Abstract
The transition from vegetative to reproductive growth is a critical process in the life cycle of higher plants. Previously, we cloned Rice Indeterminate 1 (RID1), which acts as the master switch for the transition from the vegetative to reproductive phase in rice. Although the photoperiod pathway of RID1 inducing expression of the florigen genes Hd3a and RFT1 via Ehd1 has been established, the alternative pathways for the essential flowering transition need to be further examined. Here, we identified a Suppressor of rid1 (SID1), which rescues the never-flowering phenotype of rid1. SID1 encodes an INDETERMINATE DOMAIN (IDD) transcription factor. Mutation in SID1 showed the delayed flowering phenotype. Gain-of-function of SID1, OsIDD1, or OsIDD6 could restore the rid1 to flowering. Further analyses showed SID1 and RID1 directly target the promoter regions of Hd3a and RFT1, two florigen genes in rice. Taken together, our results reveal an autonomous flowering pathway might be mediated by RID1, thereby controlling the phase transition from vegetative to reproductive development in rice.
Author summary
Transition from vegetative to reproductive phase is a critical developmental switch in the life cycle of higher plants. In rice, our previous work suggested Rice Indeterminate 1 (RID1) acts as the master switch for the transition to flowering. Mutation in RID1 results in a never-flowering phenotype. In order to uncover the molecular network regulated by RID1, a Suppressor of rid1 (SID1) was identified in this study. Both SID1 and RID1 encode a plant-specific INDETERMINATE DOMAIN (IDD) transcription factor. Overexpression of SID1, OsIDD1, or OsIDD6 could rescue the never-flowering phenotype of rid1. Molecular data indicate both SID1 and RID1 physically bind the promoters of the florigen genes Hd3a and RFT1 in rice. Thus, we propose that the transition to flowering could be regulated by RID1 through the autonomous pathway, in addition to the photoperiod pathway.
Introduction
The post-embryonic development of flowering plants can be divided into two major phases: the vegetative and reproductive growth stages. During vegetative development, shoot apical meristems continue to produce leaves for the generation of organic materials through photosynthesis. After a given number of leaves are generated, endogenous genetic factors and environmental signals control the time of flowering [1]. Molecular regulatory networks that monitor the changes in the environment and complex endogenous signals determine the timing of the developmental transition [2–4]. Great progress has been made in elucidating the molecular basis for the flowering transition in Arabidopsis, which represents a long-day (LD) plant [5–10]. Numerous genes were identified and integrated into six major pathways: the photoperiod, vernalization, age, autonomous, gibberellin, and ambient temperature pathways [11]. Rice is not only a leading cereal crop in the world, but also a representative short-day (SD) plant for flowering time (heading date) studies. As an important agronomic trait, heading date is crucial for determining the regional adaptability and grain yields [12–14].
The molecular mechanisms for flowering time control have been well studied in Arabidopsis. However, studies of heading date control in rice have almost exclusively focused on the photoperiodic pathway [15]. Although rice is regarded as a SD plant, it also has evolved its flowering pathway to induce flowering under LD conditions during artificial domestication at high latitudes [16–20]. Thus, photoperiodic flowering in rice can be artificially considered as two distinct pathways: the evolutionarily conserved OsGI-Hd1-Hd3a pathway for adaption under SD conditions, which is parallel to the GI-CO-FT module in Arabidopsis [21, 22], and the uniquely evolved Ghd7-Ehd1-Hd3a/RFT1 pathway for adaptation under LD conditions [12, 13, 15, 23]. To understand the photoperiodic control of flowering in rice more comprehensively, recent investigations have identified some flowering mutants that are insensitive to photoperiod variations. Mutants with RID1/OsID1/Ehd2, a rice ortholog of the maize INDETERMINATE1 (ID1) gene, showed a late- or never-flowering phenotype under SD or LD conditions [24–26], indicating that RID1 might function as an autonomous factor to induce the floral transition in rice [26]. Ehd3 encodes a plant homeodomain finger-containing protein [27]. Mutation in Ehd3 results in no flowering under LD conditions, suggesting that Ehd3 acts as a flowering inducer in the unique genetic pathway Ehd3-Ghd7-Ehd1 in rice [27]. In addition, Ehd4, encoding a novel CCCH-type zinc finger protein, was identified as a critical regulator promoting flowering under both SD and LD conditions [16]. ehd4 also showed a never-flowering phenotype under LD conditions [16]. All these flowering switches (RID1, Ehd3, Ehd4) thus far identified in rice and have no direct homologs in Arabidopsis [16, 26, 27]. Thus, it appears that RID1, Ehd3, and Ehd4 may participate in a rice-specific flowering transition pathway, the underlying molecular mechanisms of which are still not well understood.
RID1/OsID1/Ehd2 encodes a highly conserved zinc finger protein in plants [24–26]. The zinc fingers and its surrounding sequence compose a so-called INDETERMINATE DOMAIN (IDD), which was identified in all higher plant genomes [28]. Maize ID1 is the founding member of the IDD family and controls the transition to flowering in maize [29]. In vitro DNA binding experiments showed that ID1 binds selectively to an 11-bp DNA sequence with the consensus motif TTTGTCG/CT/CT/aT/aT via the IDD [30]. Sixteen and fifteen IDD members were identified in the genomes of Arabidopsis and rice, respectively [28]. Previous studies of IDD members in Arabidopsis revealed that IDD genes participate in multiple developmental processes. AtIDD8 is involved in photoperiodic flowering by modulating sugar transport and metabolism [31]. AtIDD8, AtIDD3, and AtIDD10, either physically or genetically interact with the GRAS domain proteins SHR and SCR to regulate root development or patterning [32–34]. AtIDD1 is required for seed maturation and germination [35]. AtIDD14, AtIDD15, and AtIDD16 play a critical role in lateral organ morphogenesis and gravitropism by regulating spatial auxin accumulation [36]. Recent investigations showed that some IDD members (AtIDD2, AtIDD3, AtIDD4, AtIDD5, AtIDD9, and AtIDD10) interact with DELLAs to control gibberellin homeostasis and signaling and modulate flowering time in Arabidopsis [37, 38]. RID1 is the only IDD member being functionally analyzed in rice. RID1 and its putative orthologs, ID1 in maize and SbID in Sorghum, are preferentially expressed in immature leaves and may exhibit conserved function for flowering transition [26, 28, 29].
In maize, ID1 is a key regulator of the transition from vegetative to reproductive growth [29, 39]. The id1 mutant has prolonged vegetative growth and retains vegetative features in the inflorescence [29]. The ID1 gene was proposed to regulate the production or transmission of a mobile florigenic signal [29, 40]. Transcript and metabolite profiles indicated that expression levels of major sucrose and starch metabolism genes were altered in the id1 mutant, suggesting that ID1 might be involved in the starch to sucrose transition and sucrose utilization within the leaf [39]. However, similar changes in transitory starch and sucrose are not observed in the photoperiodic flowering plants [39]. Thus, it appears that ID1 is likely engaged in a novel autonomous flowering pathway that is distinct from the photoperiod induction pathway [39, 41]. Our previous study showed that RID1 acts as a master switch of the flowering transition in rice [26]. Loss of function of RID1 seriously suppressed the expression of Ehd1 and florigen genes Hd3a and RFT1, suggesting that RID1 plays important roles in photoperiodic flowering promotion in rice [26]. At present, the direct target of RID1 and whether RID1 controls an autonomous flowering pathway in rice remain unclear.
In this study, a gain-of-function mutant suppressor of rid1-D (sid1-D) was identified. sid1-D restored the rid1 mutant to flowering successfully. SID1 belongs to the IDD family in rice. Loss-of-function mutants of SID1 exhibit late flowering under LD conditions. Moreover, our results show that RID1 and SID1 directly regulate the expression of Hd3a and RFT1, two florigen genes in rice. Our results indicate that the original function of RID1 might trigger the expression of florigen genes, thus controlling the flowering transition in rice.
Results
Identification of a suppressor of rid1
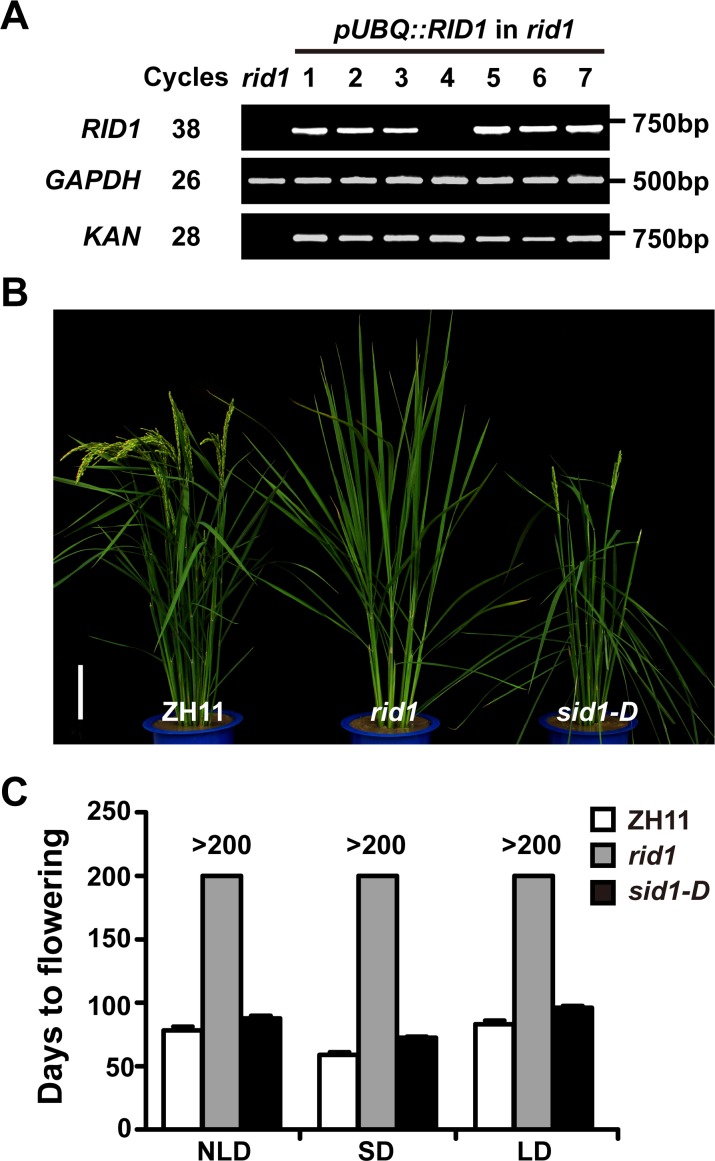
Our previous study identified a RID1 knockout mutant (rid1), which shows a never-flowering phenotype under LD or SD conditions [26]. Further examination showed that T-DNA insertion at the second intron of the RID1 gene caused the never-flowering phenotype, and a 5.7-kb genomic fragment containing the entire RID1 coding region and its promoter could successfully rescue the mutant phenotype [26]. To examine whether the full-length cDNA of RID1 could rescue the mutant phenotype, we generated genetic complementary plants transformed with construct (pUBQ::RID1) harboring the RID1 cDNA fragment driven by the Ubiquitin promoter. Among 80 independent transgenic plants, we analyzed 7 positive transgenic plants with the restored normal flowering phenotype. Surprisingly, we discovered one line (#4) in which the transcript of RID1 was undetectable but exhibited a restored flowering (Fig 1A). Its progeny of 200 plants exhibited a phenotypic segregation of flowering to never-flowering of 3:1 (143:57, χ2 = 1.13, P < 0.05), in which all flowering plants contained the selection marker Kanamycin gene. This observation suggests that the restored flowering plant results from a dominant mutation of a single gene that is likely to co-segregate with a T-DNA insertion event. Thus, we designated this mutant as suppressor of rid1-D (sid1-D) (Fig 1B).
