Abstract
Background
In nephrotic syndrome, damage to the podocytes of the kidney produces severe hypercholesterolemia for which novel treatments are urgently needed. Proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged as an important regulator of plasma cholesterol levels and therapeutic target. Here, we tested the role of PCSK9 in mediating the hypercholesterolemia of nephrotic syndrome.
Methods and Results
Both nephrotoxic serum (NTS) treatment, which induces immune-mediated damage of the podocyte, and genetic ablation of the podocyte produced hypercholesterolemia and a 7- to 24-fold induction in plasma PCSK9 in mice. Conversely, patients with nephrotic syndrome showed a decrease in plasma cholesterol and plasma PCSK9 upon remission of their disease (p<0.05, n=47-50). The induction of plasma PCSK9 in nephrotic syndrome appeared to be due to increased secretion of PCSK9 from the hepatocyte coupled with decreased clearance. Interestingly, knockout of Pcsk9 ameliorated the effects of NTS on plasma lipids. Thus, in the presence of NTS, mice lacking hepatic Pcsk9 showed a 40% to 50% decrease in plasma cholesterol and triglycerides. Moreover, the ability of NTS treatment to increase the proportion of LDL-associated cholesterol (from 9% in vehicle-treated Flox mice to 47% after NTS treatment), was lost in mice with hepatic deletion of Pcsk9 (5% in both the presence and absence of NTS).
Conclusions
Podocyte damage triggers marked inductions in plasma PCSK9, and knockout of Pcsk9 ameliorates dyslipidemia in a mouse model of nephrotic syndrome. These data suggest that PCSK9 inhibitors may be beneficial in patients with nephrotic syndrome-associated hypercholesterolemia.
Keywords: PCSK9, cholesterol, kidney, hypercholesterolemia, nephrotic syndrome, podocyte
Introduction
The association between podocyte dysfunction and hypercholesterolemia can be traced back to 1830, when it was recognized that the plasma of nephrotic patients was so hyperlipidemic as to appear milky.1 However, even today, definitive treatments for nephrotic syndrome-associated hypercholesterolemia are lacking2 and nephrotic patients suffer a six-fold increased risk of cardiovascular disease.3
The hyperlipidemia of nephrotic syndrome is complex, involving multiple mechanisms and tissues, and evolves over the course of the disease. The increase in cholesterol is due to both the increased production and decreased clearance of lipoprotein particles, particularly the ApoB-containing particles (VLDL, IDL and LDL).4-9 Hepatic LDL receptor (LDLR), which removes ApoB-containing particles from the plasma, is reduced at the protein, but not mRNA, level.10-12
Proprotein convertase subtilisin/kexin type 9 (PCSK9) has emerged over the past decade as a post-transcriptional regulator of the LDL receptor, and several studies have suggested an association between PCSK9 and renal function.12-17 Circulating PCSK9 binds LDL receptors on the surface of the hepatocyte, causing the receptor to be internalized and degraded in the lysosome. Accordingly, patients with gain of function mutations in PCSK9 have increased LDL cholesterol, patients with loss of function mutations have decreased LDL cholesterol,18 and PCSK9 inhibitors are effective in reducing LDL cholesterol.19 The goal of this study was to determine the contribution of PCSK9 to nephrotic syndrome-associated hypercholesterolemia. We found that mice in the acute phase of nephrotic syndrome showed an increase in both plasma PCSK9 and plasma cholesterol, particularly ApoB-associated cholesterol; moreover, knockout of Pcsk9 lowered plasma cholesterol and produced a more benign lipoprotein profile.
Methods
Further information can be found in Supplemental Methods.
Human samples
We identified 50 patients who were enrolled in the Nephrotic Syndrome Study Network (NEPTUNE)20 as of October 2013 and had at least one plasma sample from the initial study visit during active disease (defined as urine protein/creatinine ratio ≥ 1) as well as from first follow-up study visit during which remission was achieved (defined as urine protein/creatinine ratio < 0.5). 38% of the subjects were on immunosuppression therapy at baseline. Additionally, 24% of patients were on medications potentially affecting plasma PCSK9 levels, such as statin drugs, at some point in the study; however, exclusion of these patients did not alter our results (see Supplemental Methods for additional information). Plasma PCSK9 concentrations were measured via ELISA (CycLex CY-8079).
Mice
C57BL/6J mice were obtained from the Jackson Laboratory. Pcsk9flox/flox mice on a C57BL/6N background, which contain loxP sites in the introns between exons one & two, and exons three & four, were a generous gift from Merck & Co., and were crossed to albumin-Cre mice on a C57BL/6N background to generate mice with liver-specific knockout of Pcsk9. Pod-ATTAC mice have been previously described.21 Pcsk9-/- mice and their approximate B6129SF2/J controls were also purchased from the Jackson Laboratory and intercrossed for at least four generations.
Nephrotic models
Nephrotoxic serum was used as previously described.22 Five- to eight- week-old male mice were preimmunized with 100 μL 1:1 sheep IgG:Complete Freund's Adjuvant s.c., then injected with either 50 μL nephrotoxic serum (NTS) or normal sheep serum (Vehicle) retro-orbitally daily for three days. Mice were sacrificed in the non-fasted state three to four days after the first NTS or Vehicle injection. Pod-ATTAC mice and their controls were injected with 0.5 μg/g BW of AP20187 (MedChemExpress or Clontech), and sacrificed seven days after injection in the non-fasted state.
Plasma lipid analyses
Total plasma triglycerides and cholesterol were measured via colorimetric assays (Infinity Triglycerides and Cholesterol, Thermo). Size exclusion liquid chromatography was performed by the Lipid, Lipoprotein and Atherosclerosis Analysis Core Laboratory at Wake Forest University. Briefly, equal volumes of plasma collected at the time of sacrifice from 4-9 mice per group were pooled. The cholesterol content of the pooled plasma was measured and approximately 15 μg of total cholesterol was diluted to a final volume of 120 μL using phosphate-buffered saline containing 0.01% EDTA, and 0.01% sodium azide and loaded onto a Superose 6 10/300 GL column powered by a LaChrom Elite HPLC system (Hitachi High Technologies) with online mixing of the column effluent and cholesterol enzymatic reagent (Cholesterol Liquid Reagent Set, Pointe Scientific, Inc.). The relative cholesterol concentration, measured as the OD500nm and converted to an electrical signal (Response, measured in millivolts), was continuously monitored; raw traces of the Response are shown in the Supplemental Material. The area corresponding to each lipoprotein (VLDL, LDL, HDL and little HDL) was measured and used to calculate the percentage of the total cholesterol contained in each lipoprotein fraction. In the main text, the data are presented as pie charts, with HDL representing the sum of HDL and little HDL. Little HDL made up 3%, 1%, 2%, and 4% of cholesterol in plasma from vehicle-treated B6 mice, vehicle-treated WT mice, vehicle-treated PCSK9-KO mice, and vehicle-treated Flox mice, respectively, and was not present in other samples.
