Abstract
Several drugs have recently been reported to induce rapid antidepressant effects in clinical trials and rodent models. Although the cellular mechanisms involved remain unclear, reports suggest that increased glutamate transmission contributes to these effects. Here, we demonstrate that the antidepressant-like efficacy of three unique drugs, with reported rapid onset antidepressant properties, is coupled with a rapid transient rise in glutamate cycling in medial prefronal cortex (mPFC) of awake rats as measured by ex vivo 1H-[13C]-nuclear magnetic resonance spectroscopy. Rats were acutely pre-treated by intraperitoneal injection with a single dose of ketamine (1,3,10,30,80mg/kg), Ro 25-6981 (1,3,10mg/kg), scopolamine (5,25,100μg/kg) or vehicle (controls). At fixed times after drug injection animals received an intravenous infusion of [1,6-13C2]glucose for 8 min to enrich brain amino acid pools with 13C, followed by rapid euthanasia. The mPFC was dissected, extracted with ethanol and metabolite 13C enrichments measured. We found a clear dose dependent effect of ketamine and Ro 25-6981 on behavior and the percent of 13C-enrichment of glutamate, glutamine and GABA. Further, we also found an effect of scopolamine on both cycling and behavior. These studies demonstrate that three pharmacologically distinct classes of drugs, clinically related through their reported rapid antidepressant actions, share the common ability to rapidly stimulate glutamate cycling at doses pertinent for their antidepressant-like efficacy. We conclude that increased cycling precedes the antidepressant action at behaviorally effective doses and suggests the rapid change in cycling could be used to predict efficacy of novel agents or identify doses with antidepressant activity.
Keywords: NMDA, glutamate/glutamine cycle, ketamine, magnetic resonance spectroscopy, medial prefrontal cortex, GABA
Introduction
A growing number of studies are now reporting that a novel class of antidepressant drugs is capable of inducing a rapid antidepressant response in patients with previously treatment-resistant mood disorders1. The strongest evidence of this rapid acting antidepressant (RAAD) effect has been established for ketamine, a non-selective N-methyl-D-aspartate receptor (NMDAR) antagonist2. However, RAAD effects were also reported for other NMDAR targeting drugs, such as lanicemine, the low trapping non-subunit selective NMDA receptor channel blocker3 and CP-101,6064, a NR2B selective receptor antagonist. In addition, there is now evidence suggesting the anticholinergic drug scopolamine also possesses RAAD-like effects5.
Other findings suggest that a transient activation of AMPA receptors is a necessary event in the generation of the RAAD effects induced by NMDAR antagonists and scopolamine in rodent models6–9. In light of earlier work showing ketamine’s ability to rapidly increase glutamate efflux in rat frontal cortex10, possibly by effects on inhibitory GABAergic interneurons11, it has been hypothesized that this brief ketamine-induced surge in glutamate release is a critical event for the RAAD activity. However, the question remains whether this surge in glutamate release is a mechanism common to other drugs with RAAD-like properties, and whether the increase in glutamate release is related to the more durable behavioral antidepressant effects of these RAAD drugs.
Having previously found that sub-anesthetic doses of ketamine led to rapid increases in glutamate, GABA and glutamine cycling12, and considering previous reports of inverted-U type dose response relationships between ketamine-induced effects on glutamate efflux10, as well as antidepressant-like behavioral responses and cellular changes in rats6, we first sought to confirm and extend the dose response relationship between RAAD properties of ketamine and glutamate cycling. We next sought to determine the time dependence of ketamine’s effect on glutamate/GABA-glutamine cycling in rat medial prefrontal cortex (mPFC). Lastly, to determine whether the increase in glutamate cycling is generalizable to drugs of other classes with RAAD properties, we investigated the effects of Ro 25-6981 an NR2B selective NMDAR antagonist, and scopolamine, a muscarinic receptor M1 antagonist13 that has been shown to require AMPA activation to produce the antidepressant-like effects in rodents9, on both cycling and behavior.
1H-[13C]-nuclear magnetic resonance (NMR) spectroscopy was employed to examine the effects of the drugs on rat mPFC glutamate release and recycling into glia (glutamate cycling) and neuronal and glial energy metabolism. 13C-labeled glucose is metabolized mainly in the neuronal TCA cycle and labels neuronal glutamate and GABA, which are released and taken up by astrocytes, followed by conversion to (and labeling of) glutamine, suggesting that 13C-labeled glucose studies provide information on glutamate and GABA neurotransmitter cycling as well as neuronal (mainly) and glial (partly) cell metabolism, reflecting neurotransmitter activity12, 14, 15 (see Supplemental Figure S1). As several studies have specifically demonstrated increased rates of mPFC metabolism in rodents following treatment with sub-anesthetic doses of ketamine16–19, and similar regions are believed to mediate several of ketamine’s behavioral effects20–22, all studies were performed in rat mPFC.
MATERIALS AND METHODS
Animals
Male Sprague-Dawley rats (Charles River) weighting 180–220g were group-housed and maintained under standard conditions of constant temperature (25°C) and humidity with a 12-hour light/dark cycle, with ad libitum access to food and water. All experiments were conducted in accordance with the National Institutes of Health guidelines and protocols approved by the Yale University Animal Care and Use Committee.