Fig 1. Characterization of sid1-D, a dominant genetic suppressor of rid1.
(A) Transcript analyses of RID1 in transgenic flowering lines carrying pUBQ::RID1 in rid1 background (lanes 1 to 7). Note that no transcript of RID1 was detected in line 4 (renamed as sid1-D). rid1 served as the negative control, and GAPDH gene served as an internal control. KAN amplified from Kanamycin gene indicated the T-DNA insertion. pUBQ, maize Ubiquitin promoter. (B) Phenotypic comparison of ZH11 (control), rid1, and sid1-D plants at the heading stage. Scale bar, 15 cm. (C) Flowering times of ZH11, rid1, and sid1-D under the indicated day length conditions (n = 10). NLD, natural-long-day; SD, short-day; LD, long-day conditions.
Next, we investigated the heading date of sid1-D compared to wild-type plants. Under natural-long-day (NLD) conditions during the growing season at Wuhan, China, the heading date of sid1-D was delayed 10 days compared with the wild type. In the growth control room, the heading date of sid1-D (72.4 ± 1.1 days for SD; 101.1 ± 1.4 days for LD) was delayed about 2 weeks compared with the wild type under SD or LD conditions (Fig 1C). Furthermore, sid1-D exhibited a similar leaf emergence rate as that of rid1 under both SD and LD conditions (S1A Fig). The heterozygotes and homozygous sid1-D exhibited an indistinguishable heading date under distinct day length conditions (S1B Fig). These results indicate that sid1-D is a dominant mutant that partially rescued the never-flowering phenotype of rid1.
SID1 is the rice INDETERMINATE DOMAIN 4 (OsIDD4) transcription factor
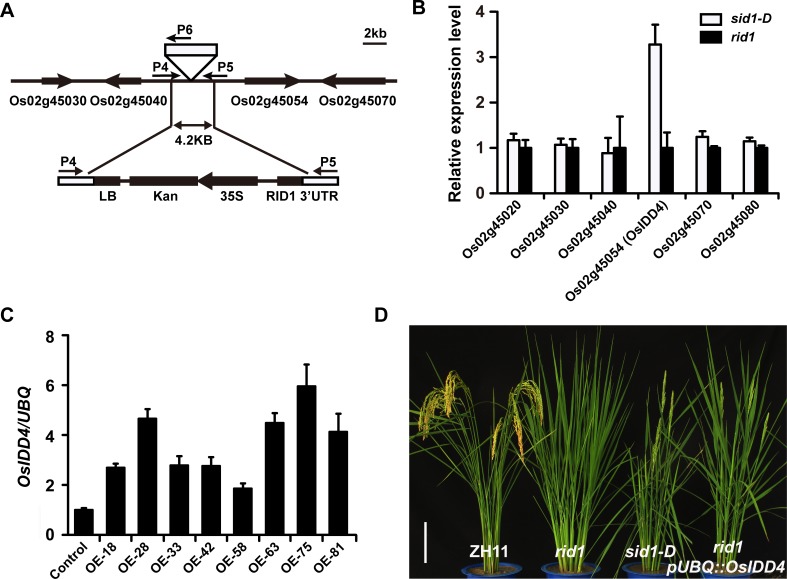
Because sid1-D is generated by a single gene mutation and co-segregates with a T-DNA insertion, the genomic sequence flanking the left border of the T-DNA insertion site was isolated by thermal asymmetric interlaced PCR [42]. BLAST analysis of the flanking sequence indicated that a T-DNA was inserted into the intergenic region between the annotated genes LOC_Os02g45054 (OsIDD4) and LOC_Os02g45040 (Fig 2A). PCR analysis using the primers P1, P2, and P3 [26] indicated that the genomic background is homozygous for rid1 (S2A Fig). We determined the genotypes of the re-introduced T-DNA insertion site by PCR amplification using the primers P4, P5, and P6 (Fig 2A and S2A Fig). All the plants homozygous or heterozygous for the T-DNA insertion showed the restored heading phenotype, whereas the plants without T-DNA insertion showed the never-flowering phenotype, like that of rid1. Our further analysis of the T-DNA sequence integrated into the genome indicated that a truncated T-DNA insertion event occurred. The truncated T-DNA only remained in the left region containing the selection marker Kanamycin driven by the CaMV 35S promoter and a sequence of the 3' untranslated region of RID1 (162 bp) (Fig 2A).
Fig 2. Molecular identification of SID1.
(A) Schematic of the genomic region flanking T-DNA insertion site and T-DNA insertion region in sid1-D. Genes are shown as thick arrows, and intergenic regions are shown as lines at the top. T-DNA left border (LB), the selection marker Kanamycin driven by the CaMV 35S promoter, and a sequence of 3' untranslated region of RID1 are indicated. P4 to P6 primers indicate the PCR primers used for genotyping the T-DNA in sid1-D. (B) Transcript analyses of the genes flanking the T-DNA in sid1-D and rid1 plants. Note that the transcript of OsIDD4 (LOC_Os02g45054) is highly elevated in sid1-D. Expression data relative to control were normalized to that of Ubiquitin (UBQ). Each bar represents the mean ± SEM. (C) Expression level of independent T0 transgenic flowering lines generated by transforming homozygous callus of rid1 with pUBQ::OsIDD4. Control, transgene-negative control plant. (D) Phenotypes of wild-type (ZH11), rid1, sid1-D, and rid1 pUBQ::OsIDD4 at heading stage. Scale bar, 15 cm.
Because sid1-D is a dominant mutant with re-introduced T-DNA inserted in the intergenic region, we examined the expression levels of genes flanking the T-DNA insertion. Quantitative reverse transcription PCR (QRT-PCR) analysis indicated that the transcript of LOC_Os02g45054 (OsIDD4) was significantly increased, while the other genes showed identical expression patterns in sid1-D and rid1 (Fig 2B). To determine whether the elevated transcript level of OsIDD4 is responsible for the rescued flowering in sid1-D, we introduced a pUBQ::OsIDD4 construct (S2B Fig) into rid1 callus. All the transgenic plants overexpressing OsIDD4 (Fig 2C) recovered the flowering of sid1-D (Fig 2D), whereas plants transformed with empty vector (negative control) retained a never-flowering phenotype similar to that of rid1 (S2C Fig). In the progenies of the rescued flowering plants, segregation of the OsIDD4 transgene coincided very well with successful flowering, whereas the negative transgenic plants did not head, similar to rid1 (S2C and S2D Fig). These results suggest that increased expression of OsIDD4 is responsible for reversing the never-flowering phenotype of rid1. Thus, OsIDD4 is the Suppressor of rid1 (SID1).
In rice, SID1 encodes a typical Cys-2/His-2 (C2H2) zinc finger protein belonging to the plant-specific IDD protein family, comprising 15 members in rice [28]. Phylogenetic analysis showed that SID1 belongs to a different clade than that of RID1 (S3A Fig). However, SID1 shares 43% identity with RID1; in particular, they have a highly conserved IDD at the N-terminal region (S3B Fig).
Overexpression of OsIDD1 or OsIDD6 also restored flowering of rid1
The conserved IDD in SID1 contains four putative zinc finger domains (S3B Fig). To investigate whether the zinc fingers of SID1 are essential to complement rid1, we generated four constructs via ectopic expression of SID1 with mutation in each zinc finger in rid1 (S4A Fig). A normal SID1 CDs overexpression construct was used as a positive control. For each transformation, at least 100 independent transgenic plants were generated. The transgenic results showed that mutating each zinc finger of SID1 abolished rescuing the never-flowering phenotype of rid1, whereas plants transformed with normal SID1 CDs overexpression construct recapitulated the phenotype of sid1-D (S4B Fig). Our results showed that the four zinc fingers of SID1 are required to restore the flowering transition in rid1.
Phylogenetic analysis showed there are 15 identifiable IDD genes in rice (S3A Fig). To investigate the possible redundancy of the OsIDD genes with SID1, we generated transgenic plants overexpressing the OsIDD genes OsIDD1, OsIDD3, OsIDD6, OsIDD10, OsIDD12, and OsIDD14, respectively. At least 100 independent transgenic plants of each transformation were generated. The overexpression of OsIDD1 or OsIDD6 in rid1 recapitulated the phenotype of sid1-D plants, showing restored flowering of rid1 (Fig 3). However, plants transformed with other OsIDD genes retained a never-flowering phenotype similar to rid1. These results suggested that OsIDD1 and OsIDD6 might have redundant function in floral transition with SID1 when they were overexpressed.
Fig 3. Characterization of transgenic plants overexpressing OsIDD1 and OsIDD6 in rid1.
(A) Expression level of independent T0 transgenic flowering lines generated by transforming homozygous callus of rid1 with pUBQ::OsIDD1. Negative, transgene-negative control plant. (B) Phenotypes of transgene-negative control plant and rid1 pUBQ::OsIDD1 at heading stage. Scale bar, 15 cm. (C) Days to flowering under natural-long-day (NLD) conditions. Black boxes, rid1 pUBQ::OsIDD1 transgenic plants; empty boxes, transgene-negative control plant (n = 10). (D) Expression level of independent T0 transgenic flowering lines generated by transforming homozygous callus of rid1 with pUBQ::OsIDD6. Negative, transgene-negative control plant. (E) Phenotypes of transgene-negative control plant and rid1 pUBQ::OsIDD6 at heading stage. Scale bar, 15 cm. (F) Days to flowering under NLD conditions. Black boxes, rid1 pUBQ::OsIDD6 transgenic plants; empty boxes, transgene-negative control plant (n = 10).