Urine analysis
Spot urine was collected the morning of sacrifice, and 2 μL was subjected to SDS-PAGE and Coomassie staining.
PCSK9 clearance assay
Pod-ATTAC and Control mice were injected retro-orbitally with 0.4 μg human recombinant PCSK9 seven days after AP20187 injection. Plasma samples were collected 1 to 121 minutes after PCSK9 injection. For each mouse, plasma concentrations of human recombinant PCSK9 were expressed as percentage of the t = 1 minute concentration, and were plotted against time. The data from each mouse were fitted to a one-phase exponential decay curve using GraphPad Prism, such that N(t)=(N0 – Plateau)e-λt + Plateau, and the half-life (t1/2) was calculated by the formula t1/2 = ln(2)/λ.
Gene Expression
Gene expression was measured by real-time PCR and normalized to Tbp.
Protein analysis
Protein levels in liver and plasma were measured by western blotting or ELISA. The Sec24a antibody used for western blotting in this study was a kind gift from Dr. David Ginsburg;23 all other antibodies were purchased from commercial sources. Mouse (CycLex CY-8078) and recombinant human PCSK9 (R&D Systems DPC900) concentrations in plasma were measured via ELISA.
Statistical Analysis
Human studies: Paired two-tailed t-tests and Wilcoxon signed-rank tests were used to assess changes from baseline to remission. Spearman correlations were used to assess correlations at baseline and correlations of changes from baseline to remission. All tests were performed at two-sided alpha-level of 0.05. IBM SPSS (version 21.0) software was used for all analyses. Data in the text are presented as mean ± St.Dev.
Mouse studies: Differences between groups were assessed by a two-tailed Mann-Whitney test at a significance level of p < 0.05; similar results were obtained using a two-tailed unequal variance Student's t-test at a significance level of p < 0.05. No adjustment was made for multiple comparisons. These tests were performed using either Microsoft Excel 2010 or GraphPad Prism 6. Data are presented as mean and S.E.M. in text and graphs (bars and error bars, respectively).
Study approval
The human studies protocol was approved by the Institutional Review Board at each patient recruiting site and at Boston Children's Hospital; all subjects gave informed consent. All mouse studies were approved by the Institutional Animal Care and Research Advisory Committee at Boston Children's Hospital.
Results
We measured plasma PCSK9 concentrations in 50 subjects (30% membranous nephropathy, 28% minimal change disease, 10% focal segmental glomerulosclerosis or 11% other, Table S1) enrolled in the Nephrotic Syndrome Study Network (NEPTUNE) at two time points: during active disease (defined as urine protein/creatinine ratio (UPCR) > 1 mg/mg) and upon remission (UPCR < 0.5 mg/mg). At baseline, patients were proteinuric, hypertriglyceridemic and hypercholesterolemic. The UPCR was significantly correlated with total cholesterol (rs = 0.496), LDL cholesterol (rs = 0.398) and HDL cholesterol (rs = 0.321, Table).
Table. Plasma PCSK9 in nephrotic syndrome.
| Clinical Variables | Baseline* | Remission* | P-value† |
|---|---|---|---|
| Urine protein/creatinine ratio (mg/mg) | 5.2 (4.9) | 0.14 (0.10) | <0.0001 |
| Total cholesterol (mg/dL) | 298.0 (107.8) | 197.5 (60.2) | <0.0001 |
| LDL cholesterol (mg/dL) | 178.2 (91.1) | 101.0 (44.7) | <0.0001 |
| HDL cholesterol (mg/dL) | 81.6 (28.1) | 69.2 (25.2) | 0.002 |
| Triglycerides (mg/dL) | 191.1 (109.1) | 136.2 (73.3) | 0.001 |
| Correlations between UPCR and lipid parameters at baseline | |||
|
| |||
| Lipid parameter | Correlation Coefficient‡ | P-value‡ | |
|
| |||
| Total cholesterol (mg/dL) | 0.496 | <0.0001 | |
| LDL cholesterol (mg/dL) | 0.398 | 0.006 | |
| HDL cholesterol (mg/dL) | 0.321 | 0.028 | |
| Triglycerides (mg/dL) | 0.189 | 0.203 | |
| Correlations between change in PCSK9 and change in lipid parameters | |||
|
| |||
| Lipid parameter | Correlation Coefficient‡ | P-value‡ | |
|
| |||
| Total cholesterol (mg/dL) | 0.384 | 0.006 | |
| LDL cholesterol (mg/dL) | 0.277 | 0.051 | |
| HDL cholesterol (mg/dL) | 0.462 | 0.001 | |
| Triglycerides (mg/dL) | 0.076 | 0.602 | |
n = 47-50
Data represent means and SD
Paired Ttests
Spearman correlations on nontransformed data
Upon remission, hyperlipidemia resolved (Table). In parallel, plasma PCSK9 decreased significantly from 348.0 ± 139.5 ng/mL at baseline to 300.5 ± 130.3 ng/mL at remission (p = 0.04, Figure 1). The change in PCSK9 was correlated with the changes in total, LDL, and HDL cholesterol, though the correlation with LDL cholesterol did not quite reach significance (p = 0.051, Table). Subgroup analysis revealed that minimal change disease was associated with the largest decreases in total cholesterol and plasma PCSK9 upon remission (data not shown).
Figure 1. Plasma PCSK9 in nephrotic patients.
PCSK9 levels in nephrotic patients at baseline (348.0 ± 139.5 ng/mL) and remission (300.5 ± 130.3 ng/mL). Boxes show 25th-75th percentiles, whiskers represent 5% - 95% confidence intervals. n = 50, paired two-sided t-test.
