Key Points
In vivo imaging reveals a NET–platelet–thrombin axis that promotes intravascular coagulation in sepsis.
Inhibition of NETs during sepsis reduces intravascular coagulation, improves microvascular perfusion, and reduces organ damage.
Abstract
Neutrophil extracellular traps (NETs; webs of DNA coated in antimicrobial proteins) are released into the vasculature during sepsis where they contribute to host defense, but also cause tissue damage and organ dysfunction. Various components of NETs have also been implicated as activators of coagulation. Using multicolor confocal intravital microscopy in mouse models of sepsis, we observed profound platelet aggregation, thrombin activation, and fibrin clot formation within (and downstream of) NETs in vivo. NETs were critical for the development of sepsis-induced intravascular coagulation regardless of the inciting bacterial stimulus (gram-negative, gram-positive, or bacterial products). Removal of NETs via DNase infusion, or in peptidylarginine deiminase-4–deficient mice (which have impaired NET production), resulted in significantly lower quantities of intravascular thrombin activity, reduced platelet aggregation, and improved microvascular perfusion. NET-induced intravascular coagulation was dependent on a collaborative interaction between histone H4 in NETs, platelets, and the release of inorganic polyphosphate. Real-time perfusion imaging revealed markedly improved microvascular perfusion in response to the blockade of NET-induced coagulation, which correlated with reduced markers of systemic intravascular coagulation and end-organ damage in septic mice. Together, these data demonstrate, for the first time in an in vivo model of infection, a dynamic NET–platelet–thrombin axis that promotes intravascular coagulation and microvascular dysfunction in sepsis.
Introduction
Severe sepsis is a syndrome of systemic inflammation in response to infection that results in multiorgan dysfunction, shock, and death in 20% to 50% of patients.1 Although inflammation is central to the pathogenesis of sepsis, many other biological systems conspire to produce pathology in this disease.2 Notably, collaboration between the innate immune system and the hemostatic system (platelets and coagulation factors) has been identified as a principle offender in the pathogenesis of sepsis. Progressive thromobocytopenia and coagulopathy are strong negative prognostic findings in severe sepsis and have recently been included in the updated definition of the disease.3 The extreme phenotype of pathological coagulation in sepsis is the development of disseminated intravascular coagulation, a devastating syndrome of systemic microvascular thrombosis and coagulation with a high mortality rate, for which there is no specific treatment.4 Understanding the cellular and molecular mechanisms that promote the development of pathological coagulation in sepsis may provide targets for therapeutic development and yield insight into the collaboration between innate immunity and coagulation in health and disease.
Neutrophils are central to the inflammatory pathogenesis of sepsis, and a number of components of the neutrophil armamentarium are known to interact with coagulation factors and platelets.5-7 In particular, recent evidence has implicated neutrophil extracellular traps (NETs) in the activation of platelets and coagulation in vitro, as well as in models of deep vein thrombosis in vivo.8,9 Individually, various components of NETs have been identified as initiators or propagators of coagulation activity, including proteolytic constituents and histones.7,10 Aggregation of platelets within the microvasculature during gram-negative bacterial sepsis is largely neutrophil dependent and is linked to the release of NETs.5,6,11 Furthermore, electron microscopy of NETs formed in vitro has shown that platelets can bind directly to NETs.9 During sepsis, neutrophils cast NETs throughout the microcirculation of multiple organs, where they participate in host defense by capturing circulating bacteria, but also contribute to the development of end-organ damage.6,12 However, the role of NETs (and their interactions with platelets and coagulation factors in vivo) in the development of sepsis-induced intravascular coagulation has not been comprehensively investigated.
Previous research on coagulation in sepsis has relied primarily on peripheral blood samples and ex vivo assays of coagulation, but understanding the dynamics of coagulation and interactions with the immune system in vivo require that this process be studied at the site of action, within the tissue microvasculature. Advancements in intravital imaging now enable multicolor visualization of complex biological processes within the microcirculation. Furthermore, intravital microscopy enables a better understanding of how infection-induced coagulation directly impacts microvascular perfusion in the live animal. Herein, using high-resolution confocal intravital microscopy, we demonstrate that collaboration between NETs, platelets, and the release of inorganic polyphosphate (PolyP) promotes disseminated activation of the coagulation system within the microvasculature during sepsis, independent of the inciting pathogen (gram-negative, gram-positive, or pure bacterial product). Dynamic perfusion imaging revealed that NET-induced intravascular coagulation caused widespread microvasculature occlusion, ultimately leading to end-organ damage, which is prevented by inhibiting or dismantling NETs. Together, our data reveal a NET–platelet–thrombin axis that is fundamental to the development of microvascular dysfunction and organ damage in sepsis.
Methods
Mice
C57Bl/6J mice were purchased from the Jackson Laboratory (Bar Harbor, ME). PAD4-deficient mice (PAD4−/−) on a C57Bl/6J background were a gift from the late Kerri Mowen (Scripps Research Institute, La Jolla, CA). Animals were maintained in a pathogen-free environment at the University of Calgary Animal Resource Centre. All experimental animal protocols were approved by the University of Calgary Animal Care Committee and were in compliance with guidelines from the Canadian Council for Animal Care (Protocol AC14-0056).
Antibodies and reagents
The following antibodies and reagents were used: DNase I (Roche), argatroban (Sigma), AF660-labeled anti-Gr1 (eBioscience), AF647-labeled anti-mouse CD49b (BioLegend), goat anti-mouse histone H2Ax (Santa Cruz Biotechnology), and sheep anti-human (with mouse cross-reactivity) fibrin/fibrinogen immunoglobulin G (IgG) (AbD Serotec). Unconjugated antibodies used for fluorescence imaging were labeled with Alexa Fluor protein labeling kits according to the manufacturer’s instructions (Thermo Fischer Scientific). The fluorescent thrombin substrate used for in vivo imaging analysis (SensoLyte internally quenched 5-FAM/QXL-520 FRET thrombin substrate) was purchased from AnaSpec. Monoclonal antibodies against histone H4 (MHIS1952) and polyphosphate (PP2055) were developed in C.T.E.’s laboratory as described previously.13
Intravital microscopy
The protocol for intravital imaging was performed as previously described.14 Specific details of the specifications of the spinning disk intravital microscope and resonance scanning confocal intravital microscope are provided in the supplemental data.
