Abstract
Objective
To determine factors important in local-regional recurrence (LRR) in patients with negative sentinel lymph nodes (SLNs) by hematoxylin and eosin (H&E) staining.
Summary Background Data
Z0010 was a prospective multicenter trial initiated in 1999 by the American College of Surgeons Oncology Group to evaluate occult disease in SLNs and bone marrow of early-stage breast cancer patients. Participants included women with biopsy-proven T1-2 breast cancer with clinically negative nodes, planned for lumpectomy and whole breast irradiation.
Methods
Women with clinical T1-2,N0,M0 disease underwent lumpectomy and SLN dissection. There was no axillary specific treatment for H&E-negative SLNs and clinicians were blinded to immunohistochemistry results. Systemic therapy was based on primary tumor factors. Univariable and multivariable analyses were performed to determine clinicopathologic factors associated with LRR.
Results
Of 5119 patients, 3904 (76.3%) had H&E-negative SLNs. Median age was 57 years (range 23–95). At median follow-up or 8.4 years there were 127 local, 20 regional, and 134 distant recurrences. Factors associated with local-regional recurrence were hormone receptor-negative disease (p=0.0004) and younger age (p=0.047). In competing risk-regression models, hormone receptor-positive disease and use of chemotherapy were associated with reduction in local-regional recurrence. When local recurrence was included in the model as a time-dependent variable, older age, T2 disease, high tumor grade and local recurrence were associated with reduced overall survival.
Conclusions
Local-regional recurrences are rare in early-stage breast cancer patients with H&E-negative SLNs. Younger age and hormone receptor-negative disease are associated with higher event rates and local recurrence is associated with reduced overall survival.
Keywords: breast cancer, sentinel lymph node biopsy, local-regional recurrence
Mini-Abstract
The ACOSOG Z0010 trial enrolled women with clinical T1,2N0 breast cancer planned for lumpectomy and SLN dissection followed by whole breast irradiation. Patients with H&E negative SLNs had no axillary specific treatment. At 8.4 years median follow-up, local-regional recurrences were rare and were associated with hormone receptor-negative disease and lymphovascular invasion.
INTRODUCTION
Sentinel lymph node (SLN) dissection has replaced axillary lymph node dissection (ALND) for staging of the regional lymph nodes in women presenting with clinically node-negative, early-stage breast cancer. Several studies have documented the decreased morbidity of SLN dissection compared with ALND and the increased detection of small volume metastases attributable to more detailed pathologic assessment of the SLNs1, 2. Multicenter trials have reported false negative rates ranging from 5% to as high as 17%, yet SLN dissection was rapidly incorporated into clinical practice prior to any long-term follow-up data documenting safety in terms of local-regional recurrence and survival3, 4. Local-regional recurrence has traditionally been considered a problem of excess tumor burden, but an increasing body of evidence suggests that tumor biology and the effectiveness of systemic therapy have a major impact on local-regional control.
Published studies from single institutions have demonstrated low axillary recurrence rates (0–4%) following a negative SLN dissection, however, only a few have reported follow-up times beyond 36 months5, 6. The National Surgical Adjuvant Breast and Bowel Project (NSABP) B-32 investigators recently published low local and regional recurrence rates in patients undergoing SLN dissection with ALND versus those undergoing SLN dissection alone7. The authors have not yet reported on factors associated with local or regional recurrences in patients undergoing SLN dissection alone or in those undergoing completion ALND. Investigators from the Sentinella trial reported a higher rate of local-regional recurrences in patients randomized to the sentinel node only arm of that study and a difference of 2.3% in 5-year disease-free survival compared with patients undergoing ALND, but this did not reach statistical significance8.
The American College of Surgeons Oncology Group (ACOSOG) Z0010 trial was a prospective evaluation of occult metastases in the SLNs and bone marrow of patients with T1-2 clinically node-negative breast cancer planned for breast conserving surgery and whole breast irradiation. Patients with H&E negative SLNs did not receive any axillary specific treatment. The overall and disease-free survival results were recently reported and found to be similar in patients with H&E negative SLNs and those with H&E negative nodes found to have occult metastases on immunohistochemical (IHC) evaluation9. In the current study, we evaluated local and regional recurrence events in Z0010 patients with H&E negative SLNs and sought to determine clinical and pathologic factors predicting for these events.
METHODS
Patients and Treatments
Z0010 was a prospective study of patients undergoing breast conserving surgery and SLN dissection, approved by the National Cancer Institute Cancer Therapy Evaluation Program and Central Institutional Review Board and the local Institutional Review Board of participating sites. Women with clinical T1-T2,N0,M0 invasive breast carcinoma planned for breast conserving surgery with whole breast irradiation were eligible. Patients were not eligible if they had neoadjuvant therapy, pre-pectoral breast implants, concurrent bilateral malignancies, multifocal or multicentric disease not amenable to a single lumpectomy, or previous axillary surgery. Informed consent was obtained prior to registration. Whole breast irradiation was specified in the protocol and excluded treatment with a third supraclavicular field. The dose to the breast was 45 to 50 Gy administered in tangential fields with a coplanar posterior border. Adjuvant systemic therapy decisions were based on primary tumor factors as assessed by treating clinicians.
Sentinel Lymph Node Dissection and Pathologic Assessment
Participating surgeons were required to perform 20 SLN procedures with completion ALND with identification and accuracy rates > 85% or provide documentation of training in SLN dissection through a postgraduate surgery training program. The technical results of SLN identification rates and factors influencing the failure to identify a SLN have been previously published10. SLNs were formalin-fixed and paraffin-embedded as per institutional protocols. Paraffin blocks were cut into 5-µm sections and assessed for metastases with standard H&E staining. For patients with H&E-negative SLNs, unstained slides were submitted to a central laboratory for IHC to cytokeratin. The results of IHC staining of the SLNs were not made available to patients or their treating clinicians. The incidence of occult metastases in patients with H&E-negative SLNs was 10.3%. There was no difference in overall survival or disease-free survival among patients with H&E-negative and IHC-negative SLNs compared with patients with H&E-negative and IHC-positive SLNs9.