Podocyte injury can be induced in mice by treatment with nephrotoxic serum (NTS), prepared from sheep immunized with rodent glomeruli. Injection of NTS into preimmunized mice produces a complex immunological attack on the mouse glomerulus. During the acute phase, NTS antibodies bind to the podocyte cell membrane, resulting in podocyte injury and severe proteinuria24, 25 that persist as the lesion progresses to glomerular inflammation and crescent formation after two to three weeks.22, 26 Changes in cholesterol metabolism also evolve during the course of nephrotic disease;10, 27 in this model, maximal hypercholesterolemia was observed four days after NTS injection (Figure S1A).
We therefore injected wildtype C57BL/6J mice (hereafter referred to as B6 mice) with NTS or vehicle and sacrificed them four days after the initial NTS injection. At this time, B6 mice were markedly proteinuric and dyslipidemic (Figure 2A-D). Plasma triglycerides were increased four-fold and plasma cholesterol levels were increased five-fold. Moreover, fractionation of the plasma by size exclusion chromatography revealed marked changes in the distribution of cholesterol among the different lipoprotein fractions. That is, although the absolute amount of VLDL, LDL and HDL cholesterol all increased, there was a disproportionate increase in LDL cholesterol, from 12% to 53% of total plasma cholesterol (Figure 2D; Fig. S1B). Consequently, the proportion of cholesterol associated with the ApoB-containing lipoproteins increased from 14% to 55%.
Figure 2. Nephrotoxic serum increases plasma PCSK9.
Six- to eight-week-old male B6 mice were injected with nephrotoxic serum (NTS) or normal sheep serum (Vehicle) and were sacrificed four days after the initial injection. 2 μL of spot urine collected the morning of sacrifice was subjected to SDS-PAGE and Coomassie Blue staining (A). Plasma taken at the time of sacrifice was used to measure triglycerides (B), total cholesterol (C), and PCSK9 (G), or subjected to size exclusion chromatography for lipoprotein analysis. Brackets indicate the percentage of total cholesterol found in ApoB-containing lipoproteins (VLDL + LDL) (D). Hepatic protein was measured by western blotting liver whole-cell lysates (E, I). Hepatic gene expression was measured by real-time PCR (F, H). n=3-8. For lipoprotein analysis, equal amounts of plasma from 4-6 mice were pooled from each group.
Consistent with prior studies,10, 11 hyperlipidemia in NTS-treated B6 mice was associated with a reduction in LDL receptor protein (Figure 2E). The degradation of the LDL receptor is regulated by both the E3 ubiquitin ligase Idol (Inducible degrader of the LDL receptor)28 and PCSK9. Interestingly, both Ldlr and Idol mRNA levels were decreased in the livers of NTS-treated mice (Figure 2F).
PCSK9, on the other hand, was increased sixteen-fold in the plasma of NTS-treated mice (Figure 2G). Plasma PCSK9 is derived primarily from the liver.29 Though hepatic Pcsk9 mRNA levels were increased (Figure 2H), the increase was modest, approximately 50%, suggesting that increased Pcsk9 transcription was unlikely to be the sole cause of elevated plasma PCSK9 levels. We therefore measured PCSK9 clearance in NTS-treated B6 mice by measuring the half-life of recombinant human PCSK9. The half-life of PCSK9 was increased by NTS treatment, but only two-fold (p = 0.009, Figure S1C). The modest changes in Pcsk9 mRNA and PCSK9 clearance compared to the large change in plasma PCSK9 suggested that PCSK9 secretion might also be increased, and that post-transcriptional regulation of PCSK9 could be involved. Consistent with this, cIAP1, which is required for PCSK9 processing and secretion,30 and Sec24a, which facilitates packaging of PCSK9 into COPII vesicles for secretion,23 were increased (Figure 2I). Sortilin, another protein which appears to enhance PCSK9 secretion,31 on the other hand, was not changed (Figure 2I).
To specifically dissect the role of the podocyte in the regulation of plasma PCSK9, we also studied Pod-ATTAC (Podocyte Apoptosis Through Targeted Activation of Caspase-8) mice. These mice carry the ATTAC transgene, which encodes a fusion protein that includes human caspase-8 and a mutant FK506 binding protein that binds the synthetic compound AP20187. AP20187 promotes dimerization of the fusion protein, activation of caspase-8 and apoptosis.32 In these mice, the transgene is driven by the podocin (Nphs2) promoter, which is specific to podocytes;21 therefore, injection of these mice with AP20187 results in selective ablation of the podocyte.
We injected AP20187 into mice heterozygous for the Pod-ATTAC transgene (Pod-ATTAC mice), as well as their wildtype littermates (Controls), and studied mice seven days later, at the point of maximal plasma cholesterol levels (data not shown). The phenotype of the Pod-ATTAC mice was remarkably similar to that of the NTS-treated mice (Figure 3). Both showed proteinuria, hypertriglyceridemia, and hypercholesterolemia, with a marked increase in the proportion of ApoB-associated cholesterol, from 14% in the controls to 48% in Pod-ATTAC mice, again due primarily to an increase in LDL-associated cholesterol (Figure 3A-D, S2). Both models also showed a decrease in hepatic LDL receptor protein, though Pod-ATTAC livers showed no changes in Ldlr, Idol, or Pcsk9 mRNA (Figure 3E, F, H). Plasma PCSK9 was increased seven-fold (Figure 3G) and PCSK9 clearance was decreased, as the half-life of injected human recombinant PCSK9 increased from 5.4 ± 0.3 minutes in control mice to 11.3 ± 1.4 minutes in Pod-ATTAC mice (p = 0.029, Figure 3I). Finally, cIAP1 and Sortilin were increased in Pod-ATTAC livers, though Sec24a was not (Figure 3J).
Figure 3. Podocyte apoptosis increases plasma PCSK9.
Five- to eleven-week-old mice with (Pod-ATTAC) or without (Control) the Pod-ATTAC transgene were injected with dimerizer AP20187 and sacrificed seven days after injection. 2 μL of spot urine collected the morning of sacrifice was subjected to SDS-PAGE and Coomassie Blue staining (A). Plasma taken at the time of sacrifice was used to measure triglycerides (B), total cholesterol (C) and PCSK9 (G), or subjected to size exclusion chromatography for lipoprotein analysis. Brackets indicate the percentage of total cholesterol found in ApoB-containing lipoproteins (VLDL + LDL) (D). Hepatic protein was measured by western blotting liver whole-cell lysates (E, J). Hepatic gene expression was measured by real-time PCR (F, H). PCSK9 clearance was measured as described in Methods (I). n=8-10. For lipoprotein analysis, equal amounts of plasma from 8-9 mice were pooled from each group.