Plasma Ketamine Measures
Separate groups of male rats (170–200g at delivery; n=4/group) were dosed IP with ketamine (1, 3, 10, 30 and 80mg/kg) and plasma samples were collected at time points corresponding to the mid-point of glucose utilization determinations in the study. Plasma aliquots (50μl) were treated with acetonitrile (200μl) followed by vortex mixing for 2min. The supernatant was then separated from the precipitated proteins by centrifugation (10min at 1479g), and 150μl was transferred to a 96-well plate. An aliquot of supernatant (5μl) was injected onto the ultra high-performance liquid chromatography (UHPLC) column for liquid chromatography (LC)/mass spectrometry (MS)/MS-based analysis.
NMR Experiments
Dose-response studies of ketamine, Ro 25-6981 and scopolamine
Rats (6–8 per group) were treated with different doses of ketamine reported to have antidepressant behavioral efficacy as well as lower and higher doses (1, 3, 10, 30, and 80mg/kg)6, 8, diluted in either saline (0.9%) or vehicle (vehicle) 10min prior to beginning of [1,6-13C2]glucose infusion. Ro 25-6981 (supplied by BMS) dissolved in vehicle was tested at doses of 1, 3, and 10mg/kg IP, based on previous reports of behavioral efficacy6, 23, 24, alongside the ketamine studies at 1, 30, and 80mg/kg. Effects of all treatments were compared to unique control groups receiving IP infusions of saline as previously described12 (for the ketamine 3 and 10mg) and vehicle (for ketamine 1, 30 and 80mg/kg and Ro 25-6981 1, 3 and 10mg/kg) since Ro 25-6981 was not soluble in saline. The vehicle consisted of 5% dimethyl sulfoxide (DMSO, Sigma)/10% propylene glycol/0.0375% methylcellulose/70% water. Scopolamine (Sigma) 25μg/kg was studied based on behavioral findings and previous reports of behavioral efficacy9. All drugs were administered intraperitoneally (ip) in volumes of 2mL/kg with exception of the highest ketamine dose (80mg/kg), which was 3mL/kg.
Time dependence of single-dose ketamine effects
Rats (6 rats/time point) received a single injection of ketamine (30mg/kg, IP) at 0, 10, 30 or 60min prior to [1,6-13C2] glucose infusion. The 30mg/kg dose of ketamine was chosen because it had the largest metabolic effect in the dose-response study. The effects of a single injection of ketamine (10mg/kg, IP) at 24hr was measured also in order to match the dosing previously shown to have significant effects on dendritic spine density at 24 hrs6, 25
[1,6-13C2]glucose infusions
Tail vein catheters were placed under brief isoflurane anesthesia and animals allowed to recover for at least 30min before drug/vehicle injections. Ten min after injection of ketamine, seven min after injection of scopolamine, and thirty min after injection of Ro 25-6981 or vehicle (times determined by the Cmax and onset of observable behavioral changes with each compound) a solution of [1,6-13C2]glucose (99 atom%; Cambridge Isotopes, Andover, Massachusetts) dissolved in water (0.75mol/L per 200g body weight) was infused for 8min as described previously12. Immediately following the 8 min infusion of [1,6-13C2]glucose the rats were quickly euthanized using focused-beam microwave irradiation as described in supplemental material.
Tissue extraction and NMR sample preparation
Metabolites in mPFC were extracted from frozen tissue (65–85mg) as described by Chowdhury et al.12 (see supplemental material). Brain and plasma samples were loaded into 5mm tubes for NMR analysis.
NMR spectroscopy analysis of 13C incorporation
Total concentrations and 13C enrichments of mPFC amino acids and metabolites and plasma glucose were determined from fully relaxed 1H-[13C]-NMR spectra acquired at 11.7 T (1H frequency of 500.13 MHz; Bruker AVANCE, Bruker Instruments, Billerica, MA) as described in Supplementary Material.
Behavioral tests
Drug administration for behavioral tests
Animals received a single IP injection of vehicle (saline and/or DMSO); ketamine at 3, 30 and 80mg/kg; Ro 25-6981 at 3 and 10mg/kg; or scopolamine at 5, 25 and 100μg/kg, 24 hr after the first day of swimming. Ketamine and scopolamine were dissolved with saline (0.9%). Ro 25-6981 was dissolved in solution mixed with saline: DMSO (v : v = 5.6 : 1). Animals for each drug treatment were tested for behavior using assays at different time points.
Forced Swim test
Antidepressant effects of ketamine, Ro 25-6981 and scopolamine were assessed using the forced swim test (FST). Each rat was placed in the plexiglass cylinder (65 cm in height and 30 cm in diameter) filled with 24–25°C water to a depth of 45 cm for 15min on day 1 and 10 min on day 3. On day 3 and 24 hr after the drug injections, animals were recorded from the side with a video camcorder. Time immobile was scored, separating 2 blocks of 5min for analysis. Immobility was defined as minimum movement to stay afloat.
Stereotypy
Effects of ketamine, Ro 25-6981 and scopolamine on stereotypic behaviors were assessed immediately after the injection on day 2. Animals were placed in a new cage and videotaped from the side. Oro-facial and aspecific stereotypy was scored by bins of 1min every 5min (i.e. minute 5 to 6, minute 10 to 11). Occurrence of each stereotypic behavior not their frequency for each minute bin was scored. The stereotypy counts for each animal represent the sum of stereotypic occurrence for the 5 bins. Orofacial stereotypy included mouth movement, bite, self-gnaw, jaw tremor and aspecific head stereotypy included head bob, head sway, taffy pull.