Women were followed for breast cancer recurrence and death. With respect to breast cancer recurrence, patients were followed until the first local, regional, or distant recurrence. The events of interest for this study were local recurrence, regional recurrence, and distant recurrence. The time to a recurrence was measured from date of study enrollment until the event. Survival was measured from the date of study enrollment until death.
Statistical Analysis
Univariable and multivariable Cox regression models were used to analyze the association between an event (local, local-regional and distant recurrences, and death) and the baseline patient or tumor characteristics. In the models, regional recurrence was not used by itself since there were so few events and it was combined into a local-regional event (i.e. the patient had a local recurrence or a regional recurrence). Multivariable models were adjusted for treatments the women received. In the Cox models, patients were censored at last follow-up, a competing recurrence of breast cancer, or death, if the breast cancer recurrence type of interest was not observed. In the multivariable model for overall survival, the local recurrence variable was treated as a time-dependent variable where a woman was in the not at risk group until the point at which she had a local recurrence and then was switched to the at risk group11.
Since women were only followed for their first breast cancer recurrence, they were censored at the time of the event for analyses that involved a different breast cancer recurrence. For example, if a woman had a local recurrence, she was censored at that time in the analysis of distant breast cancer recurrence. This serves to overestimate the incidence of the different types of breast cancer recurrences. To obtain a better estimate of the breast cancer incidences, cumulative incidence competing risk-regression models were used12. These models determined the association between the patient and tumor characteristics and the event of interest in the same manner as the Cox models. The multivariable models adjusted for the treatments patients received. These are the models that were used to estimate the incidence of local, regional, and distant breast cancer recurrence.
All tests were two-sided and p-values < 0.05 were considered significant. Statistical analyses were done using the SAS software package (version 9.1.3, SAS Institute, Cary, NC).
RESULTS
Patient and Tumor Characteristics
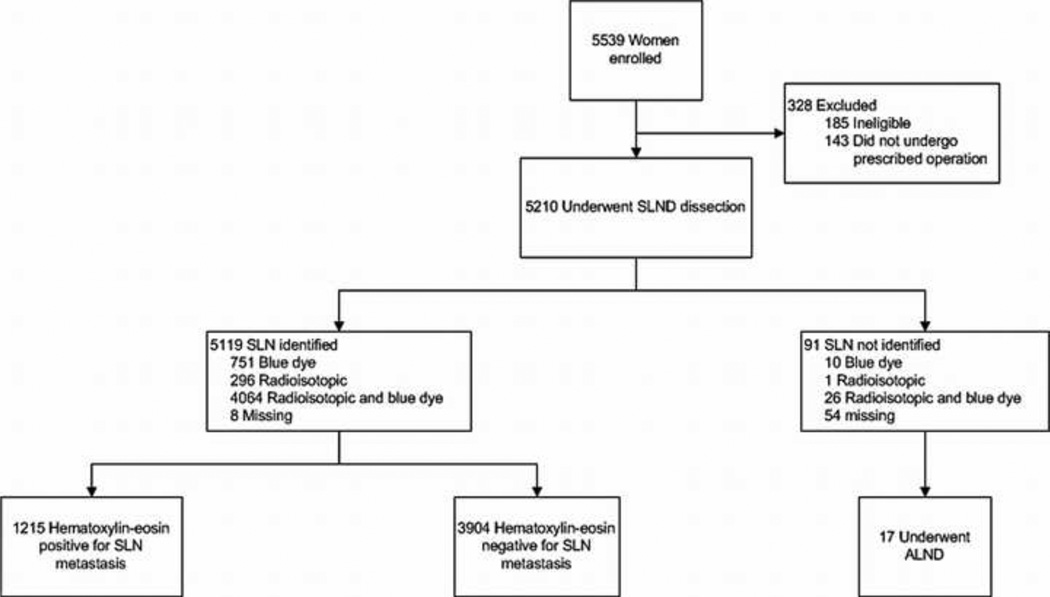
ACOSOG Z0010 opened May 10, 1999 and completed accrual May 30, 2003 with 5539 patients enrolled from 126 participating sites. Of the 5119 eligible patients who had a SLN identified at surgery, 3904 (76.3%) had H&E-negative SLNs. A CONSORT diagram of the 3904 patients included in the current study is shown in figure 1. Clinical and pathologic characteristics of the patients with H&E-negative SLNs are listed in table 1. The median age was 57 years (range 23–95 years) and median follow-up of surviving patients was 8.4 years (range 0–12.4 years). The majority of the patients had invasive ductal histology (79.4%) with clinical T1 (87.6%) tumors that were hormone receptor-positive (estrogen or progesterone receptor positive, 83.8%). Lymphovascular space invasion was reported in 396 (11.6%) patients.
Figure 1.
CONSORT diagram of study participants.
Table 1.
