ABSTRACT
Combinations of neuronal determinants and/or small-molecules such as Forskolin (Fk) can be used to convert different cell types into neurons. As Fk is known to activate cAMP-dependent pathways including CREB-activity, we aimed here to determine the role of CREB in reprogramming – including its temporal profile. We show that transient expression of the dominant-positive CREB-VP16 followed by its inactivation mediated by the dominant-negative ICER improves neuronal conversion of astrocytes mediated by the neurogenic determinant Ascl1. Contrarily, persistent over-activation by CREB-VP16 or persistent inhibition by ICER interferes with neuronal reprogramming, with the latter enhancing cell death. Taken together our work shows transient CREB activation as a key effector in neuronal reprogramming.
KEYWORDS: Bcl-2, CREB, direct reprogramming, Forskolin, ICER, immediate early genes, neurogenesis
Direct neuronal reprogramming from various cell sources has developed into a very active field since its beginning in 20021 using either neurogenic transcription factors or small molecules or a combination thereof (for recent review see ref. 2). Among the small molecules Forskolin (Fk) stands out as it was previously shown to facilitate reprogramming in different models of trans-differentiation in vitro,3,4,5 including direct neuronal reprogramming.6 Accordingly, Fk is also an invariable component of the neuronal reprogramming cocktails based on small molecules.7,8,9 We recently found that both Fk treatment and Bcl-2 expression exert a similar effect in increasing reprogramming efficiency mediated by single transcription factors and in reducing oxidative stress, a key limiting factor in direct neuronal reprogramming.10 In fact, they both potently enhance the rate of conversion and survival during neuronal reprogramming mediated by single transcription factors,10 regardless of the cell type of origin (astrocytes, fibroblasts; see refs. 6 and 10), species (human, mouse, gerbil; see refs. 6–10 and data not shown) and transcription factor (Ascl1, Neurog2, NeuroD4; see refs. 6, 10 and data not shown). While these results showed a similar potency of Fk and Bcl-2 in direct neuronal reprogramming, genome-wide expression analysis further highlighted shared down-stream effector pathways such as RXR/VDR and Nrf2,10 prompting the question of how these transcriptional effectors are activated.
Fk is well known to activate the cyclic AMP-response element-binding protein (CREB)11 which is induced by the protein kinase A (PKA) and, as expected, was also increased in the above described transcriptome of Fk effectors.10 Moreover, CREB has been related to cellular processes that are essential for the normal maintenance of brain function, such as memory formation,12,13 synaptic plasticity,14,15 adult neurogenesis,16,17,18 and neuronal differentiation of stem cells.19 Additionally, it has been described that CREB participates in the metabolic regulation of neurogenesis,20 a function that may facilitate the neuronal metabolic conversion during neuronal direct reprogramming.10 CREB also boosts cell survival,21,22 by activating transcription of genes such as Brain-Derived Neurotrophic Factor (BDNF)23 and Bcl-224 further supporting a possible link between the effects of Fk, CREB and Bcl-2 in reprogramming. Therefore we aimed here to determine the role of CREB-mediated transcription in neuronal reprogramming. Importantly, CREB is a crucial upstream regulator of the immediate early genes (IEG)25 and, hence, its activation occurs fast, transiently and does not require de novo protein synthesis. Hence, we modulated CREB activity permanently and transiently to address when and for how long CREB activity may be needed during reprogramming.
As a first step to analyze if and when CREB may mediate the effect of Fk in neuronal reprogramming we examined the dynamics of CREB phosphorylation in astrocytes transfected with Ascl1-IRES-DsRed plasmids and stained for P-CREB, β-III-tubulin and DsRed at different time points after Fk addition at one day post-transfection (DPT) (Fig. 1A, B). Phosphorylation of CREB was induced in most cells 1 h after addition of Fk, independent of whether they were immuno-reactive for DsRed (Ascl1-positive; Fig. 1A and B) or not (Ascl1-negative; Fig. 1A, upper panels). Interestingly, at yet later time points, 24 h to 72 h without Fk addition, P-CREB-immunoreactivity was higher in transfected DsRed+ cells, (Fig. 1A, left columns, and Fig. 1B) suggesting that Ascl1 potentiates or prolongs CREB phosphorylation. At 24 to 72 h of treatment with Fk, P-CREB-immunoreactivity was reduced in DsRed positive cells when compared with cultures lacking Fk(Fig. 1A, right columns, and Fig. 1B). These data indicate that exposition of astrocytes to Fk induces a short phosphorylation of CREB followed by a long dephosphorylation during reprogramming. Western blot analysis upon adding Fk for different periods of time to the astrocyte cultures showed that P-CREB and its homolog cAMP-responsive element modulator (CREM) were increased in cells treated with Fk for 30 min and 2 h, while longer periods of treatment (> 7h) decreased P-CREB/CREM below basal levels (Fig. 1C). In contrast, total levels of CREB protein were not affected by the treatment, ruling out effects via protein degradation or transcription (Fig. 1C). Interestingly, PKA activity also increases transiently as monitored by labeling for its targets with phosphorylation at Ser/Thr residues (peak at 2 hours after Fk; see Fig. 1C), but levels did not decline below baseline upon prolonged Fk exposure as observed for P-CREB (Fig. 1C). Hence, among PKA substrates only CREB phosphorylation was observed to be specifically reduced upon long periods of treatment with Fk (> 24 h). This interesting pattern of activity was similar in other cell types exposed to Fk for different lengths of time such as MEFs and neurosphere-derived cells (Supp. Figure 1) suggesting a feed-back mechanism inactivating CREB phosphorylation upon prolonged Fk exposure in many cell types. Although peculiar, this behavior is not surprising for a master regulator of IEGs.25
Figure 1.
