Abstract
Ureteral stent (UrSt) placement has been shown to be a significant independent risk factor for BK viruria, viremia and BK virus nephropathy. We assessed if this observation could be validated at our high volume kidney transplant center that has had a strong historical focus on BK virus nephropathy detection.
We performed a retrospective case-control study of adults receiving a kidney-only transplant and followed for one year between 2004 and 2011 with uniform immunosuppression and use of blood BK virus PCR screening protocol.
Among 1147 patients, 443 (38.6%) received a UrSt, and 17.2% with a UrSt had BK viremia versus 13.5% without stent (odds ratio 1.33; 95% CI 1.00–1.78). We confirmed a previously reported association between immediate graft function (IGF) and higher rate of BK viremia (15.7% versus 5.9% in patients without IGF). On multivariable competing risks Cox regression in patients with IGF, UrSt (adjusted hazard ratio [aHR] 1.35;95% CI 1.04–1.75) and African-American race (aHR 1.47;95% CI 1.04–2.09) significantly increased the risk for BK viremia.
In the largest sample size to date, we confirmed that UrSt placement during kidney transplant surgery is a risk factor for BK viremia within the first year post-transplant and that IGF is associated with BK viremia.
Introduction
BK virus has been recognized as a major cause of chronic renal allograft failure with recent studies describing the diagnosis of BK virus nephropathy (BKVN) in up to 7% of kidney transplant recipients [1]. In most cases, there is a progression from BK viruria to viremia to nephropathy and elevated BK viremia titers have been shown to be highly predictive of BK virus nephropathy on biopsy [2, 3]. Efforts to prevent BK virus nephropathy have led investigators to explore the mechanisms by which BK virus causes nephropathy and identify modifiable risk factors that predispose kidney transplant patients to BK virus nephropathy [4]. Known risk factors for BK virus infection include male gender [5], older age [5], donor positive BK virus serostatus [6], pre-transplant recipient positive BK virus serostatus [3, 6, 7], ATG (anti-thymocyte globulin) induction therapy [8], tacrolimus and/or mycophenolate mofetil maintenance therapy [5, 8], corticosteroid therapy [3, 5], and acute rejection [3]. Several studies have identified the use of ureteral stent during kidney transplant surgery as an independent risk factor for BKVN [2, 9, 10]. More recent studies have shown a similar association of ureteral stent placement with BK viremia and viruria [11–13]. The advantage of using ureteral stents to prevent major post-operative urologic complications after kidney transplantation was shown in a Cochrane review meta-analysis [14]. However the studies reviewed were performed prior to reports describing the increased risk of BK viremia in patients with ureteral stents, which may alter the risk-benefit profile.
Our goal in this study was to identify risk factors for BK viremia and assess if prior results of association between the use of ureteral stents and a higher rate of BK viremia at other centers could be replicated at Washington University in St. Louis, a high volume kidney transplant center with a long-standing interest and focus in early BK virus nephropathy detection. We report the largest retrospective review to date describing risk factors for BK viremia within the first year after kidney transplant.
Patients and Methods
After approval by the Institutional Review Board of Washington University in St. Louis School of Medicine, we performed a retrospective review of all first kidney-only transplants in adult recipients performed at Barnes-Jewish Hospital, with a minimum of 12-month follow up. The study time period of January 1, 2004 to November 30, 2011 incorporated the implementation of a uniform steroid-sparing immunosuppression protocol and initiation of a uniform BK viremia screening protocol. Pediatric patients were excluded from this study as surgeon preference to use ureteral stents resulted in stenting of virtually all pediatric patients. Patients receiving a second kidney transplant, combined kidney-liver and kidney-pancreas transplants were excluded in order to ensure uniformity of the patient population. Patients were excluded from the study if the operative report was not available electronically for review or if blood BK virus PCR testing was missing. Graft loss and death within the first year after transplant were included as competing risk factors for BK viremia. The decision to place a stent was based on surgeon preference. The stent used was always a 6 French 12 cm double-J type indwelling stent. The stents were removed at approximately 6 weeks post-transplant. Throughout the 7-year period of the study, the respective surgeons maintained their practice of either placing a stent or not placing a stent.