Patient and tumor characteristics (N = 3904 women)
| Characteristic | |
|---|---|
| Age, years | |
| median (min, max) | 57 (23, 95) |
| ≤ 50, n (%) | 1030 (26.4%) |
| > 50, n (%) | 2874 (73.6%) |
| Tumor histology, n (%) | |
| ductal | 3094 (79.4%) |
| lobular | 319 (8.2%) |
| both | 97 (2.5%) |
| other | 389 (10.0%) |
| missing, n | 5 |
| Lymphovascular invasion, n (%) | |
| absent | 3024 (88.4%) |
| present | 396 (11.6%) |
| missing, n | 484 |
| Tumor size, cm | |
| median (min, max) | 1.4 (0.0, 19.0) |
| ≤ 1.0, n (%) | 1597 (43.6%) |
| 1.1 – 2.0, n (%) | 1609 (44.0%) |
| > 2.0, n(%) | 455 (12.4%) |
| missing, n | 243 |
| HR status, n (%) | |
| ER or PR positive | 3084 (83.8%) |
| both negative | 596 (16.2%) |
| missing, n | 224 |
| Clinical stage, n (%) | |
| T1 | 3206 (87.6%) |
| T2 | 447 (12.2%) |
| T3 | 8 (0.2%) |
| missing, n | 243 |
| Tumor Grade, n (%) | |
| I | 1225 (33.8%) |
| II | 1479 (40.8%) |
| III | 918 (25.4%) |
| Missing, n | 282 |
HR – hormone receptor; ER – estrogen receptor; PR – progesterone receptor
Surgical and Adjuvant Treatments
A summary of the surgical and adjuvant treatments received by the 3904 patients are listed in table 2. All patients were planned for breast conserving surgery with SLN dissection followed by whole breast irradiation. There were 78 (2.0%) patients who underwent mastectomy as the final surgical procedure due to inability to obtain negative margins with breast conservation. There were 106 (2.7%) patients with H&E-negative SLNs who underwent ALND. Radiation treatment records were incomplete on 657 patients. Of the remaining patients, 2993 (92.2%) completed whole breast irradiation. There were 1432 (43.5%) patients who received adjuvant systemic chemotherapy and 2227 (67.7%) who received adjuvant hormonal therapy.
Table 2.
Summary of treatments patients received (N = 3904 women)
| Treatment | |
|---|---|
| Surgery, n(%) | |
| BCT | 3784 (98.0%) |
| Mastectomy | 78 (2.0%) |
| Other surgery | 1 (0.02%) |
| missing, n | 41 |
| Chemotherapy, n (%) | |
| yes | 1432 (43.5%) |
| no | 1857 (56.5%) |
| missing, n | 615 |
| Hormonal therapy, n (%) | |
| yes | 2227 (67.7%) |
| no | 1062 (32.3%) |
| missing, n | 615 |
| Radiation therapy, n (%) | |
| yes | 2993 (92.2%) |
| no | 254 (7.8%) |
| missing, n | 657 |
BCT–breast conserving therapy
Local, Regional and Distant Recurrences
At a median follow-up time of 8.4 years (range 0–12.4 years) there were 127 (3.2%) local, 20 (0.5%) regional, and 134 (3.4%) distant recurrences reported (table 3). There were 317 deaths reported. We did not find a difference in local, regional or distant recurrences in the patients with H&E-negative, IHC-negative SLNs compared with those who had H&E-negative, IHC-positive SLNs. The remaining analyses report on local-regional recurrence, distant recurrence and overall survival for the entire population of patients with H&E-negative SLNs irrespective of IHC results. Clinical and pathologic factors were assessed in univariable and multivariable Cox models to predict local, local-regional and distant recurrences and overall survival (table 4). While several factors were associated with local recurrence on univariable analysis, only older age (HR 0.98, 95% CI 0.96–1.00, p=0.018) and positive hormone receptor status (HR 0.34, 95% CI 0.20–0.58), p<0.0001) predicted for reduced local failure on multivariable analysis. Factors predicting for reduced local-regional recurrence events on multivariable analysis were older age (HR 0.98, 95% CI 0.96–1.00, p=0.047) and positive hormone receptor status (HR 0.39, 95% CI 0.23–0.66, p=0.0004). The presence of lymphovascular invasion (HR 2.00, 95% CI 1.20–3.31, p=0.008) and grade II (HR 2.44, 95% CI 1.31–4.56, p=0.005) and grade III disease (HR 3.65, 95% CI 1.82–7.34, p=0003) predicted for distant recurrences. There were 317 deaths in this patient group. Factors predicting for reduced overall survival on multivariable analysis were older age (HR 1.07, 95% CI 1.06–1.08, p <0.0001), increasing tumor size (HR 1.18, 95% CI 1.07–1.31, p=0.0013), and grade III disease (HR 2.65, 95% CI 1.77–3.96, p<0.0001).
Table 3.
Summary of patient outcomes. Median follow-up for patients who are alive is 8.4 years.
| Outcome | Number of women | 3 year cumulative incidence |
5-year cumulative incidence |
|---|---|---|---|
| local recurrence | 127 | 0.013 | 0.024 |
| regional recurrence | 20 | 0.003 | 0.005 |
| distant recurrence | 134 | 0.017 | 0.028 |
Table 4.