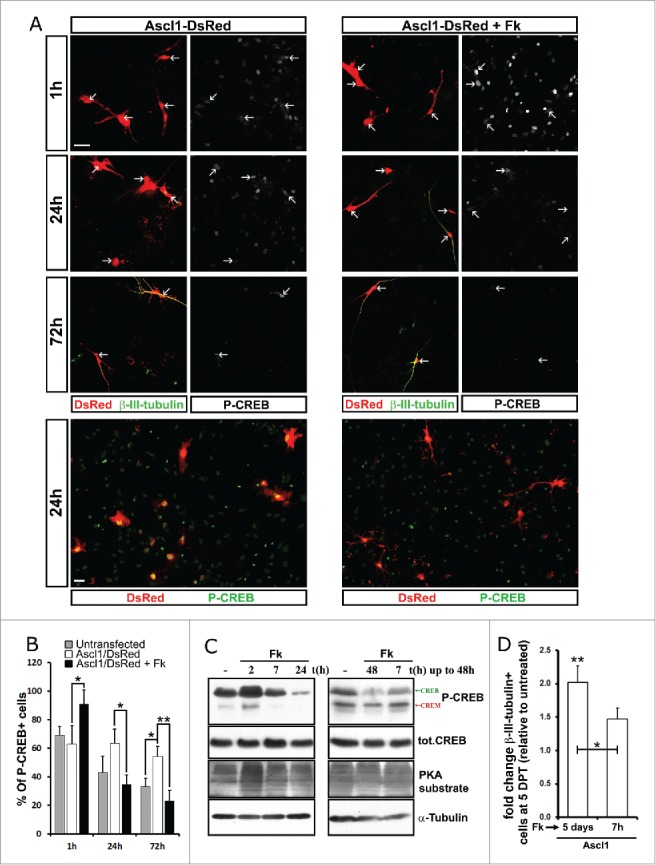
Fk induces a fast transient phosphorylation followed by a persistent de-phosphorylation of CREB. (A) Micrographs of astroglial cultures transfected with the Ascl1/DsRed-encoding vector and treated 24 h later with Fk (20 μM, right panels) or maintained untreated (left panels). High magnification pictures (upper panels) show the expression of DsRed (Red; white arrows depict transfected cells), β-III-tubulin (green) and P-CREB (see white in splitted channels) by immunostaining at different time points after Fk was supplemented. Low magnification fields in the lower panels show the expression of DsRed (Red) and P-CREB (green) at the 24 h time point. (B) Quantification of cells depicted in (A) shows the percentage of astrocytes immunoreactive for P-CREB among cells transfected with Ascl1 and maintained untreated (white bars) or treated with Fk (black bars) and untransfected cells maintained untreated (gray bars). (C) Radiographs show P-CREB, total CREB, PKA substrate and α-tubulin detected in un-transfected astroglia cultures by immunobloting of western blots with specific antibodies. On the left lysates from postnatal astroglia cultures incubated with Fk (20μM) for 2 h, 7 h, 24 h or maintained untreated. On the right cultures were maintained untreated or treated with Fk for the first 7 h or during the entire duration of the experiment (48 h). (D) Histogram shows fold increase of β-III-tubulin immunopositive neurons among Ascl1/DsRed transfected astroglial cells in vitro when 24 h later were treated with Fk only during the first 7 h or during the entire duration of the experiment (7 days). Scale bars represent 40 µm. Error bars indicate ± SD. *p<0.05, **p<0.01; ANOVA with Tukey's post hoc test.
If the hypothesis that CREB mediates the effect of Fk on neuronal reprogramming is correct, we have to consider 3 different options: a) The short activation of CREB is enough to enhance reprogramming b) Persistent reduction of CREB activity is beneficial for reprogramming rather than its activation c) Increase in reprogramming requires the dynamic behavior of transient short CREB phosphorylation followed by persistent dephosphorylation. To address the options a and b, astroglial cultures were transfected as before with Ascl1/DsRed encoding plasmids and 24 h later, the cultures were maintained untreated or treated with Fk for 7 h or for the entire duration of the experiment. The cells were fixed 48 h or 5 d later and the levels of P-CREB, CREB and PKA (see WB in the Fig. 1C, right panels) and reprogramming efficiency (Fig. 1D) was analyzed respectively. We observed that reduction of P-CREB levels required a persistent treatment with Fk (Fig. 1C, right panels). Moreover, while short treatment with Fk faintly increased reprogramming efficiency, only prolonged exposure to Fk resulted in doubling of neuronal reprogramming efficiencies (Fig. 1D). These data together suggest that the long-term Fk treatment and, hence, the long-term decrease in P-CREB levels may be beneficial for reprogramming.