Taken together, these data showed that injury to the podocyte, either via NTS treatment or genetic ablation of the podocyte, could produce striking changes in plasma PCSK9 that were correlated with reduced levels of LDL receptor protein and an increased proportion of ApoB-associated cholesterol. To dissect the role of PCSK9 in nephrotic syndrome-associated hypercholesterolemia, we administered NTS to mice with global or liver-specific knockout of Pcsk9.
Mice with global deletion of Pcsk9 (hereafter referred to as PCSK9-KO mice) were compared to their wild-type controls (hereafter referred to as WT mice) on the same mixed genetic background. NTS treatment of WT mice led to marked proteinuria, with a seven-fold increase in plasma triglycerides, a five-fold increase in plasma cholesterol, and an eight-fold increase in plasma PCSK9, with no change in liver Pcsk9 mRNA (Figure S3A, 4A-D). The proportion of ApoB-associated cholesterol increased, but in this strain the effect was more modest, increasing from 11% in vehicle-treated WT mice to 21% in NTS-treated WT mice. Moreover, this increase was due primarily to an increase in VLDL, rather than LDL, cholesterol (Figure 4E, S3B). Nonetheless, plasma levels of ApoB100 and ApoB48 increased markedly upon NTS treatment in WT mice (Figure 4F). In parallel, LDL receptor protein was decreased (Figure 4G).
Figure 4. Knockout of Pcsk9 reduces plasma cholesterol in the presence and absence of NTS.
Five- to eight-week-old wild type (WT) or Pcsk9 global knockout (PCSK9-KO) mice were injected with nephrotoxic serum (NTS) or normal sheep serum (Vehicle) and were sacrificed three to four days after the initial injection. Plasma taken at the time of sacrifice was used to measure triglycerides (A), cholesterol (B) and PCSK9 (C) or subjected to size exclusion chromatography for lipoprotein analysis. Brackets indicate the percentage of total cholesterol found in ApoB-containing lipoproteins (VLDL + LDL) (E). Hepatic gene expression was measured by real-time PCR (D, H). Protein levels were measured by western blotting plasma (F) or liver whole-cell lysates (G). n=4-7; * p < 0.05. N.M. not measured. For lipoprotein analysis, equal amounts of plasma from 4-7 mice were pooled from each group.
Knockout of Pcsk9 had no effect on renal histology or urine protein levels, either in the presence or absence of NTS treatment (Figure S3A and data not shown). On the other hand, PCSK9-KO mice showed profound changes in lipoprotein metabolism, with reductions in total cholesterol, ApoB100, and ApoE (Figure 4B, F), consistent with prior studies.33
The effects of NTS treatment on plasma lipids were reduced, but not abolished, in PCSK9-KO mice. Thus, plasma triglycerides and total cholesterol were still significantly increased by NTS in PCSK9-KO mice (Figure 4A, B). However, the ability of the NTS to increase the proportion of cholesterol in ApoB-containing lipoproteins and plasma ApoB100/ApoB48 protein levels, as well as decrease LDL receptor protein levels, was blunted (Figure 4E-G, S3B). Ldlr mRNA and Idol mRNA were not significantly changed by NTS treatment in either WT or PCSK9-KO mice (Figure 4H).
In parallel, we examined mice with liver-specific knockout of Pcsk9 (Cre+/-Pcsk9flox/flox, hereafter referred to as PCSK9 L-KO mice) on the C57BL/6 background, generated using Cre recombinase under the control of the albumin promoter. These mice were compared with their Cre-/-Pcsk9flox/flox littermates (hereafter referred to as Flox mice).
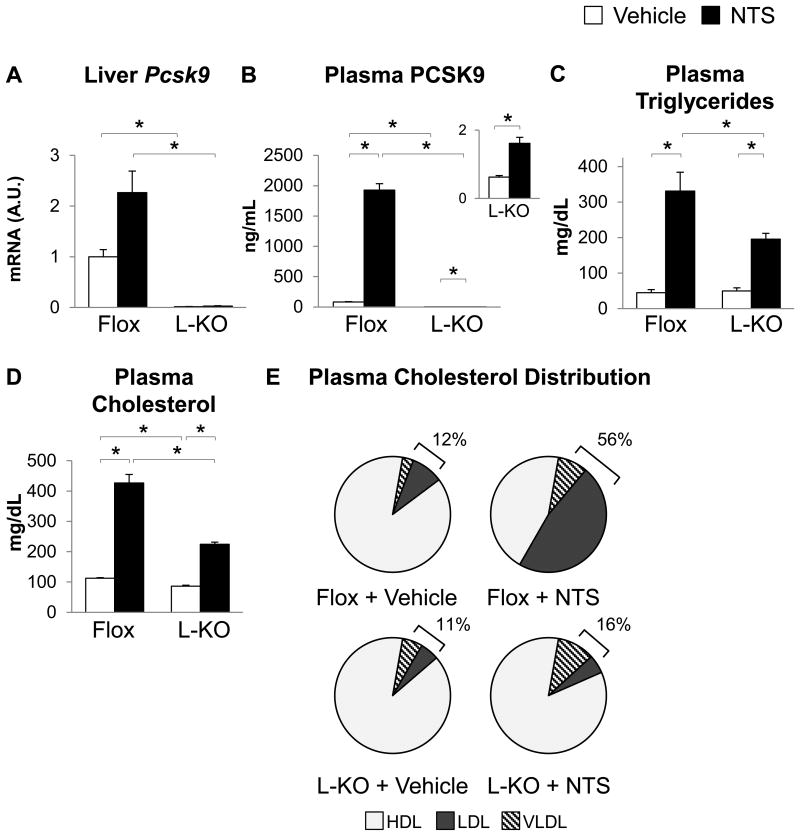
NTS treatment of Flox mice produced a two-fold increase in Pcsk9 mRNA levels that did not reach significance, a 24-fold increase in plasma PCSK9, a seven-fold increase in plasma triglycerides, and a four-fold increase in plasma cholesterol (Figure 5A-D). Here, as in wildtype B6 mice, NTS treatment produced a profound redistribution of plasma cholesterol, with LDL-associated cholesterol increased from 9% to 47% (Figure 5E, S4A). LDL receptor protein was decreased by NTS treatment, but Ldlr and Idol mRNA levels were not (Figure S4B, S4C).