Statistical Analysis
The statistical significance of differences in a concentrations and 13C enrichments between control and ketamine, Ro 25-6981, or scopolamine treated rats were assessed using ANOVA followed by Dunnett’s multiple comparisons procedure where each treatment is compared with a single control group. Overall ANOVA effects were considered significant at p<0.05 and post-hoc tests significant at adjp≤0.05 using Dunnett’s test. FST immobility times were separated into two bins (first 5min and last 5min), and were analyzed using linear models with treatment assignment included as a between-subjects factor and time as a within-subjects factor. The group by time interaction was modeled. Stereotypic behavioral outcomes were analyzed using one-way ANOVA followed by Dunnett’s test.
RESULTS
Plasma levels for all doses of ketamine at each time point examined are shown in Supplemental table 1
Glucose concentrations were similar in plasma samples from all groups (ranging from mean±s.e.m.; 15.1±1.0 mmol l−1 to 17.8±0.5 mmol l−1, n=6–8 per group). Similar results were found for the percentage 13C enrichments: range mean±s.e.m.; 45.7±1.5% to 49.4±2.4%). In addition, total levels of amino acids and metabolites were determined in the different groups by averaging the respective [1,6-13C2]glucose infusion data; total mPFC concentrations of the metabolites examined were similar in all animal groups (See Supplemental Tables S1 and S2). These results indicate that changes in mPFC levels of the 13C-labeled amino acids found in this study are not related to general metabolic alterations or changes in total amino-acid concentrations.
Dose-response effects of ketamine and Ro 25-6981 on rat mPFC glutamate/GABA-glutamine cycling
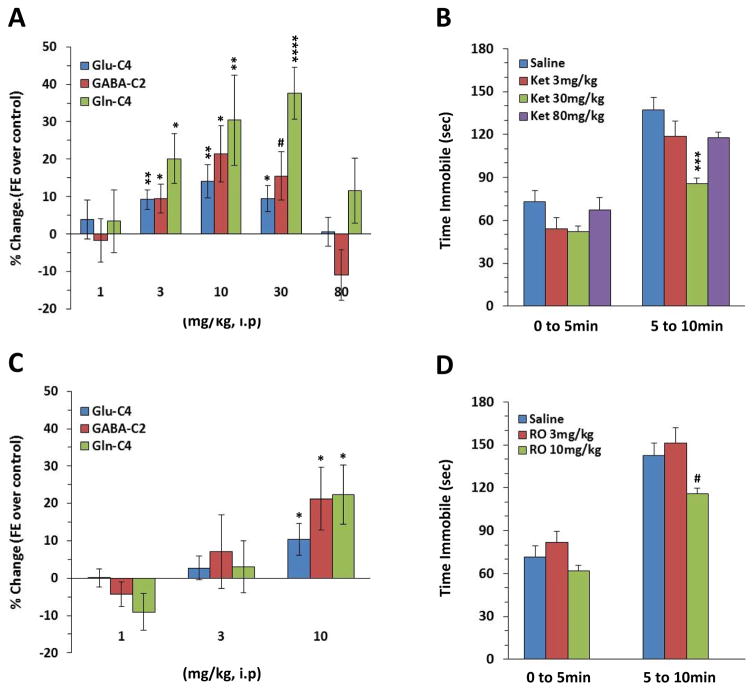
Figures 1A and 1C illustrate the dose response relationships between the percent 13C enrichment of treatment relative to controls for ketamine and R0 25-6981 respectively. Lower doses of ketamine and Ro 25-6981 (1mg/kg each) had no effect on 13C-label incorporation into glutamate-C4 (F(2,21)=0.53, p=0.60), GABA-C2 (F(2,21)=0.35, p=0.71), or glutamine-C4 (F(2,21)=1.78, p=0.19) (Supplemental Figure S2A). At the mid dose there was a significant main effect of treatment on both glutamate-C4 (F(2,20)=3.5, p<0.05) and glutamine-C4 13C enrichment (F(2,20)=15.0, p<0.0001) (Supplemental Figure S2B). Specifically, ketamine at 30mg/kg increased glutamate-C4 enrichment by 9% above vehicle [adjp <0.05], and increased glutamine-C4 enrichment by 38% above vehicle [adjp <0.0001]. There was a trend suggesting the mid doses altered GABA-C2 enrichment (F(2,20)=2.68, p=0.09), with the 30mg/kg dose of ketamine increasing enrichment by 22% [adjp =0.06]. R0 25-6981 at 3mg/kg did not show any effect on 13C enrichment of any of the amino acids [adjp >0.6, for all]. There was a significant main effect of treatment on the 13C-enrichment of all three amino acids, glutamate-C4 (F(2,23)=3.92, p<0.05), GABA-C2 (F(2,23)=8.16, p<0.01) and glutamine-C4 enrichment (F(2,23)=3.56, p<0.05) at the highest dose of the drugs tested (Supplemental Figure S2C). Here it was R0 25-6981 10mg/kg that significantly increased 13C enrichment [adjp <0.05] of all 3 amino acids, while the higher anesthetic dose of ketamine (80mg/kg) showed no effect on the enrichment [adjp ≥0.3, for all].