Expression patterns of SID1 and its transcriptional activity
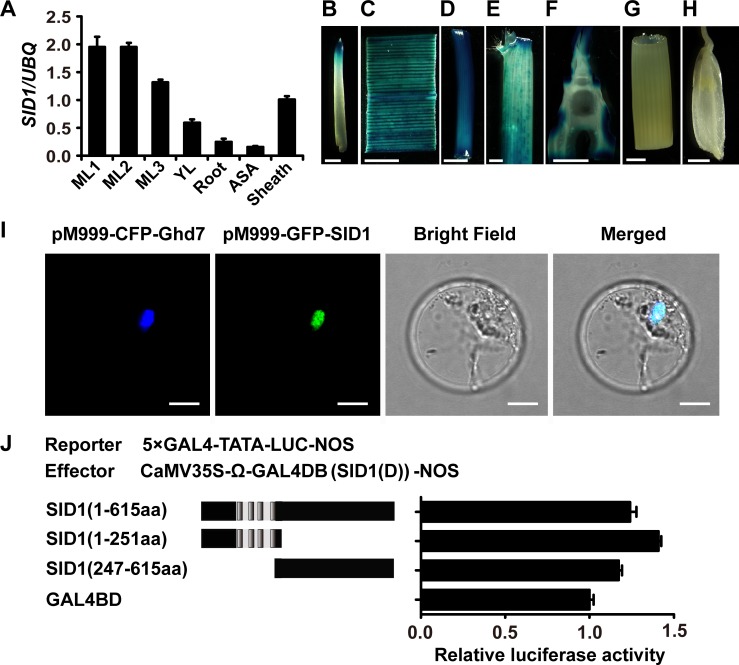
To determine the spatial expression profile of SID1, we examined the expression level of SID1 in various tissues by qRT-PCR at seedling stage (S5A Fig). The analysis showed that SID1 was preferentially expressed in vegetative tissues (Fig 4A). We also made a construct pSID1::GUS and generated transgenic plants to precisely examine SID1 expression patterns. GUS staining was detected in mature leaves, young leaves, sheath, and root tips and was most abundant in mature leaves and young leaves (Fig 4B to 4H). To examine SID1 expression during the vegetative stage, we harvested young and expanding leaves from wild-type plants every 5 days from day 15 until floral transition. SID1 showed an expression pattern similar to that of RID1, with continual expression in all examined points of young leaves (S5B Fig). In expanding leaves, the transcription level of SID1 was higher than that of RID1 in less than 30-day-old seedling, and then they decreased gradually during the remaining vegetative stage (S5C Fig). Like RID1, the expression of SID1 did not show a diurnal expression pattern under either SD or LD conditions (S5D Fig). This expression of SID1 and RID1 in leaf blades indicated that their roles in flowering control for reproductive transition in rice.
Fig 4. Expression patterns of SID1 and its transcriptional activation.
(A) Transcript levels of SID1 in the indicated organs at seedling stage. The data are means ± SEM of three independent experiments. (B–H) GUS staining (blue) of distinct organs in pSID1::GUS transgenic plants: (B) root; (C) expanding leaf; (D) young leaf; (E) sheath; (F) longitudinal section of the shoot apical meristem; (G) stem; (H) floret. Scale bar, 2 mm. (I) Subcellular localization of SID1. Full-length SID1 fused with green fluorescent protein (GFP); the nuclear protein Ghd7 fused with cyan fluorescent protein (CFP) served as a nuclear marker. The two proteins were co-expressed in rice protoplasts. A bright-field image and the merged image are shown on the right. Scale bar, 10 μm. (J) Relative luciferase activities of rice protoplasts co-transfected with reporter and distinct effectors. Schemes of deletion mutants of SID1 are shown at left. Gray bars in the N-terminal region of SID1 indicate four zinc finger motifs. All luciferase activities are expressed relative to the value of GAL4 BD alone. Values represent means of three independent experiments.
Considering that SID1 encodes a C2H2-type zinc finger transcription factor, we also assayed the subcellular localization of SID1. The construct 35S::SID1::GFP was transiently transformed into rice protoplasts. The SID1-GFP exclusively co-localized with the Ghd7-CFP fusion protein (an established nuclear marker; [43]) (Fig 4I), indicating that SID1 is localized in the nucleus. We further examined the transcriptional activity of SID1 in rice protoplasts using a dual luciferase reporter (DLR) assay system. All fragments of SID1, especially its N terminus (amino acids 1-251aa) enhanced the relative luciferase activity compared with the GAL4 binding domain negative control (Fig 4J). These results suggest that SID1 is a nuclear protein showing transcription activation activity.
SID1 is required for normal heading date in rice
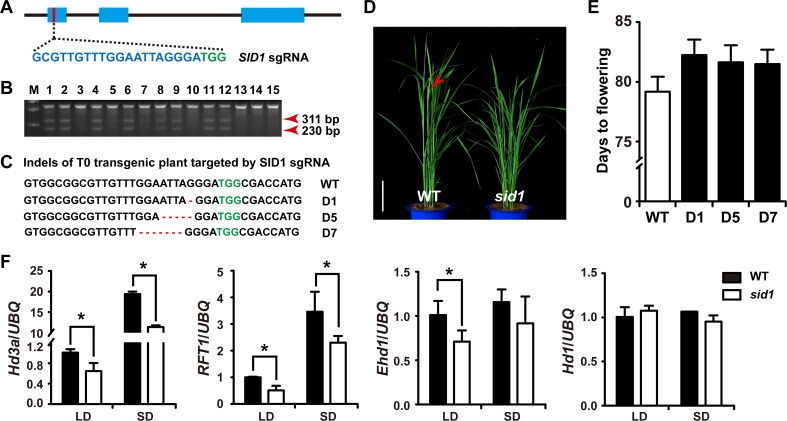
To examine the function of SID1 in rice, we generated sid1 mutants using the CRISPR-Cas9 system [44]. The construct containing the Cas9 and sgRNA targeting the first exon of SID1 was designed, and 97 transgenic plants were generated (Fig 5A). PCR amplification products containing the target region were digested by CELI enzyme to detect potential mutations (Fig 5B). Confirmation of the mutations by sequencing showed that the target region had small deletions of 1–7 bp and three mutant lines were used for further analysis (Fig 5C). T1 family of 40 homozygotes for each sid1 plants (D1, D5, D7) presented a small but statistically significant (P < 0.05) delayed flowering in NLD (82.1±1.4 days for D1; 81.6±1.4 days for D5; 81.5±1.2 days for D7) compared to the wild type (79.2±1.3 days) (Fig 5D and 5E). These results suggest that mutation of SID1 results in late heading.
Fig 5. Generation and analysis of sid1 mutants.
(A) Sites within a non-conserved region of the first exon of SID1 targeted by the CRISPR-Cas9 system. The PAM sequence (TGG) of the sgRNA target is green and the sgRNA target is cyan. (B) Outcome of CELI assay to detect CRISPR-induced mutations in 15 representative T0 transgenic rice plants. Red arrowheads indicate the fragments digested by CELI. (C) Representative sequences of mutant alleles identified from transgenic plants of the SID1 sgRNA target. D1, deletion of 1 bp; D5, deletion of 5 bp; D7, deletion of 7 bp. (D) One representative T1 transgenic plant of the SID1 sgRNA target showing a late-flowering phenotype at heading stage. Red arrowheads indicate the panicle. Scale bar, 15 cm. (E) Flowering time of sid1 and WT plants under NLD conditions. Three independent mutant lines (D1, D5, and D7) were used for analysis. Data are means ± SD (n = 40). Student’s t-test was applied to determine significant differences (P < 0.05). (F) Quantitative RT-PCR analysis of Hd3a, RFT1, Ehd1, and Hd1 in sid1 and corresponding wild type (WT) under short-day (SD) and long-day (LD) conditions. The transcript levels of each gene were normalized to the rice UBQ gene. Values are shown as means ± SEMs of three biological replicates and each with two technical repeats. Asterisks denote significant differences (P < 0.05, Student’s t test).
A previous study reported that the expression of Ehd1, Hd3a, and RFT1 were suppressed in rid1 under both SD and LD conditions [26]. Therefore, we performed qRT-PCR analysis to detect the expression levels of Ehd1, Hd3a, RFT1, and Hd1 in sid1 under SD and LD conditions (Fig 5F). Hd1 showed an almost identical expression level in sid1 and the wild type under both conditions, suggesting that SID1 had no effect on the expression of Hd1. In sid1, the transcript levels of Ehd1 were partially reduced under LD conditions. Transcript levels of Hd3a and RFT1 were largely reduced in the sid1 mutants under both conditions. These results suggest that SID1 might be involved in flowering regulation through modulation of the expression of Ehd1, Hd3a, and RFT1.
To investigate the possible regulation of Hd3a and RFT1 by SID1 and RID1, we generated transgenic plants overexpressing SID1 or RID1, respectively. The independent transgenic plants overexpressing SID1 showed a similar heading date as that in wild type (S6A to S6C Fig). Similarly, no significant changes in heading date were detected between plants with enhanced RID1 expression and wild-type plants (S6D to S6F Fig), although the transcript levels of Hd3a and RFT1 were slightly increased in some of overexpressing plants with SID1 or RID1, respectively (S6G to S6J Fig). These findings indicate that overexpression of SID1 or RID1 would not result in early flowering in rice.
Gain-of-function SID1 performs the role of flowering transition in rid1
Because overexpression of SID1 rescued the failed flowering transition in rid1, we wondered whether overexpression of SID1 would recover flowering pathways activated by RID1. As shown in Fig 6, the transcript levels of Hd3a, RFT1, and Ehd1 were completely suppressed in rid1 under either LD or SD conditions, which were the same results as in our previous investigation [26]. However, in sid1-D background, with the overexpression of SID1, the expression of Hd3a, RFT1, and Ehd1 were elevated and diurnal under both SDs and LDs at all-time points examined during the 24 h period (Fig 6). Because RID1 had only a slight effect on the expression of Hd1 [26], Hd1 showed identical expression patterns in rid1 and sid1-D under both conditions (Fig 6). Thus, overexpression of SID1 might take over the role of initiating the flowering transition in RID1-dependent photoperiodic flowering pathways in sid1-D.
Fig 6. Expression patterns of flowering-related genes in sid1-D.
The expression of Hd3a, RFT1, and Ehd1, but not Hd1, were elevated and diurnal in sid1-D plants under both long-day (LD) and short-day (SD) conditions. The open and filled boxes at the bottom represent the light and dark periods, respectively. The rice UBQ gene was used as the internal control. Values are shown as means ± SEMs of three biological replicates and each with two technical repeats.