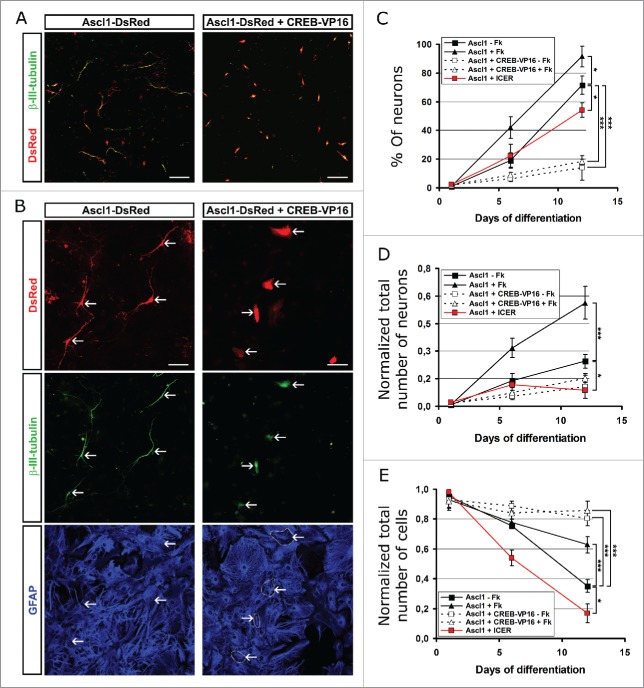
If the above hypothesis is correct, a maintained CREB-mediated transcription should interfere with reprogramming. To test this prediction, we used the dominant positive CREB-VP16 fusion protein, which constitutively activates transcription of cAMP response elements (CRE) through the transcriptional activation domain of the herpes virus VP16 protein.26 Astrocyte cultures were co-transfected with the Ascl1-DsRed and CREB-VP16 or control vector plasmids, treated or not with Fk and neuronal reprogramming was examined at 1, 6 or 12 DPT (Fig. 2). The beneficial effect of Fk was already visible in cells transduced only with Ascl1 at 6 DPT with 20% into β-III-tubulin+/GFAP- neurons without Fk and 40% with Fk (Fig. 2C). Neuronal conversion continued to increase in both conditions, but the cells cultured with Fk had generated significantly more neurons (over 90%) than those without (around 75%). In contrast, when Ascl1 and CREB-VP16 were co-transduced only 20% neurons were detectable at 12 DPT (Fig. 2C). Interestingly, cells co-transfected with CREB-VP16 were immunoreactive for β-III-tubulin but neither had neuronal morphology nor managed to turn off glial hallmarks such as GFAP (Fig. 2A, B). Thus, CREB-VP16 blocks astrocyte conversion to neurons. As expected, Fk addition or not made no difference any longer when cells were co-transduced with Ascl1 and CREB-VP16 (Fig. 2C, D). Conversely, CREB-VP16 co-transduction increased the total number of cells suggesting that it increases survival of the cells which do not successfully reprogram and normally die (Fig. 2E), thereby separating the effect of Fk treatment on reprogramming from effects on cell survival.
Figure 2.
Expression of constitutive activated CREB recombinant protein or the natural dominant negative ICER both reduce neuronal conversion of astrocytes. (A) Micrographs show expression of β-III-tubulin (green) in astroglial cultures transfected with the Ascl1/DsRed-encoding plasmid alone (red cells; left panel) or in combination with the CREB-VP16-encoding plasmid (red cells; right panel) at 6 DPT. Note that cells co-expressing Ascl1/DsRed and CREB-VP16 are immunopositive for β-III-tubulin, but do not acquire neuronal morphology. (1) Higher magnification of the above cells shows that cells transfected with the Ascl1/DsRed-encoding plasmid (arrows; left column) acquire neuronal morphology (red; upper panel), induced expression of β-III-tubulin (green; middle panel) and down-regulate the astrocytic hallmark GFAP (blue; lower panel). Co-expression of Ascl1/DsRed and CREB-VP16 (arrows; right column) induces expression of β-III-tubulin (green; middle panel) in astrocytes but the cells neither acquire neuronal processes (red; upper panel) nor switch off GFAP expression (blue; lower panel). (C-E) Graphs show cell fate outcome at different time points (6 and 12 DPT) of postnatal astroglial cultures transfected with plasmids encoding Ascl1-DsRed, Ascl1-DsRed+CREB-VP16 or Ascl1-DsRed+Icer, that were maintained untreated or incubated with Fk (20 μM) for the duration of the experiment. Quantifications show the percentage of neurons (β-III-tubulin+/GFAP-) among the total number of DsRed immunopositive cells (C) and the absolute number neurons (D) or total number of cells (E) immunoreactive for DsRed and normalized by the number of transfected cells at 1DPT. Scale bars represent 100 µm in (A) and 50 µm in (B). Error bars indicate ± SD. *p<0.05, **p<0.01, ***p<0.001; ANOVA with Tukey's post hoc test.