Figure 1. Effects of ketamine and Ro 25-6981 on mPFC glutamate/GABA-glutamine metabolism and FST behavior.
(A) Percent change in PFC glutamate-C4, GABA-C2, and glutamine-C4 fractional enrichments over an interval from 10 to 18min after i.p. doses of 1, 3, 10, 30 and 80mg/kg compared to vehicle-injected animals. (B) Ketamine 30mg/kg decreased average time immobile in the forced swim test (FST) but not 3 and 10mg/kg. The effect was more pronounced in the last 5min of the test. (C) Percent change in PFC glutamate-C4, GABA-C2, and glutamine-C4 enrichments over an interval from 30 to 38min after i.p. doses of 1, 3 and 10mg/kg Ro 25-6981 relative to vehicle-treated controls. (D) Averaged over the two blocks of 5min, the Ro 25-6981 10mg/kg animal group shows a trend for decreased time immobile with a more pronounced effect for the last 5min (#punadj=0.05 pBonferroni =0.1). Interrogation time is the 8min interval after ketamine injection during which the 13C-glucose was infused. (#p<0.1 *p<0.05; **p<0.01; ***p<0.001; ****p<0.0001).
Two additional studies examining intermediate ketamine doses of 3 (Supplemental Figure S2.D) and 10mg/kg (Supplemental Figure S2E) demonstrated effects of treatment on the 13C enrichment of glutamate-C4 (p <0.01 for both), GABA-C2 (p<0.05 for both), and glutamine-C4 (p <0.05 and p<.01 respectively) compared to their respective saline control.
Dose-response effects of ketamine and Ro 25-6981 on behavior
We were further able to align the changes in cycling with the RAAD effects of ketamine by demonstrating that the measured antidepressant-like performances in the FST 24hr after the ketamine administration mirrors the dose-dependent effects on cycling (figure 1.B). A significant overall ketamine effect was observed (F(3,25)=5.08, p<.01) owing to decreased average time immobile in the FST at 30mg/kg (adjp =0.002) but not 3 and 80mg/kg (all adjp >0.14). There was a significant interaction between group and time ((F3,25)=5.37, p<0.01) where the observed ketamine 30mg/kg effects were more pronounced during the last 5 min (pBonferroni <0.001).
An ANOVA analysis of the effects of Ro 25-6981 in the FST test revealed a significant group effect (F(2,42)=5.02, p<0.05). Averaged over the two blocks of 5min, Ro 25-6981 at 10mg/kg animal group showed a trend difference from control (punadj=0.04, adjp =0.08) with a more pronounced effect for the last 5 min (punadj=0.05 pBonferroni =0.1) (Fig. 1D).
Time dependence of ketamine effect on glutamate/GABA-glutamine cycling in rat mPFC and behavior
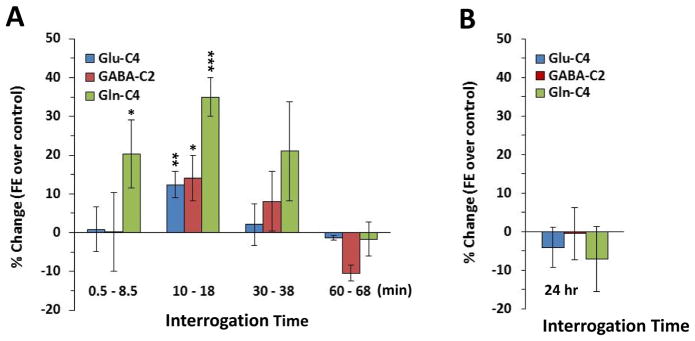
To examine the duration of ketamine’s effects on cycling we chose to examine the effects of a single 30mg/kg i.p. injection of ketamine, the dose showing the largest magnitude of effect in the dose response study above. Ketamine injection led to an elevation in glutamine-C4 13C enrichment over the 8min interval from 0.5 to 8.5min [p<0.05], 10 to 18 min [p<0.001] and 30 to 38 min [n.s., p=0.17] compared to vehicle injected rats, but not the 60 to 68min [p=0.81] time point (Figure 2A). 13C labeling of glutamate-C4 [p<0.01] and GABA-C2 [p<0.05] was significantly increased only at the 10 to 18min time point (see supplemental Figure 3 for individual 13C fractional enrichments). Thus, this post-injection period of increased 13C labeling of mPFC amino acids was transient, with a peak rise occurring in <30min and disappearing within 1 hr.
Figure 2. Effects of time after ketamine injection on glutamate, GABA and glutamine 13C labeling compared to control animals.
(A) The figure depicts the percent change in fractional enrichment compared to vehicle-injected animals over time. Amino acid labeling reflects the interval from 0.5 to 8.5min, 10 to 18min, 30 to 38min, and 60 to 68min after i.p. injection of ketamine 30mg/kg. (B) There was no significant effect of a single 10mg/kg i.p. dose of ketamine on glutamate, GABA and glutamine 13C labeling from 13C-glucose when interrogated for 8mins at 24h. (#p<0.1; *p<0.05; **p<0.01; ***p<0.001).
There was no significant effect of a single 10mg/kg i.p. dose of ketamine (dose most frequently shown to have delayed behavioral effects in tests of antidepressant action) at 24 hrs on glutamate-C4, GABA–C2 and glutamine-C4 13C labeling from 13C-glucose (Figure 2B, supplemental figure 4 for individual fractional enrichments).