Image analysis
NET components were visualized using AF555-anti-H2Ax antibody (5 μg/mouse) and, in some experiments, AF647-anti-neutrophil elastase (0.6 μg/mouse) and Sytox green DNA stain (10 μL of 50 pM solution per mouse). Platelets and neutrophils were visualized using AF647-anti-CD49b antibody (2 μg per mouse) and AF660 or 488-anti-Gr-1 antibody (4 μg per mouse), respectively. Thrombin activity was visualized using SensoLyte internally-quenched 5-FAM/QXL-520 FRET thrombin substrate (2 μL per mouse) administered IV 2 minutes before imaging. All antibodies were injected IV 15 minutes prior to imaging unless otherwise noted. Image analysis methodology was performed as previously described6,15,16 and as outlined in the supplemental data.
Experimental protocols
Mice were administered lipopolysaccharide (LPS) intraperitoneally (i.p.; 1 mg/kg purified LPS from Escherichia coli 0111:B4, List Biologicals) 4 hours prior to intravital microscopy to induce endotoxemia as described previously.6,14 In some experiments, mice were treated with 2000 international units of DNase I (Roche) IV 30 minutes prior to LPS. In argatroban experiments, mice were treated with 10 mg/kg i.p. 30 minutes prior to LPS or E coli infection. For E coli infection experiments, bacteria (Xen14 strain) was grown to midlog phase in Luria-Bertani with kanamycin broth, washed, resuspended in saline, and injected i.p. (3 × 107 CFU). For Staphylococcus aureus infection experiments, bacteria (USA300-2406) were grown to midlog phase in brain-heart infusion–chloramphenicol broth, washed, resuspended in saline, and injected IV (2 × 107 CFU).
Enzyme-linked immunosorbent assay and biochemical assays
Plasma was obtained in the usual fashion from whole blood collected from anesthetized mice via cardiac puncture. A commercially available enzyme-linked immunosorbent assay kit was used to measure levels of thrombin-antithrombin complexes (TATs; Abcam) and plasminogen activator inhibitor-1 (PAI-1) (Molecular Innovations). Commercially available colorimetric assays were used to measure plasma levels of alanine aminotransferase (ALT), creatinine, and lactate (Sigma).
Statistical analysis
All data are presented as mean values ± standard error of the mean (SEM). A Student t test (for parametric data) or Mann-Whitney U test (for nonparametric data) was used to determine the significance between population means when 2 groups were compared. When >2 groups were compared, a one-way analysis of variance with post-hoc Bonferroni test was used for multiple comparisons. Statistical significance was set at P < .05.
Results
Intravascular coagulation and thrombosis in the liver microcirculation during sepsis
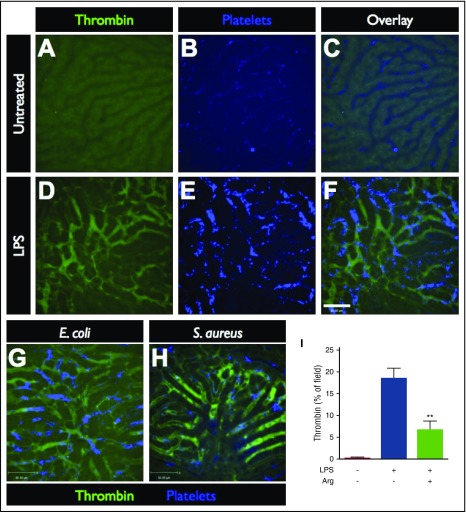
Using multichannel spinning-disk confocal intravital microscopy (SD-IVM) or resonance-scanning confocal intravital microscopy, the liver microcirculation was visualized in untreated control mice (Figure 1A-C) and septic/endotoxemic mice after the i.p. administration of 1 mg/kg LPS (Figure 1D-F), 3 × 107 CFU E coli (Figure 1G) or 2 × 107 CFU S aureus (Figure 1H). To visualize the activity of the coagulation system within the vasculature, a quenched-fluorescence peptide substrate of thrombin, the terminal enzyme of the coagulation cascade, was introduced into the circulation. When cleaved by endogenous active thrombin, green fluorescence activity is detected within the vasculature (Figure 1D,G-H; supplemental Video 1). The specificity of the peptide probe for thrombin was confirmed by treating mice with specific thrombin inhibitors, argatroban or hirudin (data not shown) and demonstrating significant attenuation of the fluorescent signal (Figure 1I; supplemental Video 2), as well as the lack of the thrombin probe signal in nonseptic (untreated) controls (Figure 1I; supplemental Video 3). Striking activation of intravascular thrombin activity was observed throughout the liver microvasculature of septic mice, regardless of the inciting infection (Figure 1D,G-H; supplemental Figures 1 and 2, supplemental Video 1).
Figure 1.
Imaging intravascular coagulation in sepsis. Representative SD-IVM images of the liver microcirculation in untreated (A-C), endotoxemic (D-F), E coli–infected (G), and S aureus–infected (H) mice. Thrombin probe fluorescence is shown in green (note the level of background autofluorescence of the liver parenchyma shown in panel A), and platelets are shown in blue (AF647 anti-CD49b). Bar represents 50 μm. (I) Quantitative analysis of thrombin probe fluorescence within the liver microcirculation of untreated control mice, LPS-treated mice, or LPS-treated mice that received a direct thrombin inhibitor (argatroban). Data are shown as mean ± SEM. **P < .01; N = 3-6 mice per group.