Univariable and multivariable Cox models
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Local recurrence | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age, years | 0.97 (0.95– 0.98) | <0.0001 | 0.98 (0.96 – 1.00) | 0.018 |
| Tumor size | 1.13 (1.00 – 1.27) | 0.042 | 1.03 (0.84 – 1.26) | 0.79 |
| Clinical stage | ||||
| T1 | 1.00 (ref) | --- | --- | |
| T2 | 1.75 (1.13– 2.72) | 0.012 | ||
| LVI | ||||
| absent | 1.00 (ref) | 1.00 (ref) | ||
| present | 1.62 (0.99 – 2.64) | 0.056 | 1.47 (0.85 – 2.55) | 0.17 |
| Tumor Grade | ||||
| I | 1.00 (ref) | |||
| II | 1.41 (0.90 – 2.20) | 0.14 | 1.11 (0.65 – 1.87) | 0.71 |
| III | 2.69 (1.66 – 4.05) | <0.0001 | 1.31 (0.69 – 2.47) | 0.41 |
| HR status | ||||
| negative | 1.00 (ref) | 1.00 (ref) | ||
| positive | 0.32 (0.22 – 0.46) | <0.0001 | 0.34 (0.20 – 0.58) | <0.0001 |
| Chemotherapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 1.42 (0.99 –2.05) | 0.056 | 0.64 (0.39 – 1.03) | 0.068 |
| Hormonal therapy | ||||
| no | 1.00 (ref) | --- | --- | |
| yes | 0.45 (0.31 – 0.65) | <0.0001 | ||
| Radiation therapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 0.89 (0.45 – 1.76) | 0.74 | 0.83 (0.40 – 1.72) | 0.62 |
| Local-Regional recurrence | ||||
| Age, years | 0.97 (0.95 – 0.98) | <0.0001 | 0.98 (0.96 – 1.00) | 0.047 |
| Tumor size | 1.14 (1.02 – 1.27) | 0.017 | 1.04 (0.86 – 1.26) | 0.66 |
| Clinical stage | ||||
| T1 | 1.00 (ref) | --- | --- | |
| T2 | 1.90 (1.27 – 2.86) | 0.002 | ||
| LVI | ||||
| absent | 1.00 (ref) | 1.00 (ref) | ||
| present | 1.82 (1.16 – 2.86) | 0.010 | 1.62 (0.88 – 2.71) | 0.062 |
| Tumor Grade | ||||
| I | 1.00 (ref) | |||
| II | 1.37 (0.89 – 2.12) | 0.15 | 1.03 (0.62 – 1.70) | 0.91 |
| III | 2.68 (1.75 – 4.09) | <0.0001 | 1.42 (0.78 – 2.59) | 0.26 |
| HR status | ||||
| negative | 1.00 (ref) | 1.00 (ref) | ||
| positive | 0.33 (0.23 – 0.48) | <0.0001 | 0.39 (0.23 – 0.66) | 0.0004 |
| Chemotherapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 1.41 (1.00 – 2.00) | 0.051 | 0.66 (0.42 – 1.05) | 0.080 |
| Hormonal therapy | ||||
| no | 1.00 (ref) | --- | --- | |
| yes | 0.45 (0.32 – 0.64) | <0.0001 | ||
| Radiation therapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 0.87 (0.46 – 1.66) | 0.67 | 0.90 (0.44 – 1.86) | 0.78 |
| Distant recurrence | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age, years | 0.99 (0.97 – 1.00) | 0.099 | 1.00 (0.98 – 1.02) | 0.76 |
| Tumor size | 1.19 (1.09 – 1.30) | <0.0001 | 1.16 (1.00 – 1.35) | 0.054 |
| Clinical stage | ||||
| T1 | 1.00 (ref) | --- | --- | |
| T2 | 1.80 (1.17–2.76) | 0.008 | ||
| LVI | ||||
| absent | 1.00 (ref) | 1.00 (ref) | ||
| present | 2.25 (1.46 – 3.49) | 0.0003 | 2.00 (1.20 – 3.31) | 0.008 |
| Tumor Grade | ||||
| I | 1.00 (ref) | |||
| II | 1.73 (1.10 – 2.74) | 0.019 | 2.44 (1.31 – 4.56) | 0.005 |
| III | 3.42 (2.18 – 5.36) | <0.0001 | 3.65 (1.82 – 7.34) | 0.0003 |
| HR status | ||||
| negative | 1.00 (ref) | 1.00 (ref) | ||
| positive | 0.51 (0.34 – 0.76) | 0.0009 | 1.11 (0.62 – 2.00) | 0.73 |
| Chemotherapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 1.69 (1.16 – 2.45) | 0.006 | 1.02 (0.62 – 1.66) | 0.95 |
| Hormonal therapy | ||||
| no | 1.00 (ref) | --- | --- | |
| yes | 0.72 (0.49 – 1.05) | 0.08 | ||
| Radiation therapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 0.54 (0.30 – 0.94) | 0.029 | 0.57 (0.29 – 1.10) | 0.094 |
| Survival | ||||
| Age, years | 1.06 (1.05 – 1.07) | <0.0001 | 1.07 (1.06 – 1.08) | <0.0001 |
| Tumor size | 1.14 (1.06– 1.22) | 0.0004 | 1.18 (1.07 – 1.31) | 0.0013 |
| Clinical stage | ||||
| T1 | 1.00 (ref) | --- | --- | |
| T2 | 1.78 (1.36 – 2.33) | <0.0001 | ||
| LVI | ||||
| absent | 1.00 (ref) | 1.00 (ref) | ||
| present | 1.60 (1.17– 2.18) | 0.003 | 1.27 (0.85 – 1.88) | 0.24 |
| Tumor Grade | ||||
| I | 1.00 (ref) | |||
| II | 1.32 (1.00– 1.74) | 0.052 | 1.21 (0.85 – 1.73) | 0.30 |
| III | 2.39 (1.81 – 3.15) | <0.0001 | 2.65 (1.77 – 3.96) | <0.0001 |
| HR status | ||||
| negative | 1.00 (ref) | 1.00 (ref) | ||
| positive | 0.59 (0.45– 0.77) | 0.0001 | 0.96 (0.64 – 1.44) | 0.86 |
| Chemotherapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 0.86 (0.67 – 1.11) | 0.24 | 0.91 (0.66 −1.26) | 0.56 |
| Hormonal therapy | ||||
| no | 1.00 (ref) | --- | --- | |
| yes | 0.64 (0.50 – 0.82) | 0.0005 | ||
| Radiation therapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 0.96 (0.60 – 1.53) | 0.86 | 1.00 (0.56 – 1.72) | 0.99 |
| Local recurrence (time dependent) |
||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 4.90 (3.27 – 7.36) | <0.0001 | 6.73 (4.26 – 10.63) | <0.0001 |
LVI – lymphovascular invasion; HR – hormone receptor
Clinical Outcomes Using Competing Risk-Regression Models
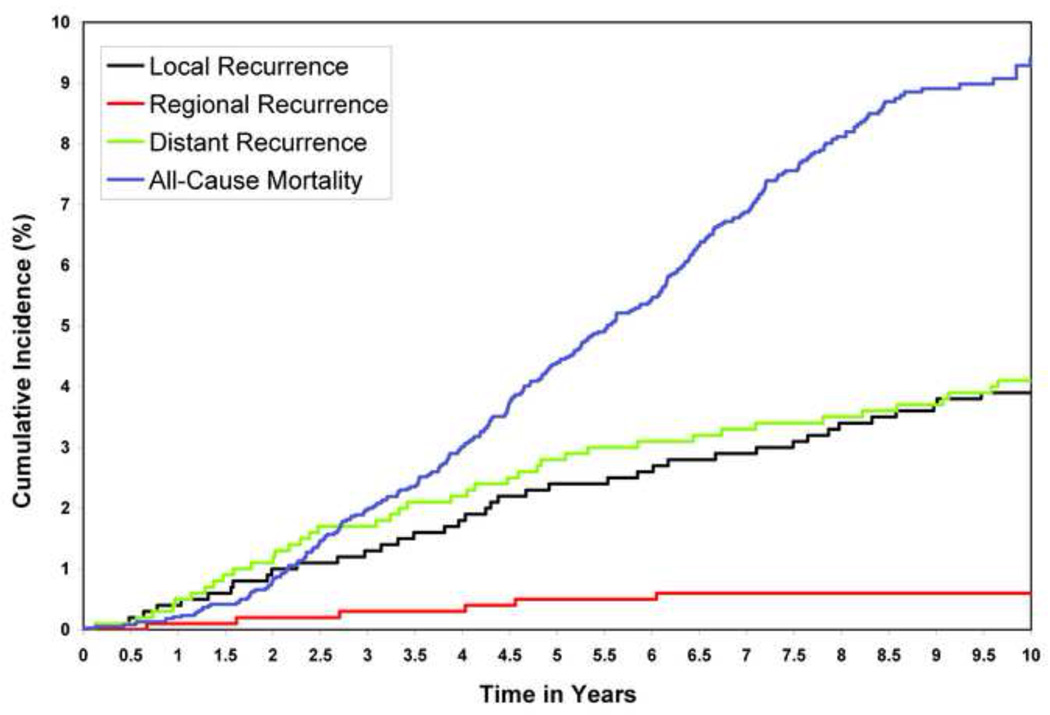