Since Fk induces P-CREB by increasing PKA activity, we next analyzed whether this enzyme may have a positive effect on reprogramming. We transfected astroglial cultures with the plasmid encoding Ascl1/DsRed, in combination with a second plasmid encoding either the catalytic subunit-α of PKA (PKAα) or a control plasmid. Constitutive expression of PKAα also dramatically reduced reprogramming in astroglial cells. When cells were examined at 7 DPT we observed a 20x reduction in the induced neurons, from 44% neurons among the Ascl1-only transduced cells to less than 2% among Ascl1 and PKA transduced cells (Supp. Figure 2). Thus, similarly to the effect of CREB-VP16, PKA co-transduction also resulted in a reduction of reprogrammed neurons.
Considering these results, we next assessed whether a reduction of CREB-mediated transcription has the opposite effect and enhances reprogramming. To test this, we co-transfected astroglial cells with vectors encoding Ascl1/DsRed and the inducible cAMP early repressor (ICER), which is a natural dominant negative form of CREB.27 Interestingly, ICER co-expression dramatically reduced survival of cells during reprogramming with only 20% left after 12 DPT (Fig. 2E). While the proportion of induced neurons was lower than the control transfected with Ascl1 only at 12 DPT (∼55% +ICER; ∼75% -ICER; Fig. 2C), but still higher than upon Ascl1 and CREB-VP16 co-transduction (around 15%). However, when considering the death of cells and quantifying the total number of neurons this was lowest for cells co-transduced with Ascl1 and ICER at 12 DPT (Fig. 2D). Taken together, these data indicate that constant levels of increased CREB-mediated transcription increases cell survival while constant block of CREB by ICER decreases survival. Conversion into neurons, however was worse than the control for both cases as well as PKA co-transduction. These results thus suggest that the dynamic regulation with first increased and later decreased levels of P-CREB (see option c above) may be the key to improve the efficiency of neuronal conversion of astrocytes.
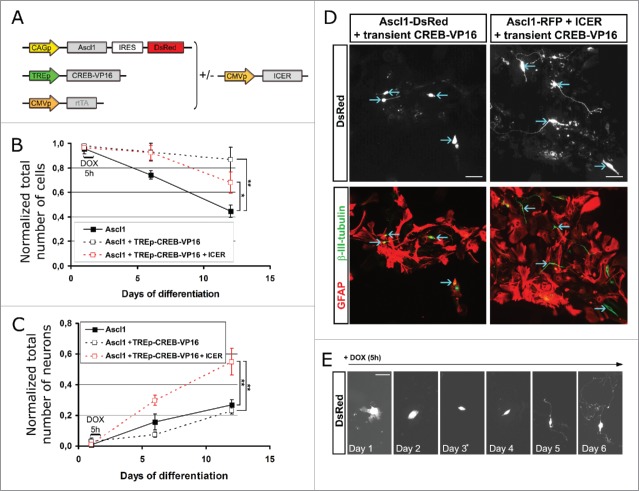
To test this hypothesis we designed a vector, TREp-CREB-VP16 (Fig. 3A), based on the Tet-On system in which the cDNA encoding CREB-VP16 protein is driven by the tetracycline response element (TRE), that induces transcription of CREB-VP16 only in presence of doxycycline (DOX) and the reverse tetracycline-controlled transactivator (rtTA). Astroglial cells were co-transfected with the plasmids encoding Ascl1/DsRed and rtTA alone or in combination with the vector TREp-CREB-VP16. Twenty-four hours after transfection, the expression of CREB-VP16 was briefly induced by addition of DOX in the differentiation medium during a period of 5 h and the cells were maintained without DOX until the end of the experiment. In a separate series of experiments, the plasmid encoding ICER was also included in the transfection together with TREp-CREB-VP16 and Ascl1/DsRed and rtTA coding plasmids (ratio 3:1 respectively for the CREB-VP16:ICER encoding plasmids) to accelerate the reduction of CREB-VP16 activity after treatment with DOX (Fig. 3A). We observed that transient expression of CREB-VP16 significantly increases cell viability at 6 as well as 12 DPT (Fig. 3B). However, most of the cells were not successfully reprogrammed into neurons (Fig. 3C) as they were immuno-positive for both, β-III-tubulin and GFAP and did not acquire neuronal morphology (Fig. 3D, left panels). On the contrary, cells co-transfected with the vector TREp-CREB-VP16 and the plasmid encoding ICER successfully reduced GFAP expression (Fig. 3D, right panels) and acquired neuronal morphology along of the time in culture (Fig. 3E). Indeed, transient expression of CREB-VP16 in combination with stable expression of ICER increased both, cell survival (1,7 ± 0.12 x compared with Ascl1 only expressing cells at 6 d after transfection; Fig. 3B) and total number of neurons (1,87 ± 0.2 and 2,12 ± 0.15 x compared with Ascl1 expressing cells respectively at 6 and 12 DPT; Fig. 3C). Thus, the efficient and fast shut off of P-CREB is essential for reprogramming, while initial activation is essential for survival. These results thus clearly demonstrate that a short “on-off” switch of CREB activity improves Ascl1-mediated conversion of postnatal astroglial cells.
Figure 3.