Together with the disseminated activation of intravascular thrombin, we also observed profound aggregation of platelets in the liver microcirculation in response to endotoxemia, E coli, and S aureus (Figure 1E,G-H). In contrast, within the livers of untreated control mice, platelets flowed freely through the microvasculature, occasionally undergoing transient touch-and-go interactions with the endothelium, but not forming any durable aggregates (Figure 1B). This is in keeping with previous observations of extensive intravascular platelet aggregation within liver sinusoids during sepsis.6,11
NETs promote intravascular coagulation during sepsis
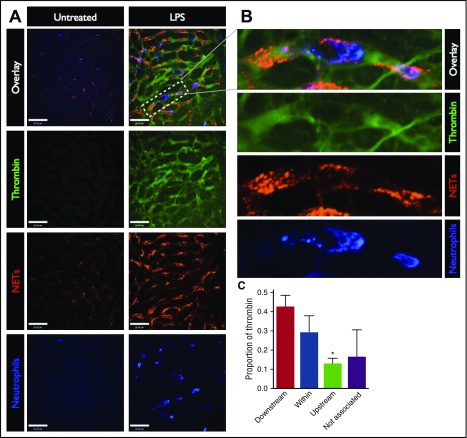
To test the hypothesis that the activation of intravascular coagulation is related to the presence of NETs, we visualized the spatial relationship between these processes within the liver microcirculation (Figure 2). NETs were identified as extracellular structures composed of DNA, histones, and neutrophil proteins (such as neutrophil elastase), and were quantified based on histone H2Ax, as previously described6,16,17 (supplemental Figure 3). As previously reported by us and others,6,12,16 a vast distribution of NETs was observed in the liver vasculature of endotoxemic mice (Figure 2A, red). Similar NET production was observed in response to E coli (supplemental Figure 1), and S aureus (supplemental Figure 2). Inspection of the spatial relationship between intravascular NETs and thrombin activity revealed colocalization between NETs and areas of dense thrombin activity (Figure 2A-B). Similarly, visualization of the terminal product of coagulation, fibrin, demonstrated colocalization with NETs (supplemental Figure 4). It should be noted that this fluorescent thrombin substrate is soluble and as such can dissipate from its site of activation. Analysis of the location of the maximal thrombin signal within each vessel in relation to the closest NET revealed that 71.2% of thrombin activity was within or immediately downstream of NETs, whereas only 12.6% was observed upstream of NETs, and 16.2% was not associated with NETs (>100 μm from the nearest NET) (Figure 2C). Together, these data demonstrate that the majority of intravascular thrombin is found within or immediately downstream of NETs.
Figure 2.
Spatial relationship between thrombin activity and NETs in the microvasculature. (A) Representative SD-IVM images of the liver microcirculation of untreated and LPS-treated mice. Thrombin probe fluorescence is in green, NETs are shown in red (AF555 anti-H2Ax), and neutrophils are shown in blue (AF647 anti-Gr1). Bars represent 50 μm. (B) Magnified inset showing a liver sinusoid containing an adherent neutrophil (blue), surrounding NETs (red), and the associated thrombin activity (green). (C) The regions of the highest thrombin probe signal within each liver sinusoid (per field of view) were identified in LPS-treated mice and categorized according to the spatial relationship with the nearest NET relative to the direction of blood flow: downstream, within NET, upstream, or not associated (>100 μm from the nearest NET). Data are represented as mean ± SEM. *P < .05; N = 4 mice per group.
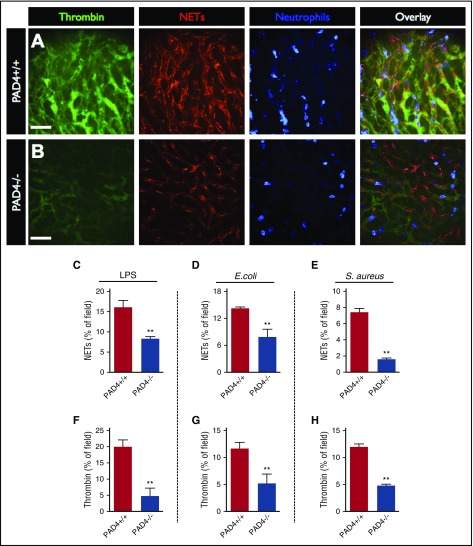
Having identified a clear spatial relationship between intravascular coagulation and NETs, we aimed to investigate the functional relationship between these processes through the inhibition of NETs production using PAD4−/− mice. Neutrophils from PAD4−/− mice are recruited to sites of infection/inflammation and appear to be activated normally, but fail to produce NETs in vitro and have a severe defect in NET release in vivo.8,18-20 Compared with wild-type (PAD4+/+) control mice, PAD4−/− animals produced significantly less NETs within the liver vasculature in response to LPS, E coli, and S aureus infection (Figure 3A-E) despite no difference in the quantity of recruited neutrophils (supplemental Figures 1, 2, and 7). Quantification of intravascular thrombin activity revealed that PAD4−/− mice had significantly lower levels of intravascular thrombin activity compared with wild-type (PAD4+/+) controls (Figure 3A-B, F-H).
Figure 3.
Reducing NET production in PAD4-deficient mice reduces intravascular coagulation. Representative SD-IVM images of the liver microcirculation of wild-type (A, PAD4+/+) and PAD4−/− (B) mice 4 hours after LPS administration. Thrombin probe fluorescence is in green, NETs are shown in red (AF555 anti-H2Ax), and neutrophils are shown in blue (AF647 anti-Gr1). Bars represent 50 μm. Quantitative analysis of NETs (C-E) and thrombin probe fluorescence (F-H) within the liver microvasculature of wild-type (PAD4+/+) and PAD4−/− mice after administration of LPS, E coli infection, or S aureus infection. Data are represented as mean ± SEM. **P < .01; N = 3-6 mice per group.
To control for the possibility that PAD4−/− mice have an intrinsic defect in coagulation, we used a second experimental approach to investigate the effect of NET inhibition on intravascular coagulation. In these experiments, septic mice (LPS, E coli, or S aureus) were treated with IV DNase to dismantle the structure of NETs (supplemental Figure 5A-C), as previously described.6,16,21 Indeed, treatment with IV DNase significantly reduced intravascular thrombin activity compared with control-treated animals (supplemental Figure 5D-F). The low level of thrombin activity in PAD4−/− mice was unchanged when they were administered IV DNase (supplemental Figure 6A-D).
