 302 | 296 | 363 | 407 | 443 | 427 |
Numbers are band sizes in base pairs. The 12 loci detected correspond to 10 variable microsatellite loci and two mating type loci, MATa and MATα, as described in Richards et al. (2009).
Data availability
All strains are available upon request. Supplemental Material: Table S1 contains the values for the kinetic parameters for all individuals. Table S2 contains the list of ORFs identified either side of each LOD >3 peak marker. File S1 contains the LOD scores for all individuals across the five fermentation parameters. File S2 contains Clustal alignments.
Results
Fermentation of 121 mapped F1 progeny identified genes linked to fermentation rate and lag phase
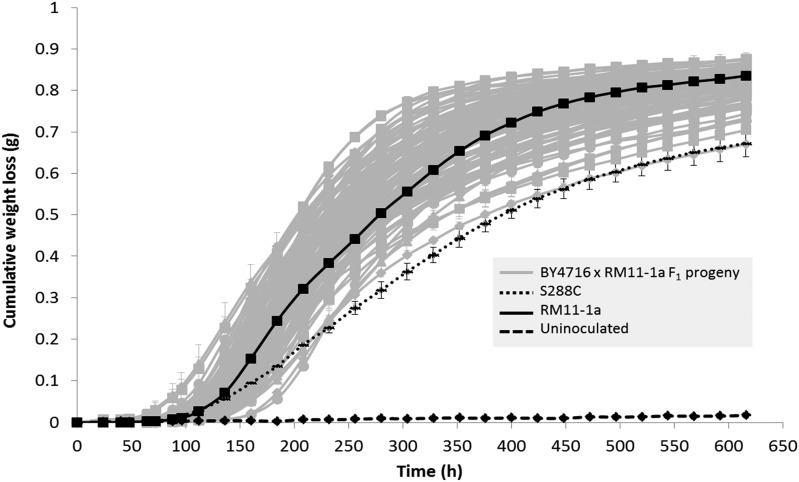
Cumulative weight loss was measured for 616 hr throughout the fermentation of 121 BY4716 × RM11-1a F1 progeny at 12.5° (Figure 1). As expected, the RM11-1a parental strain demonstrated superior fermentation performance compared to the S288C parental reference. F1 progeny demonstrated sufficient phenotypic variation for genetic mapping with fermentation curves covering the full range between both parents. Positive heterosis was also evident, with some F1 progeny exhibiting improved fermentation performance compared to RM11-1a. After removal of outliers (triplicate fermentations that deviated by >10% weight loss), 6/121 of the F1 progeny were analyzed only in duplicate (3D, 5B, 6A, 8G, 10G, and 3A-2), while two F1 progeny were completely excluded from the analysis (5A-1 and 11F-1). Five fermentation-related kinetic variables were derived from the weight loss data: Vmax, Amax, length of lag phase, final weight loss, and AF time (Table S1). These parameters were used for QTL mapping.
Figure 1.
Average cumulative weight loss (g) of 121 BY4716 × RM11-1a F1 progeny and parental reference strains S288C and RM11-1a. Strains were fermented in Sauvignon Blanc juice at 12.5°. BY4716 × RM11-1a F1 progeny = gray. S288C = black, small dashed line. RM11-1a = black solid line. Uninoculated = black dashed line, n = 3, error bars represent 95% C.I.s.
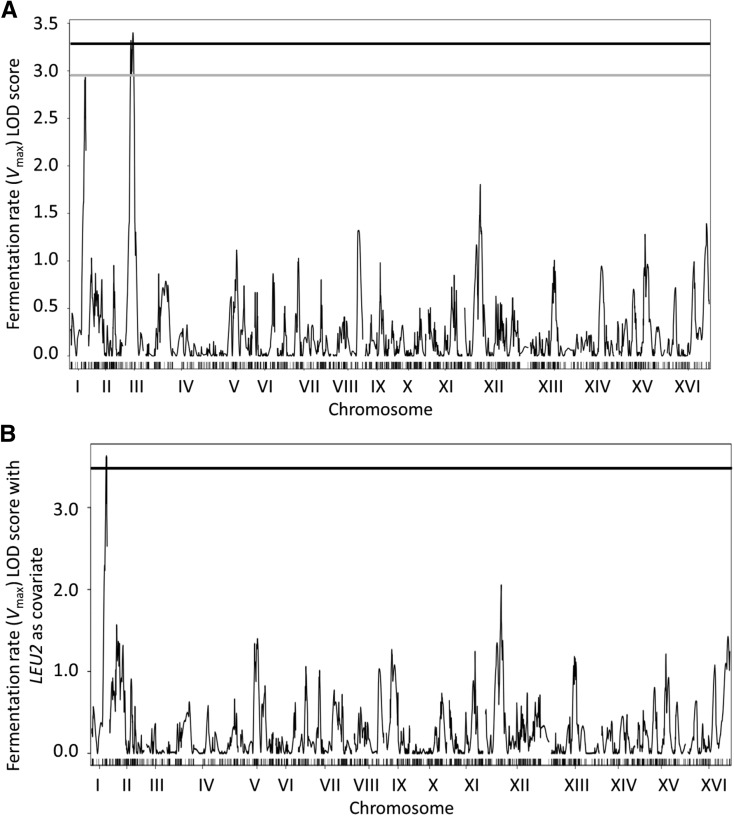
LOD scores were generated from the phenotypic data from the remaining 119 F1 progeny, which resulted in the identification of three regions across the genome with LOD scores > 3 (see File S1). A region on chromosome III was linked to Vmax, whereas regions on chromosomes VII and XIII were linked to lag phase. No loci had significant linkages to Amax, final weight loss, or AF time.
Closer inspection of the chromosome III region linked to Vmax on SGD indicated that the linkage was due to the inclusion of the LEU2 locus, which is deleted in RM11-1a. Removal of the effect of LEU2 on the dataset eliminated the chromosome III peak and resulted in a significant LOD score for the QTL at the subtelomeric end of chromosome I. Figure 2 shows LOD score plots for Vmax before (Figure 2A) and after (Figure 2B) the effect of LEU2 was removed by using the LEU2 genotype of F1 progeny as a covariate in a linear model of phenotype. LOD score data shows that the advantage for the Vmax trait on chromosome I is derived from the BY4716 allele, and not the RM11-1a allele (File S1). This was somewhat unexpected, given that the parental fermentation data showed that S288C progressed throughout fermentation much slower than RM11-1a, although the phenomenon of “low parents” in terms of transgressive segregation has been described previously (Ehrenreich et al. 2009).