Scopolamine effects on rat mPFC glutamate/GABA-glutamine behavior and cycling
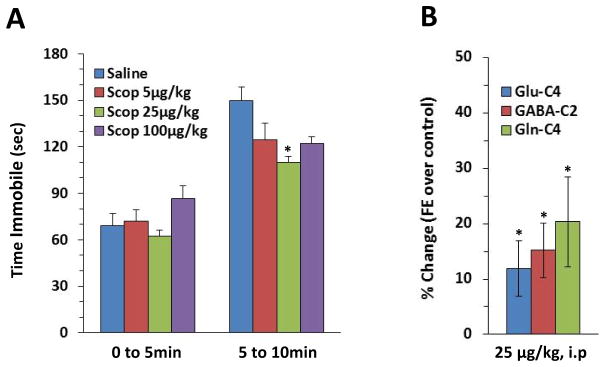
We first examined the antidepressant-like effects of scopolamine in the FST using 3 doses. Scopolamine decreased average time immobile in the FST at 25μg/kg but not 5 and 100μg/kg. There was a significant interaction between drug and time (F(3,48)=4.03, p<0.05) with a more pronounced effect with scopolamine (Scop) 25μg/kg during the last 5 min (punadj=0.007 pBonferroni =0.04) (Fig. 3A).
Figure 3. Effects of Scopolamine on FST immobility and glutamate, GABA and glutamine 13C labeling compared to control animals.
(A) Scopolamine decreased average time immobile in the FST at 25μg/kg but not 5 and 100μg/kg. Averaged over the two blocks of 5 min, there was a significant interaction between drug and time (F3,48=4.03, p<0.05) with a more pronounced effect with scopolamine (Scop) 25μg/kg for the last 5 min (*pBonferroni <0.05) when compared to the vehicle-injected animal group. (B) Effects of a single 25μg/kg i.p. dose of scopolamine on magnitude change of glutamate, GABA and glutamine 13C labeling from glucose over control (*p <0.05 when compared to vehicle-injected animal group).
A single 25μg/kg i.p. dose of scopolamine increased 13C enrichment of glutamate-C4, GABA-C2, and glutamine-C4 over control from 13C-glucose for all 3 amino acids (p<0.05 for all) (Figure 3.B, see Supplemental figure 5 for individual fractional enrichments).
Effect of ketamine, Ro 25-6981 and scopolamine on orofacial and aspecific head stereotypy
The effect of rapid acting antidepressant drugs on orofacial and head stereotypy is described in Supplemental figure S6. Ketamine increased stereotypy counts within 30min after injection; the effect was significant at the dose of 30mg/kg adjp <0.05 when compared to the vehicle-injected group. It is important to note that animals receiving ketamine at 80mg/kg were practically asleep 20 min after the injection, however they showed occurrence of more diverse stereotypic behaviors during the first few bins. In contrast, Ro 25-6981 injections had no effect on stereotypy, while scopolamine’s slight numeric increase stereotypic behaviors at the dose of 25μg/kg was not significant.
DISCUSSION
This study provides the first experimental evidence directly suggesting a transient surge in amino acid neurotransmitter cycling is associated with the induction of RAAD-like effects. The study found evidence of inverted U-shaped dose response relationship between ketamine and amino acid neurotransmitter cycling. We also found similar evidence of an inverted U-shaped dose response relationship between ketamine and immobility on the FST. These findings are generally similar to previously established dose response relationships for ketamine-induced effects on glutamate efflux10, ketamine associated antidepressant-like behavioral responses, and a variety of accompanying cellular changes in rats6. The plasma levels obtained at the 14min time point for 10mg/kg ketamine dose are consistent with the levels reported in Li et al.6 for the same IP dose adjusted for time, and are in the similar range of the peak concentrations seen in the human studies dosing at 0.5mg/kg (typically around 100–200ng/mL at the 40min time point, see26). However, the 30mg/kg dose of ketamine, which induces clear increases in labeling and antidepressant-like effects in the rats, produces plasma levels that are well above that typically seen with doses used in the treatment of mood disorders, and more in line with anesthetic doses as reported by Domino et al. 198227. In spite of this, the 30mg/kg dose did not produce hypnotic effects in the rats, indicating there are species-specific dose effects related to the hypnotic and anesthetic properties of ketamine. Interpretation of the plasma level results at the lower dose range are limited by the variability of the individual measures, making it difficult to show meaningful differences in the plasma levels between the 1mg/kg and 3mg/kg doses. Together, these studies provide evidence that sub-anesthetic doses of ketamine are associated with rapid increases in amino acid neurotransmitter cycling and antidepressant-like effects on the FST, while higher anesthetic doses fail to have the same effects on either measure.
We further examined the relationship between glutamate cycling and antidepressant-like behavior by also analyzing the effects of an NR2B selective NMDA receptor antagonist, Ro 25-6981. Although the dose range with Ro 25-6981 was truncated by the limited solubility of the drug, there was a dose-dependent increase in cycling and to a lesser extent, behavior in the FST. Only the highest dose of Ro 25-6981 (10mg/kg) induced significant increases in glutamate-C4, GABA-C2, and glutamine-C4 13C enrichment and immobility on the FST over vehicle-treated animals. The dose effect of Ro 25-6981 in the FST was similar to that previously reported in mice23, with the largest behavioral response being seen at the highest dose, 10mg/kg. These studies demonstrate that both ketamine and Ro 25-6981 can dose dependently induce changes in amino acid neurotransmitter cycling, and suggest that NMDAR antagonist-induced increases in mPFC glutamate release and cycling are critical events in generating the antidepressant-like response of the drugs.