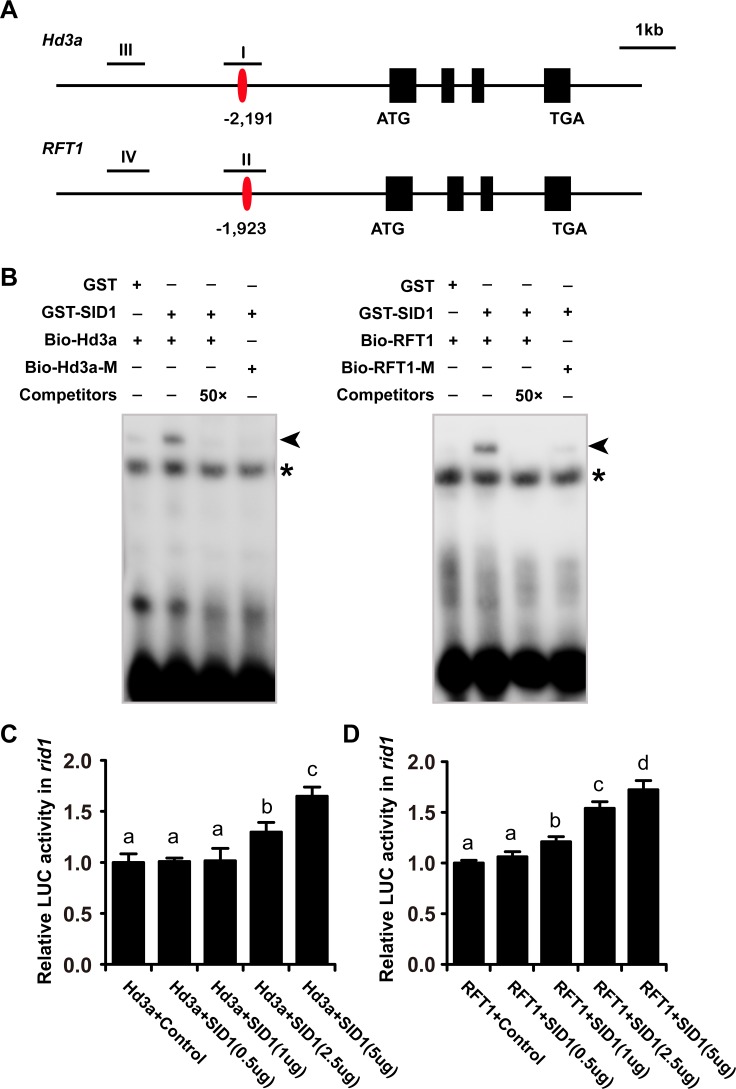
Because SID1 has a highly conserved IDD belonging to members of the plant-specific zinc finger protein family, we speculated that they might exhibit the same DNA binding characteristic. Previous experiments demonstrated maize ID1 selectively binds to the consensus motifs TTTGTCG/CT/CT/aT/aT and TTTTGTCG/C by IDD in vitro, but the consensus motifs without T in the 5' position did not affect the binding affinity of ID1 [30]. To identify possible targets of the SID1, we surveyed the consensus motifs in the promoter regions of genes controlling flowering time in rice. As shown in Fig 7A, a core sequence containing TTTGTC was found at –2191 (I region) and –1923 (II region) in the promoter regions of Hd3a and RFT1, respectively. To examine whether SID1 could directly bind to these fragments, we performed electrophoresis mobility shift assays (EMSA) to assess the potential binding ability in vitro. The recombinant SID1 protein was able to bind to the fragments containing the consensus motif TTTGTC in the promoter regions of Hd3a or RFT1, respectively (Fig 7B). However, the negative controls with mutation in TTTGTC (bio-Hd3a-M or bio-RFT1-M) abolished these binding evens (Fig 7B), indicating that SID1 could specially bind to the TTTGTC motif. The results suggest that SID1 might have the ability to drive the expression of Hd3a and RFT1.
Fig 7. SID1 is able to activate Hd3a and RFT1 expression.
(A) Schematic representation of putative loci of SID1 binding sites in the promoter of Hd3a and RFT1. The red bars represent the core sequence containing TTTGTC. The numbers indicate the position relative to the start codon. The precipitated chromatin fragments were analyzed by qPCR using four primer sets amplifying Hd3a and RFT1 regions (I, II, III, and IV) as indicated, respectively. (B) EMSA showed that SID1 could bind to the core sequence containing TTTGTC in the Hd3a and RFT1 promoter in vitro. The Hd3a and RFT1 promoter fragments containing the core sequence were incubated with GST and GST-SID1 protein in vitro. Unlabeled Hd3a and RFT1 promoter fragments were used to compete for SID1 binding. The fragment with mutated core cis-element served as the negative control. Triangles and asterisks indicate shifted bands and nonspecific binding, respectively. (C) and (D) Ratio of firefly luciferase (LUC) to Renilla luciferase (REN) activity in rid1 protoplasts transformed with varies dosage of SID1. Data represent means ± SDs (n = 4). Statistical analyses are based on a two-way analysis of variance.
We further examined the transcriptional activity of SID1 using a DLR assay system in rid1 protoplasts. Hd3a or RFT1 promoter driving the firefly luciferase gene was used as reporter and transfected into protoplasts of rid1, respectively. The construct harboring the SID1 gene driven by CaMV 35S promoter was used as the effector. With the increasing effector construct containing the SID1 gene, the luciferase activity was gradually enhanced (Fig 7C and 7D). This result further confirms that a considerable amount of SID1 has the transcriptional activation ability to drive the expression of Hd3a and RFT1 when RID1 was abolished.
RID1 directly activates the expression of Hd3a and RFT1
RID1 is also a member of the IDD family in rice, and QRT-PCR results demonstrated that transcription of Hd3a and RFT1 were seriously reduced in the rid1 mutant [26]. Likewise, we performed EMSA to test the potential interactions between RID1 and the promoters of Hd3a and RFT1. EMSA competition experiments demonstrated that the recombinant RID1 protein could bind to the fragments containing the consensus motif TTTGTC (Fig 8A). The binding activities of RID1 were abolished when the consensus motif was mutated to TTAATC (bio-Hd3a-M or bio-RFT1-M), indicating RID1 could specially bind to the fragments containing the consensus motif TTTGTC in the promoter regions of Hd3a or RFT1, respectively (Fig 8A). Next, we generated the construct ProRID1::RID1:FLAG:HA and introduced it into the rid1 mutant background by Agrobacterium-mediated transformation. The transgenic plants successfully rescued the flowering of rid1 (S7 Fig). Using the ProRID1::RID1:FLAG:HA transgenic plants, a chromatin immunoprecipitation (ChIP)-QPCR assays was carried out in the young leaves using HA antibody. As expected, the selected regions I and II of the Hd3a and RFT1 promoters were significantly enriched in young leaves (Fig 8B). These results indicate that RID1 may initiate the flowering transition through its direct targets Hd3a and RFT1.
Fig 8. RID1 directly bind to the promoter regions of Hd3a and RFT1.
(A) Gel shift assays of His and His-RID1 recombinant proteins interacting with promoter region of Hd3a and RFT1. Escherichia coli–produced recombinant RID1 protein were incubated with biotin-labeled Hd3a and RFT1 in the absence or presence of 100- or 500-fold molar excess of the unlabeled probes as competitor for the electrophoretic mobility shift assay (EMSA) reaction and analyzed by electrophoresis. The fragment with mutated core cis-element served as the negative control. (B) ChIP analysis of transgenic plants expressing RID1-FLAG-HA fusion protein. Nuclei from RID1-FLAG-HA transgenic plants’ leaves were immune precipitated by anti-HA. The precipitated chromatin fragments were analyzed by qPCR using four primer sets amplifying Hd3a and RFT1 regions (I, II, III, and IV), as indicated in Fig 7A. The input (without antibody precipitation) chromatin was analyzed and used as the control. The ChIP experiments were repeated two times using independent biological replicates with similar results, and one representative data set is shown.
Overexpressing Hd3a restored the flowering of rid1
Because the two florigen genes Hd3a and RFT1 were shown to be the direct targets of RID1, we generated transgenic plants overexpressing Hd3a to investigate whether they can rescue the flowering transition in rid1. We introduced the p35S::Hd3a construct into rid1 and obtained more than 40 transgenic plants overexpressing Hd3a (Fig 9A). Interestingly, all the positive transgenic plants reached flowering at the seedling stage (Fig 9B). Thus, overexpression of Hd3a caused early flowering in rid1.
Fig 9. Overexpressing of Hd3a and Ehd1 in rid1 plants.
(A) Expression analyses of Hd3a in p35S::Hd3a transgenic plants. Three independent transformed lines were analyzed. rid1 served as the negative control. (B) Hd3a, driven by the CaMV 35S promoter (p35S::Hd3a), rescued the never-flowering phenotype of rid1 and heading at seedling stage (T0 plants, n = 40). Top insets show magnifications of the panicles surrounded by dashed lines. Scale bar, 2 cm. (C) Expression analyses of Ehd1 in pUBQ::Ehd1 transgenic plants. Three independent transformed lines were analyzed. rid1 was used as a negative control. (D) Overexpression of Ehd1 in rid1 (pUBQ::Ehd1) could not reverse the never-flowering phenotype of rid1 (T0 plants, n = 80). All plants were grown under natural-long-day conditions. Scale bar, 15 cm.
Our previous investigation demonstrated that the expression of Ehd1 and Hd3a were completely repressed in the rid1 mutants [26]. Subsequently, we generated transgenic plants with overexpression of Ehd1 in rid1 (Fig 9C). All of the transgenic plants exhibited the never-flowering phenotype, similar to rid1 (Fig 9D). This observation shows that overexpression of Ehd1 is not sufficient to restore flowering transition in rid1. Our results further confirm that RID1-Ehd1-Hd3a/RFT1 is not the sole pathway for floral induction mediated by RID1 in rice [26].
Discussion
Our previous investigation established the rough photoperiodic flowering pathway mediated by RID1 in rice [26], but the detailed molecular mechanism of RID1 initiating the flowering transition remained unclear. In Arabidopsis and yeast, dosage suppression genetic interaction has been known to apply extensively to map functional connections among genes [45–47]. To our knowledge, this is the first report to identify a suppressor functionally related to a certain gene in rice. SID1, a suppressor of rid1, was identified as a rice flowering promoter in this study. Gain of function of SID1 led to rescue of the never-flowering phenotype in rid1. SID1 and RID1 showed the binding ability with the promoter regions of Hd3a and RFT1 to drive expression of Hd3a and RFT1. Our new findings indicate that RID1 and SID1 might be involved in the autonomous flowering pathway regulating the transition to flowering in rice.