Figure 2.
LOD scores plots of 2957 genetic markers across the 16 yeast chromosomes for Vmax values across 119 BY4716 × RM11-1a F1 progeny. (A) LODplot including the effects of LEU2. The gray and black horizontal lines represent the 10 and 5% significance levels, respectively (determined from 1000 permutations of each trait). (B) LODplot using LEU2 as covariate in a normal model to remove its effect. The black horizontal line represents the 5% significance level. LOD, Logarithm (base 10) of odds.
Identification of two candidate genes in the chromosome I region linked to Vmax and multiple ORFs on chromosomes VII and XIII in the regions linked to lag phase
Genes were identified in regions with LOD scores > 3 with C.I.s set at one LOD unit drop either side of a peak marker. GBrowse maps were used to identify and visualize all ORFs within the defined areas for Vmax and lag phase on chromosomes I, VII, and XIII (ORFs listed in Table S2), and the presence of nucleotide differences between the parental strains was also considered as an additional criterion for candidate ORFs. Of the six ORFs in the region linked to Vmax on chromosome I, two were considered to be potentially relevant to low temperature fermentation based on their respective functions: FLO1 (YAR050W), encoding a cell wall lectin-like protein that binds mannose and is involved in flocculation (Miki et al. 1982); and SWH1 (YAR042W, previously known as OSH1), encoding an oxysterol binding protein (Schmalix and Bandlow 1994). S. cerevisiae swh1 mutants exhibit phenotypes similar to viable mutants defective in sterol biosynthesis and show a reduction in membrane ergosterol levels, which also results in low temperature sensitivity in a tryptophan auxotroph (Jiang et al. 1994; Daum et al. 1999). However, sequence alignment analyses using Clustal found very few allelic differences between SWH1 in S288C and RM11-1a (99% similarity and deletion of two amino acids in S288C, see File S2). In contrast to SWH1, FLO1 has a very repetitive gene structure and the allele from RM11-1a has multiple large deletions compared to S288C (see File S2). Additionally, FLO1 is very highly expressed during fermentation at 12.5° in an F1 hybrid constructed by crossing another wine strain, Enoferm M2, with S288C (Deed et al. 2015). According to standard understanding, FLO1 is not expressed in S288C because FLO8, encoding its transcriptional regulator, has a nonsense mutation and is nonfunctional; however, there are reports of FLO1 being activated in a Flo8p-independent manner (Bester et al. 2006; Shen et al. 2006; Fichtner et al. 2007).
Ten ORFs were within the C.I.s near the LOD score peak for lag phase on chromosome VII (Table S2), including two genes encoding B-type cyclins involved in cell cycle progression, CLB1 (YGR108W) and CLB6 (YGR109C) (Surana et al. 1991; Schwob and Nasmyth 1993). Two neighboring peak markers with LOD scores > 3 were identified on chromosome XIII in the region linked to lag phase. Either side of these two peak markers, 34 ORFs were identified (Table S2). Genes of interest include RCF1 (YML030W), encoding a cytochrome c oxidase subunit that is required for growth under hypoxic conditions (Strogolova et al. 2012; Vukotic et al. 2012), and YOX1 (YML027W), encoding a transcriptional repressor involved in the regulation of cell cycle genes (Kaufmann 1993; Horak et al. 2002). Due to the difficulty of reproducibly phenotyping lag phase between different experiments in grape juice and the sheer number of potential candidate genes within the regions linked to lag phase, it was decided to concentrate on the identification of the locus influencing fermentation rate on chromosome I.
The FLO1 gene is linked to Vmax
To determine the effect of the FLO1 and SWH1 loci on Vmax, deletions of FLO1 and SWH1 were constructed in S288C and RM11-1a and hybrids were created to perform RHA. Fermentations at 12.5 and 25° in Sauvignon Blanc juice were performed using the original S288C and RM11-1a strains (renamed S-FS and R-FS to indicate strain name and FLO1/SWH1 genotype), the haploid flo1 and swh1 S288C and RM11-1a deletion strains (renamed S-Fs, S-fS, R-Fs, and R-fS), and the five diploid RHA F1 hybrids constructed by crossing combinations of S288C and RM11-1a wild-type, flo1, and swh1 deletion strains (R-FS × S-FS, R-Fs × S-FS, R-fS × S-FS, R-FS × S-Fs, and R-FS × S-fS, see Table 2).