Since patients were followed for breast cancer recurrence only until the first recurrence and patients may die prior to having a breast cancer recurrence, competing risk models are more appropriate for ascertaining the incidence of the different types of breast cancer recurrence prior to death. Univariable and multivariable competing risk models are presented in Table 5 and the curves are presented in Figure 2. The competing risk models identified the same risk factors for local recurrence, local-regional recurrence, and distant recurrence as did the Cox time-to-event models, which did not account for competing risks. In addition, the competing risk-regression models revealed that use of chemotherapy predicted for reduced local (HR 0.58, 95% CI 0.35–0.95, p=0.030) and local-regional (HR 0.60, 95% CI 0.37–0.97, p=0.0039) recurrences. The five year incidence rates for local recurrence, regional recurrence, and distant recurrence were 2.4%, 0.5%, and 2.8%, respectively.
Table 5.
Univariable and multivariable competing risk models
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Local recurrence | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age, years | 0.96 (0.95 – 0.98) | <0.0001 | 0.98 (0.96 – 0.99) | 0.020 |
| Tumor size | 1.13 (1.00 – 1.27) | 0.045 | 0.99 (0.78 – 1.25) | 0.93 |
| Clinical stage | ||||
| T1 | 1.00 (ref) | --- | --- | |
| T2 | 1.68 (1.05 – 2.69) | 0.031 | ||
| LVI | ||||
| absent | 1.00 (ref) | 1.00 (ref) | ||
| present | 1.60 (0.98 – 2.62) | 0.062 | 1.33 (0.74 – 2.38) | 0.34 |
| Tumor Grade | ||||
| I | 1.00 (ref) | 1.00 (ref) | ||
| II | 1.77 (1.06 – 2.94) | 0.028 | 1.24 (0.70 – 2.16) | 0.45 |
| III | 3.25 (1.96 – 5.39) | <0.0001 | 1.59 (0.81 – 3.12) | 0.18 |
| HR status | ||||
| negative | 1.00 (ref) | 1.00 (ref) | ||
| positive | 0.32 (0.22 – 0.46) | <0.0001 | 0.35 (0.20 – 0.62) | 0.0003 |
| Chemotherapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 1.43 (0.99 – 2.05) | 0.054 | 0.58 (0.35 – 0.95) | 0.030 |
| Hormonal therapy | ||||
| no | 1.00 (ref) | --- | --- | |
| yes | 0.45 (0.31 – 0.65) | <0.0001 | ||
| Radiation therapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 0.89 (0.45 – 1.75) | 0.73 | 0.78 (0.38 – 1.62) | 0.51 |
| Local-Regional recurrence | ||||
| Age, years | 0.97 (0.95 – 0.98) | <0.0001 | 0.98 (0.96 – 1.00) | 0.049 |
| Tumor size | 1.14 (1.02 – 1.27) | 0.019 | 1.01 (0.82 – 1.25) | 0.91 |
| Clinical stage | ||||
| T1 | 1.00 (ref) | --- | --- | |
| T2 | 1.87 (1.29 – 2.88) | 0.005 | ||
| LVI | ||||
| absent | 1.00 (ref) | 1.00 (ref) | ||
| present | 1.80 (1.14 – 2.83) | 0.011 | 1.49 (0.87 – 2.55) | 0.14 |
| Tumor Grade | ||||
| I | 1.00 (ref) | 1.00 (ref) | ||
| II | 1.66 (1.02 – 2.69) | 0.040 | 1.12 (0.66 – 1.91) | 0.68 |
| III | 3.21 (1.99 – 5.16) | <0.0001 | 1.66 (0.88 – 3.13) | 0.11 |
| HR status | ||||
| negative | 1.00 (ref) | 1.00 (ref) | ||
| positive | 0.33 (0.23 – 0.48) | <0.0001 | 0.42 (0.24 – 0.71) | 0.0014 |
| Chemotherapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 1.42 (1.00 – 2.01) | 0.049 | 0.60 (0.37 – 0.97) | 0.0039 |
| Hormonal therapy | ||||
| no | 1.00 (ref) | --- | --- | |
| yes | 0.45 (0.32 – 0.64) | <0.0001 | ||
| Radiation therapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 0.87 (0.45 – 1.65) | 0.66 | 0.85 (0.41 – 1.76) | 0.67 |
| Distant recurrence | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age, years | 0.99 (0.97 – 1.00) | 0.083 | 0.99 (0.98 – 1.01) | 0.57 |
| Tumor size | 1.19 (1.09 – 1.30) | <0.0001 | 1.16 (0.99 – 1.35) | 0.064 |
| Clinical stage | ||||
| T1 | 1.00 (ref) | --- | --- | |
| T2 | 1.90 (1.22 – 2.97) | 0.005 | ||
| LVI | ||||
| absent | 1.00 (ref) | 1.00 (ref) | ||
| present | 2.23 (1.44 – 3.45) | 0.0003 | 2.03 (1.22 – 3.37) | 0.0064 |
| Tumor Grade | ||||
| I | 1.00 (ref) | 1.00 (ref) | ||
| II | 2.00 (1.20 – 3.34) | 0.0078 | 2.33 (1.22 – 4.42) | 0.0099 |
| III | 3.92 (2.37 – 6.48) | <0.0001 | 3.43 (1.67 – 7.04) | 0.0008 |
| HR status | ||||
| negative | 1.00 (ref) | 1.00 (ref) | ||
| positive | 0.51 (0.35 – 0.76) | 0.001 | 1.08 (0.60 – 1.96) | 0.79 |
| Chemotherapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 1.69 (1.16 – 2.46) | 0.006 | 0.97 (0.59 – 1.59) | 0.91 |
| Hormonal therapy | ||||
| no | 1.00 (ref) | --- | --- | |
| yes | 0.72 (0.49 – 1.05) | 0.087 | ||
| Radiation therapy | ||||
| no | 1.00 (ref) | 1.00 (ref) | ||
| yes | 0.53 (0.30 – 0.93) | 0.028 | 0.56 (0.29 – 1.08) | 0.083 |
HR– hormone receptor; LVI– lymphovascular invasion
Figure 2.
Cumulative incidence curves: local recurrence, local-regional recurrence, distant recurrence, and death.
DISCUSSION