The studies also revealed the effect of the treatment on amino acid neurotransmitter cycling to be transient. Consistent with the dose-response studies, the effect of ketamine (30mg/kg) on cycling was highly significant over the first 18 min after drug injection. Glutamine enrichment is increased within the first 8min, and the effect is maintained for at least 18min post injection. However, the effect dissipates over the course of one hour, and no differences in cycling was found at 24 hours, a time point when the antidepressant-like activity of ketamine was seen and repeatedly documented in rodent models6, 7, 25, 28. These findings are consistent with other reports demonstrating rapid changes in phencyclidine-induced effects on glutamine levels29, and metabolism30 in rodents that normalize or even reverse by 24 hours, and a report suggesting that glutamine levels are rapidly but transiently increased in healthy control subjects receiving a sub-anesthetic dose of ketamine31. The fact that no effects on cycling were observed an hour after the administration of 30mg/kg ketamine, despite having blood levels consistent with that found 14mins after 10mg/kg dose that did increase cycling, suggest the existence of a hysteresis-like effect. In sum, the findings suggest that although a transient effect on glutamate cycling is associated with initiation of the antidepressant response, maintenance of the effect is not required for the delayed antidepressant-like effects of the treatments.
Another recent study found that scopolamine increases glutamate efflux, and induces synaptogenesis through AMPA receptor activation in rodent models9, suggesting that the surge in glutamate release is also involved in the drugs mechanism of RAAD action. Consistent with that report we found the 25μg/kg dose to produce an antidepressant-like effect and demonstrated that the same dose of scopolamine increased glutamate cycling rates. These results support the hypothesis that the rapid transient induction of cycling is common to drugs with RAAD properties.
Although the highest stereotypy counts were seen at the doses of ketamine and scopolamine that were associated with the greatest antidepressant-like effects, this effect was not seen with Ro 25-6981. This suggests that the neuronal mechanisms underlying the onset of stereotypy, behaviors considered related to the psychotomimetic effects of NMDAR antagonists32, are not necessarily the same as those underlying the antidepressant-like action.
There are several factors that limit the interpretation of the findings presented in this manuscript. Measures of 13C enrichment are not direct measures of glutamate release. However, the fact that the results align so closely with previous reports of glutamate efflux, suggest the method is reflecting changes in amino acid release. Moreover, it was not possible to examine the effects of higher doses of Ro 25-6981 due to its limited solubility, precluding the determination of a full dose-response curve for this compound. We also did not examine the time dependent effects of Ro 25-6981 and scopolamine, limiting conclusions on the duration of their effects on glutamate cycling. Lastly, although the temporal and dose response relationships between the increase in amino acid neurotransmitter cycling and the cellular6 and electrophysiological33 effects of NMDAR antagonists suggest the rapid increase in cycling is a critical step in the initiation of the antidepressant response, the study does not provide direct mechanistic evidence for this relationship.
CONCLUSION
In sum, these findings are consistent with the hypothesis that a transient glutamate surge is critical in initiating RAAD action. This work defines a timeline of signaling events whereby RAAD drugs initially stimulate a rapid increase in synaptic glutamate release and cycling, but suggests the effects on amino acid cycling is not required for the maintenance of the antidepressant-like effects. Rather, the enduring effects of these agents could be related to the sustained effects on synapse number and function that is dependent on glutamate signaling34. The novel use of 13C-Nuclear Magnetic Resonance Spectroscopy outlined in the present study can be relatively easily translated to human studies and may be helpful in future attempts to optimize dosing for treatment response in clinical populations.
Supplementary Material
Acknowledgments
We thank Amy Newton and Yulia Benitex of Bristol-Myers Squibb for the execution of the rat ketamine PK studies, and Terry Nixon, Peter Brown, and Scott McIntyre for their support in maintaining the NMR spectrometer. This work was supported by National Institute of Mental Health R01-MH095104 and R01-MH081211, NARSAD, QNMR Core Center P30-NS052519, The VA National Center for PTSD and funding from Bristol-Myers Squib.
Footnotes
CONFLICT OF INTEREST
Dr. Sanacora has received consulting fees from AstraZeneca, Avanier Pharmaceuticals, Bristol-Myers Squibb, Eli Lilly & Co., Hoffman La-Roche, Merck, Navigen, Naurex, Noven Pharmaceuticals, Servier Pharmaceuticals, Takeda, Teva and Vistagen therapeutics over the last 24 months. He has also received additional research contracts from AstraZeneca, Bristol-Myers Squibb, Eli Lilly & Co., Johnson & Johnson, Hoffman La-Roche, Merck & Co., Naurex and Servier over the last 24 months. Free medication was provided to Dr. Sanacora for an NIH sponsored study by Sanofi-Aventis. In addition he holds shares in BioHaven Pharmaceuticals Holding Company and is a co-inventor on a US patent (#8,778,979) held by Yale University.
Dr. Duman has received consulting fees from Taisho, Naurex, Sunovion, Johnson & Johnson, and investigator initiated grants from Forest, Naurex, Sunovion, and Eli Lilly & Co.