Minor contribution of NETs to microvascular platelet aggregation
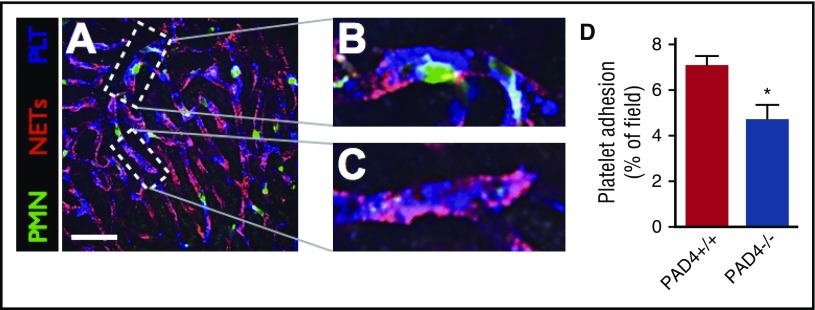
Platelets are critical to effective hemostasis and contribute to the generation of pathological coagulation in a variety of diseases, but are also essential for the release of NETs by neutrophils during gram-negative sepsis.5 Given that platelets contribute to both coagulation and NET function, we sought to investigate the interaction between platelets, NETs, and intravascular coagulation in sepsis. First, visualization of the platelet response within the microvasculature of septic mice revealed profound aggregation within sinusoids (Figure 4A). The majority of platelets were observed to aggregate on the surface of neutrophils, undergoing dynamic adhesion and aggregation (Figure 4B; supplemental Video 4). We have previously shown that platelet aggregation on the surface of neutrophils is dependent on the adhesion molecule leukocyte function-associated antigen-1 (LFA-1).6 However, some platelets were observed to adhere within the vasculature remote from neutrophils, within areas of NETs (Figure 4C). Furthermore, a small but significant reduction in the quantity of adherent platelets was observed in PAD4−/− mice, despite no difference in the number of adherent neutrophils (Figure 4D; supplemental Figure 7A). The number of circulating platelets was unchanged by the dismantling NETs with IV DNase (supplemental Figure 7B), again suggesting only a minor contribution of platelet-NET interaction to the systemic consumption of platelets. Platelet aggregation is pathogen-independent, as E coli and S aureus failed to induce platelet aggregation in vitro (data not shown). These data suggest that the majority of the microvascular platelet aggregation that occurs in sepsis is the result of platelet-neutrophil interactions, whereas only a minority of platelets (∼≤30%) undergo direct platelet-NET binding in the microvasculature.
Figure 4.
Minor contribution of NET-platelet interaction to microvascular platelet adhesion. (A) Representative SD-IVM image of the liver microvasculature of an endotoxemic wild-type (PAD4+/+) mouse showing neutrophils (green, AF488 anti-Gr1), NETs (red, AF555 anti-H2Ax), and platelets (blue, AF647 anti-CD49b). Bar represents 50 μm. (B) Magnified inset showing platelets (blue) aggregating on the surface of an adherent neutrophil (green). (C) Magnified inset showing platelets (blue) adhering to a NET (red). (D) Quantitative analysis of platelet adhesion within the liver sinusoids of wild-type (PAD4+/+) and PAD4−/− mice. Data are represented as mean ± SEM. *P < .05; N = 4-6 mice per group.
Histone H4 and PolyP promote intravascular coagulation, but not platelet sequestration or NET production
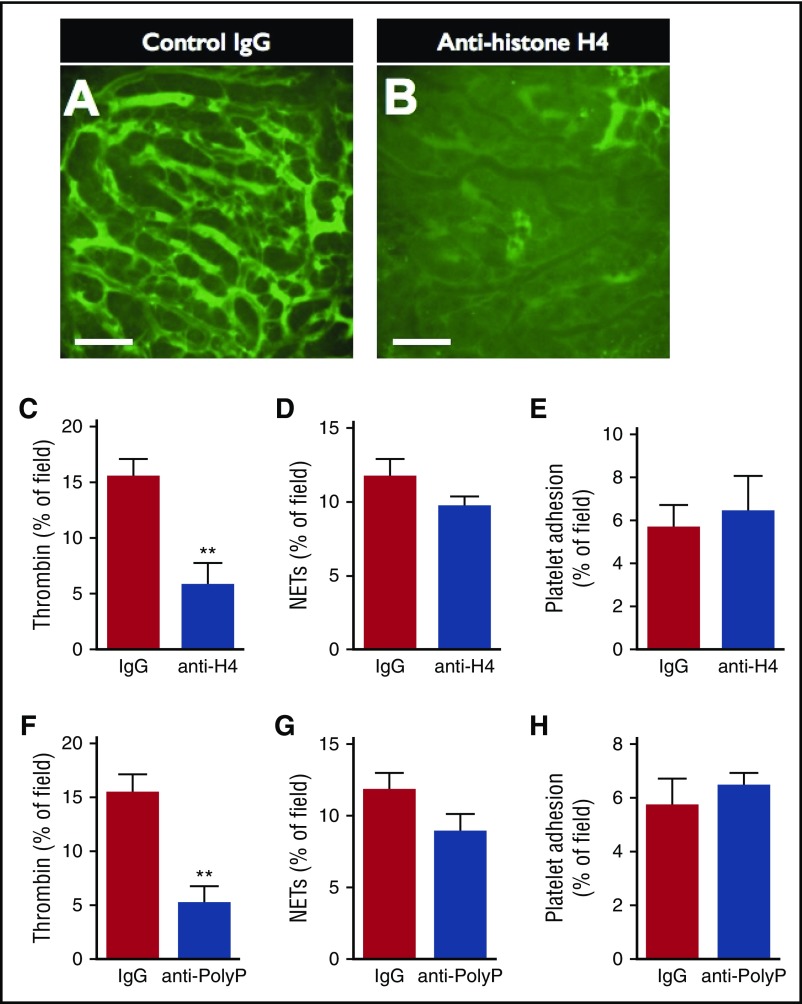
Histone H4 contributes to organ damage and mortality when released into the extracellular environment in bacterial sepsis.13 In addition, in vitro studies have demonstrated that histone proteins can activate the coagulation cascade via a number of mechanisms.10,22 Given that NETs are a major source of extracellular histones during sepsis, we hypothesized that extracellular histones, specifically H4, contribute to the activation of intravascular coagulation in sepsis. To test this hypothesis, LPS-treated mice were administered a monoclonal H4-blocking antibody (MHIS1952), or isotype-control IgG, and the quantity of intravascular thrombin activity was measured (Figure 5). Mice receiving control IgG demonstrated diffuse intravascular thrombin activity, whereas treatment with the H4-blocking antibody dramatically inhibited the amount of active thrombin observed within liver sinusoids (Figure 5A-B). Furthermore, quantification of thrombin probe fluorescence confirmed a marked reduction in the quantity of intravascular thrombin activity in anti-H4–treated mice compared with control-treated mice (Figure 5C). Notably, neutralization of histone H4 did not affect the quantity of NETs (Figure 5D) nor platelet adhesion within the vasculature (Figure 5E).