We reviewed each transplant surgery operative report to determine whether a ureteral stent was placed during kidney transplant surgery. We collected transplant recipient data (age, gender, race, CMV serostatus), donor source (living or deceased), organ donor CMV serostatus, transplant surgery data (organ ischemia time, immediate vs. delayed graft function), episodes of rejection (at discharge, 6 months, and 1 year), and induction medication used for immunosuppression. Delayed graft function (DGF) was defined as need for hemodialysis within the first 7 days post-transplant. Acute rejection was defined as the presence of evidence of acute rejection on biopsy according to the Banff 2007 grading system [15].
Immunosuppression regimen included either rabbit anti-thymocyte globulin (Thymoglobulin, Genzyme, Cambridge, MA, USA) 6 mg/kg IV total dose (2 mg/kg intraoperatively and 2 mg/kg on post-operative days 1 and 2) until 2009, then after 2009, 5 mg/kg IV total dose (1 mg/kg intra-operative and 2 mg/kg post-transplant days 1 and 2) or basiliximab 20 mg IV pre-operatively then again on post-transplant day 4 or alemtuzumab 30 mg IV once intraoperatively. Corticosteroid dosing included prednisone 1 mg/kg PO in the first week post-transplant which was tapered to 5 mg daily PO at 1 month post-transplant. Maintenance medication included corticosteroid (prednisone 5 mg PO daily from 1 month post-transplant onwards) and calcineurin inhibitor (either tacrolimus or cyclosporine), and antimetabolite (either mycophenolate mofetil or mycophenolic acid). Tacrolimus dosing was 5 mg PO twice daily, with goal level 7–10 ng/ml during first month post-transplant and goal level 3–7 ng/ml after first month post-transplant. Cyclosporine dosing was 8 mg/kg/day PO divided into twice daily dosing with goal level 250–300 ng/ml during first month post-transplant, then after first month post-transplant dosing adjusted to achieve goal 12 hour trough level of 75–150 ng/ml, and goal 90 minute peak level 400–600 ng/ml. Antimetabolite dosing for mycophenolate mofetil was 1000 mg twice daily PO and reduction to 500 mg twice daily if necessary for WBC<5,000/mm3 or severe diarrhea and dosing for mycophenolic acid was 720 mg twice daily. Mycophenolate mofetil was reduced to 500 mg PO twice daily and mycophenolic acid was reduced to 360 mg twice daily by post-operative day 5 in most cases. In the setting of DGF, there was no specific adjustment in immunosuppression strategy.
All blood BK virus testing was performed at the adjoining St. Louis Children’s Hospital Virology Laboratory which uses a quantitative real-time PCR assay for BK virus DNA with a lower limit of detection of 3,200 copies/ml using the Lightcycler platform. The primers used amplify a 176 base pair segment of BK virus genome using the following hybridized probes: (1) PEP-1: AGT-CTT-TAG-GGT-CTT-CTA-CC; (2) PEP-2: GGT-GCC-AAC-CTA-TGG-AAC-AG; (3) BKF: TTG-CCA-TGA-AGA-TAT-GTT-TGC-CAG-TGA-TGA-FITC; (4) BKR: LCRed640-GAA-GCA-ACA-GCA-GAT-TCT-CA. The testing protocol included plasma BK PCR measurements monthly for 6 months, and then at 9 months and 12 months and for cause at any time after transplant. The primary endpoint was first detection of BK viremia detected within the first year post-transplant. Identification of BK viremia prompted a reduction of the maintenance immunosuppression dose initially with immediate and, in most cases, indefinite withdrawal of the antimetabolite. If viremia did not resolve, tacrolimus level was further reduced. In certain instances, cidofovir was administered, however data was not collected to describe these interventions.