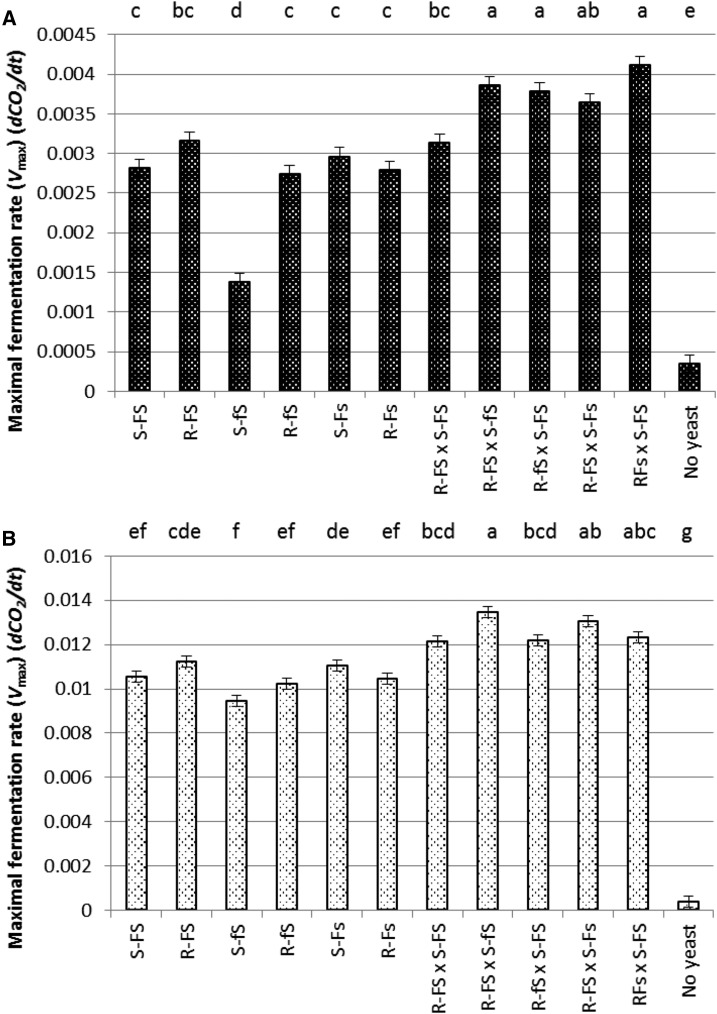
Vmax data are presented in Figure 3. At 12.5° (Figure 3A), there was no significant difference between the fermentation rates of S-FS or R-FS compared to three of the deletion mutants: S-Fs, R-Fs, or R-fS. However, the Vmax of the S288C flo1 mutant, S-fS, was reduced by ∼50% compared to the wild-type S-FS strain and the other deletion mutants. This result indicates that the FLO1 allele is important for low temperature fermentation in S288C, but not in RM11-1a. There was no difference in Vmax between the R-FS × S-FS F1 hybrid and the original parent strains; however, R-FS × S-FS had a slightly but significantly lower Vmax than three of the RHA F1 hybrids: R-Fs × S-FS, R-fS × S-FS, and R-FS × S-fS. The significance of this result is not clear, but may involve uncharacterized cis or trans effects in the different strain background.
Figure 3.
Maximal fermentation rates (Vmax) (dCO2/dt) of S288C (S-FS), RM11-1a (R-FS), S288C, and RM11-1a flo1 and swh1 gene knockouts (S-fS, R-fS, S-Fs, and R-Fs), and five F1 hybrids for RHA (reciprocal hemizygosity analysis) (R-FS × S-FS, R-FS × S-fS, R-fS × S-FS, R-FS × S-Fs, and R-Fs × S-FS) in Sauvignon Blanc juice. (A) 12.5°. (B) 25°. Significant differences were identified using Tukey’s HSD (honest significant difference); samples not connected by the same letter, as displayed at the top of each graph, are significantly different; n = 9.
At 25° (Figure 3B), there was no significant difference between the fermentation rates of S288C (S-FS) and its flo1 or swh1 mutants (S-fS or S-Fs). There was also no difference between RM11-1a (R-FS) compared to its two deletion mutants, R-fS or R-Fs. However, the Vmax of the S288C swh1 mutant, S-Fs, was slightly but significantly higher than that of S-Fs. These data suggest that the deletion of the flo1 locus does not have a significant effect on the maximal fermentation rate at higher temperature. The five diploid RHA hybrids showed only minor differences in fermentation rate, with no clear pattern emerging.
The reduction in maximal fermentation rate in the S288C flo1 deletion strain, S-fS, strongly suggests that FLO1 is linked to low temperature fermentation and most likely corresponds to the high LOD score region on chromosome I. However, RHA between S288C and RM11-1a FLO1 gene variants did not identify any easily explained effects on maximal fermentation rate in the cold. In particular, the R-FS × S-fS F1 hybrid did not ferment poorly compared to the other hybrids, which would be expected if the presence of the S288C FLO1 allele was promoting a more rapid fermentation at 12.5°.
Discussion
Genetic linkage analysis, using a set of completely mapped 119 BY4716 × RM11-1a F1 progeny, identified a strong linkage between maximal fermentation rate at low temperature, and the FLO1 gene on chromosome I. Mapping data indicated that the beneficial allele was derived from the “low parent” BY4716 and not from RM11-1a. The linkage of the BY4716 variant of FLO1 to Vmax was validated based on a 50% reduction in Vmax in a cold-fermented S288C flo1 mutant; as expected, there was no difference in Vmax between the RM11-1a flo1 deletion strain compared to RM11-1a. However, RHA between S288C and RM11-1a FLO1 alleles did not confirm the prediction that the S288C version of FLO1 was promoting more rapid fermentation in a different strain background.
FLO1 has a role in stress tolerance during low temperature fermentation
The 4.6 kb FLO1 gene encodes a cell wall surface protein that aggregates cells into “flocs” by binding to mannose sugar chains on the surfaces of other cells (Miki et al. 1982; Teunissen and Steensma 1995), and on substrates during glucose starvation (Fichtner et al. 2007). FLO1 is one of four subtelomeric and structurally similar FLO genes possessed by S. cerevisiae (the others are FLO5, FLO9, and FLO10), and together they control the flocculation phenotype of different S. cerevisiae strains (Teunissen and Steensma 1995). Previous studies strongly suggest that the floc formation by S. cerevisiae is a protective mechanism against environmental and nutritional stress, since flocculation is typically induced in response to high ethanol, antifungal agents (Teunissen and Steensma 1995; Smukalla et al. 2008; Beauvais et al. 2009), and nutrient limitation [particularly carbon and/or nitrogen, see Rose (1984), Sampermans et al. (2005) and Stratford (1992)]. FLO1-expressing industrial strains also have improved fermentation performance under acetic acid stress compared to strains not expressing FLO1 (Du et al. 2015), and consume hexose sugars more efficiently than nonexpressing strains in the presence of fermentation inhibitors (Westman et al. 2014). The subtelomeric location of FLO1 is also in agreement with the observation that an especially high proportion of variable genes located at chromosomal telomeres are involved in fermentation (Argueso et al. 2009; Cubillos et al. 2011). The protection provided by the formation of flocs is not only due to the physical shielding of the cells in the center of the floc, but also due to an increased overall resistance to stress (Smukalla et al. 2008). We hypothesize that the induction of the transcriptional flocculation response not only has a role in protecting cells from chemical stressors, but also plays a role during low temperature fermentation. Smukalla et al. (2008) have shown that S288C cells engineered to express a Flo1+ flocculation phenotype also upregulate genes involved in cell wall, lipid, and sterol metabolism, which are also induced during the stress response to low temperatures (Beney et al. 2001; Gasch and Werner-Washburne 2002; Beltran et al. 2008; Redón et al. 2011; Deed et al. 2015). Additionally, genes within the DAN/TIR and PAU gene families, which have long been associated with the transcriptional response of S. cerevisiae to low temperature (Kondo and Inouye 1991; Abramova et al. 2001; Homma et al. 2003; Schade et al. 2004), including during fermentation at low temperatures (Beltran et al. 2006; Deed et al. 2015), are also induced in flocculating cells (Smukalla et al. 2008). Low fermentation temperatures may also favor flocculation due to reduced turbulence from the lower metabolic rate and slower CO2 formation (Soares 2011). FLO1-expressing cells preferentially stick to one another, regardless of genetic relatedness across the rest of the genome, suggesting a level of cooperativeness (Smukalla et al. 2008). This cooperation toward other cells expressing the same gene suggests that FLO1 is one of very few “green beard genes” for altruistic social interactions. Rossouw et al. (2015) have taken this idea one step further by showing that FLO genes allow S. cerevisiae to form large ecological networks with non-Saccharomyces species, including both flocculant and nonflocculant strains. Additionally, different members of the FLO gene family either promote or repress certain combinations of mixed species and/or strain adhesion.