Figure 5.
Neutralization of histone H4 or PolyP reduces intravascular thrombin activity, but not platelet adhesion or NET production. Representative SD-IVM images of thrombin probe activity (green) within the liver microcirculation of endotoxemic wild-type mice treated with (A) control IgG versus (B) anti-histone H4 IgG. Bars represent 50 μm. (C-D) Quantitative analysis of (C) thrombin probe fluorescence, (D) NETs, and (E) platelet adhesion within the liver sinusoids of endotoxemic wild-type mice treated with control IgG or anti-histone H4 IgG. (F-H) Quantitative analysis of (F) thrombin probe fluorescence, (G) NETs, and (H) platelet adhesion within the liver sinusoids of endotoxemic wild-type mice treated with control IgG or antipolyphosphate IgG. Data are represented as mean ± SEM. **P < .01; N = 5 mice per group.
Semeraro and colleagues10 have suggested that histone H4 contributes to the activation of the coagulation cascade through a mechanism involving PolyP release from platelets. To test this hypothesis in vivo, septic mice were treated with a monoclonal blocking antibody against PolyP. Indeed, neutralization of polyphosphate significantly reduced intravascular thrombin activity, without affecting the quantity of NETs or platelets within the microvasculature (Figure 5F-H). Furthermore, others have suggested that the extracellular histones may contribute to coagulation through augmentation of tissue factor expression. We observed increased levels of tissue factor within the microvasculature of septic mice within areas of NET and thrombin activity (supplemental Figure 8). Together, our in vivo data support previous in vitro findings of a key collaboration between histone H4 in NETs, platelets, and PolyP, and possibly tissue factor, in the development of disseminated intravascular coagulation in sepsis.
Blocking NET-induced intravascular coagulation restores microvascular perfusion in sepsis
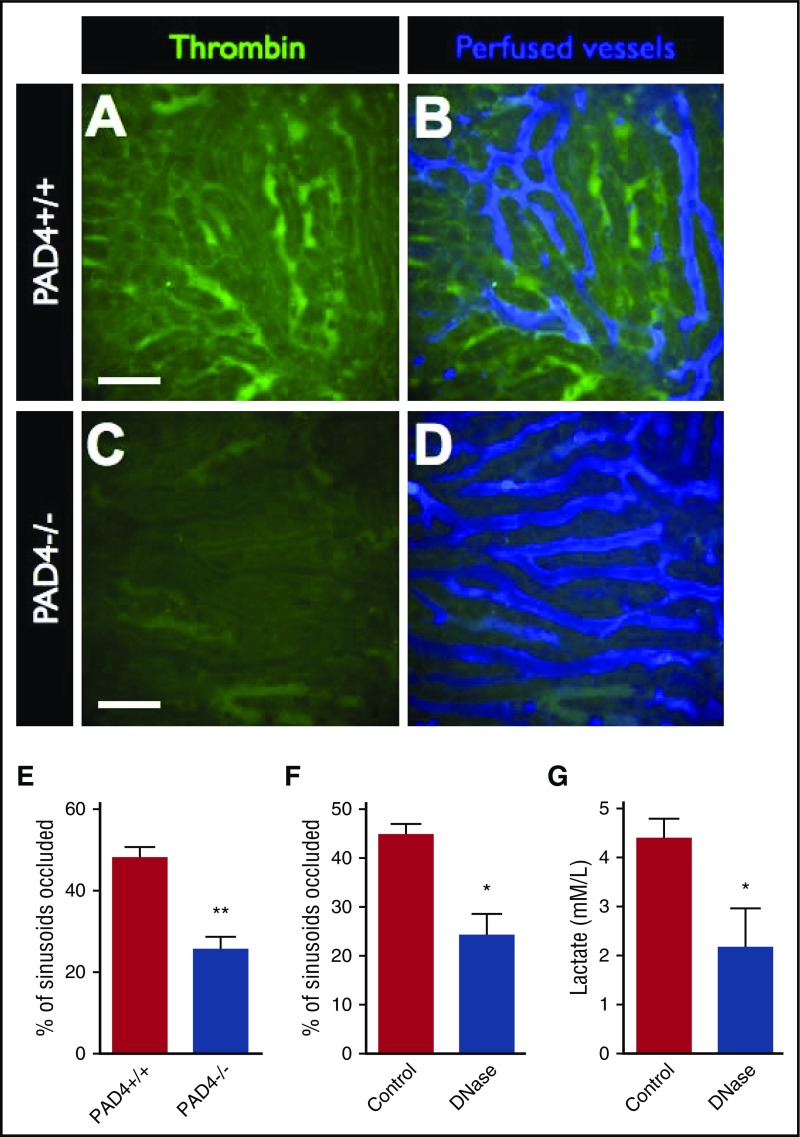
Microvascular hypoperfusion is a key pathologic feature of severe sepsis and septic shock.23 Although this phenomenon can be difficult to assess clinically, the use of intravital microscopy allows us to directly assess microvascular perfusion in live animals in real time. Using a fluorescent contrast dye (AF647-labeled albumin), we investigated the perfusion of liver sinusoids in relation to intravascular thrombin activity. We observed that ∼50% of sinusoids were occluded in septic mice and that, indeed, occluded vessels were those that contained high amounts of active thrombin (Figure 6A-B). Furthermore, the reduction in intravascular thrombin activity observed in PAD4−/− mice (Figure 6C-E) or mice treated with IV DNase (Figure 6F) resulted in significantly reduced microvascular occlusion compared with control animals. Similarly, inhibiting NET-mediated intravascular coagulation by treating septic mice with IV DNase resulted in significantly lower levels of serum lactate compared with control animals (Figure 6G). Together, these data support the conclusion that NET-induced intravascular coagulation is a fundamental contributor to microvascular hypoperfusion in sepsis.
Figure 6.