The use of SLN dissection in early-stage breast cancer patients has allowed hundreds of thousands of women to avoid the morbidity of ALND while still providing the anatomic staging information that has traditionally been important in determining prognosis and guiding treatment decisions. However, data from prospective registries and randomized trials have demonstrated that there is a false negative rate with SLN dissection, even in experienced hands, ranging from 5% to as high as 10%. Several studies have reported low axillary failure rates after a negative SLN dissection but the follow-up times have been relatively short. In the current study, we evaluated local-regional recurrence rates in women participating in the ACOSOG Z0010 trial that completed accrual in 2003. Of 3904 patients with H&E negative SLNs, only 127 local, 20 regional, and 134 distant recurrences were reported at a median follow-up time of 8.4 years. Clinicopathologic factors associated with local-regional recurrences in patients with H&E-negative SLNs were hormone receptor-negative disease and younger age. When local recurrence was included in the model as a time-dependent variable, older age, T2 disease, high tumor grade and local recurrence were associated with reduced overall survival. In addition, in competing risk-regression models, use of chemotherapy was associated with a reduction in local and local-regional recurrences.
Axillary failure rates have generally been reported in the range of 1–2% in patients with early-stage breast cancer treated with ALND. Similarly, the incidence of axillary recurrences following a negative SLN dissection have been low, typically less than 1%, although the median follow-up times have been shorter, on the order of 24–36 months. One exception is a Japanese study from Imoto et al. where they found a 3.6% axillary recurrence rate at a median follow-up time of 52 months13. Thirty years ago Fisher et al. observed in NSABP B-04 that the axillary first failure rate in patients randomized to no axillary dissection was only 50% of what would have been expected based on the incidence of nodal metastases in those randomized to axillary dissection, even in the absence of systemic therapy or radiotherapy14. More recently, data from the NSABP B-32 trial revealed no differences in regional failure rates at a mean follow-up time of 95.6 months between patients randomized to SLN dissection alone compared to those who underwent SLN dissection followed by completion ALND (0.7% vs. 0.4%)7. These data would suggest that not all patients who have disease left behind in the axilla due to a false negative SLN will manifest clinically relevant disease in terms of axillary nodal recurrence or death due to breast cancer. This is congruent with the recent findings from the ACOSOG Z0011 trial where there was no difference in regional recurrences or disease-free or overall survival outcomes in patients with one or two positive SLNs whether they were randomized to undergo completion ALND or SLN dissection alone15, 16. It is likely that some of the residual nodal disease in the axilla is eradicated by systemic adjuvant therapy or by treatment of the nodes through the tangential breast irradiation fields and in some cases the disease may remain clinically dormant and not warrant aggressive therapies.
It has become increasingly clear that biologic factors other than tumor size and nodal status are important in prognosis and treatment decisions for breast cancer patients. Gene expression profiling has been shown to separate patients with similar anatomic stages and histologies into distinct subtypes that demonstrate differences in survival outcomes17. The molecular subtypes of breast cancer can be approximated through immunohistochemical staining for estrogen receptor (ER), progesterone receptor (PR) and HER-2. Nguyen and colleagues have shown that these approximated subtypes are associated with differences in both local and distant recurrence after breast conserving therapy18. Voduc et al. used an immunohistochemical panel to stain tissue microarrays and reported that the HER-2-enriched and triple receptor negative subtypes had an increased risk for local and regional recurrences in patients treated with breast conservation19. However, a similar analysis of patients participating in the Danish Breast Cancer Group randomized trials of postmastectomy radiotherapy also showed an increased risk of chest wall recurrence in the triple negative and HER2 overexpressing subgroups, and 2 recent retrospective comparisons of local recurrence rates in triple negative breast cancers treated by mastectomy or breast conserving therapy demonstrated no differences between groups20–22. In aggregate, these findings indicate that negative estrogen receptor status is a marker of biologically aggressive tumors, but not a selection factor for mastectomy.