Figure 5. Liver-specific knockout of Pcsk9 prevents increased LDL cholesterol after NTS treatment.
Five- to eight-week-old male Pcsk9flox/flox (Flox) and liver-specific knockout (L-KO) mice were injected with nephrotoxic serum (NTS) or normal sheep serum (Vehicle) and sacrificed four days after the initial NTS injection. Hepatic gene expression was measured by real-time PCR (A). Plasma collected at the time of sacrifice was used to measure PCSK9 (B), triglycerides (C), and cholesterol (D), or subjected to size exclusion chromatography for lipoprotein analysis. Brackets indicate the percentage of total cholesterol found in ApoB-containing lipoproteins (VLDL + LDL) (E). n= 4-8; * p < 0.05. For lipoprotein analysis, equal amounts of plasma from 4-8 mice were pooled from each group.
In PCSK9 L-KO mice, plasma PCSK9 was barely detectable in vehicle-treated mice, consistent with prior reports that plasma PCSK9 is derived primarily from the liver.29 Interestingly, NTS treatment of PCSK9 L-KO mice nonetheless produced a three-fold increase in plasma PCSK9 (p=0.004) (Figure 5B insert). To explore this further, we profiled Pcsk9 mRNA in the other tissues known to express Pcsk9: brain, intestine, and kidney. We found that NTS treatment of B6 mice had no effect in the brain or intestine, but produced a three-fold increase in renal Pcsk9. Interestingly, in situ hybridization revealed that Pcsk9 was induced not in podocytes or other intra-glomerular cells, but rather in a subset of tubule cells (Figure S5A, B).
Despite this, the effects of liver-specific and global Pcsk9 knockout were quite similar, and both showed a significant 50-60% reduction in plasma cholesterol in the presence of NTS (Figure 4B, 5D). Additionally, Flox mice showed a dramatic increase in ApoB-associated cholesterol, from 12% to 56% upon NTS treatment, whereas PCSK9 L-KO mice showed only a modest increase in ApoB-associated cholesterol, from 11% to 16% upon NTS treatment (Figure 5E, S4A). In parallel, the reduction in LDL receptor protein observed in Flox mice was blunted in PCSK9 L-KO mice (Figure S4B).
Discussion
Our data reveal a novel podocyte/hepatic axis that contributes to the regulation of plasma lipid levels via PCSK9. NTS-treated mice and Pod-ATTAC mice showed increased levels of PCSK9, while patients with nephrotic syndrome showed decreased levels of PCSK9 upon remission. Moreover, knockout of Pcsk9 in NTS-treated mice reduced plasma triglycerides and cholesterol, particularly ApoB-associated cholesterol.
Our studies in mice showed plasma PCSK9 to be increased up to 24-fold in nephrotic syndrome models. Prior cross-sectional studies in humans showed plasma PCSK9 to be increased 50-60% in proteinuric/nephrotic patients compared to control subjects.13, 14 Similarly, our longitudinal human studies showed a 14% decrease in plasma PCSK9 upon remission of nephrotic syndrome. These data demonstrate a consistent association between nephrotic syndrome and PCSK9 in humans. However, the changes in PCSK9 observed in humans with nephrotic syndrome were much more modest than those observed in mouse models. In our study, this may have been due in part to the fact that one third of the patients were already undergoing immunosuppressive therapy to treat nephrotic syndrome at baseline. It is also possible that PCSK9 levels vary during the course of nephrotic disease and that our mouse studies, performed during the acute phase of the disease, reflected a time point with greater changes in PCSK9.
There appeared to be multiple mechanisms by which podocyte injury induced PCSK9, and these varied somewhat between the models we studied. In NTS-treated B6 mice, hepatic Pcsk9 mRNA was slightly increased, suggesting an increase in Pcsk9 transcription. The drivers of Pcsk9 transcription include Sterol Regulatory Element Binding Protein 2 (SREBP2) and Hepatocyte Nuclear Factor 1α (HNF1α).34-36 Gene expression analysis in NTS-treated B6 livers revealed an increase in Srebp2 and in some, but not all, of its targets, as well as an increase in the HNF1α targets Alb, Fgb, and Serpina1, though not in Hnf1a itself (Figure S1D). Thus, both SREBP2 and HNF1α may have contributed to the induction in Pcsk9 mRNA in NTS-treated B6 livers. However, hepatic Pcsk9 mRNA was not significantly changed in the other models studied.
PCSK9 clearance was also decreased in both NTS-treated and Pod-ATTAC mice. Curiously, the LDL receptor, a target of PCSK9, participates in the clearance of PCSK9 from the plasma.37 Moreover, LDL seems to impair PCSK9 binding to and uptake by the LDL receptor.38 Thus, the decrease in PCSK9 clearance could be secondary to the decrease in LDL receptor and increase in LDL cholesterol.
The facts that PCSK9 clearance was reduced only two-fold after podocyte injury, and that Pcsk9 mRNA was not usually increased, imply the existence of a post-transcriptional mechanism by which podocyte injury increases PCSK9 secretion. This mechanism may involve cIAP1, as both NTS-treated and Pod-ATTAC mice showed an increase in hepatic cIAP1, which promotes PCSK9 secretion.30 How exactly the injured podocyte signals to the liver to increase cIAP1 has yet to be determined. One possible mediator is TNFα, which can be secreted by the podocyte,39 is increased in nephrotic syndrome plasma,40, 41 and is known to induce cIAP1.42 It is also possible that the increase in plasma PCSK9 observed in nephrotic syndrome is secondary to one of the systemic effects of podocyte injury, such as protein loss into the urine. Indeed, protein loss in the context of peritoneal dialysis is also associated with elevated levels of plasma PCSK9.14
Our studies in mice with both global and liver-specific knockout of Pcsk9 showed that Pcsk9 ablation has beneficial effects in NTS-treated mice. While PCSK9-KO and PCSK9 L-KO mice were on different genetic backgrounds that varied somewhat in their response to NTS, it was clear that either global or liver-specific knockout of Pcsk9 could reduce plasma triglycerides and cholesterol by 40-60%. Moreover, both global and liver-specific knockout of Pcsk9 decreased the proportion of ApoB-associated cholesterol, and increased the proportion of HDL-associated cholesterol.