Clinical data analyzed included placement of ureteral stent, positive testing for BK virus PCR in blood, age group (18–50, >50 years), recipient gender, race (Caucasian, African American, and other), donor source (living or deceased), cold ischemia time (<12 hours, 12–24 hours, >24 hours), graft function (immediate or delayed), induction agent (Thymoglobulin or others).
Statistical analyses
The chi-square test was used to analyze associations between categorical variables. A one-tailed chi-square test was used to characterize the association between ureteral stent placement and presence of BK viremia as multiple prior studies have described this direction of association. A two-tailed chi-square test was used to analyze associations between other categorical variables. The Z test, or t-test, as appropriate, was used to analyze continuous variables. Risk factors that were found to be significant on univariate analysis were then included in two separate multivariable Cox regression analyses (without and with censoring for the competing risks of patient death or allograft failure within the first year post-transplant) to identify independent risk factors. Results of univariate analysis were reviewed to consider plausible confounders to include in multivariable analysis. If the patient did not experience an event (BK viremia, death or graft loss) then they were censored at 365 days. The log-rank test was used to compare time of onset of BK viremia in patients with and without stent placement and a Kaplan-Meier plot was generated to compare BK virus infection free survival, with censoring for the competing risks of patient death or allograft failure within the first year post-transplant. P-values and confidence intervals were estimated using the Wald statistic. All analyses were performed using SAS software, version 9.2 or 9.4 (SAS Institute, Inc., Cary, North Carolina, USA).
Results
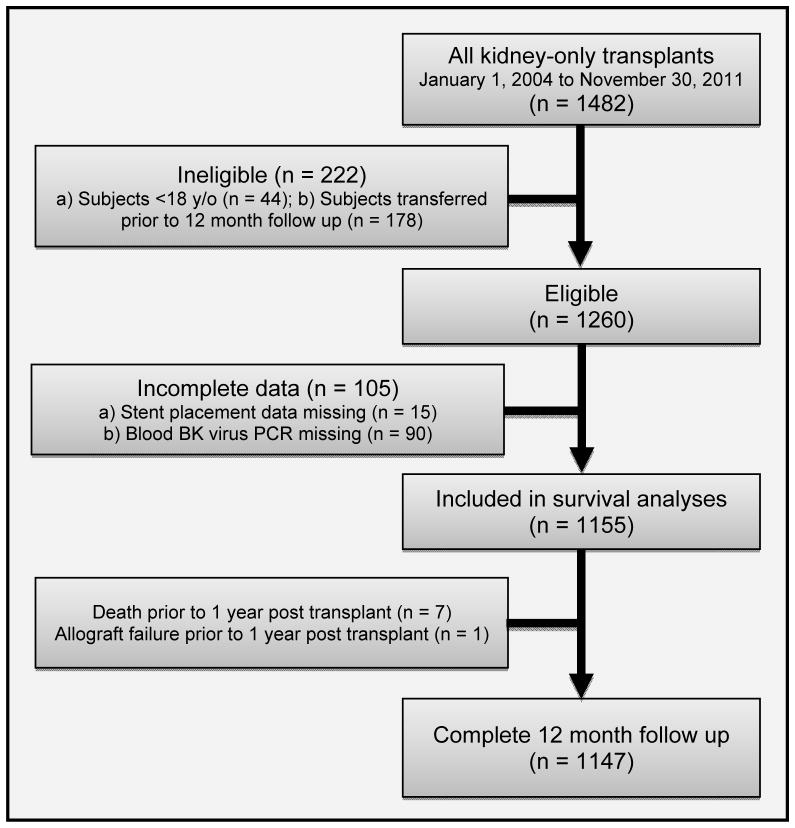
A total of 1482 kidney-only transplants were performed at Washington University in St. Louis from January 1, 2004 to November 30, 2011. After excluding patients who were younger than 18 years of age at the time of transplant, or who transferred to a different institution preventing 12 month follow up, 1260 were left eligible. In 15 subjects we were unable to determine whether stent was placed during kidney transplant surgery and in 90 subjects blood BK virus PCR results were missing. Of the remaining 1155, there were 7 patient deaths and 1 allograft failure prior to 12 months. Thus, 1147 subjects had complete data. (Figure 1). BK viremia occurred in 171/1147 patients (14.9%).