The positive influence of the S288C FLO1 allele has never before been described for fermentation rate and this effect appeared to be enhanced significantly at low temperature (see Figure 3A). It is widely assumed that FLO1 in S288C is not expressed, because its transcriptional regulator, Flo8p, is nonfunctional in S288C due to a nonsense mutation (Liu et al. 1996). In RM11-1a, the FLO8 gene is functional and Brem et al. (2002) found that one-quarter of the F1 progeny from the BY4716 × RM11-1a cross showed a flocculation phenotype (Flo1+ and Flo8+). Gene expression microarray data from Deed et al. (2015) show that FLO1 transcripts in a M2 × S288C F1 hybrid are dramatically upregulated during low temperature fermentation compared to the M2 parental reference. FLO1 was upregulated 73-fold at early fermentation (2% weight loss) and 182-fold at midlate fermentation (70% weight loss). Typically, FLO1-dependent flocculation requires activation by Flo8p, in conjunction with another transcription factor, Mss11p, which also coregulate the MUC1/FLO11 flocculin (Kobayashi et al. 1996; Bester et al. 2006; Fichtner et al. 2007). However, there are reports of FLO1 being activated in a Flo8p-independent manner. For example, the overexpression of MSS11, encoding a transcription factor, can overcome the flo8 deletion in S288C (Bester et al. 2006). There is evidence that MUC1/FLO11 and MSS11 have temperature-dependent regulation and can only facilitate trait expression at lower temperatures, strengthening the case for temperature-specific roles for cell surface proteins such as Flo1p (Lee et al. 2016; Taylor et al. 2016). Additionally, Shen et al. (2006) found that another transcription factor, Gts1p, could induce FLO1 in a flo8 mutant strain of W303-1A by binding to the Sfl1p repressor. This research supports the idea that specific environmental signals, initiating a stress response, may allow for FLO1 to be induced in S288C, independent of Flo8p, via other transcriptional regulators. Since Flo1p tends to support cell–substrate interactions under specific environmental conditions (Fichtner et al. 2007), the fermentation environment may induce FLO1 in a Flo8p-independent manner, resulting in cell–substrate adhesion, rather than flocculation per se. If this is the case, the ability of the S288C FLO1::KanMX (S-fS) mutant to form attachments to substrates within the grape solids could be visualized vs. the wild type using a technique such as atomic force microscopy (Canetta et al. 2006). Increased adhesion of yeast cells to substrates such as nutrients or grape solids may result in a higher fermentation rate at low temperature, as shown by cells that ferment while immobilized onto supports made of cellulose, gluten, corn starch, or wheat grains, in numerous studies (Mallouchos et al. 2003, 2007; Kandylis et al. 2008, 2010; Lainioti et al. 2011). This finding is in line with current literature demonstrating a role of Flo1p in protecting yeast from environmental stresses and improving fermentation performance under industrial conditions, including improved resistance to ethanol, acetic acid, and antimicrobial compounds (Queller 2008; Smukalla et al. 2008; Soares 2011; Westman et al. 2014; Du et al. 2015; Rossouw et al. 2015; Cheng et al. 2016).
The positive effect on Vmax was not visible in the RHA F1 hybrids constructed from crosses between RM11-1a and S288C parent and deletion strains. The R-FS × S-fS RHA strain contained one FLO1 allele from RM11-1a and the flo1 deletion from S288C. The absence of the S288C FLO1 allele was predicted to result in a lower Vmax compared to the other RHA hybrids that possessed the wild-type version of this same allele. However, there were no significant differences between the RHA hybrids. One possible explanation is that the positive influence on Vmax only occurs in haploid strains and not in diploids. The RHA F1 hybrids were the only diploid strains analyzed, since the two parents and all the 119 F1 progeny were haploid. Fichtner et al. (2007) state that FLO1-dependent flocculation is haploid-specific and that diploids display invasive or pseudohyphal growth via a nonsubtelomeric FLO gene, MUC1/FLO11, encoding a GPI-anchored cell surface glycoprotein required for pseudohyphal formation (Kobayashi et al. 1999; Guo et al. 2000). Haploids and diploids often differ in their tolerance to stress, even with the same genetic backgrounds, which extends to fermentation-related stressors such as ethanol (Katou et al. 2008; Li et al. 2010). Since the induction of flocculation has an impact on the ethanol resistance of S. cerevisiae, perhaps the influence of FLO1 on Vmax is haploid-specific and works by providing additional ethanol tolerance.