Of course, knockout of Pcsk9 did not entirely prevent NTS-induced hyperlipidemia. This is consistent with the multiple mechanisms at play in nephrotic syndrome-associated dyslipidemia, which include increased cholesterol synthesis, increased synthesis and secretion of ApoB-containing lipoproteins, defective HDL maturation, and impaired triglyceride clearance.11, 43-45 For example, acetyl-Coenzyme A acetyltransferase (ACAT)-2, which promotes cholesterol ester formation, is increased in nephrotic syndrome and required for the development of hypercholesterolemia.11, 46, 47 Similarly, scavenger receptor class B, member 1 (SR-B1), a component of the reverse cholesterol transport system which resides on the surface of the hepatocyte and removes HDL cholesterol from the plasma, is reduced in nephrotic syndrome.48, 49 The fact that hypercholesterolemia and hypertriglyceridemia still occur in the absence of PCSK9 indicates that many of these processes may be PCSK9-independent. Thus, the correlation we observed between the change in PCSK9 and the change in HDL upon remission in our nephrotic syndrome patients was likely due to the fact that the resolution of nephrotic syndrome was associated with the normalization of HDL metabolism, as well as a reduction in PCSK9.
In summary, our data show that PCSK9, though not solely responsible for nephrotic syndrome-associated dyslipidemia, is nonetheless an important participant. Plasma PCSK9 is increased in nephrotic syndrome, and Pcsk9 ablation not only reduces the magnitude of the hypercholesterolemia associated with podocyte injury, but also produces a more atheroprotective lipoprotein profile. These data raise the possibility that PCSK9 inhibitors, which were recently approved by the FDA for the treatment of hypercholesterolemia, may be the long-sought treatment for the dyslipidemia of nephrotic syndrome.
Supplementary Material
Clinical Perspective.
What is new?
Nephrotic syndrome increases plasma PCSK9 up to 24-fold in mice.
Pcsk9 ablation reduces plasma triglycerides and cholesterol by 40-60% in mice with nephrotic syndrome.
Pcsk9 ablation abolishes the dramatic increase in the ratio of LDL to HDL cholesterol observed upon the induction of nephrotic syndrome.
What are the clinical implications?
These studies suggest that PCSK9 inhibitors may be useful in treating nephrotic syndrome-associated hypercholesterolemia.
Acknowledgments
We thank Dr. Henry Feldman for excellent statistical support, Dr. David Ginsburg for the Sec24a antibody, and Merck and Co. for Pcsk9flox/flox mice. Nephrotic patient samples were provided by the Nephrotic Syndrome Study Network Consortium (NEPTUNE).
Sources of Funding: This work was funded by NIH grants R01HL109650 (SBB & MEH), 5K12DK094721-04 (AEL), R01DK090029 (DJS), American Heart Association grant 12SDG12050287 (JMR), and by a Department of Defense National Defense Science & Engineering Graduate (NDSEG) fellowship to MEH. The Nephrotic Syndrome Study Network Consortium (NEPTUNE) was funded by NIH grant U-54-DK083912.
Footnotes
Disclosures: SBB has received consulting fees from Novo Nordisk.
References
- 1.Christison R. On the cause of the milky and whey-like appearances sometimes observed in the blood. Edin Med Surg J. 1830;33:274–280. [PMC free article] [PubMed] [Google Scholar]
- 2.Kong X, Yuan H, Fan J, Li Z, Wu T, Jiang L. Lipid-lowering agents for nephrotic syndrome. The Cochrane database of systematic reviews. 2013;12:CD005425. doi: 10.1002/14651858.CD005425.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ordonez JD, Hiatt RA, Killebrew EJ, Fireman BH. The increased risk of coronary heart disease associated with nephrotic syndrome. Kidney international. 1993;44:638–642. doi: 10.1038/ki.1993.292. [DOI] [PubMed] [Google Scholar]
- 4.Aguilar-Salinas CA, Barrett PH, Kelber J, Delmez J, Schonfeld G. Physiologic mechanisms of action of lovastatin in nephrotic syndrome. Journal of lipid research. 1995;36:188–199. [PubMed] [Google Scholar]
- 5.Warwick GL, Packard CJ, Demant T, Bedford DK, Boulton-Jones JM, Shepherd J. Metabolism of apolipoprotein b-containing lipoproteins in subjects with nephrotic-range proteinuria. Kidney international. 1991;40:129–138. doi: 10.1038/ki.1991.190. [DOI] [PubMed] [Google Scholar]
- 6.de Sain-van der Velden MG, Kaysen GA, Barrett HA, Stellaard F, Gadellaa MM, Voorbij HA, Reijngoud DJ, Rabelink TJ. Increased vldl in nephrotic patients results from a decreased catabolism while increased ldl results from increased synthesis. Kidney international. 1998;53:994–1001. doi: 10.1111/j.1523-1755.1998.00831.x. [DOI] [PubMed] [Google Scholar]
- 7.Warwick GL, Caslake MJ, Boulton-Jones JM, Dagen M, Packard CJ, Shepherd J. Low-density lipoprotein metabolism in the nephrotic syndrome. Metabolism: clinical and experimental. 1990;39:187–192. doi: 10.1016/0026-0495(90)90074-m. [DOI] [PubMed] [Google Scholar]
- 8.Demant T, Mathes C, Gutlich K, Bedynek A, Steinhauer HB, Bosch T, Packard CJ, Warwick GL. A simultaneous study of the metabolism of apolipoprotein b and albumin in nephrotic patients. Kidney international. 1998;54:2064–2080. doi: 10.1046/j.1523-1755.1998.00204.x. [DOI] [PubMed] [Google Scholar]
- 9.Vega GL, Toto RD, Grundy SM. Metabolism of low density lipoproteins in nephrotic dyslipidemia: Comparison of hypercholesterolemia alone and combined hyperlipidemia. Kidney international. 1995;47:579–586. doi: 10.1038/ki.1995.73. [DOI] [PubMed] [Google Scholar]
- 10.Vaziri ND, Liang KH. Down-regulation of hepatic ldl receptor expression in experimental nephrosis. Kidney international. 1996;50:887–893. doi: 10.1038/ki.1996.388. [DOI] [PubMed] [Google Scholar]