SID1 is a flowering promoter
Although the identification of SID1 could be due to mere chance in the transgenic events, our genetic and molecular analyses clearly suggested that SID1 is required for promoting flowering in rice. SID1 encodes an IDD-type zinc finger transcription factor, preferentially expressed in mature leaves (Fig 4A), where floral inductive cues are perceived or initiated [22, 29]. Mutation in SID1 caused delayed flowering time compared to that of the wild type (Fig 5D and 5E). Overexpression of SID1 could successfully restore the flowering transition in rid1 (Fig 2C and 2D). In addition, the expression levels of Hd3a and RFT1 were greatly suppressed in sid1 (Fig 5F). However, the expression of Ehd1 was slightly reduced in sid1 plants (Fig 5F), but was almost completely repressed in the rid1 mutants [26], suggesting that the Ehd1-mediated flowering pathways may differ between rid1 and sid1 mutants. This observation coincides with evidence that rid1 shows the strongest phenotype, never flowering, whereas sid1 shows only slightly delayed flowering (Fig 5D and 5E).
RID1 acts as a master switch for floral transition. SID1 and RID1 might exert their function in the flowering transition with RID1 having priority for driving the expression of Hd3a and RFT1. When the function of RID1 is abolished, only the normal expressing level of SID1 may not enough to trigger the expression of Hd3a and RFT1, or due to non-overlapping expression patterns between SID1 and RID1. Thus, rid1 plants remain in the vegetative growth stage. However, increasing or ectopic expressing SID1 transcripts is responsible for reverting rid1 to the phase of flowering. Subsequently, we demonstrated that florigen genes, Hd3a and RFT1, are up-regulated in sid1-D (Fig 6). Furthermore, SID1 binds Hd3a and RFT1 promoter region in vitro (Fig 7B), and the LUC activity enhanced with increasing levels of SID1 in rid1 protoplasts (Fig 7C and 7D). These evidences support the function of SID1 recovering, at least in part, the SID1-Hd3a/RFT1 pathway to elicit flowering when RID1 is abolished. Expression of Ehd1 was also elevated in sid1-D plants (Fig 6), suggests that other pathways regulated by RID1 may also be activated by overexpression of SID1.
Roles of IDD protein in promoting flowering transition
Both SID1 and RID1 are IDD zinc finger proteins. Proteins containing an IDD comprise a family of zinc finger transcription factors that are unique to plants [28]. The recognition of the DNA consensus sequence is likely to be mediated by the zinc finger modules located in the IDD [30]. The highly conserved IDD is composed of four putative zinc finger domains with spacer sequences between them [28, 30]. In vitro DNA binding experiments showed that the second and third zinc fingers in the IDD are required for interaction with the DNA consensus motif [30]. Moreover, a different spacer between these zinc fingers modules in the IDD does not alter DNA binding specificity [30]. In this study, genetic evidences suggest that overexpression of SID1, OsIDD1 or OsIDD6 could restore the rid1 mutant to flowering successfully. We propose the function of SID1, OsIDD1, and OsIDD6 are redundant and that overexpression any of them could take the place of RID1 to initiate the flowering transition when RID1 is absent. However, because mutation of any of zinc fingers of SID1 abolished rescuing the never-flowering phenotype of rid1 (S4A and S4B Fig), suggesting that the first and fourth zinc fingers in the IDD also have critical roles in mediating DNA-protein interactions. Given that SID1, OsIDD1, and OsIDD6 are some co-expression (S8A and S8B Fig) and sid1 null mutants displayed moderate late flowering phenotypes (Fig 5D and 5E), future research is needed to develop the double and triple mutants to understand whether these OsIDDs coordinately modulate flowering time or not.
RID1 may participate in flowering transition through an autonomous pathway in rice
Our previous investigation indicated that RID1 activates the expression of florigen genes (Hd3a, RFT1) mainly by regulating the expression of Ehd1 and Hd1 [26]. These findings suggest that RID1 is involved in two independent photoperiod pathways, mediated by Ehd1 and Hd1, respectively. However, plants harboring nonfunctional alleles of Hd1 and Ehd1 still flower under either SD or LD conditions [48], demonstrating that RID1 may play a role in alternative flowering pathway(s) for the flowering transition. In this study, overexpression of Ehd1 could not reverse the never-flowering phenotype of rid1 (Fig 9C and 9D), further verifying this speculation.
A striking finding of our study is that Hd3a and RFT1 are the direct targets of RID1 (Fig 8). Transcription of Hd3a and RFT1 was completely repressed in rid1 [26], and genetic analysis showed that ectopic expression of Hd3a could reverse the never-flowering phenotype of rid1 (Fig 9A and 9B), indicating that florigen genes indeed act downstream of RID1. Furthermore, ChIP investigations indicated that RID1 could bind the promoter region of Hd3a and RFT1 in young leaves (Fig 8B), suggested that RID1 initiates the expression of Hd3a and RFT1 most likely occurred in the early vegetative stages. Hd3a and RFT1, two florigens in rice, be synthesized in leaves and transported to the shoot apex, and induced flowering [49–51]. As previously reported, photoperiod variation, temperature, gibberellin, age, and nutrition have been implicated in floral induction [11, 52]; thus, the transition to flowering is complex and involves the convergence of multiple signals onto the florigen genes. RID1 acts as the master switch of phase transition and may function in initiating the expression of florigen genes (Hd3a and RFT1) at the early stage, and then several floral induction cues converge to accumulate florigens to promote floral transition.
Indeed, RID1 expression was detected most abundantly in young leaves at early seedling stages and is unaffected by photoperiod, indicating that RID1 may regulate an autonomous signal for flowering transition. Recent transcription and metabolism analyses showed that maize ID1 actually affects primary carbohydrate metabolism–related genes’ function and is closely associated with florigen production in maize mature leaves [39]. Although no clear ortholog of RID1 exists in Arabidopsis, the AtIDD8 was found to regulate sugar transport and metabolism contributing to photoperiodic flowering time [31]. Therefore, mediation of autonomous floral induction by RID1 may involve coordinating the state of carbohydrate metabolism in rice. Characterizing the transcript and metabolite signature changes in rid1 would further provide clues to help us further understand the mechanism(s) underlying the vegetative–reproductive phase transition in rice.
Materials and methods
Plant materials and growth conditions
The rice variety used in this study was Oryza sativa subsp. japonica ‘Zhonghua11’ (ZH11). Plants were grown under NLD conditions in the experimental field during the rice growing season of Huazhong Agriculture University in Wuhan, China, and in a greenhouse during the winter. All transgenic plants were grown under similar growth conditions. Plants were grown in controlled-growth chambers (Conviron) under SD (10 h light at 26°C/14 h dark at 24°C) or LD (14 h light at 26°C/10 h dark at 24°C) conditions with a relative humidity of 70%. The light intensity was 800 μmol m-2 s-1.
Plasmid construction and rice transformation
To generate pUBQ::OsIDDs transgenic plants, the OsIDD genomic DNA sequence was amplified and then cloned into pU2301, which was modified from pC2301 vector with the maize Ubiquitin promoter, and then verified by sequencing. An empty pU2301 vector was used as a negative control. For overexpression of Ehd1, the Ehd1 genomic DNA sequence was amplified with primer pair Ehd1-OX-F/Ehd1-OX-R and then cloned into pU2301 by KpnI-BamHI digestion. For overexpression of Hd3a, the Hd3a genomic DNA sequence was amplified with primer pair Hd3a-OX-F/Hd3a-OX-R and then cloned into pS2300 by XbaI-KpnI digestion. To obtain ProRID1::RID1:FLAG:HA transgenic plants, genomic fragments containing the RID1 promoter and coding region lacking a stop codon were amplified with primer pair PFA2300-RID1-F/PFA2300-RID1-R and then cloned in frame into pFA2300 (kindly provided by Saifeng Cheng, Huazhong Agricultural University) by KpnI digestion. The constructs were introduced into Agrobacterium tumefaciens EHA105 and homozygous callus from rid1 was used as the transformation recipient.
To generate pUBQ::SID1(cDNA) and pUBQ::RID1(cDNA) transgenic plants, the coding sequences were amplified by RT-PCR and ligated into the pEASY-T3 vector (TransGen Biotech), and then verified by sequencing. The resulting plasmids were used as templates. Full-length cDNA of SID1 were amplified with primer pair SID1(CDs)-OX-F/SID1(CDs)-OX-R and then cloned into pU2301 by KpnI-BamHI digestion; full-length cDNA of RID1 was amplified with primer pair RID1(cDNA)-OX-F/RID1(cDNA)-OX-R and then cloned into pU2301 by KpnI-BamHI digestion. To investigate the tissue-specific expression of SID1, approximately 3-kb promoter fragments of SID1 were amplified from genomic DNA and then cloned into pC2300-EX-GUS [53] to create pSID1::GUS. To introduce targeted mutations in SID1 protein, sgRNA:Cas9 expression vector of the SID1 gene was constructed as described previously [44]. The constructs were introduced into A. tumefaciens EHA105 and transformed into the callus derived from ZH11. All primers used for genotyping and vector construction are listed in S1 Table.
Site-directed mutagenesis
Site-directed mutagenesis was introduced by three-step PCR. Full-length SID1 CDs in pEASY-T3 vector were used as templates in the first and second PCR amplifications. In the first PCR, the forward primer SID1(CDs)-OX-F and reverse primers containing the desired mutation were used. In the second PCR, the forward primers containing the desired mutation, which was the complement sequence of the first PCR reverse primer, and reverse primer SID1(CDs)-OX-R were used. The first and second PCR products were purified, and this mixture was used as a template for the final PCR amplification with primers SID1(CDs)-OX-F/SID1(CDs)-OX-R. The final products were inserted into the pU2301 vector and confirmed by sequence analyses. The resulting plasmids were introduced into A. tumefaciens EHA105 and homozygous callus from rid1 was used as the transformation recipient. All primers for site-directed mutagenesis are listed in S1 Table.
Identification mutants by CRISPR-Cas9 system
Genomic DNA from individual transgenic plants was extracted for PCR analysis. The CELI assay was used to identify the potential mutations. The PCR products amplified with SID1-specific primers SID1-CE-F/SID1-CE-R from individual mutant plants were cloned into pEASY-T3 vector (TransGen Biotech) for sequencing.
GUS assay
GUS staining and imaging were carried out as described previously [26].
RT-PCR and qRT-PCR analyses
Total RNA was extracted using TRIzol reagent (Invitrogen). RNA (2 μg) was treated with RNase-free DNaseI (Invitrogen), and first-strand cDNA was synthesized by M-MLV reverse transcriptase (Invitrogen) in a volume of 150 μl. For RT-PCR analysis, 3 μl of the first-strand cDNA described above was used as a template for PCR in a reaction volume of 20 μl. GAPDH served as a control for mRNA levels. qRT-PCR was run in a total volume of 10 μl containing 4.4 μl of the reverse-transcribed product described above, 0.3 μM gene-specific primers, and 5 μl FastStart Universal SYBR Green Master (Rox) superMIX (Roche) on an Applied Biosystems ViiA 7 Real-Time PCR system or the ABI PRISM 7500 sequence detection system according to the manufacturer’s instructions. Rice Ubiquitin was set as an internal control. The measurements were obtained using the relative quantification method. All primers are listed in S1 Table.