The ACOSOG Z0010 trial was initiated in 1999 prior to routine HER-2 testing on all clinical breast cancer samples and therefore we cannot approximate the molecular subtypes in this patient population. However, we did find that negative hormone receptor status was associated with higher local, regional and distant recurrence rates and reduced overall survival. The triple receptor negative subtype has a higher recurrence rate compared with other subtypes but it is also associated with a slightly lower nodal positivity rate overall19. This suggests that improved systemic therapies, as opposed to more aggressive use of ALND or mastectomy would have a greater impact on survival outcomes.
The negative impact of higher tumor grade and lymphovascular invasion was evident in the Z0010 cohort in predicting for increased distant recurrence events and grade III disease was associated with reduced overall survival. Rakha et al. recently showed that LVI was an independent predictor of breast cancer-specific survival and distant metastasis-free survival in their population of patients across all stage groupings and approximated molecular subtypes23. While the presence of LVI predicts for a higher rate of recurrences, it is not clear that any specific local-regional or systemic treatment strategies are able to reduce this risk. Rakha and colleagues also found that grade was a strong predictor of outcome in patients and suggested that it be incorporated in a breast cancer staging system.24, 25 Tumor grade is currently part of staging systems for prostate cancer, soft tissue sarcomas, and some bone tumors and Wasif et al. recently made a case for incorporating grade into the AJCC staging system for pancreas cancer.26 Several groups have suggested that biologic factors such as estrogen receptor status, tumor grade and LVI be added to the AJCC staging system for breast cancer in order to provide improved prognostic information over what is currently available using anatomic staging with tumor size and nodal status23, 27.
Although the five year local recurrence rate was only 2.4% in the Z0010 cohort, local recurrence was associated with reduced survival. The factors predicting for reduced local recurrence were older age and positive hormone receptor status. Use of adjuvant chemotherapy was not associated with a reduction in local recurrences in the standard Cox models but was statistically significant in the competing risk-regression models. There is now data from multiple studies demonstrating that systemic chemotherapy and hormonal therapy reduce local recurrences in women with breast cancer. We did not have complete radiation therapy records on all of the patients and therefore cannot be certain that omission of radiation in the adjuvant setting did not have an impact on local recurrence rates. The meta-analysis from the Early Breast Cancer Trialists’ Collaborative Group has demonstrated that the addition of radiation therapy to the conserved breast reduces the in-breast recurrence rate by half which in turn reduces deaths due to breast cancer by about one-sixth28.
We examined the 3904 patients with H&E negative SLNs from the ACOSOG Z0010 trial to determine factors important in local-regional recurrence. These patients were intended for breast conserving surgery and whole breast irradiation with no axillary specific treatment. We found that regional nodal recurrences were rare and that hormone receptor-negative disease and younger age predicted for higher rates of local-regional recurrence. Older age, larger tumor size, grade III disease and local recurrence were associated with reduced overall survival. SLN dissection alone is safe and avoids the morbidity of ALND in early-stage breast cancer patients with H&E-negative SLNs.
Acknowledgments
Supported by a grant from the National Cancer Institute: U10-CA76001-15
Contributor Information
Kelly K. Hunt, Department of Surgical Oncology, MD Anderson Cancer Center
Karla V. Ballman, Division of Biomedical Statistics and Informatics, Mayo Clinic, Rochester
Linda M. McCall, Duke Cancer Institute
Judy C. Boughey, Department of Surgery, Mayo Clinic, Rochester
Elizabeth A. Mittendorf, Department of Surgical Oncology, MD Anderson Cancer Center
Charles E. Cox, Department of Surgery, USF Health
Pat W. Whitworth, Nashville Breast Center
Peter D. Beitsch, Dallas Surgical Group
A.Marilyn Leitch, Department of Surgery, UT Southwestern Medical Center
Thomas A. Buchholz, Department of Radiation Oncology, MD Anderson Cancer Center
Monica A. Morrow, Department of Surgery, Memorial Sloan Kettering Cancer Center
Armando E. Giuliano, Department of Surgery, Cedars Sinai Medical Center
REFERENCES
- 1.Giuliano AE, Dale PS, Turner RR, et al. Improved axillary staging of breast cancer with sentinel lymphadenectomy. Ann Surg. 1995;222(3):394–399. doi: 10.1097/00000658-199509000-00016. discussion 399–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mansel RE, Fallowfield L, Kissin M, et al. Randomized multicenter trial of sentinel node biopsy versus standard axillary treatment in operable breast cancer: the ALMANAC trial. J Natl Cancer Inst. 2006;98:599–609. doi: 10.1093/jnci/djj158. [DOI] [PubMed] [Google Scholar]
- 3.Krag DN, Anderson SJ, Julian TB, et al. Technical outcomes of sentinel-lymph-node resection and conventional axillary-lymph-node dissection in patients with clinically node-negative breast cancer: results from the NSABP B-32 randomised phase III trial. Lancet Oncol. 2007;8:881–888. doi: 10.1016/S1470-2045(07)70278-4. [DOI] [PubMed] [Google Scholar]