Transient activation of CREB allows to increase conversion of postnatal astrocytes into iNs. (A) Scheme of the vectors used to transiently induce transcription mediated by CREB: the expression of CREB-VP16 is driven by the inducible promoter TRE which is transcriptionally active only in the presence of the coactivator rtTA and DOX. (B, C) Graphs show the outcome of cells co-transfected with the indicated combination of plasmids 20–30 h after plating. At 1 DPT, cultures were incubated with DOX for a period of time of 5 h, and then left untreated for 6 or 12 d in culture. Quantifications show the percentage of transfected cells (DsRed+) classified as neurons (β-III-tubulin+/GFAP-) normalized to the number of transfected cells at 1 DPT (B) and the absolute number of cells immunoreactive for DsRed also normalized to the number of transfected cells at 1 DPT (C). (D) Micrographs show cells immunoreactive for DsRed (upper panels; blue arrows) which were transfected with the combination of plasmids Ascl1-DsRed/transient CREB-VP16 (left column) or Ascl1-DsRed/transient CREB-VP16/Icer (right column). The neuronal outcome was analyzed by immunostaining (lower panels) for β-III-tubulin (green) and GFAP (red). Note that only cells co-expressing Icer upregulated β-III-tubulin, switched off GFAP expression and exhibited a complex neuronal morphology. (E), Still images from video-time lapse show a cell transfected (DsRed expression in white) with Ascl1-DsRed, transient CREB-VP16 and Icer acquiring neuronal morphology along of 7 d in culture. Scale bars represent 50 µm. Error bars indicate ± SD. *p<0.05, **p<0.01; ANOVA with Tukey's post hoc test.
As mentioned above, Bcl-2 is a known direct target of CREB. Since the expression of Bcl-2 enhances survival and conversion efficiency during neuronal reprogramming similarly as Fk does,10 we hypothesized that Fk treatment and CREB-transient activity may partially mediate its beneficial effect in reprogramming by rising endogenous levels of Bcl-2. Indeed, treatment of astrocytes and fibroblasts with Fk caused the progressive accumulation of Bcl-2 protein, as detected by Western Blot (Supp. Figure 3A, B). Moreover, postnatal astrocytes co-transfected with Ascl1/DsRed and CREB-VP16 encoding plasmids exhibited a higher immunoreactivity for Bcl-2 compared with only Ascl1/DsRed transfected controls (Supp. Figure 3C), demonstrating that CREB-activity increases Bcl-2 protein levels during reprogramming. These results strongly suggest that the effect of Fk and CREB activation in reprogramming may be mediated by Bcl-2, at least, partially.
In summary, our work provides direct evidence that transcription mediated by CREB is beneficial for direct neuronal reprogramming. However, this pathway must be tightly regulated to allow an optimal efficient conversion. We demonstrated here that CREB-mediated transcription always correlates to an increase of cells surviving to reprogramming, however, either persistent blockage or persistent boost of CREB activity result in a reduction in the number of neurons derived from astrocytes. Previous results in mouse models of adult neurogenesis in the hippocampus also highlighted the importance of a precise control of CREB activity for the expansion and differentiation of neuronal progenitors. Thus, a potent reduction of CREB activity mediated by a dominant-negative form of CREB exhibited reduced proliferation of neuronal progenitors in the adult hippocampus16 while a mild reduction in CREB deficient mice (CREBαΔ) that preserve the homologous gene CREM showed increased proliferation and concomitant neuronal differentiation of progenitors.28 These results indicate that CREB-mediated transcription can also lead to opposite effects on neuronal precursors in vivo and, therefore, suggest that its activation has to be precisely controlled during natural neurogenesis as well. Moreover, the dramatic effect of CREB-VP16 impeding the acquisition of neuronal features in our model of astrocyte-to-neuron conversion is not completely unexpected considering that CREB can activate a plethora of non-neuronal genes29 that may interfere with a successful neurogenesis program. Therefore, all together, previous data in vivo, the results of this study and the nature of CREB as fast transient effector regulating IEG pathways suggest that short temporal activation of CREB is most likely the correct road to improve neurogenesis in both natural differentiation and forced reprogramming paradigms.
As mentioned above, besides the effect of CREB-transcription on neuronal reprogramming, CREB protein targets a vast number of genes that are not necessarily linked to a neuronal identity (∼1350 genes in mouse and ∼1663 in the human; see ref. 29). Consequently, Fk also activates molecular cascades that are involved in many different cellular contexts.10 These data suggest that CREB and Fk may also play roles in other non-neuronal reprogramming paradigms. Supporting this, it has been shown that Fk treatment allows trans-differentiation of bone marrow-derived neurons into epithelial cells.4 Therefore, it will be interesting to explore the extent to which Fk and transient activation of CREB may contribute to direct reprogramming of somatic cells into non-neuronal lineages such as, for example, cardiomyocytes,30 endocrine pancreatic β cells31 and glial cells.32 Finally, the results of our previous study showing a beneficial of Bcl-2 in direct neuronal reprogramming10 as well as the results of this study confirming that both Fk treatment and CREB-mediated transcription induce Bcl-2 protein levels, strongly suggest that Bcl-2 may be a crucial mediator of the effect of Fk in cell fate re-specification of different lineages by molecular cues that are not completely elucidated yet.