Blocking NETs-induced intravascular coagulation restores microvascular perfusion. Representative SD-IVM images of the liver microcirculation of endotoxemic wild-type (PAD4+/+) (A-B) and PAD4−/− (C-D) mice. Thrombin probe activity is shown in green. Mice were then injected with AF647-albumin (blue) as a contrast material to identify perfused versus occluded vessels. Bars represent 50 μm. The proportion of occluded vessels was quantified per field of view in (E) endotoxemic wild-type (PAD4+/+) versus PAD4−/− mice and (F) endotoxemic mice treated with IV DNase versus vehicle control. (G) Serum lactate levels were quantified in blood samples from septic mice (24 hours after i.p. infection with E coli) treated with IV DNase versus vehicle control. Data are represented as mean ± SEM. **P < .01, *P < .05; N = 3-6 mice per group.
Inhibition of NETs attenuates intravascular coagulation and end-organ damage in bacterial sepsis
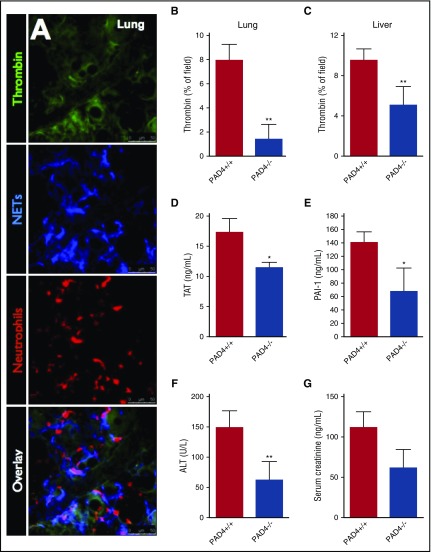
To determine if our observations within the liver microvasculature were also occurring within other organs, we performed in vivo imaging of the microvasculature in the lungs (Figure 7A), spleen (supplemental Figure 9A), and mesentery (supplemental Figure 9B). Indeed, similar to the hepatic microvasculature, we observed the colocalization of NETs and intravascular thrombin activity (Figure 7A; supplemental Figure 9). To investigate the role of NETs in intravascular coagulation and end-organ damage on a systemic level, septic wild-type (PAD4+/+) control and PAD4−/− mice were evaluated for systemic markers of intravascular coagulation (intravascular thrombin activity within the lungs and liver, plasma TAT levels, and plasma PAI-1 levels) as well as biomarkers of organ dysfunction (ALT and creatinine) were quantified. Compared with wild-type (PAD4+/+) controls, PAD4−/− mice displayed significantly reduced levels of intravascular thrombin activity, TAT, and PAI-1 (Figure 7B-E). Furthermore, PAD4-deficiency also markedly attenuated end-organ damage compared with wild-type (PAD4+/+) controls, with a 59% reduction in serum ALT and a 45% reduction in creatinine (Figure 7F-G). Taken together, these results indicate that NETs are key pathological mediators in systemic intravascular coagulation and subsequent end-organ damage in bacterial sepsis.
Figure 7.
Inhibition of NETs attenuates intravascular coagulation and end-organ damage in sepsis. (A) Representative resonance-scanning confocal intravital microscopy images of the lung microvasculature of endotoxemic mice showing intravascular thrombin activity (green), NETs (blue, AF647 anti-NE), and neutrophils (red, PE-anti-Gr1). Microvascular thrombin probe fluorescence was quantified in the lung microvasculature in response to intratracheal LPS (B), and liver microvasculature in response to i.p. E coli (C) in PAD+/+ and PAD4−/− mice. (D) Plasma TAT levels were quantified in PAD4+/+ and PAD4−/− mice 6 hours after IV S aureus infection. Plasma levels of (E) PAI-1, (F) ALT, and (G) creatinine were measured in PAD4+/+ and PAD4−/− mice with E coli peritonitis (24 hours postinfection). Data are represented as mean ± SEM. **P < .01, *P < .05; N = 5-10 mice per group.
Given that NETs are complex structures composed of multiple potential pathological mediators,24 it is possible that the diminished end-organ damage observed in PAD4−/− mice is the result of multiple factors in addition to reduced intravascular coagulation. To investigate this possibility, we aimed to elucidate the contribution of coagulation alone to end-organ damage in sepsis. Septic mice were treated with the direct thrombin inhibitor argatroban to block intravascular coagulation (Figure 1). As anticipated, direct inhibition of thrombin with argatroban significantly reduced markers of intravascular coagulation (supplemental Figure 10A-B). However, no significant changes in serum ALT or creatinine were observed in argatroban-treated animals compared with controls (supplemental Figure 10C-D). Together, these data suggest that inhibition of NETs disrupts both intravascular coagulation and organ damage, whereas anticoagulation alone does not significantly impact organ pathology in gram-negative sepsis.