- 4.Veronesi U, Viale G, Paganelli G, et al. Sentinel lymph node biopsy in breast cancer: ten-year results of a randomized controlled study. Ann Surg. 2010;251:595–600. doi: 10.1097/SLA.0b013e3181c0e92a. [DOI] [PubMed] [Google Scholar]
- 5.Bergkvist L, de Boniface JP-EJ, et al. Axillary Recurrence Rate After Negative Sentinel Node Biopsy in Breast Cancer. Three-Year Follow-Up of the Swedish Multicenter Cohort Study. Ann Surg. 2008;247:150–156. doi: 10.1097/SLA.0b013e318153ff40. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Son BH, Park EW, et al. Axillary recurrence after negative sentinel lymph node biopsy. Breast Cancer Res Treat. 2009;114:301–305. doi: 10.1007/s10549-008-9994-4. [DOI] [PubMed] [Google Scholar]
- 7.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zavagno G, De Salvo GL, Scalco G, et al. A Randomized Clinical Trial on Sentinel Lymph Node Biopsy Versus Axillary Lymph Node Dissection in Breast Cancer. Results of the Sentinella/GIVOM Trial. Ann Surg. 2008;247:207–213. doi: 10.1097/SLA.0b013e31812e6a73. [DOI] [PubMed] [Google Scholar]
- 9.Giuliano AE, Hawes D, Ballman KV, et al. Association of Occult Metastases in Sentinel Lymph Nodes and Bone Marrow with Survival Among Women with Early-Stage Invasive Breast Cancer. JAMA. 2011;306:385–393. doi: 10.1001/jama.2011.1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Posther KE, McCall LM, Blumencranz PW, et al. Sentinel node skills verification and surgeon performance: data from a multicenter clinical trial for early-stage breast cancer. Ann Surg. 2005;242(4):593–599. doi: 10.1097/01.sla.0000184210.68646.77. discussion 599–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher LD, Lin DY. Time-dependent covariates in the Cox proportional-hazards regression model. Annu Rev Public Health. 1999;20:145–157. doi: 10.1146/annurev.publhealth.20.1.145. [DOI] [PubMed] [Google Scholar]
- 12.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risk: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 13.Imoto S, Wada N, Murakami K, et al. Prognosis of breast cancer patients treated with sentinel node biopsy in Japan. Jpn J Clin Oncol. 2004;34:452–456. doi: 10.1093/jjco/hyh077. [DOI] [PubMed] [Google Scholar]
- 14.Fisher B, Wolmark N, Redmond C, et al. Findings from NSABP Protocol No. B-04: comparison of radical mastectomy with alternative treatments. II. The clinical and biologic significance of medial-central breast cancers. Cancer. 1981;48(8):1863–1872. doi: 10.1002/1097-0142(19811015)48:8<1863::aid-cncr2820480825>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 15.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252(3):426–432. doi: 10.1097/SLA.0b013e3181f08f32. discussion 432–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Perou CM, Sorlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 18.Nguyen PL, Taghian AG, Katz MS, et al. Breast Cancer Subtype Approximated by Estrogen Receptor, Progesterone Receptor, and HER-2 is Associated wtih Local and Distant Recurrence After Breast-Conserving Therapy. J Clin Oncol. 2008;14:2373–2378. doi: 10.1200/JCO.2007.14.4287. [DOI] [PubMed] [Google Scholar]
- 19.Voduc KD, Cheang MCU, Tyldesley S, et al. Breast Cancer Subtypes and the Risk of Local and Regional Relapse. J Clin Oncol. 2010;28(10):1684–1691. doi: 10.1200/JCO.2009.24.9284. [DOI] [PubMed] [Google Scholar]
- 20.Kyndi M, Sorensen FB, Knudsen H, et al. Estrogen receptor, progesterone receptor, HER-2, and response to postmastectomy radiotherapy in high-risk breast cancer: the Danish Breast Cancer Cooperative Group. J Clin Oncol. 2008;26:1419–1426. doi: 10.1200/JCO.2007.14.5565. [DOI] [PubMed] [Google Scholar]
- 21.Abdulkarim BS, Cuartero J, Hanson J, et al. Increased risk of locoregional recurrence for women with T1-2N0 triple-negative breast cancer treated with modified radical mastectomy without adjuvant radiation therapy compared with breast-conserving therapy. J Clin Oncol. 2011;29:2852–2858. doi: 10.1200/JCO.2010.33.4714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ho AY, Gupta G, King TA, et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer. 2012 doi: 10.1002/cncr.27480. epub. [DOI] [PubMed] [Google Scholar]
- 23.Rakha EA, Martin S, Lee AH, et al. The prognostic significance of lymphovascular invasion in invasive breast carcinoma. Cancer. 2011 doi: 10.1002/cncr.26711. Epub. [DOI] [PubMed] [Google Scholar]
- 24.Rakha EA, El-Sayed ME, Lee AH, et al. Prognostic significance of Nottingham histologic grade in invasive breast carcinoma. J Clin Oncol. 2008;26(19):3153–3158. doi: 10.1200/JCO.2007.15.5986. [DOI] [PubMed] [Google Scholar]
- 25.Rakha EA, Reis-Filho JS, Baehner F, et al. Breast cancer prognostic classification in the molecular era: the role of histological grade. Breast Cancer Res. 2010;12(4):207. doi: 10.1186/bcr2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wasif N, Ko CY, Farrell J, et al. Impact of tumor grade on prognosis in pancreatic cancer: should we include grade in AJCC staging? Ann Surg Oncol. 2010;17(9):2312–2320. doi: 10.1245/s10434-010-1071-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yi M, Mittendorf EA, Cormier JN, et al. Novel staging system for predicting disease-specific survival in patients with breast cancer treated with surgery as the first intervention: time to modify the current American Joint Committee on Cancer staging system. J Clin Oncol. 2011;29:4654–4661. doi: 10.1200/JCO.2011.38.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.EBCTCG. Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10801 women in 17 randomised trials. Lancet. 2011;378:1707–1716. doi: 10.1016/S0140-6736(11)61629-2. [DOI] [PMC free article] [PubMed] [Google Scholar]