Methods and materials
Cell cultures of astroglia from the postnatal mouse cerebral cortex
Astrocytes were cultured as described in Heins et al. (2002). After removal of the meninges, gray matter tissue was dissected from P5-P7 cerebral cortex of C57BL/6J mice and dissociated mechanically. Subsequently, cells were centrifuged for 5 min at 1000 rpm, re-suspended, and plated in medium consisting of DMEM/F12 (Gibco), 3.5 mM glucose (Sigma), 10% fetal calf serum (Gibco), 5% horse serum (Gibco), penicillin/streptomycin (Gibco) and supplemented with B27 (Gibco), 10 ng/mL epidermal growth factor (EGF, Roche) and 10 ng/mL fibroblast growth factor 2 (FGF2, Roche). After one week expansion cells were harvested using trypsin/EDTA (Gibco) and plated onto poly-D-lysine (Sigma-Aldrich) coated glass coverslips in 24-well plates (BD Biosciences) or directly onto plastic for time lapse experiments at a density of 60,000 cells per well in the same medium.
Transfection of mouse postnatal astroglia cultures
Cells were transfected always 24 h after plating. For transfection DNA-liposome complexes were prepared in Optimem medium (Invitrogen) using the retroviral plasmids described below and Lipofectamine 2000 (Invitrogen). Astrocyte cultures were exposed to DNA-liposome complexes at a concentration of 0.5 µg DNA per 400 µl of Optimem medium for 4 hours and cultured after that in the differentiation medium as above. (See ref. 33). For survival analysis of transfected cells after immunostaining we counted the total number of DsRed+ cells at 5 DPT and normalized by the total number of DsRed+ cells counted at day 1. To quantify the ratio of neurons/initial transfected cells the proportion of DsRed+/β-III-tubulin+ cells was assessed at 5 DPT and normalized to the population of DsRed+ cells at 1 DPT.
Cell cultures of mouse embryonic fibroblasts (MEFs)
MEFs were obtained from mouse embryos at day 14 gestation. Head, spine and visceras were removed and discarded.34 The remaining tissue was dissociated with 0.15% of trypsin, centrifuged for 5 min at 1300 rpm, re-suspended, and plated in a medium consisting of DMEM high glucose (3.5mM) with GlutaMAX (Gibco), 10% fetal calf serum (Gibco) and penicillin/streptomycin (Gibco) in 5% CO2 and normoxygenated conditions. Fibroblasts were used for experiments after minimum 3 passages. For reprogramming experiments cells were harvested and replated as described above for astrocytes.
Neurosphere cultures
For culturing neurosphere cells from postnatal cerebral cortex we followed the protocol described by Johansson et al. (1999). After 5–7 d in culture, neurosphere cells were enzymatically dissociated with trypsin (Invitrogen) and plated on poly-D-lysine (Sigma-Aldrich) coated coverslips at a density of 10 × 104 cells per coverslip (in 24-well plates, BD Biosciences) in a medium consisting of DMEM/F12 supplemented with B27, EGF, FGF2, penicillin/streptomycin, and buffered with HEPES (i.e. neurosphere medium).
Supplementary Material
Disclosure of potential conflicts of interest
No potential conflicts of interest were disclosed.
Acknowledgments
We would like to thank Prof. Dr. David D. Ginty for kindly providing the CREB-VP16 encoding plasmid and Tatjana Ebert and Carmen Meyer for excellent technical assistance.
Funding
This work was supported by the SFB 870, SPP 1751 and the advanced ERC grant ChroNeuroRepair.
References
- [1].Heins N, Malatesta P, Cecconi F, Nakafuku M, Tucker KL, Hack MA, Chapouton P, Barde YA, Götz M. Glial cells generate neurons: the role of the transcription factor Pax6. Nature neuroscience 2002; 5(4):308-315; PMID: 11896398; http://dx.doi.org/ 10.1038/nn828 [DOI] [PubMed] [Google Scholar]
- [2].Masserdotti G, Gascon S, Gotz M. Direct neuronal reprogramming: learning from and for development. Development 2016; 143(14):2494-2510; PMID: 27436039; http://dx.doi.org/ 10.1242/dev.092163 [DOI] [PubMed] [Google Scholar]
- [3].Jang S, Cho HH, Cho YB, Park JS, Jeong HS. Functional neural differentiation of human adipose tissue-derived stem cells using bFGF and forskolin. BMC cell biology 2010; 11:25; PMID: 20398362; http://dx.doi.org/ 10.1186/1471-2121-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Liu Y, Jiang X, Yu MK, Dong J, Zhang X, Tsang LL, Chung YW, Li T, Chan HC. Switching from bone marrow-derived neurons to epithelial cells through dedifferentiation and translineage redifferentiation. Cell biology international 2010; 34(11):1075-1083; PMID: 20939829; http://dx.doi.org/ 10.1042/CBI20100516 [DOI] [PubMed] [Google Scholar]