Discussion
This study reports a number of significant advancements in our understanding of the pathophysiology of sepsis-induced intravascular coagulation. First, our data support the conclusion that NETs promote the development of intravascular coagulation and microvascular dysfunction during bacterial sepsis. Inhibition of NET production or breakdown of NET structure, either genetically using PAD4−/− mice or enzymatically with IV DNase, inhibited the development of intravascular coagulation. This translated into improved microvascular perfusion and decreased end-organ damage. The role of NETs was independent of the bacterial stimulus, confirming that sepsis-induced coagulation is a complication of the dysregulated host response rather than the inciting pathogen. NETs contain multiple factors that are capable of crosstalk with the coagulation cascade; therefore, activation of intravascular coagulation by NETs likely occurs via multiple pathways. For example, various proteolytic components of NETs activate coagulation in vitro and propagate coagulation in vivo. Serine proteases, such as neutrophil elastase, promote coagulation through the degradation of tissue factor pathway inhibitor,7 whereas neutrophil elastase and cathepsin G activate platelets through protease-activated receptors.25,26 Furthermore, studies of NETs within arterial thrombi of humans have identified tissue factor within the structure of NETs, which is consistent with our observations within the liver microvasculature, suggesting a direct link between NETs and the extrinsic pathway of coagulation.27 In addition, extracellular histones within NETs can precipitate coagulation in a pleiotropic fashion, via the contact pathway, tissue factor upregulation, as well as indirectly via platelet activation.10,28 Our results extend this to the stetting of sepsis-induced coagulation, as neutralization of histone H4 within NETs dramatically attenuated intravascular coagulation and microvascular dysfunction. Furthermore, our data suggest that the structural integrity of NETs is critical to their ability to promote coagulation, given that destruction of the DNA backbone with IV DNase was sufficient to impair intravascular coagulation. Previous work using a model of S aureus–induced sepsis revealed that IV DNase dismantles the DNA backbone of NETs, but only partially removes other components, such as histones from the vasculature.20 Taken together, our results indicate that the release of NETs into the microvasculature during sepsis creates an expansive catalytic scaffold decorated with multiple procoagulant factors that promote diffuse activation of thrombin and the development of intravascular coagulation. Furthermore, our findings indicate that these catalytic elements must be spatially organized in the intact NET scaffold, as disruption of the DNA backbone with DNase is sufficient to block coagulation and prevent vascular occlusion, despite the fact that a number of NET components remain within the vasculature.20
Our findings also expand the previously underappreciated role of platelets in the pathophysiology of sepsis and intravascular coagulation. Platelets are critical to the production of NETs during gram-negative sepsis through their ability to bind to neutrophils and induce NET release.5,6,29 In doing so, platelets form aggregates on the surface of neutrophils, resulting in their sequestration within the microvasculature and the development of sepsis-induced thrombocytopenia.5,6 Although platelet-neutrophil interactions account for the vast majority of microvascular platelet consumption, some of the sequestered platelets were observed to bind directly to NETs, consistent with previous observations from models of venous thrombosis.9 Multiple components of NETs are known to activate platelets, including DNA, multiple histone proteins (in a redundant manner), and neutrophil proteases (via protease-activated receptors).30,31 Together, these findings suggest a vicious cycle of microvascular thrombosis, wherein platelets accumulate on the surface of neutrophils and, in doing so, activate NET release, which then precipitates further platelet aggregation directly. This dramatic formation of platelet–neutrophil–NET macrostructure within the vasculature fuels the activation and propagation of intravascular coagulation. Recently, PolyP (released from the dense granules of platelets) has been implicated as a potent activator of thrombin, primarily via the contact pathway through FXII activation.32 In fact, a primary mechanism by which histone H4 activates thrombin in vitro is via stimulation of PolyP release from platelets.10 Indeed, we observed a significant reduction in intravascular thrombin activity after neutralization of PolyP, implicating this molecule as a keystone of the platelet–NET–coagulation axis. Further refinement of the cellular and molecular interactions that support this axis represents an important avenue for future research and may yield new targets for the development of anticoagulants.
Lastly, our findings add to a growing body of literature highlighting NETs as a potential therapeutic target in sepsis, with our data suggesting a potential role for NET inhibition in the treatment of disseminated intravascular coagulation. Small cohort studies of patients admitted to the intensive care unit with septic shock have found that increasing levels of circulating NET biomarkers (free DNA/myeloperoxidase complexes) correlate with disease severity scores (sequential organ failure assessment) and multiorgan dysfunction.33 Animal models of severe bacterial sepsis have found that treatment with IV DNase decreases end-organ damage and improves survival.6,20,33,34 Our data not only replicate these observations, but also extend them to provide further insight into the mechanisms by which targeting NETs may be of therapeutic benefit. We observed that inhibiting NETs drastically improved perfusion through liver sinusoids and, on a systemic level, reduced serum lactate levels. In fact, we found that treatment with IV DNase reduced lactate levels by ∼50% in septic animals without fluid resuscitation or antibiotics. Interestingly, although targeting NETs yielded significant attenuation of end-organ damage, treatment with a direct thrombin inhibitor (argatroban) did not. Indeed, NETs pose additional threats to host cells beyond their ability to activate coagulation, including the fact that multiple components of NETs can be directly cytotoxic to host cells.24 As our understanding of the biology of NET production expands, further strategies to disrupt this pathological axis will be revealed. For example, targeting platelet-neutrophil interactions may yield additional advantages to targeting NETs alone, as this could attenuate both microvascular thrombosis and NET production, as well as the ensuing intravascular coagulation. Furthermore, compared with conventional systemic anticoagulant therapy, targeting NET-mediated coagulation may allow for selective blockade of pathological infection-related coagulation in the microcirculation, while preserving physiological hemostasis. The ability to functionally uncouple coagulopathy from hemostasis is very attractive clinically, as it would enable the prevention of pathological coagulation without the side-effect of increased bleeding.
Despite our evolving understanding of pathological mechanisms in sepsis, current treatments remain primitive (including antibiotics, IV fluids, and vasopressors). Furthermore, these treatments are titrated to macrovascular endpoints (such as blood pressure and urine output), without addressing the fundamental pathology occurring at the level of the microvasculature. The many failed clinical trials of putative targeted therapies in sepsis clearly demonstrate the pathological redundancy of individual molecular mechanisms of disease. However, evolving evidence implicates NETs as a centerpiece linking many of these pathological mechanisms, now including microvascular coagulation and thrombosis, making NETs an attractive target for therapeutic development in sepsis and possibly other disorders involving disseminated intravascular coagulation.
Acknowledgments
The authors thank the late Kerri Mowen for providing the PAD4−/− mice and Bas Surewaard for his assistance with platelet counts.
This work was supported by grants from the Canada Foundation for Innovation John R. Evan Leaders Fund, the Natural Sciences and Engineering Research Council of Canada, a Heart and Stroke Foundation of Canada grant-in-aid, and the Critical Care Strategic Clinical Network of Alberta Health Services (C.N.J.) and by National Institutes of Health, Trans-Agency Research Consortium for Trauma-Induced Coagulopathy grant HL120877 (C.T.E). C.N.J. is supported by the Canada Research Chairs program. E.K. is supported by National Science Centre of Poland grant 2014/15/B/NZ6/02519, Opus 8.
Footnotes
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: B.M. designed and conducted experiments, analyzed data, and wrote the manuscript; C.N.J. provided overall supervision, analyzed data, and wrote the manuscript; R.P.D., S.-J.K., and M.T. conducted experiments and analyzed data; C.T.E. provided experimental reagents and contributed to experimental design; and E.K. contributed to experimental design.
Conflict-of-interest-disclosure: The authors declare no competing financial interests.
Correspondence: Braedon McDonald, Snyder Translational Laboratory in Critical Care Medicine, Department of Critical Care Medicine, University of Calgary, Health Research Innovation Centre 4C51, 3230 Hospital Dr NW, Calgary, AB T2N 4N1, Canada; e-mail: braedon.mcdonald@icloud.com.