Dr. Bristow is an employee of Bristol-Myers Squibb. Dr. Schaeffer was an employee Bristol-Myers Squibb at the time the research was completed and is currently an employee of Janssen Research & Development. Dr. Banasr has received research contracts from BioHaven Pharmaceuticals and Servier Pharmaceuticals. Dr. Behar holds common stock in Pfizer.
Supplementary information is available at Molecular Psychiatry’s website
References
- 1.Martinowich K, Jimenez DV, Zarate CA, Jr, Manji HK. Rapid antidepressant effects: moving right along. Molecular psychiatry. 2013;18(8):856–863. doi: 10.1038/mp.2013.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newport DJ, Carpenter LL, McDonald WM, Potash JB, Tohen M, Nemeroff CB, et al. Ketamine and Other NMDA Antagonists: Early Clinical Trials and Possible Mechanisms in Depression. The American journal of psychiatry. 2015;172(10):950–966. doi: 10.1176/appi.ajp.2015.15040465. [DOI] [PubMed] [Google Scholar]
- 3.Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, et al. Lanicemine: a low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Molecular psychiatry. 2014;9(9):978–985. doi: 10.1038/mp.2013.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW. An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. Journal of clinical psychopharmacology. 2008;28(6):631–637. doi: 10.1097/JCP.0b013e31818a6cea. [DOI] [PubMed] [Google Scholar]
- 5.Drevets WC, Zarate CA, Jr, Furey ML. Antidepressant effects of the muscarinic cholinergic receptor antagonist scopolamine: a review. Biological psychiatry. 2013;73(12):1156–1163. doi: 10.1016/j.biopsych.2012.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, et al. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science. 2010;329(5994):959–964. doi: 10.1126/science.1190287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, et al. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 2011;475(7354):91–95. doi: 10.1038/nature10130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koike H, Iijima M, Chaki S. Involvement of AMPA receptor in both the rapid and sustained antidepressant-like effects of ketamine in animal models of depression. Behav Brain Res. 2011;224(1):107–111. doi: 10.1016/j.bbr.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 9.Voleti B, Navarria A, Liu RJ, Banasr M, Li N, Terwilliger R, et al. Scopolamine Rapidly Increases Mammalian Target Of Rapamycin Complex 1 Signaling, Synaptogenesis, and Antidepressant Behavioral Responses. Biological psychiatry. 2013 doi: 10.1016/j.biopsych.2013.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moghaddam B, Adams B, Verma A, Daly D. Activation of glutamatergic neurotransmission by ketamine: a novel step in the pathway from NMDA receptor blockade to dopaminergic and cognitive disruptions associated with the prefrontal cortex. Journal of Neuroscience. 1997;17(8):2921–2927. doi: 10.1523/JNEUROSCI.17-08-02921.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Homayoun H, Moghaddam B. NMDA receptor hypofunction produces opposite effects on prefrontal cortex interneurons and pyramidal neurons. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2007;27(43):11496–11500. doi: 10.1523/JNEUROSCI.2213-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chowdhury GM, Behar KL, Cho W, Thomas MA, Rothman DL, Sanacora G. (1)H-[(1)(3)C]-nuclear magnetic resonance spectroscopy measures of ketamine’s effect on amino acid neurotransmitter metabolism. Biological psychiatry. 2012;71(11):1022–1025. doi: 10.1016/j.biopsych.2011.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Witkin JM, Overshiner C, Li X, Catlow JT, Wishart GN, Schober DA, et al. M1 and m2 muscarinic receptor subtypes regulate antidepressant-like effects of the rapidly acting antidepressant scopolamine. The Journal of pharmacology and experimental therapeutics. 2014;351(2):448–456. doi: 10.1124/jpet.114.216804. [DOI] [PubMed] [Google Scholar]
- 14.Chowdhury GM, Patel AB, Mason GF, Rothman DL, Behar KL. Glutamatergic and GABAergic neurotransmitter cycling and energy metabolism in rat cerebral cortex during postnatal development. J Cereb Blood Flow Metab. 2007;27(12):1895–1907. doi: 10.1038/sj.jcbfm.9600490. [DOI] [PubMed] [Google Scholar]
- 15.Hyder F, Patel AB, Gjedde A, Rothman DL, Behar KL, Shulman RG. Neuronal-glial glucose oxidation and glutamatergic-GABAergic function. J Cereb Blood Flow Metab. 2006;26(7):865–877. doi: 10.1038/sj.jcbfm.9600263. [DOI] [PubMed] [Google Scholar]
- 16.Breier A, Malhotra AK, Pinals DA, Weisenfeld NI, Pickar D. Association of ketamine-induced psychosis with focal activation of the prefrontal cortex in healthy volunteers. The American journal of psychiatry. 1997;154(6):805–811. doi: 10.1176/ajp.154.6.805. [DOI] [PubMed] [Google Scholar]