Subcellular localization of SID1
To construct the subcellular localization plasmids, the full-length CDs of SID1 were amplified with primers SID1-pM999-F and SID1-pM999-R with EcoRI-KpnI digestion sites and inserted into pM999-GFP for fusion with the reporter gene. Rice protoplasts were isolated from 13-day-old etiolated seedlings and transformed with the tested pairs of constructs. Fluorescence in the transformed protoplasts was imaged using a confocal laser scanning microscope (TCS SP2; Leica) after incubation at 23°C for 12–16 h.
Transcriptional activity analysis
The transcriptional activity of SID1 was analyzed using the DLR assay system in rice protoplasts prepared from etiolated seedlings [54]. The firefly luciferase gene driven by the minimal TATA box of the CaMV 35S promoter following five copies of the GAL4 binding element was used as a reporter. The Renilla luciferase gene driven by CaMV 35S was used as an internal control. The different deletion fragments of SID1 were amplified and then fused with the yeast GAL4 DNA-binding domain as effectors, driven by CaMV 35S followed by the translational enhancer Ω from tobacco mosaic virus. For each assay, 2.5 μg reporter plasmid DNA, 2.5 μg effector plasmid DNA, and 0.5 μg internal control plasmid DNA were co-transfected. After incubating for 12–16 h at 23°C, the relative luciferase activity was measured using the DLR assay system and the TECAN Infinite M200 microplate reader.
To assess the specific binding and activity of Hd3a and RFT1 promoters, protoplasts were prepared from 2-week-old fully green tissue of rid1 [55]. The coding sequence of SID1 was amplified and then fused into the NONE vector, an effector plasmid driven by the CaMV 35S promoter followed with the translational enhancer Ω sequence. To generate the Hd3a::LUC and RFT1::LUC reporter genes, the Hd3a and RFT1 promoters were amplified (specific primers are listed in S1 Table) and inserted into 190-LUC vector, respectively. The Renilla luciferase gene driven by CaMV 35S was used as a normalization control. The recovery assays were performed with Hd3a::LUC or RFT1::LUC plus 35S::SID1 at various dosages, respectively.
Electrophoretic mobility shift assays
To express the RID1 protein in Escherichia coli, the CDs of RID1 were amplified with primers 32a-RID1-F and 32a-RID1-R cloned into the BamHI-EcoRI sites of the pET-32a expression vector (Novagen) and then introduced into Transetta (DE3) cells (TransGen Biotech). The target protein was purified with Ni-NTA agarose (Qiagen). To express the SID1 protein in E. coli, the CDs of SID1 were amplified with primers pGEX-4T-SID1-F and pGEX-4T-SID1-R cloned into the BamHI-EcoRI sites of the pGEX-4T-1 expression vector (GE Healthcare) and then introduced into Transetta (DE3) cells (TransGen Biotech). The target protein was purified with GST Fast Flow (GE Healthcare).
The Hd3a promoter (including the consensus motif TTTGTC), Hd3a-M promoter (with nucleotide TTAATC replacement in the consensus motifs), RFT1 promoter (including the consensus motif TTTGTC), and RFT1-M promoter (with nucleotide TTAATC replacement in the consensus motifs) were produced by annealing of oligonucleotides with biotin 5'-end labeled Hd3a-EMSA-F/R, Hd3a-EMSA-MF/MR, RFT1-EMSA-F/R, and RFT1-EMSA-MF/MR, respectively. For each reaction, 50 fmol biotin-labeled probes were incubated with the His-RID1 or GST-SID1 protein in the binding buffer (10 mM Tris, 50 mM KCl, 10 μM ZnCl2, 1 mM DTT, 1 μg/μl poly(dI-dC), 0.1% BSA, 2.5% glycerol, and 0.05% NP-40) for 30 min on ice using the LightShift Chemiluminescent EMSA kit. After incubation, the DNA–protein complex was separated by 6% native polyacrylamide gel electrophoresis. After separation, the signal of biotin was developed using the Chemiluminescent Nucleic Acid Detection Module (Thermo, USA) according to the manufacturer’s protocol. Images were visualized on Tanon-5200 Chemiluminescent Imaging System (Tanon Science and Technology).
Chromatin co-immunoprecipitation assay
Chromatin co-immunoprecipitations (ChIPs) were performed as described previously [56]. In brief, the young leaves of ProRID1::RID1:FLAG:HA transgenic plants were fixed in formaldehyde in a vacuum. The chromatin solution was sonicated and the soluble chromatin fragments were obtained from isolated nuclei. Pre-adsorption with Dynabeads protein G (Invitrogen) was performed to remove nonspecific binding DNA. Immunoprecipitation with anti-HA specific antibody (Pierce HA Tag IP/Co-IP; #26180) and IgG were performed as described previously [56]. Immunoprecipitated DNA was analyzed by qRT-PCR, and the primers are listed in S1 Table online.
Supporting information
(A) Comparison of leaf emergence rates between rid1 and sid1-D plants under both short-day (SD) and long-day (LD) conditions during development (mean ± SD, n = 8). Arrow indicates the flowering time of sid1-D plants. (B) Flowering time of sid1-D and heterozygote (HETE) plants under distinct day length conditions (n = 10). NLD, natural long day.
(TIF)
(A) Linkage analysis of the T-DNA and sid1-D phenotype. P1 to P3 primers, which were described by Wu et al. [26], were used to ensure the tested plants are in the rid1 mutant background. P4 to P6 primers indicate the PCR primers used for genotyping the re-introduced T-DNA in sid1-D. (B) Schematic representation of the construct used for overexpression of OsIDD4; the construct was named pUBQ::OsIDD4. (C) Plants transformed with empty vector (negative control) retained a never-flowering phenotype similar to that of rid1. All positive transgenic T1 plants (left) derived from a transgenic T0 line can flower normally, whereas all negative segregants never flowered (right). Scale bar, 15 cm. (D) Co-segregation between flowering time and the transgenic fragment in T1 segregants derived from a single copy restored line (T0).
(TIF)
(A) A phylogenetic tree of rice IDD family proteins based on IDD domain sequences. Phylogenetic analysis was conducted using MEGA 5.1. Fifteen IDD proteins were selected for establishing a bootstrap neighbor-joining phylogenetic tree and 1000 replicates were conducted to determine the statistical support for each node. (B) Alignment of amino acid sequences of RID1and SID1 proteins. The identical amino acids are shown with white text on a black background. Underlines show the position of putative zinc finger domain (ID domain). The putative nuclear localization signal motifs (NLS) are shown by blue bars.
(TIF)
(A) Schematic diagram of mutant SID1 protein used in transgenic experiments. Structure of the ID domain to SID1 is shown on top. Zinc fingers (Z1 to Z4) are indicated as colored boxes. C and H indicate cysteines and histidines that define putative zinc fingers. Numbers indicate the amino acid position of C and H residues of the SID1 protein. Zinc fingers were disrupted by replacing the first cysteine pair of each module with two alanine residues: Z1M (C97A, C100A), Z2M (C139A, C144A), Z3M (C174A, C177A), and Z4M (C201A, C203A) represent ID domain proteins with mutant versions of Z1, Z2, Z3, and Z4, respectively. X indicates each putative zinc finger was disrupted. (B) Mutating each zinc finger of SID1 could not rescue the never-flowering phenotype of rid1. A normal SID1 CDs overexpression plant served as the positive control. Scale bar, 15 cm.
(TIF)
(A) 35-day-old wild-type plants (Zhonghua 11) grown under natural-long-day (NLD) conditions were used for qRT-PCR. ML1, newly emerging leaf; ML2, expanding leaf; ML3, fully expanded leaf; ML1, ML2, and ML3 collectively referred to as mature leaf (ML); YL, young leaf; ASA, around the shoot apex. Scale bar, 15 cm. (B) and (C) Expression analyses of RID1 and SID1 in young and mature leaves under NLD conditions during vegetative stage. (D) Rhythmic expression of SID1. The rice Ubiquitin (UBQ) gene served as the internal control. Values are shown as means ± SEMs of three independent experiments. The open and filled bars at the top represent the light and dark periods, respectively.
(TIF)
(A) Transcript analyses of SID1 in SID1-OX lines. Samples were harvested from 35-day-old plants under natural-long-day (NLD) conditions. Ubiquitin served as a control. Values are means ± SEMs of three replicate samples. (B) Days to flowering under long-day (LD, left) and short-day (SD, right) conditions. Black boxes, segregating wild type (WT); empty boxes, SID1-overexpressing lines (n = 10). (C) Phenotypes of segregating WT (left) and SID1-OX (right) plants at heading stage. Scale bar, 15 cm. (D) Transcript analyses of RID1 in RID1-OX lines. Samples were harvested from 35-day-old plants under NLD conditions. Ubiquitin served as a control. Values are means ± SEMs of three replicate samples. (E) Days to flowering under NLD conditions. Black boxes, segregating WT; empty boxes, SID1-overexpressing lines (n = 10). (F) Phenotypes of segregating WT (left) and RID1-OX (right) plants at heading stage. Scale bar, 15 cm. (G–J) Quantitative RT-PCR analysis of Hd3a and RFT1 in SID1 and RID1 overexpressing plants under NLD conditions. The transcript levels of each gene were normalized to the rice UBQ gene. Values are shown as means ± SEMs of three independent experiments.
(TIF)
(A) Phenotypes of rid1 and ProRID1::RID1:FLAG:HA transgenic plants at heading stage. Scale bar, 15 cm. (B) Co-segregation between flowering time and the transgenic fragment in T1 segregants derived from a single copy restored line (T0). (C) Protein level of RID1 in rid1 and ProRID1::RID1:FLAG:HA transgenic plants.
(TIF)
(A) Transcript levels of OsIDD1 in the indicated organs (S5A Fig). The data shown are the means ± SEMs of three independent experiments. (B) Transcript levels of OsIDD6 in the indicated organs. The data shown are the means ± SEMs of three independent experiments.