References
- 1.Dombrovskiy VY, Martin AA, Sunderram J, Paz HL. Rapid increase in hospitalization and mortality rates for severe sepsis in the United States: a trend analysis from 1993 to 2003. Crit Care Med. 2007;35(5):1244-1250. [DOI] [PubMed] [Google Scholar]
- 2.Rittirsch D, Flierl MA, Ward PA. Harmful molecular mechanisms in sepsis. Nat Rev Immunol. 2008;8(10):776-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dempfle C-E. Coagulopathy of sepsis. Thromb Haemost. 2004;91(2):213-224. [DOI] [PubMed] [Google Scholar]
- 5.Clark SR, Ma AC, Tavener SA, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13(4):463-469. [DOI] [PubMed] [Google Scholar]
- 6.McDonald B, Urrutia R, Yipp BG, Jenne CN, Kubes P. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe. 2012;12(3):324-333. [DOI] [PubMed] [Google Scholar]
- 7.Massberg S, Grahl L, von Bruehl ML, et al. Reciprocal coupling of coagulation and innate immunity via neutrophil serine proteases. Nat Med. 2010;16(8):887-896. [DOI] [PubMed] [Google Scholar]
- 8.Martinod K, Demers M, Fuchs TA, et al. Neutrophil histone modification by peptidylarginine deiminase 4 is critical for deep vein thrombosis in mice. Proc Natl Acad Sci USA. 2013;110(21):8674-8679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fuchs TA, Brill A, Duerschmied D, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci USA. 2010;107(36):15880-15885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Semeraro F, Ammollo CT, Morrissey JH, et al. Extracellular histones promote thrombin generation through platelet-dependent mechanisms: involvement of platelet TLR2 and TLR4. Blood. 2011;118(7):1952-1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jenne CN, Wong CHY, Petri B, Kubes P. The use of spinning-disk confocal microscopy for the intravital analysis of platelet dynamics in response to systemic and local inflammation. PLoS One. 2011;6(9):e25109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka K, Koike Y, Shimura T, et al. In vivo characterization of neutrophil extracellular traps in various organs of a murine sepsis model. PLoS One. 2014;9(11):e111888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu J, Zhang X, Pelayo R, et al. Extracellular histones are major mediators of death in sepsis. Nat Med. 2009;15(11):1318-1321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDonald B, McAvoy EF, Lam F, et al. Interaction of CD44 and hyaluronan is the dominant mechanism for neutrophil sequestration in inflamed liver sinusoids. J Exp Med. 2008;205(4):915-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McDonald B, Pittman K, Menezes GB, et al. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science. 2010;330(6002):362-366. [DOI] [PubMed] [Google Scholar]
- 16.Jenne CN, Wong CHY, Zemp FJ, et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe. 2013;13(2):169-180. [DOI] [PubMed] [Google Scholar]
- 17.Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532-1535. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y, Li M, Stadler S, et al. Histone hypercitrullination mediates chromatin decondensation and neutrophil extracellular trap formation. J Cell Biol. 2009;184(2):205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li P, Li M, Lindberg MR, Kennett MJ, Xiong N, Wang Y. PAD4 is essential for antibacterial innate immunity mediated by neutrophil extracellular traps. J Exp Med. 2010;207(9):1853-1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kolaczkowska E, Jenne CN, Surewaard BGJ, et al. Molecular mechanisms of NET formation and degradation revealed by intravital imaging in the liver vasculature. Nat Commun. 2015;6:6673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yipp BG, Petri B, Salina D, et al. Infection-induced NETosis is a dynamic process involving neutrophil multitasking in vivo. Nat Med. 2012;18(9):1386-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ammollo CT, Semeraro F, Xu J, Esmon NL, Esmon CT. Extracellular histones increase plasma thrombin generation by impairing thrombomodulin-dependent protein C activation. J Thromb Haemost. 2011;9(9):1795-1803. [DOI] [PubMed] [Google Scholar]
- 23.Vincent J-L, De Backer D. Circulatory shock. N Engl J Med. 2013;369(18):1726-1734. [DOI] [PubMed] [Google Scholar]
- 24.Kaplan MJ, Radic M. Neutrophil extracellular traps: double-edged swords of innate immunity. J Immunol. 2012;189(6):2689-2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sambrano GR, Huang W, Faruqi T, Mahrus S, Craik C, Coughlin SR. Cathepsin G activates protease-activated receptor-4 in human platelets. J Biol Chem. 2000;275(10):6819-6823. [DOI] [PubMed] [Google Scholar]
- 26.Mihara K, Ramachandran R, Renaux B, Saifeddine M, Hollenberg MD. Neutrophil elastase and proteinase-3 trigger G protein-biased signaling through proteinase-activated receptor-1 (PAR1). J Biol Chem. 2013;288(46):32979-32990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stakos DA, Kambas K, Konstantinidis T, et al. Expression of functional tissue factor by neutrophil extracellular traps in culprit artery of acute myocardial infarction. Eur Heart J. 2015;36(22):1405-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.von Brühl ML, Stark K, Steinhart A, et al. Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. J Exp Med. 2012;209(4):819-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Caudrillier A, Kessenbrock K, Gilliss BM, et al. Platelets induce neutrophil extracellular traps in transfusion-related acute lung injury. J Clin Invest. 2012;122(7):2661-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Semple JW, Italiano JE Jr, Freedman J. Platelets and the immune continuum. Nat Rev Immunol. 2011;11(4):264-274. [DOI] [PubMed] [Google Scholar]
- 31.Fuchs TA, Bhandari AA, Wagner DD. Histones induce rapid and profound thrombocytopenia in mice. Blood. 2011;118(13):3708-3714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mutch NJ. Polyphosphate as a haemostatic modulator. Biochem Soc Trans. 2016;44(1):18-24. [DOI] [PubMed] [Google Scholar]
- 33.Czaikoski PG, Mota JMSC, Nascimento DC, et al. Neutrophil extracellular traps induce organ damage during experimental and clinical sepsis. PLoS One. 2016;11(2):e0148142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Meng W, Paunel-Görgülü A, Flohé S, et al. Depletion of neutrophil extracellular traps in vivo results in hypersusceptibility to polymicrobial sepsis in mice. Crit Care. 2012;16(4):R137. [DOI] [PMC free article] [PubMed] [Google Scholar]