As discussed in Sinha et al. (2006), QTL architecture can be very complex. In this case, there may be the requirement for a second gene modifier compensator (XR/XS) that works along with FLO1 in order for the benefit for Vmax to be present. For instance, a second gene modifier may be FLO8, encoding the transcriptional inducer of FLO1; MUC1/FLO11; another member of the FLO gene family with high sequence homology, such as FLO5, FLO9, or FLO10; or one of three pseudogenes, YAL065C, YAR061W (merged with YAR062W), or YHR213W, with sequence similarity to other flocculin genes (Teunissen and Steensma 1995). If the advantage of S288C FLO1 for improved fermentation performance at low temperature is Flo8p-independent, perhaps the complementation of FLO8 via hybridization of S288C with RM11-1a prevented the S288C version of FLO1 from having any effect on maximal fermentation rate. However, analyses of the LOD score data for FLO8, MUC1/FLO11, and the six FLO homologs did not identify any significant peaks corresponding to Vmax. FLO genes are particularly difficult to work with due to their highly repetitive nature and tandem repeats, high sequence homology, complex patterns of regulation, and high genetic instability (Stratford 1994; Bidard et al. 1995; Sato et al. 2001; Verstrepen et al. 2003; Fichtner et al. 2007; Liu et al. 2009; Van Mulders et al. 2010; Yue et al. 2013). Flocculation phenotypes also differ immensely between strains (Govender et al. 2008, 2010). Further research that could be performed to determine why the RHA strains had no significant differences in fermentation rate include sporulating the R-FS × S-fS RHA hybrid and measuring the maximal fermentation rate at low temperature of segregating F1 progeny containing the S288C flo1 deletion. Additionally, FLO5, FLO9, and FLO10 could be deleted in S288C to see whether these loci influence Vmax at low temperature. Potential differences between the maximal fermentation rate of flo1 haploids and diploids could also be investigated.
Conclusions
We have identified a QTL linked to Vmax and two QTL linked to lag phase in S. cerevisiae. Deletion of candidate genes confirmed that the gene on chromosome I linked to Vmax in S288C is FLO1, encoding a yeast flocculin. Deletion of FLO1 in the haploid S288C strain resulted in a large decrease in fermentation rate at 12.5°, but no change at 25°. A greater understanding of the role of the FLO family in stress tolerance will allow easier manipulation and/or selection of S. cerevisiae strains to improve Vmax and provide growth advantages during the low temperature fermentation of foods and beverages.
Supplementary Material
Supplemental material is available online at www.g3journal.org/lookup/suppl/doi:10.1534/g3.116.037630/-/DC1.
Acknowledgments
The authors thank Erin Smith and Leonid Kruglyak (Princeton University) for gifting them the 121 BY4716 × RM11-1a segregating F1 progeny, Joshua Bloom and Justin Gerke (Princeton University) for the quantitative trait loci mapping and logarithm of odds plot construction, Kristine Boxen (Genomics Unit, School of Biological Sciences, University of Auckland) for processing microsatellite samples, and Andy Frost (Pernod Ricard NZ Ltd) for supplying the Sauvignon Blanc grape juice. Rebecca Deed was a recipient of the Bright Futures Top Achiever Doctoral Scholarship and the University of Auckland Doctoral Scholarship. This work was funded in part by a grant from the Foundation for Research Science and Technology in New Zealand (contract UOAX0404), with the support of New Zealand Winegrowers. The authors declare that they have no conflicts of interest.
Footnotes
Communicating editor: B. A. Cohen
Literature Cited
- Abramova N., Sertil O., Mehta S., Lowry C. V., 2001. Reciprocal regulation of anaerobic and aerobic cell wall mannoprotein gene expression in Saccharomyces cerevisiae. J. Bacteriol. 183(9): 2881–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilera J., Randez-Gil F., Prieto J. A., 2007. Cold response in Saccharomyces cerevisiae: new functions for old mechanisms. FEMS Microbiol. Rev. 31(3): 327–341. [DOI] [PubMed] [Google Scholar]
- Argueso J. L., Carazzolle M. F., Mieczkowski P. A., Duarte F. M., Netto O. V. C., et al. , 2009. Genome structure of a Saccharomyces cerevisiae strain widely used in bioethanol production. Genome Res. 19(12): 2258–2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais A., Loussert C., Prevost M. C., Verstrepen K., Latgé J. P., 2009. Characterization of a biofilm-like extracellular matrix in FLO1-expressing Saccharomyces cerevisiae cells. FEMS Yeast Res. 9(3): 411–419. [DOI] [PubMed] [Google Scholar]
- Beltran G., Novo M., Leberre V., Sokol S., Labourdette D., et al. , 2006. Integration of transcriptomic and metabolic analyses for understanding the global responses of low-temperature winemaking fermentations. FEMS Yeast Res. 6(8): 1167–1183. [DOI] [PubMed] [Google Scholar]
- Beltran G., Novo M., Guillamon J. M., Mas A., Rozes N., 2008. Effect of fermentation temperature and culture media on the yeast lipid composition and wine volatile compounds. Int. J. Food Microbiol. 121(2): 169–177. [DOI] [PubMed] [Google Scholar]
- Bely M., Sablayrolles J. M., Barre P., 1990. Description of alcoholic fermentation kinetics: its variability and significance. Am. J. Enol. Vitic. 41(4): 319–324. [Google Scholar]
- Beney L., Marechal P. A., Gervais P., 2001. Coupling effects of osmotic pressure and temperature on the viability of Saccharomyces cerevisiae. Appl. Microbiol. Biotechnol. 56(3–4): 513–516. [DOI] [PubMed] [Google Scholar]