Figure 1.
Eligibility and inclusion criteria
The demographics and clinical characteristics of the patients with stent placement were compared to those of the patients without stent placement (Table 1). Among the 1147 patients included in the study, UrSt was placed in 443 patients (38.6%). BK viremia was detected in 17.2% of patients receiving stent placement, compared to 13.5% of patients without stent placement (P = 0.045) (Table 1). We also found that IGF was associated with BK viremia. Among patients with IGF, BK virus was detected in 15.7% of patients, compared to 5.9% in patients with DGF (P = 0.007). We found that there was no significant difference in rate of DGF between patients receiving stent (9.5%) compared to patients without stent placement (7.9%), (P = 0.351). We found no statistically significant difference among the two groups in gender, age or race on univariate analysis, though recipient race was near to significance (P = 0.078). Also, we found no difference between the two groups in the proportion receiving a kidney transplant from a living donor, donor CMV positive status, recipient CMV positive status, anti-thymocyte globulin status, acute rejection at 6 months after transplant, or acute rejection at 12 months after transplant when comparing those with and without BK viremia.
Table 1.
Patient demographic data and clinical characteristics of patients with and without stent placement
| Overall (N=1147) | Without Stent (N=704) | With Stent (N=443) | P value | ||
|---|---|---|---|---|---|
| Age > 50 years old, %(n) | 59.2 (679) | 60.2 (424) | 57.6 (255) | 0.371 | |
| Female, %(n) | 39.7 (455) | 37.4 (263) | 43.3 (192) | 0.044 | |
| Race, %(n) | Caucasian | 72.7 (834) | 74.6 (525) | 69.8 (309) | 0.13 |
| African-American | 19.6 (225) | 17.8 (125) | 22.6 (100) | ||
| Other | 7.7 (88) | 7.7 (54) | 7.7 (34) | ||
| Ischemia Time, %(n) | 0 – 12 hours | 71.5 (820) | 70.9 (499) | 72.5 (321) | 0.127 |
| 12 – 24 hours | 27.2 (312) | 27.2 (192) | 27.1 (120) | ||
| >24 hours | 1.3 (15) | 1.9 (13) | 0.5 (2) | ||
| Living donor, %(n) | 36.7 (416) | 39.8 (278) | 31.7 (138) | 0.005 | |
| CMV positive donor, %(n) | 77.2 (858) | 77.5 (531) | 76.6 (327) | 0.717 | |
| CMV positive recipient, %(n) | 60.6 (687) | 59.3 (411) | 62.7 (276) | 0.251 | |
| Anti-thymocyte globulin, %(n) | 89.4 (966) | 90.0 (593) | 88.6 (373) | 0.47 | |
| Delayed graft function, %(n) | 8.9 (102) | 9.5 (67) | 7.9 (35) | 0.351 | |
| Acute rejection at 6 months after TX, %(n) | 3.3 (37) | 3.5 (24) | 3.0 (13) | 0.66 | |
| Acute rejection at 12 months after TX, %(n) | 1.9 (21) | 1.6 (11) | 2.3 (10) | 0.373 | |