- 17.Nishizawa N, Nakao S, Nagata A, Hirose T, Masuzawa M, Shingu K. The effect of ketamine isomers on both mice behavioral responses and c-Fos expression in the posterior cingulate and retrosplenial cortices. Brain research. 2000;857(1–2):188–192. doi: 10.1016/s0006-8993(99)02426-9. [DOI] [PubMed] [Google Scholar]
- 18.Littlewood CL, Jones N, O’Neill MJ, Mitchell SN, Tricklebank M, Williams SC. Mapping the central effects of ketamine in the rat using pharmacological MRI. Psychopharmacology. 2006;186(1):64–81. doi: 10.1007/s00213-006-0344-0. [DOI] [PubMed] [Google Scholar]
- 19.Stone JM, Erlandsson K, Arstad E, Squassante L, Teneggi V, Bressan RA, et al. Relationship between ketamine-induced psychotic symptoms and NMDA receptor occupancy: a [(123)I]CNS-1261 SPET study. Psychopharmacology. 2008;197(3):401–408. doi: 10.1007/s00213-007-1047-x. [DOI] [PubMed] [Google Scholar]
- 20.Miyamoto S, Leipzig JN, Lieberman JA, Duncan GE. Effects of ketamine, MK-801, and amphetamine on regional brain 2-deoxyglucose uptake in freely moving mice. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22(4):400–412. doi: 10.1016/S0893-133X(99)00127-X. [DOI] [PubMed] [Google Scholar]
- 21.Miyamoto S, Mailman RB, Lieberman JA, Duncan GE. Blunted brain metabolic response to ketamine in mice lacking D(1A) dopamine receptors. Brain research. 2001;894(2):167–180. doi: 10.1016/s0006-8993(01)01991-6. [DOI] [PubMed] [Google Scholar]
- 22.Duncan GE, Moy SS, Knapp DJ, Mueller RA, Breese GR. Metabolic mapping of the rat brain after subanesthetic doses of ketamine: potential relevance to schizophrenia. Brain research. 1998;787(2):181–190. doi: 10.1016/s0006-8993(97)01390-5. [DOI] [PubMed] [Google Scholar]
- 23.Maeng S, Zarate CA, Jr, Du J, Schloesser RJ, McCammon J, Chen G, et al. Cellular mechanisms underlying the antidepressant effects of ketamine: role of alpha-amino-3-hydroxy-5-methylisoxazole-4-propionic acid receptors. Biological psychiatry. 2008;63(4):349–352. doi: 10.1016/j.biopsych.2007.05.028. [DOI] [PubMed] [Google Scholar]
- 24.Haller J, Nagy R, Toth M, Pelczer KG, Mikics E. NR2B subunit-specific NMDA antagonist Ro25-6981 inhibits the expression of conditioned fear: a comparison with the NMDA antagonist MK-801 and fluoxetine. Behavioural pharmacology. 2011;22(2):113–121. doi: 10.1097/FBP.0b013e328343d7b2. [DOI] [PubMed] [Google Scholar]
- 25.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological psychiatry. 2011;69(8):754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaffer CL, Osgood SM, Smith DL, Liu J, Trapa PE. Enhancing ketamine translational pharmacology via receptor occupancy normalization. Neuropharmacology. 2014;86:174–180. doi: 10.1016/j.neuropharm.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 27.Domino EF, Zsigmond EK, Domino LE, Domino KE, Kothary SP, Domino SE. Plasma levels of ketamine and two of its metabolites in surgical patients using a gas chromatographic mass fragmentographic assay. Anesthesia and analgesia. 1982;61(2):87–92. [PubMed] [Google Scholar]
- 28.Lucki I. The forced swimming test as a model for core and component behavioral effects of antidepressant drugs. Behavioural pharmacology. 1997;8(6–7):523–532. doi: 10.1097/00008877-199711000-00010. [DOI] [PubMed] [Google Scholar]
- 29.Iltis I, Koski DM, Eberly LE, Nelson CD, Deelchand DK, Valette J, et al. Neurochemical changes in the rat prefrontal cortex following acute phencyclidine treatment: an in vivo localized (1)H MRS study. NMR in biomedicine. 2009;22(7):737–744. doi: 10.1002/nbm.1385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gao XM, Shirakawa O, Du F, Tamminga CA. Delayed regional metabolic actions of phencyclidine. European journal of pharmacology. 1993;241(1):7–15. doi: 10.1016/0014-2999(93)90926-9. [DOI] [PubMed] [Google Scholar]
- 31.Rowland LM, Bustillo JR, Mullins PG, Jung RE, Lenroot R, Landgraf E, et al. Effects of ketamine on anterior cingulate glutamate metabolism in healthy humans: a 4-T proton MRS study. The American journal of psychiatry. 2005;162(2):394–396. doi: 10.1176/appi.ajp.162.2.394. [DOI] [PubMed] [Google Scholar]
- 32.Homayoun H, Jackson ME, Moghaddam B. Activation of metabotropic glutamate 2/3 receptors reverses the effects of NMDA receptor hypofunction on prefrontal cortex unit activity in awake rats. J Neurophysiol. 2005;93(4):1989–2001. doi: 10.1152/jn.00875.2004. [DOI] [PubMed] [Google Scholar]
- 33.Nagy D, Stoiljkovic M, Menniti FS, Hajos M. Differential Effects of an NR2B NAM and Ketamine on Synaptic Potentiation and Gamma Synchrony: Relevance to Rapid-Onset Antidepressant Efficacy. Neuropsychopharmacology. doi: 10.1038/npp.2015.298. online publication 21 October 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duman RS, Aghajanian GK. Synaptic dysfunction in depression: potential therapeutic targets. Science. 2012;338(6103):68–72. doi: 10.1126/science.1222939. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.