- 11.Vaziri ND, Sato T, Liang K. Molecular mechanisms of altered cholesterol metabolism in rats with spontaneous focal glomerulosclerosis. Kidney international. 2003;63:1756–1763. doi: 10.1046/j.1523-1755.2003.00911.x. [DOI] [PubMed] [Google Scholar]
- 12.Liu S, Vaziri ND. Role of pcsk9 and idol in the pathogenesis of acquired ldl receptor deficiency and hypercholesterolemia in nephrotic syndrome. Nephrol Dial Transplant. 2014;29:538–543. doi: 10.1093/ndt/gft439. [DOI] [PubMed] [Google Scholar]
- 13.Kwakernaak AJ, Lambert G, Slagman MC, Waanders F, Laverman GD, Petrides F, Dikkeschei BD, Navis G, Dullaart RP. Proprotein convertase subtilisin-kexin type 9 is elevated in proteinuric subjects: Relationship with lipoprotein response to antiproteinuric treatment. Atherosclerosis. 2013;226:459–465. doi: 10.1016/j.atherosclerosis.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 14.Jin K, Park BS, Kim YW, Vaziri ND. Plasma pcsk9 in nephrotic syndrome and in peritoneal dialysis: A cross-sectional study. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2014;63:584–589. doi: 10.1053/j.ajkd.2013.10.042. [DOI] [PubMed] [Google Scholar]
- 15.Konarzewski M, Szolkiewicz M, Sucajtys-Szulc E, Blaszak J, Lizakowski S, Swierczynski J, Rutkowski B. Elevated circulating pcsk-9 concentration in renal failure patients is corrected by renal replacement therapy. Am J Nephrol. 2014;40:157–163. doi: 10.1159/000365935. [DOI] [PubMed] [Google Scholar]
- 16.Abujrad H, Mayne J, Ruzicka M, Cousins M, Raymond A, Cheesman J, Taljaard M, Sorisky A, Burns K, Ooi TC. Chronic kidney disease on hemodialysis is associated with decreased serum pcsk9 levels. Atherosclerosis. 2014;233:123–129. doi: 10.1016/j.atherosclerosis.2013.12.030. [DOI] [PubMed] [Google Scholar]
- 17.Sucajtys-Szulc E, Szolkiewicz M, Swierczynski J, Rutkowski B. Up-regulation of liver pcsk9 gene expression as a possible cause of hypercholesterolemia in experimental chronic renal failure. Mol Cell Biochem. 2016;411:281–287. doi: 10.1007/s11010-015-2590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lambert G, Sjouke B, Choque B, Kastelein JJ, Hovingh GK. The pcsk9 decade. Journal of lipid research. 2012;53:2515–2524. doi: 10.1194/jlr.R026658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Robinson JG, Farnier M, Krempf M, Bergeron J, Luc G, Averna M, Stroes ES, Langslet G, Raal FJ, El Shahawy M, Koren MJ, Lepor NE, Lorenzato C, Pordy R, Chaudhari U, Kastelein JJ, Investigators OLT. Efficacy and safety of alirocumab in reducing lipids and cardiovascular events. The New England journal of medicine. 2015;372:1489–1499. doi: 10.1056/NEJMoa1501031. [DOI] [PubMed] [Google Scholar]
- 20.Gadegbeku CA, Gipson DS, Holzman LB, Ojo AO, Song PX, Barisoni L, Sampson MG, Kopp JB, Lemley KV, Nelson PJ, Lienczewski CC, Adler SG, Appel GB, Cattran DC, Choi MJ, Contreras G, Dell KM, Fervenza FC, Gibson KL, Greenbaum LA, Hernandez JD, Hewitt SM, Hingorani SR, Hladunewich M, Hogan MC, Hogan SL, Kaskel FJ, Lieske JC, Meyers KE, Nachman PH, Nast CC, Neu AM, Reich HN, Sedor JR, Sethna CB, Trachtman H, Tuttle KR, Zhdanova O, Zilleruelo GE, Kretzler M. Design of the nephrotic syndrome study network (neptune) to evaluate primary glomerular nephropathy by a multidisciplinary approach. Kidney international. 2013;83:749–756. doi: 10.1038/ki.2012.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rutkowski JM, Wang ZV, Park AS, Zhang J, Zhang D, Hu MC, Moe OW, Susztak K, Scherer PE. Adiponectin promotes functional recovery after podocyte ablation. Journal of the American Society of Nephrology : JASN. 2013;24:268–282. doi: 10.1681/ASN.2012040414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lloyd C, Gutierrez-Ramos JC. Murine model of crescentic nephritis. Methods Mol Biol. 2000;138:311–318. doi: 10.1385/1-59259-058-6:311. [DOI] [PubMed] [Google Scholar]
- 23.Chen XW, Wang H, Bajaj K, Zhang P, Meng ZX, Ma D, Bai Y, Liu HH, Adams E, Baines A, Yu G, Sartor MA, Zhang B, Yi Z, Lin J, Young SG, Schekman R, Ginsburg D. Sec24a deficiency lowers plasma cholesterol through reduced pcsk9 secretion. Elife. 2013;2:e00444. doi: 10.7554/eLife.00444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chugh S, Yuan H, Topham PS, Haydar SA, Mittal V, Taylor GA, Kalluri R, Salant DJ. Aminopeptidase a: A nephritogenic target antigen of nephrotoxic serum. Kidney international. 2001;59:601–613. doi: 10.1046/j.1523-1755.2001.059002601.x. [DOI] [PubMed] [Google Scholar]
- 25.Salant DJ, Cybulsky AV. Experimental glomerulonephritis. Methods Enzymol. 1988;162:421–461. doi: 10.1016/0076-6879(88)62096-9. [DOI] [PubMed] [Google Scholar]
- 26.George B, Verma R, Soofi AA, Garg P, Zhang J, Park TJ, Giardino L, Ryzhova L, Johnstone DB, Wong H, Nihalani D, Salant DJ, Hanks SK, Curran T, Rastaldi MP, Holzman LB. Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J Clin Invest. 2012;122:674–692. doi: 10.1172/JCI60070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaziri ND, Liang KH. Hepatic hmg-coa reductase gene expression during the course of puromycin-induced nephrosis. Kidney international. 1995;48:1979–1985. doi: 10.1038/ki.1995.500. [DOI] [PubMed] [Google Scholar]
- 28.Zelcer N, Hong C, Boyadjian R, Tontonoz P. Lxr regulates cholesterol uptake through idol-dependent ubiquitination of the ldl receptor. Science. 2009;325:100–104. doi: 10.1126/science.1168974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zaid A, Roubtsova A, Essalmani R, Marcinkiewicz J, Chamberland A, Hamelin J, Tremblay M, Jacques H, Jin W, Davignon J, Seidah NG, Prat A. Proprotein convertase subtilisin/kexin type 9 (pcsk9): Hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 2008;48:646–654. doi: 10.1002/hep.22354. [DOI] [PubMed] [Google Scholar]