- [5].Onorati M, Camnasio S, Binetti M, Jung CB, Moretti A, Cattaneo E. Neuropotent self-renewing neural stem (NS) cells derived from mouse induced pluripotent stem (iPS) cells. Molecular and cellular neurosciences 2010; 43(3):287-295; PMID: 20026276; http://dx.doi.org/ 10.1016/j.mcn.2009.12.002 [DOI] [PubMed] [Google Scholar]
- [6].Liu ML, Zang T, Zou Y, Chang JC, Gibson JR, Huber KM, Zhang CL. Small molecules enable neurogenin 2 to efficiently convert human fibroblasts into cholinergic neurons. Nature communications 2013; 4:2183; PMID: 2387330626253202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Hu W, Qiu B, Guan W, Wang Q, Wang M, Li W, Gao L, Shen L, Huang Y, Xie G, et al.. Direct Conversion of Normal and Alzheimer's Disease Human Fibroblasts into Neuronal Cells by Small Molecules. Cell stem cell 2015; 17(2):204-212; PMID:26253202;http://dx.doi.org/ 10.1016/j.stem.2015.07.006 [DOI] [PubMed] [Google Scholar]
- [8].Li X, Zuo X, Jing J, Ma Y, Wang J, Liu D, Zhu J, Du X, Xiong L, Du Y et al.. Small-Molecule-Driven Direct Reprogramming of Mouse Fibroblasts into Functional Neurons. Cell stem cell 2015; 17(2):195-203; PMID: 26253201; http://dx.doi.org/ 10.1016/j.stem.2015.06.003 [DOI] [PubMed] [Google Scholar]
- [9].Zhang L, Yin JC, Yeh H, Ma NX, Lee G, Chen XA, Wang Y, Lin L, Chen L, Jin P, et al.. Small Molecules Efficiently Reprogram Human Astroglial Cells into Functional Neurons. Cell stem cell 2015; 17(6):735-747; PMID: 26481520; http://dx.doi.org/ 10.1016/j.stem.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Gascon S, Murenu E, Masserdotti G, Ortega F, Russo GL, Petrik D, Deshpande A, Heinrich C, Karow M, Robertson SP, et al.. Identification and Successful Negotiation of a Metabolic Checkpoint in Direct Neuronal Reprogramming. Cell stem cell 2015; 18(3):396-409. [DOI] [PubMed] [Google Scholar]
- [11].Montminy MR, Bilezikjian LM. Binding of a nuclear protein to the cyclic-AMP response element of the somatostatin gene. Nature 1987; 328(6126):175-178; PMID: 2885756; http://dx.doi.org/ 10.1038/328175a0 [DOI] [PubMed] [Google Scholar]
- [12].Yin JC, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell 1994; 79(1):49-58; PMID: 7923376; http://dx.doi.org/ 10.1016/0092-8674(94)90399-9 [DOI] [PubMed] [Google Scholar]
- [13].Balschun D, Wolfer DP, Gass P, Mantamadiotis T, Welzl H, Schutz G, Frey JU, Lipp HP. Does cAMP response element-binding protein have a pivotal role in hippocampal synaptic plasticity and hippocampus-dependent memory? The Journal of neuroscience : the official journal of the Society for Neuroscience 2003; 23(15):6304-6314; PMID: 12867515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jagasia R, Steib K, Englberger E, Herold S, Faus-Kessler T, Saxe M, Gage FH, Song H, Lie DC. GABA-cAMP response element-binding protein signaling regulates maturation and survival of newly generated neurons in the adult hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009; 29(25):7966-7977; PMID: 19553437; http://dx.doi.org/ 10.1523/JNEUROSCI.1054-09.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Rudiger R, Binder E, Tsarovina K, Schmidt M, Reiff T, Stubbusch J, Rohrer H. In vivo role for CREB signaling in the noradrenergic differentiation of sympathetic neurons. Molecular and cellular neurosciences 2009; 42(2):142-151; PMID: 19545628; http://dx.doi.org/ 10.1016/j.mcn.2009.06.007 [DOI] [PubMed] [Google Scholar]
- [16].Nakagawa S, Kim JE, Lee R, Malberg JE, Chen J, Steffen C, et al.. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. The Journal of neuroscience : the official journal of the Society for Neuroscience 2002; 22(9):3673-3682; PMID: 11978843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Nakagawa S, Kim JE, Lee R, Chen J, Fujioka T, Malberg J, Tsuji S, Duman RS. Localization of phosphorylated cAMP response element-binding protein in immature neurons of adult hippocampus. The Journal of neuroscience : the official journal of the Society for Neuroscience 2002; 22(22):9868-9876; PMID: 12427843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Giachino C, De Marchis S, Giampietro C, Parlato R, Perroteau I, Schutz G, Fasolo A, Peretto P. cAMP response element-binding protein regulates differentiation and survival of newborn neurons in the olfactory bulb. The Journal of neuroscience : the official journal of the Society for Neuroscience 2005; 25(44):10105-10118; PMID: 16267218; http://dx.doi.org/ 10.1523/JNEUROSCI.3512-05.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Shan ZY, Shen JL, Li QM, Wang Y, Huang XY, Guo TY, Liu HW, Lei L, Jin LH. pCREB is involved in neural induction of mouse embryonic stem cells by RA. Anatomical record 2008; 291(5):519-526; http://dx.doi.org/ 10.1002/ar.20686 [DOI] [PubMed] [Google Scholar]