- Bester M. C., Pretorius I. S., Bauer F. F., 2006. The regulation of Saccharomyces cerevisiae FLO gene expression and Ca2+-dependent flocculation by Flo8p and Mss11p. Curr. Genet. 49(6): 375–383. [DOI] [PubMed] [Google Scholar]
- Bidard F., Bony M., Blondin B., Dequin S., Barre P., 1995. The Saccharomyces cerevisiae FLO1 flocculation gene encodes for a cell surface protein. Yeast 11(9): 809–822. [DOI] [PubMed] [Google Scholar]
- Brachmann C. B., Davies A., Cost G. J., Caputo E., Li J., et al. , 1998. Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast 14(2): 115–132. [DOI] [PubMed] [Google Scholar]
- Brem R. B., Yvert G., Clinton R., Kruglyak L., 2002. Genetic dissection of transcriptional regulation in budding yeast. Science 296(5568): 752–755. [DOI] [PubMed] [Google Scholar]
- Broman K. W., Wu H., Sen Ś., Churchill G. A., 2003. R/qtl: QTL mapping in experimental crosses. Bioinformatics 19(7): 889–890. [DOI] [PubMed] [Google Scholar]
- Canetta E., Walker G. M., Adya A. K., 2006. Correlating yeast cell stress physiology to changes in the cell surface morphology: atomic force microscopic studies. Sci. World J. 6: 777–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenchai C., Fleet G. H., Henschke P. A., 1998. Effects of temperature, pH, and sugar concentration on the growth rates and cell biomass of wine yeasts. Am. J. Enol. Vitic. 49(3): 283–288. [Google Scholar]
- Cheng C., Zhao X., Bai F., 2016. Effects of cell flocculation and zinc sulfate addition on acetic acid stress tolerance of Saccharomyces cerevisiae. Chin. J. Appl. Environ. Biol. 22(1): 116–119. [Google Scholar]
- Chiva R., López-Malo M., Salvadó Z., Mas A., Guillamón J. M., 2012. Analysis of low temperature-induced genes (LTIG) in wine yeast during alcoholic fermentation. FEMS Yeast Res. 12(7): 831–843. [DOI] [PubMed] [Google Scholar]
- Coleman M. C., Fish R., Block D. E., 2007. Temperature-dependent kinetic model for nitrogen-limited wine fermentations. Appl. Environ. Microbiol. 73(18): 5875–5884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cubillos F. A., Billi E., Zörgö E., Parts L., Fargier P., et al. , 2011. Assessing the complex architecture of polygenic traits in diverged yeast populations. Mol. Ecol. 20(7): 1401–1413. [DOI] [PubMed] [Google Scholar]
- Daum G., Tuller G., Nemec T., Hrastnik C., Balliano G., et al. , 1999. Systematic analysis of yeast strains with possible defects in lipid metabolism. Yeast 15(7): 601–614. [DOI] [PubMed] [Google Scholar]
- Deed R. C., Deed N. K., Gardner R. C., 2015. Transcriptional response of Saccharomyces cerevisiae to low temperature during wine fermentation. Antonie Van Leeuwenhoek 107(4): 1029–1048. [DOI] [PubMed] [Google Scholar]
- Du Z., Cheng Y., Zhu H., He X., Zhang B., 2015. Improvement of acetic acid tolerance and fermentation performance of industrial Saccharomyces cerevisiae by overexpression of flocculent gene FLO1 and FLO1c. Sheng Wu Gong Cheng Xue Bao 31(2): 231–241. [PubMed] [Google Scholar]
- Ehrenreich I. M., Gerke J. P., Kruglyak L., 2009. Genetic dissection of complex traits in yeast: insights from studies of gene expression and other phenotypes in the BY×RM cross. Cold Spring Harb. Symp. Quant. Biol. 74: 145–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fichtner L., Schulze F., Braus G. H., 2007. Differential Flo8p-dependent regulation of FLO1 and FLO11 for cell-cell and cell-substrate adherence of S. cerevisiae S288c. Mol. Microbiol. 66(5): 1276–1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasch A. P., Werner-Washburne M., 2002. The genomics of yeast responses to environmental stress and starvation. Funct. Integr. Genomics 2(4–5): 181–192. [DOI] [PubMed] [Google Scholar]
- Govender P., Domingo J. L., Bester M. C., Pretorius I. S., Bauer F. F., 2008. Controlled expression of the dominant flocculation genes FLO1, FLO5, and FLO11 in Saccharomyces cerevisiae. Appl. Environ. Microbiol. 74(19): 6041–6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender P., Bester M., Bauer F. F., 2010. FLO gene-dependent phenotypes in industrial wine yeast strains. Appl. Microbiol. Biotechnol. 86(3): 931–945. [DOI] [PubMed] [Google Scholar]
- Guo B., Styles C. A., Feng Q., Fink G. R., 2000. A Saccharomyces gene family involved in invasive growth, cell-cell adhesion, and mating. Proc. Natl. Acad. Sci. USA 97(22): 12158–12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Homma T., Iwahashi H., Komatsu Y., 2003. Yeast gene expression during growth at low temperature. Cryobiology 46(3): 230–237. [DOI] [PubMed] [Google Scholar]
- Horak C. E., Luscombe N. M., Qian J., Bertone P., Piccirrillo S., et al. , 2002. Complex transcriptional circuitry at the G1/S transition in Saccharomyces cerevisiae. Genes Dev. 16(23): 3017–3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B., Brown J. L., Sheraton J., Fortin N., Bussey H., 1994. A new family of yeast genes implicated in ergosterol synthesis is related to the human oxysterol binding protein. Yeast 10(3): 341–353. [DOI] [PubMed] [Google Scholar]
- Kandylis P., Goula A., Koutinas A. A., 2008. Corn starch gel for yeast cell entrapment. A view for catalysis of wine fermentation. J. Agric. Food Chem. 56(24): 12037–12045. [DOI] [PubMed] [Google Scholar]
- Kandylis P., Drouza C., Bekatorou A., Koutinas A. A., 2010. Scale-up of extremely low temperature fermentations of grape must by wheat supported yeast cells. Bioresour. Technol. 101(19): 7484–7491. [DOI] [PubMed] [Google Scholar]
- Katou T., Kitagawa H., Aka T., Shimoi H., 2008. Brewing characteristics of haploid strains isolated from the sake yeast Kyokai no. 7. Yeast 25: 799–807. [DOI] [PubMed] [Google Scholar]
- Kaufmann E., 1993. In vitro binding to the leucine tRNA gene identifies a novel yeast homeobox gene. Chromosoma 102(3): 174–179. [DOI] [PubMed] [Google Scholar]