(TIF)
(DOCX)
Acknowledgments
We thank Prof. Shouyi Chen for providing the DLR assay system, Prof. Jiankang Zhu for providing the CRISPR/Cas9 system, Yong Qiu for providing the sid1-D mutant plants, Lun Zhao for providing the plasmid pS2300 and pC2300-EX-GUS, Saifeng Cheng for providing the plasmid pFA2300, Yanfen Zhu for providing the plasmid pM999-GFP, Lei Wang for providing the plasmid pM999-CFP-Ghd7. We also thank Drs Xingwang Li and Lun Zhao for helpful discussion and suggestion.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was funded by the National Key Research and Development Program of China (2016YFD0100903), National Natural Science Foundation of China (31370222, 31425018), National Program on the Development of Basic Research (2013CB126900), Specialized Research Fund for the Doctoral Program of Higher Education (20130146110012) and Fundamental Research Funds for the Central Universities (2015PY003, 2013PY062). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Colasanti J, Sundaresan V. ‘Florigen’ enters the molecular age: long-distance signals that cause plants to flower. Trends Biochem Sci. 2000; 25(5):236–40. [DOI] [PubMed] [Google Scholar]
- 2.Baurle I, Dean C. The timing of developmental transitions in plants. Cell. 2006;125(4):655–64. 10.1016/j.cell.2006.05.005 [DOI] [PubMed] [Google Scholar]
- 3.Greenup A, Peacock WJ, Dennis ES, Trevaskis B. The molecular biology of seasonal flowering-responses in Arabidopsis and the cereals. Ann Bot. 2009; 103(8):1165–72. 10.1093/aob/mcp063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amasino RM, Michaels SD. The timing of flowering. Plant Physiol. 2010; 154(2):516–20. 10.1104/pp.110.161653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abe M, Kobayashi Y, Yamamoto S, Daimon Y, Yamaguchi A, Ikeda Y, et al. FD, a bZIP protein mediating signals from the floral pathway integrator FT at the shoot apex. Science. 2005; 309(5737):1052–6. 10.1126/science.1115983 [DOI] [PubMed] [Google Scholar]
- 6.Wigge PA, Kim MC, Jaeger KE, Busch W, Schmid M, Lohmann JU, et al. Integration of spatial and temporal information during floral induction in Arabidopsis. Science. 2005; 309(5737):1056–9. 10.1126/science.1114358 [DOI] [PubMed] [Google Scholar]
- 7.Corbesier L, Vincent C, Jang S, Fornara F, Fan Q, Searle I, et al. FT protein movement contributes to long-distance signaling in floral induction of Arabidopsis. Science. 2007; 316(5827):1030–3. 10.1126/science.1141752 [DOI] [PubMed] [Google Scholar]
- 8.Jaeger KE, Wigge PA. FT protein acts as a long-range signal in Arabidopsis. Curr Biol. 2007; 17(12):1050–4. 10.1016/j.cub.2007.05.008 [DOI] [PubMed] [Google Scholar]
- 9.Zeevaart JA. Leaf-produced floral signals. Curr Opin Plant Biol. 2008; 11(5):541–7. 10.1016/j.pbi.2008.06.009 [DOI] [PubMed] [Google Scholar]
- 10.Wahl V, Ponnu J, Schlereth A, Arrivault S, Langenecker T, Franke A, et al. Regulation of flowering by trehalose-6-phosphate signaling in Arabidopsis thaliana. Science. 2013; 339(6120):704–7. 10.1126/science.1230406 [DOI] [PubMed] [Google Scholar]
- 11.Fornara F, de Montaigu A, Coupland G. SnapShot: Control of flowering in Arabidopsis. Cell. 2010; 141(3):550, e1–2. 10.1016/j.cell.2010.04.024 [DOI] [PubMed] [Google Scholar]
- 12.Tsuji H, Taoka K, Shimamoto K. Regulation of flowering in rice: two florigen genes, a complex gene network, and natural variation. Curr Opin Plant Biol. 2011; 14(1):45–52. 10.1016/j.pbi.2010.08.016 [DOI] [PubMed] [Google Scholar]
- 13.Tsuji H, Taoka K, Shimamoto K. Florigen in rice: complex gene network for florigen transcription, florigen activation complex, and multiple functions. Curr Opin Plant Biol. 2013; 16(2):228–35. 10.1016/j.pbi.2013.01.005 [DOI] [PubMed] [Google Scholar]
- 14.Shrestha R, Gomez-Ariza J, Brambilla V, Fornara F. Molecular control of seasonal flowering in rice, Arabidopsis and temperate cereals. Ann Bot. 2014; 114(7):1445–58. 10.1093/aob/mcu032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Song YH, Shim JS, Kinmonth-Schultz HA, Imaizumi T. Photoperiodic flowering: time measurement mechanisms in leaves. Annu Rev Plant Biol. 2015; 66:441–64. 10.1146/annurev-arplant-043014-115555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao H, Zheng XM, Fei G, Chen J, Jin M, Ren Y, et al. Ehd4 encodes a novel and Oryza-genus-specific regulator of photoperiodic flowering in rice. PLoS Genet. 2013; 9(2):e1003281 10.1371/journal.pgen.1003281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujino K, Yamanouchi U, Yano M. Roles of the Hd5 gene controlling heading date for adaptation to the northern limits of rice cultivation. Theor Appl Genet. 2013; 126(3):611–8. 10.1007/s00122-012-2005-5 [DOI] [PubMed] [Google Scholar]
- 18.Gomez-Ariza J, Galbiati F, Goretti D, Brambilla V, Shrestha R, Pappolla A, et al. Loss of floral repressor function adapts rice to higher latitudes in Europe. J Exp Bot. 2015; 66(7):2027–39. 10.1093/jxb/erv004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang B, Liu X, Xu W, Chang J, Li A, Mao X, et al. Novel function of a putative MOC1 ortholog associated with spikelet number per spike in common wheat. Sci Rep. 2015; 5:12211 10.1038/srep12211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Izawa T. Adaptation of flowering-time by natural and artificial selection in Arabidopsis and rice. J Exp Bot. 2007; 58(12):3091–7. 10.1093/jxb/erm159 [DOI] [PubMed] [Google Scholar]
- 21.Hayama R, Yokoi S, Tamaki S, Yano M, Shimamoto K. Adaptation of photoperiodic control pathways produces short-day flowering in rice. Nature. 2003; 422(6933):719–22. 10.1038/nature01549 [DOI] [PubMed] [Google Scholar]
- 22.Turck F, Fornara F, Coupland G. Regulation and identity of florigen: FLOWERING LOCUS T moves center stage. Annu Rev Plant Biol. 2008; 59:573–94. 10.1146/annurev.arplant.59.032607.092755 [DOI] [PubMed] [Google Scholar]
- 23.Xue W, Xing Y, Weng X, Zhao Y, Tang W, Wang L, et al. Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet. 2008; 40(6):761–7. 10.1038/ng.143 [DOI] [PubMed] [Google Scholar]
- 24.Matsubara K, Yamanouchi U, Wang ZX, Minobe Y, Izawa T, Yano M. Ehd2, a rice ortholog of the maize INDETERMINATE1 gene, promotes flowering by up-regulating Ehd1. Plant Physiol. 2008; 148(3):1425–35. 10.1104/pp.108.125542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park SJ, Kim SL, Lee S, Je BI, Piao HL, Park SH, et al. Rice Indeterminate 1 (OsId1) is necessary for the expression of Ehd1 (Early heading date 1) regardless of photoperiod. Plant J. 2008; 56(6):1018–29. 10.1111/j.1365-313X.2008.03667.x [DOI] [PubMed] [Google Scholar]
- 26.Wu C, You C, Li C, Long T, Chen G, Byrne ME, et al. RID1, encoding a Cys2/His2-type zinc finger transcription factor, acts as a master switch from vegetative to floral development in rice. Proc Natl Acad Sci USA. 2008; 105(35):12915–20. 10.1073/pnas.0806019105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matsubara K, Yamanouchi U, Nonoue Y, Sugimoto K, Wang ZX, Minobe Y, et al. Ehd3, encoding a plant homeodomain finger-containing protein, is a critical promoter of rice flowering. Plant J. 2011; 66(4):603–12. 10.1111/j.1365-313X.2011.04517.x [DOI] [PubMed] [Google Scholar]
- 28.Colasanti J, Tremblay R, Wong AY, Coneva V, Kozaki A, Mable BK. The maize INDETERMINATE1 flowering time regulator defines a highly conserved zinc finger protein family in higher plants. BMC Genomics. 2006; 7:158 10.1186/1471-2164-7-158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Colasanti J, Yuan Z, Sundaresan V. The indeterminate gene encodes a zinc finger protein and regulates a leaf-generated signal required for the transition to flowering in maize. Cell. 1998; 93(4):593–603. [DOI] [PubMed] [Google Scholar]
- 30.Kozaki A, Hake S, Colasanti J. The maize ID1 flowering time regulator is a zinc finger protein with novel DNA binding properties. Nucleic Acids Res. 2004; 32(5):1710–20. 10.1093/nar/gkh337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seo PJ, Ryu J, Kang SK, Park CM. Modulation of sugar metabolism by an INDETERMINATE DOMAIN transcription factor contributes to photoperiodic flowering in Arabidopsis. Plant J. 2011; 65(3):418–29. 10.1111/j.1365-313X.2010.04432.x [DOI] [PubMed] [Google Scholar]
- 32.Cui H, Levesque MP, Vernoux T, Jung JW, Paquette AJ, Gallagher KL, et al. An evolutionarily conserved mechanism delimiting SHR movement defines a single layer of endodermis in plants. Science. 2007; 316(5823):421–5. 10.1126/science.1139531 [DOI] [PubMed] [Google Scholar]
- 33.Welch D, Hassan H, Blilou I, Immink R, Heidstra R, Scheres B. Arabidopsis JACKDAW and MAGPIE zinc finger proteins delimit asymmetric cell division and stabilize tissue boundaries by restricting SHORT-ROOT action. Gene Dev. 2007; 21(17):2196–204. 10.1101/gad.440307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassan H, Scheres B, Blilou I. JACKDAW controls epidermal patterning in the Arabidopsis root meristem through a non-cell-autonomous mechanism. Development. 2010; 137(9):1523–9. 10.1242/dev.048777 [DOI] [PubMed] [Google Scholar]
- 35.Feurtado JA, Huang D, Wicki-Stordeur L, Hemstock LE, Potentier MS, Tsang EW, et al. The Arabidopsis C2H2 zinc finger INDETERMINATE DOMAIN1/ENHYDROUS promotes the transition to germination by regulating light and hormonal signaling during seed maturation. Plant Cell. 2011; 23(5):1772–94. 10.1105/tpc.111.085134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cui D, Zhao J, Jing Y, Fan M, Liu J, Wang Z, et al. The Arabidopsis IDD14, IDD15, and IDD16 cooperatively regulate lateral organ morphogenesis and gravitropism by promoting auxin biosynthesis and transport. PLoS Genet. 2013; 9(9):e1003759 10.1371/journal.pgen.1003759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fukazawa J, Teramura H, Murakoshi S, Nasuno K, Nishida N, Ito T, et al. DELLAs function as coactivators of GAI-ASSOCIATED FACTOR1 in regulation of gibberellin homeostasis and signaling in Arabidopsis. Plant Cell. 2014; 26(7):2920–38. 10.1105/tpc.114.125690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshida H, Hirano K, Sato T, Mitsuda N, Nomoto M, Maeo K, et al. DELLA protein functions as a transcriptional activator through the DNA binding of the indeterminate domain family proteins. Proc Natl Acad Sci USA. 2014; 111(21):7861–6. 10.1073/pnas.1321669111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Coneva V, Guevara D, Rothstein SJ, Colasanti J. Transcript and metabolite signature of maize source leaves suggests a link between transitory starch to sucrose balance and the autonomous floral transition. J Exp Bot. 2012; 63(14):5079–92. 10.1093/jxb/ers158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wong AY, Colasanti J. Maize floral regulator protein INDETERMINATE1 is localized to developing leaves and is not altered by light or the sink/source transition. J Exp Bot. 2007; 58(3):403–14. 10.1093/jxb/erl206 [DOI] [PubMed] [Google Scholar]