- [20].Fusco S, Leone L, Barbati SA, Samengo D, Piacentini R, Maulucci G, Toietta G, Spinelli M, McBurney M, Pani G, et al.. A CREB-Sirt1-Hes1 Circuitry Mediates Neural Stem Cell Response to Glucose Availability. Cell reports 2016; 14(5):1195-1205; PMID: 26804914; http://dx.doi.org/ 10.1016/j.celrep.2015.12.092 [DOI] [PubMed] [Google Scholar]
- [21].Lonze BE, Riccio A, Cohen S, Ginty DD. Apoptosis, axonal growth defects, and degeneration of peripheral neurons in mice lacking CREB. Neuron 2002; 34(3):371-385; PMID: 11988169; http://dx.doi.org/ 10.1016/S0896-6273(02)00686-4 [DOI] [PubMed] [Google Scholar]
- [22].Mantamadiotis T, Lemberger T, Bleckmann SC, Kern H, Kretz O, Martin Villalba A, Tronche F, Kellendonk C, Gau D, Kapfhammer J, et al.. Disruption of CREB function in brain leads to neurodegeneration. Nature genetics 2002; 31(1):47-54; PMID: 11967539; http://dx.doi.org/ 10.1038/ng882 [DOI] [PubMed] [Google Scholar]
- [23].Tao X, Finkbeiner S, Arnold DB, Shaywitz AJ, Greenberg ME. Ca2+ influx regulates BDNF transcription by a CREB family transcription factor-dependent mechanism. Neuron 1998; 20(4):709-726; PMID: 9581763; http://dx.doi.org/ 10.1016/S0896-6273(00)81010-7 [DOI] [PubMed] [Google Scholar]
- [24].Wilson BE, Mochon E, Boxer LM. Induction of bcl-2 expression by phosphorylated CREB proteins during B-cell activation and rescue from apoptosis. Molecular and cellular biology 1996; 16(10):5546-5556; PMID: 8816467; http://dx.doi.org/ 10.1128/MCB.16.10.5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Tullai JW, Schaffer ME, Mullenbrock S, Sholder G, Kasif S, Cooper GM. Immediate-early and delayed primary response genes are distinct in function and genomic architecture. The Journal of biological chemistry 2007; 282(33):23981-23995; PMID: 17575275; http://dx.doi.org/ 10.1074/jbc.M702044200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Riccio A, Ahn S, Davenport CM, Blendy JA, Ginty DD. Mediation by a CREB family transcription factor of NGF-dependent survival of sympathetic neurons. Science 1999; 286(5448):2358-2361; PMID: 10600750; http://dx.doi.org/ 10.1126/science.286.5448.2358 [DOI] [PubMed] [Google Scholar]
- [27].Mioduszewska B, Jaworski J, Kaczmarek L. Inducible cAMP early repressor (ICER) in the nervous system–a transcriptional regulator of neuronal plasticity and programmed cell death. Journal of neurochemistry 2003; 87(6):1313-1320; PMID: 14713288; http://dx.doi.org/ 10.1046/j.1471-4159.2003.02116.x [DOI] [PubMed] [Google Scholar]
- [28].Gur TL, Conti AC, Holden J, Bechtholt AJ, Hill TE, Lucki I, Malberg JE, Blendy JA. cAMP response element-binding protein deficiency allows for increased neurogenesis and a rapid onset of antidepressant response. The Journal of neuroscience: the official journal of the Society for Neuroscience 2007; 27(29):7860-7868; PMID: 17634380; http://dx.doi.org/ 10.1523/JNEUROSCI.2051-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Conkright MD, Guzman E, Flechner L, Su AI, Hogenesch JB, Montminy M. Genome-wide analysis of CREB target genes reveals a core promoter requirement for cAMP responsiveness. Molecular cell 2003; 11(4):1101-1108; PMID: 12718894; http://dx.doi.org/ 10.1016/S1097-2765(03)00134-5 [DOI] [PubMed] [Google Scholar]
- [30].Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell 2010; 142(3):375-386; PMID: 20691899; http://dx.doi.org/ 10.1016/j.cell.2010.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature 2008; 455(7213):627-632; PMID: 18754011; http://dx.doi.org/ 10.1038/nature07314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Caiazzo M, Giannelli S, Valente P, Lignani G, Carissimo A, Sessa A, Colasante G, Bartolomeo R, Massimino L, Ferroni S, et al.. Direct conversion of fibroblasts into functional astrocytes by defined transcription factors. Stem cell reports 2015; 4(1):25-36; PMID: 25556566; http://dx.doi.org/ 10.1016/j.stemcr.2014.12.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Heinrich C, Gascon S, Masserdotti G, Lepier A, Sanchez R, Simon-Ebert T, Schroeder T, Götz M, Berninger B. Generation of subtype-specific neurons from postnatal astroglia of the mouse cerebral cortex. Nature protocols 2011; 6(2):214-228; PMID: 21293461; http://dx.doi.org/ 10.1038/nprot.2010.188 [DOI] [PubMed] [Google Scholar]
- [34].Vierbuchen T, Ostermeier A, Pang ZP, Kokubu Y, Sudhof TC, Wernig M. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010; 463(7284):1035-1041; PMID: 20107439; http://dx.doi.org/ 10.1038/nature08797 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.