- Killian E., Ough C. S., 1979. Fermentation esters — formation and retention as affected by temperature. Am. J. Enol. Vitic. 30(4): 301–305. [Google Scholar]
- Kobayashi O., Suda H., Ohtani T., Sone H., 1996. Molecular cloning and analysis of the dominant flocculation gene FLO8 from Saccharomyces cerevisiae. Mol. Gen. Genet. 251(6): 707–715. [DOI] [PubMed] [Google Scholar]
- Kobayashi O., Yoshimoto H., Sone H., 1999. Analysis of the genes activated by the FLO8 gene in Saccharomyces cerevisiae. Curr. Genet. 36(5): 256–261. [DOI] [PubMed] [Google Scholar]
- Kondo K., Inouye M., 1991. TIP1, a cold shock-inducible gene of Saccharomyces cerevisiae. J. Biol. Chem. 266: 17537–17544. [PubMed] [Google Scholar]
- Lainioti G. C., Kapolos J., Koliadima A., Karaiskakis G., 2011. The study of the effect of fermentation temperature on the growth kinetics of Saccharomyces cerevisiae yeast strain, in the presence or absence of support, by chromatographic techniques. J. Liq. Chromatogr. Relat. Technol. 34(3): 195–208. [Google Scholar]
- Lee J. T., Taylor M. B., Shen A., Ehrenreich I. M., 2016. Multi-locus genotypes underlying temperature sensitivity in a mutationally induced trait. PLoS Genet. 12(3): 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B. Z., Cheng J. S., Ding M. Z., Yuan Y. J., 2010. Transcriptome analysis of differential responses of diploid and haploid yeast to ethanol stress. J. Biotechnol. 148(4): 194–203. [DOI] [PubMed] [Google Scholar]
- Liu H., Styles C. A., Fink G. R., 1996. Saccharomyces cerevisiae S288C has a mutation in FLO8, a gene required for filamentous growth. Genetics 144(3): 967–978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N., Wang D. L., Wang Z. Y., He X. P., Zhang B. R., 2009. Deletion of tandem repeats causes flocculation phenotype conversion from Flo1 to NewFlo in Saccharomyces cerevisiae. J. Mol. Microbiol. Biotechnol. 16(3–4): 137–145. [DOI] [PubMed] [Google Scholar]
- Llauradó J. M., Rozès N., Bobet R., Mas A., Constantí M., 2002. Low temperature alcoholic fermentations in high sugar concentration grape musts. J. Food Sci. 67(1): 268–273. [Google Scholar]
- Mallouchos A., Komaitis M., Koutinas A., Kanellaki M., 2003. Wine fermentations by immobilized and free cells at different temperatures. Effect of immobilization and temperature on volatile by-products. Food Chem. 80(1): 109–113. [Google Scholar]
- Mallouchos A., Paul L., Argyro B., Koutinas A., Komaitis M., 2007. Ambient and low temperature winemaking by immobilized cells on brewer’s spent grains: effect on volatile composition. Food Chem. 104(3): 918–927. [Google Scholar]
- Marks V. D., Ho Sui S. J., Erasmus D., Van Der Merwe G. K., Brumm J., et al. , 2008. Dynamics of the yeast transcriptome during wine fermentation reveals a novel fermentation stress response. FEMS Yeast Res. 8(1): 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marullo P., Bely M., Masneuf-Pomarède I., Pons M., Aigle M., et al. , 2006. Breeding strategies for combining fermentative qualities and reducing off-flavor production in a wine yeast model. FEMS Yeast Res. 6(2): 268–279. [DOI] [PubMed] [Google Scholar]
- Miki B. L. A., Poon N. H., James A. P., Seligy V. L., 1982. Possible mechanism for flocculation interactions governed by gene FLO1 in Saccharomyces cerevisiae. J. Bacteriol. 150(2): 878–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A. M., Swiegers J. H., Varela C., Pretorius I. S., Agosin E., 2007. Influence of wine fermentation temperature on the synthesis of yeast-derived volatile aroma compounds. Appl. Microbiol. Biotechnol. 77(3): 675–687. [DOI] [PubMed] [Google Scholar]
- Mortimer R., Romano P., Suzzi G., Polsinelli M., 1994. Genome renewal: a new phenomenon revealed from a genetic study of 43 strains of Saccharomyces cerevisiae derived from natural fermentation of grape musts. Yeast 10(12): 1543–1552. [DOI] [PubMed] [Google Scholar]
- Pizarro F. J., Jewett M. C., Nielsen J., Agosin E., 2008. Growth temperature exerts differential physiological and transcriptional responses in laboratory and wine strains of Saccharomyces cerevisiae. Appl. Environ. Microbiol. 74(20): 6358–6368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queller D. C., 2008. Behavioural ecology: the social side of wild yeast. Nature 456(7222): 589–590. [DOI] [PubMed] [Google Scholar]
- Redón M., Guillamón J. M., Mas A., Rozès N., 2011. Effect of growth temperature on yeast lipid composition and alcoholic fermentation at low temperature. Eur. Food Res. Technol. 232(3): 517–527. [Google Scholar]
- Richards K. D., Goddard M. R., Gardner R. C., 2009. A database of microsatellite genotypes for Saccharomyces cerevisiae. Antonie Van Leeuwenhoek 96(3): 355–359. [DOI] [PubMed] [Google Scholar]
- Rose A. H., 1984. Physiology of cell aggregation. Flocculation in Saccharomyces cerevisiae as a model system, pp. 323–335 in Microbial Adhesion and Aggregation, edited by Marshall K. C. Springer-Verlag, Berlin, Heidelberg. [Google Scholar]
- Rossouw D., Bagheri B., Setati M. E., Bauer F. F., 2015. Co-flocculation of yeast species, a new mechanism to govern population dynamics in microbial ecosystems. PLoS One 10(8): e0136249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahara T., Goda T., Ohgiya S., 2002. Comprehensive expression analysis of time-dependent genetic responses in yeast cells to low temperature. J. Biol. Chem. 277(51): 50015–50021. [DOI] [PubMed] [Google Scholar]
- Sampermans S., Mortier J., Soares E. V., 2005. Flocculation onset in Saccharomyces cerevisiae: the role of nutrients. J. Appl. Microbiol. 98(2): 525–531. [DOI] [PubMed] [Google Scholar]