- 41.Coneva V, Zhu T, Colasanti J. Expression differences between normal and indeterminate1 maize suggest downstream targets of ID1, a floral transition regulator in maize. J Exp Bot. 2007; 58(13):3679–93. 10.1093/jxb/erm217 [DOI] [PubMed] [Google Scholar]
- 42.Liu YG, Mitsukawa N, Oosumi T, Whittier RF. Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J. 1995; 8(3):457–63. [DOI] [PubMed] [Google Scholar]
- 43.Li X, Gao X, Wei Y, Deng L, Ouyang Y, Chen G, et al. Rice APOPTOSIS INHIBITOR5 coupled with two DEAD-box adenosine 5'-triphosphate-dependent RNA helicases regulates tapetum degeneration. Plant Cell. 2011; 23(4):1416–34. 10.1105/tpc.110.082636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feng Z, Zhang B, Ding W, Liu X, Yang DL, Wei P, et al. Efficient genome editing in plants using a CRISPR/Cas system. Cell Res. 2013; 23(10):1229–32. 10.1038/cr.2013.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Wen J, Lease KA, Doke JT, Tax FE, Walker JC. BAK1, an Arabidopsis LRR receptor-like protein kinase, interacts with BRI1 and modulates brassinosteroid signaling. Cell. 2002; 110(2):213–22. [DOI] [PubMed] [Google Scholar]
- 46.Xiao S, Dai L, Liu F, Wang Z, Peng W, Xie D. COS1: an Arabidopsis coronatine insensitive1 suppressor essential for regulation of jasmonate-mediated plant defense and senescence. Plant Cell. 2004; 16(5):1132–42. 10.1105/tpc.020370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Magtanong L, Ho CH, Barker SL, Jiao W, Baryshnikova A, Bahr S, et al. Dosage suppression genetic interaction networks enhance functional wiring diagrams of the cell. Nat Biotechnol. 2011; 29(6):505–11. 10.1038/nbt.1855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Doi K, Izawa T, Fuse T, Yamanouchi U, Kubo T, Shimatani Z, et al. Ehd1, a B-type response regulator in rice, confers short-day promotion of flowering and controls FT-like gene expression independently of Hd1. Gene Dev. 2004; 18(8):926–36. 10.1101/gad.1189604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tamaki S, Matsuo S, Wong HL, Yokoi S, Shimamoto K. Hd3a protein is a mobile flowering signal in rice. Science. 2007;316(5827):1033–6. 10.1126/science.1141753 [DOI] [PubMed] [Google Scholar]
- 50.Komiya R, Yokoi S, Shimamoto K. A gene network for long-day flowering activates RFT1 encoding a mobile flowering signal in rice. Development. 2009; 136(20):3443–50. 10.1242/dev.040170 [DOI] [PubMed] [Google Scholar]
- 51.Taoka K, Ohki I, Tsuji H, Furuita K, Hayashi K, Yanase T, et al. 14-3-3 proteins act as intracellular receptors for rice Hd3a florigen. Nature. 2011; 476(7360):332–5. 10.1038/nature10272 [DOI] [PubMed] [Google Scholar]
- 52.Srikanth A, Schmid M. Regulation of flowering time: all roads lead to Rome. Cell Mol Life Sci. 2011; 68(12):2013–37. 10.1007/s00018-011-0673-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhao L, Jing X, Chen L, Liu Y, Su Y, Liu T, et al. Tribenuron-methyl induces male sterility through anther-specific inhibition of acetolactate synthase leading to autophagic cell death. Mol Plant. 2015; 8(12):1710–24. 10.1016/j.molp.2015.08.009 [DOI] [PubMed] [Google Scholar]
- 54.Hao YJ, Song QX, Chen HW, Zou HF, Wei W, Kang XS, et al. Plant NAC-type transcription factor proteins contain a NARD domain for repression of transcriptional activation. Planta. 2010; 232(5):1033–43. 10.1007/s00425-010-1238-2 [DOI] [PubMed] [Google Scholar]
- 55.Bart R, Chern M, Park CJ, Bartley L, Ronald PC. A novel system for gene silencing using siRNAs in rice leaf and stem-derived protoplasts. Plant Methods. 2006; 2:13 10.1186/1746-4811-2-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao Y, Cheng S, Song Y, Huang Y, Zhou S, Liu X, et al. The interaction between rice ERF3 and WOX11 promotes crown root development by regulating gene expression involved in cytokinin signaling. Plant Cell. 2015; 27(9):2469–83. 10.1105/tpc.15.00227 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Comparison of leaf emergence rates between rid1 and sid1-D plants under both short-day (SD) and long-day (LD) conditions during development (mean ± SD, n = 8). Arrow indicates the flowering time of sid1-D plants. (B) Flowering time of sid1-D and heterozygote (HETE) plants under distinct day length conditions (n = 10). NLD, natural long day.
(TIF)
(A) Linkage analysis of the T-DNA and sid1-D phenotype. P1 to P3 primers, which were described by Wu et al. [26], were used to ensure the tested plants are in the rid1 mutant background. P4 to P6 primers indicate the PCR primers used for genotyping the re-introduced T-DNA in sid1-D. (B) Schematic representation of the construct used for overexpression of OsIDD4; the construct was named pUBQ::OsIDD4. (C) Plants transformed with empty vector (negative control) retained a never-flowering phenotype similar to that of rid1. All positive transgenic T1 plants (left) derived from a transgenic T0 line can flower normally, whereas all negative segregants never flowered (right). Scale bar, 15 cm. (D) Co-segregation between flowering time and the transgenic fragment in T1 segregants derived from a single copy restored line (T0).
(TIF)
(A) A phylogenetic tree of rice IDD family proteins based on IDD domain sequences. Phylogenetic analysis was conducted using MEGA 5.1. Fifteen IDD proteins were selected for establishing a bootstrap neighbor-joining phylogenetic tree and 1000 replicates were conducted to determine the statistical support for each node. (B) Alignment of amino acid sequences of RID1and SID1 proteins. The identical amino acids are shown with white text on a black background. Underlines show the position of putative zinc finger domain (ID domain). The putative nuclear localization signal motifs (NLS) are shown by blue bars.
(TIF)
(A) Schematic diagram of mutant SID1 protein used in transgenic experiments. Structure of the ID domain to SID1 is shown on top. Zinc fingers (Z1 to Z4) are indicated as colored boxes. C and H indicate cysteines and histidines that define putative zinc fingers. Numbers indicate the amino acid position of C and H residues of the SID1 protein. Zinc fingers were disrupted by replacing the first cysteine pair of each module with two alanine residues: Z1M (C97A, C100A), Z2M (C139A, C144A), Z3M (C174A, C177A), and Z4M (C201A, C203A) represent ID domain proteins with mutant versions of Z1, Z2, Z3, and Z4, respectively. X indicates each putative zinc finger was disrupted. (B) Mutating each zinc finger of SID1 could not rescue the never-flowering phenotype of rid1. A normal SID1 CDs overexpression plant served as the positive control. Scale bar, 15 cm.
(TIF)
(A) 35-day-old wild-type plants (Zhonghua 11) grown under natural-long-day (NLD) conditions were used for qRT-PCR. ML1, newly emerging leaf; ML2, expanding leaf; ML3, fully expanded leaf; ML1, ML2, and ML3 collectively referred to as mature leaf (ML); YL, young leaf; ASA, around the shoot apex. Scale bar, 15 cm. (B) and (C) Expression analyses of RID1 and SID1 in young and mature leaves under NLD conditions during vegetative stage. (D) Rhythmic expression of SID1. The rice Ubiquitin (UBQ) gene served as the internal control. Values are shown as means ± SEMs of three independent experiments. The open and filled bars at the top represent the light and dark periods, respectively.
(TIF)
(A) Transcript analyses of SID1 in SID1-OX lines. Samples were harvested from 35-day-old plants under natural-long-day (NLD) conditions. Ubiquitin served as a control. Values are means ± SEMs of three replicate samples. (B) Days to flowering under long-day (LD, left) and short-day (SD, right) conditions. Black boxes, segregating wild type (WT); empty boxes, SID1-overexpressing lines (n = 10). (C) Phenotypes of segregating WT (left) and SID1-OX (right) plants at heading stage. Scale bar, 15 cm. (D) Transcript analyses of RID1 in RID1-OX lines. Samples were harvested from 35-day-old plants under NLD conditions. Ubiquitin served as a control. Values are means ± SEMs of three replicate samples. (E) Days to flowering under NLD conditions. Black boxes, segregating WT; empty boxes, SID1-overexpressing lines (n = 10). (F) Phenotypes of segregating WT (left) and RID1-OX (right) plants at heading stage. Scale bar, 15 cm. (G–J) Quantitative RT-PCR analysis of Hd3a and RFT1 in SID1 and RID1 overexpressing plants under NLD conditions. The transcript levels of each gene were normalized to the rice UBQ gene. Values are shown as means ± SEMs of three independent experiments.
(TIF)
(A) Phenotypes of rid1 and ProRID1::RID1:FLAG:HA transgenic plants at heading stage. Scale bar, 15 cm. (B) Co-segregation between flowering time and the transgenic fragment in T1 segregants derived from a single copy restored line (T0). (C) Protein level of RID1 in rid1 and ProRID1::RID1:FLAG:HA transgenic plants.
(TIF)
(A) Transcript levels of OsIDD1 in the indicated organs (S5A Fig). The data shown are the means ± SEMs of three independent experiments. (B) Transcript levels of OsIDD6 in the indicated organs. The data shown are the means ± SEMs of three independent experiments.
(TIF)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.