- 30.Xu W, Liu L, Hornby D. C-iap1 binds and processes pcsk9 protein: Linking the c-iap1 in a tnf-α pathway to pcsk9-mediated ldlr degradation pathway. Molecules. 2012;17:12086–12101. doi: 10.3390/molecules171012086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gustafsen C, Kjolby M, Nyegaard M, Mattheisen M, Lundhede J, Buttenschøn H, Mors O, Bentzon JF, Madsen P, Nykjaer A, Glerup S. The hypercholesterolemia-risk gene sort1 facilitates pcsk9 secretion. Cell Metab. 2014;19:310–318. doi: 10.1016/j.cmet.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 32.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE. Fat apoptosis through targeted activation of caspase 8: A new mouse model of inducible and reversible lipoatrophy. Nat Med. 2005;11:797–803. doi: 10.1038/nm1262. [DOI] [PubMed] [Google Scholar]
- 33.Rashid S, Curtis DE, Garuti R, Anderson NN, Bashmakov Y, Ho YK, Hammer RE, Moon YA, Horton JD. Decreased plasma cholesterol and hypersensitivity to statins in mice lacking pcsk9. Proc Natl Acad Sci U S A. 2005;102:5374–5379. doi: 10.1073/pnas.0501652102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jeong HJ, Lee HS, Kim KS, Kim YK, Yoon D, Park SW. Sterol-dependent regulation of proprotein convertase subtilisin/kexin type 9 expression by sterol-regulatory element binding protein-2. J Lipid Res. 2008;49:399–409. doi: 10.1194/jlr.M700443-JLR200. [DOI] [PubMed] [Google Scholar]
- 35.Li H, Dong B, Park SW, Lee HS, Chen W, Liu J. Hepatocyte nuclear factor 1alpha plays a critical role in pcsk9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. The Journal of biological chemistry. 2009;284:28885–28895. doi: 10.1074/jbc.M109.052407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Costet P, Cariou B, Lambert G, Lalanne F, Lardeux B, Jarnoux AL, Grefhorst A, Staels B, Krempf M. Hepatic pcsk9 expression is regulated by nutritional status via insulin and sterol regulatory element-binding protein 1c. The Journal of biological chemistry. 2006;281:6211–6218. doi: 10.1074/jbc.M508582200. [DOI] [PubMed] [Google Scholar]
- 37.Tavori H, Fan D, Blakemore JL, Yancey PG, Ding L, Linton MF, Fazio S. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: Evidence for a reciprocal regulation. Circulation. 2013;127:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kosenko T, Golder M, Leblond G, Weng W, Lagace TA. Low density lipoprotein binds to proprotein convertase subtilisin/kexin type-9 (pcsk9) in human plasma and inhibits pcsk9-mediated low density lipoprotein receptor degradation. The Journal of biological chemistry. 2013;288:8279–8288. doi: 10.1074/jbc.M112.421370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rosa AC, Rattazzi L, Miglio G, Collino M, Fantozzi R. Angiotensin ii induces tumor necrosis factor-α expression and release from cultured human podocytes. Inflamm Res. 2012;61:311–317. doi: 10.1007/s00011-011-0412-8. [DOI] [PubMed] [Google Scholar]
- 40.Bustos C, Gonzalez E, Muley R, Alonso JL, Egido J. Increase of tumour necrosis factor alpha synthesis and gene expression in peripheral blood mononuclear cells of children with idiopathic nephrotic syndrome. European journal of clinical investigation. 1994;24:799–805. doi: 10.1111/j.1365-2362.1994.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 41.Suranyi MG, Guasch A, Hall BM, Myers BD. Elevated levels of tumor necrosis factor-alpha in the nephrotic syndrome in humans. American journal of kidney diseases : the official journal of the National Kidney Foundation. 1993;21:251–259. doi: 10.1016/s0272-6386(12)80742-6. [DOI] [PubMed] [Google Scholar]
- 42.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS., Jr Nf-kappab antiapoptosis: Induction of traf1 and traf2 and c-iap1 and c-iap2 to suppress caspase-8 activation. Science. 1998;281:1680–1683. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 43.Tsimihodimos V, Dounousi E, Siamopoulos KC. Dyslipidemia in chronic kidney disease: An approach to pathogenesis and treatment. Am J Nephrol. 2008;28:958–973. doi: 10.1159/000144024. [DOI] [PubMed] [Google Scholar]
- 44.Vaziri ND. Hdl abnormalities in nephrotic syndrome and chronic kidney disease. Nat Rev Nephrol. 2016;12:37–47. doi: 10.1038/nrneph.2015.180. [DOI] [PubMed] [Google Scholar]
- 45.Clement LC, Macé C, Avila-Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin-like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46. doi: 10.1038/nm.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vaziri ND, Liang K. Up-regulation of acyl-coenzyme a:Cholesterol acyltransferase (acat) in nephrotic syndrome. Kidney Int. 2002;61:1769–1775. doi: 10.1046/j.1523-1755.2002.00319.x. [DOI] [PubMed] [Google Scholar]
- 47.Vaziri ND, Liang KH. Acyl-coenzyme a:Cholesterol acyltransferase inhibition ameliorates proteinuria, hyperlipidemia, lecithin-cholesterol acyltransferase, srb-1, and low-denisty lipoprotein receptor deficiencies in nephrotic syndrome. Circulation. 2004;110:419–425. doi: 10.1161/01.CIR.0000136023.70841.0F. [DOI] [PubMed] [Google Scholar]
- 48.Liang K, Vaziri ND. Down-regulation of hepatic high-density lipoprotein receptor, sr-b1, in nephrotic syndrome. Kidney Int. 1999;56:621–626. doi: 10.1046/j.1523-1755.1999.00585.x. [DOI] [PubMed] [Google Scholar]
- 49.Vaziri ND, Gollapudi P, Han S, Farahmand G, Yuan J, Rahimi A, Moradi H. Nephrotic syndrome causes upregulation of hdl endocytic receptor and pdzk-1-dependent downregulation of hdl docking receptor. Nephrol Dial Transplant. 2011;26:3118–3123. doi: 10.1093/ndt/gfr136. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.