- Sato M., Watari J., Shinotsuka K., 2001. Genetic instability in flocculation of bottom-fermenting yeast. J. Am. Soc. Brew. Chem. 59(3): 130–134. [Google Scholar]
- Schade B., Jansen G., Whiteway M., Entian K. D., Thomas D. Y., 2004. Cold adaptation in budding yeast. Mol. Biol. Cell 15(12): 5492–5502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiestl R. H., Gietz R. D., 1989. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr. Genet. 16(5–6): 339–346. [DOI] [PubMed] [Google Scholar]
- Schmalix W. A., Bandlow W., 1994. SWH1 from yeast encodes a candidate nuclear factor containing ankyrin repeats and showing homology to mammalian oxysterol-binding protein. Biochim. Biophys. Acta 1219(1): 205–210. [DOI] [PubMed] [Google Scholar]
- Schwob E., Nasmyth K., 1993. CLB5 and CLB6, a new pair of B cyclins involved in DNA replication in Saccharomyces cerevisiae. Genes Dev. 7(7A): 1160–1175. [DOI] [PubMed] [Google Scholar]
- Shen H., Iha H., Yaguchi S. I., Tsurugi K., 2006. The mechanism by which overexpression of Gts1p induces flocculation in a FLO8-inactive strain of the yeast Saccharomyces cerevisiae. FEMS Yeast Res. 6(6): 914–923. [DOI] [PubMed] [Google Scholar]
- Sinha H., Nicholson B. P., Steinmetz L. M., McCusker J. H., 2006. Complex genetic interactions in a quantitative trait locus. PLoS Genet. 2(2): 140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smukalla S., Caldara M., Pochet N., Beauvais A., Guadagnini S., et al. , 2008. FLO1 is a variable green beard gene that drives biofilm-like cooperation in budding yeast. Cell 135(4): 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soares E. V., 2011. Flocculation in Saccharomyces cerevisiae: a review. J. Appl. Microbiol. 110(1): 1–18. [DOI] [PubMed] [Google Scholar]
- Steinmetz L. M., Sinha H., Richards D. R., Spiegelman J. I., Oefner P. J., et al. , 2002. Dissecting the architecture of a quantitative trait locus in yeast. Nature 416(6878): 326–330. [DOI] [PubMed] [Google Scholar]
- Stratford M., 1992. Yeast flocculation: a new perspective. Adv. Microb. Physiol. 33: 1–71. [PubMed] [Google Scholar]
- Stratford M., 1994. Genetic aspects of yeast flocculation: in particular, the role of FLO genes in the flocculation of Saccharomyces cerevisiae. Colloids Surf. B Biointerfaces 2(1–3): 151–158. [Google Scholar]
- Strogolova V., Furness A., Micaela M. R., Garlich J., Stuart R. A., 2012. Rcf1 and Rcf2, members of the hypoxia-induced gene 1 protein family, are critical components of the mitochondrial cytochrome bc1-cytochrome c oxidase supercomplex. Mol. Cell. Biol. 32(8): 1363–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surana U., Robitsch H., Price C., Schuster T., Fitch I., et al. , 1991. The role of CDC28 and cyclins during mitosis in the budding yeast S. cerevisiae. Cell 65(1): 145–161. [DOI] [PubMed] [Google Scholar]
- Tai S. L., Daran-Lapujade P., Walsh M. C., Pronk J. T., Daran J. M., 2007. Acclimation of Saccharomyces cerevisiae to low temperature: a chemostat-based transcriptome analysis. Mol. Biol. Cell 18(12): 5100–5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor M. B., Phan J., Lee J. T., McCadden M., Ehrenreich I. M., 2016. Diverse genetic architectures lead to the same cryptic phenotype in a yeast cross. Nat. Commun. 7: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teunissen A. W., Steensma H. Y., 1995. Review: the dominant flocculation genes of Saccharomyces cerevisiae constitute a new subtelomeric gene family. Yeast 11(11): 1001–1013. [DOI] [PubMed] [Google Scholar]
- Torija M. J., Rozes N., Poblet M., Guillamon J. M., Mas A., 2003. Effects of fermentation temperature on the strain population of Saccharomyces cerevisiae. Int. J. Food Microbiol. 80(1): 47–53. [DOI] [PubMed] [Google Scholar]
- Treu L., Toniolo C., Nadai C., Sardu A., Giacomini A., et al. , 2014. The impact of genomic variability on gene expression in environmental Saccharomyces cerevisiae strains. Environ. Microbiol. 16(5): 1378–1397. [DOI] [PubMed] [Google Scholar]
- Uchimoto D., Cruess W. V., 1952. Effect of temperature on certain products of vinous fermentation. J. Food Sci. 17(1–6): 361–366. [Google Scholar]
- Van Mulders S. E., Ghequire M., Daenen L., Verbelen P. J., Verstrepen K. J., et al. , 2010. Flocculation gene variability in industrial brewer’s yeast strains. Appl. Microbiol. Biotechnol. 88(6): 1321–1331. [DOI] [PubMed] [Google Scholar]
- Verstrepen K. J., Derdelinckx G., Verachtert H., Delvaux F. R., 2003. Yeast flocculation: what brewers should know. Appl. Microbiol. Biotechnol. 61(3): 197–205. [DOI] [PubMed] [Google Scholar]
- Vukotic M., Oeljeklaus S., Wiese S., Vögtle F. N., Meisinger C., et al. , 2012. Rcf1 mediates cytochrome oxidase assembly and respirasome formation, revealing heterogeneity of the enzyme complex. Cell Metab. 15(3): 336–347. [DOI] [PubMed] [Google Scholar]
- Westman J. O., Mapelli V., Taherzadeh M. J., Franzén C. J., 2014. Flocculation causes inhibitor tolerance in Saccharomyces cerevisiae for second-generation bioethanol production. Appl. Environ. Microbiol. 80(22): 6908–6918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue F., Du Z., Guo X., He X., Zhang B., 2013. Effect of tandem repeats adjacent to 3′-terminal of FLO1 on the flocculation function of Saccharomyces cerevisiae. Acta Microbiol. Sin. 53(12): 1276–1284. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All strains are available upon request. Supplemental Material: Table S1 contains the values for the kinetic parameters for all individuals. Table S2 contains the list of ORFs identified either side of each LOD >3 peak marker. File S1 contains the LOD scores for all individuals across the five fermentation parameters. File S2 contains Clustal alignments.