| BK viremia % (n) | 14.9% (171) | 13.5% (95) | 17.2% (76) | 0.045 | |
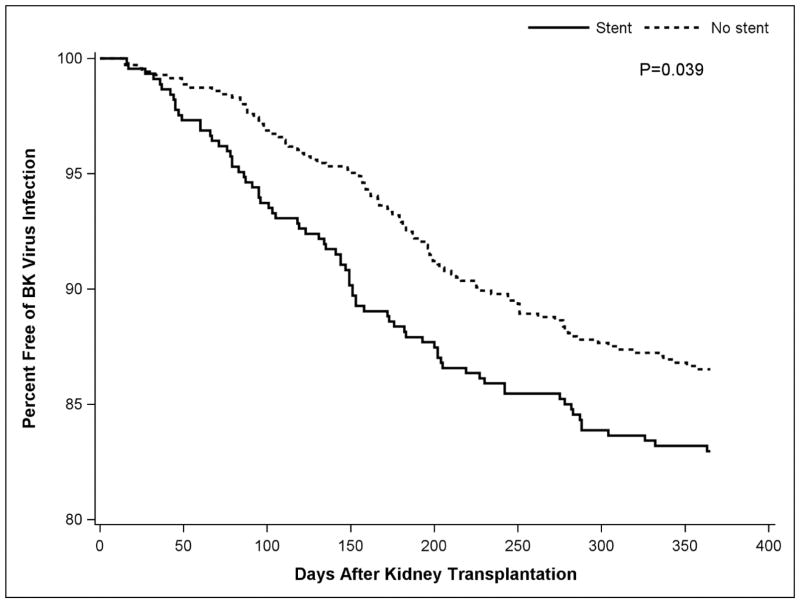
In a time to event analysis that censored for the competing events of patient death or allograft failure prior to 12 months post-transplant, the median time to initial detection of BK viremia in patients with stent was 143 days, compared to 175 days in patients without stent placement, which was found to be statistically significant on log-rank test (P = 0.039), as shown on a Kaplan-Meier plot (Figure 2).
Figure 2.
Kaplan-Meier plot demonstrating BK Virus infection free survival, incorporating the 7 patient deaths and 1 allograft failure as competing risks
When we evaluated stent placement and graft function together, only 35 patients with stent placement went on to demonstrate DGF and none of these patients developed BK viremia. However, 407 patients with stent placement went on to demonstrate IGF, and 76 developed BK viremia (Table 2).
Table 2.
Stent use and BK viremia among subjects with delayed and immediate graft function
| Overall, %,(n) | Subjects Without BK viremia | Subjects With BK viremia | P value | |
|---|---|---|---|---|
| Stent Use among DGF | 34.3 (35) | 100 (35) | 0 | 0.046 |
| Stent Use among IGF | 39 (407) | 81.3 (331) | 18.7 (76) | 0.021 |
Multivariable Cox regression was performed on only the subjects with IGF, due to the zero cell count when assessing the stent and DGF interaction. The initial model, incorporating the 1147 subjects with complete data out to 12 months in all subjects, identified two independent and statistically significant risk factors for BK viremia: stent use (adjusted hazard ratio 1.36; 95% CI 1.05–1.76, P = 0.024) and African American race (adjusted hazard ratio 1.51; 95% CI 1.06–2.15, P = 0.022) (Table 3). A second model, incorporating the 7 patient deaths and 1 allograft failure prior to 12 months as competing risks, also identified the same two independent risk factors for BK viremia with almost identical results: stent use (adjusted hazard ratio 1.35; 95% CI 1.04–1.75, P = 0.028) and African American race (adjusted hazard ratio 1.47; 95% CI 1.04–2.09, P = 0.031) (Table 3).
Table 3.
Cox regression models among subjects with immediate graft function
| Regression Model | Stent Use | African American |
|---|---|---|
| Multivariable Cox Regression Model | Hazard Ratio 1.36 CI 1.05 – 1.76 P = 0.024 |
Hazard Ratio 1.51 CI 1.06 – 2.15 P = 0.022 |
| Competing Risks Cox Regression Model | Hazard Ratio 1.35 CI 1.04 – 1.75 P = 0.028 |
Hazard Ratio 1.47 CI 1.04 – 2.09 P = 0.031 |
Discussion
As use of modern immunosuppression has successfully decreased the incidence of acute rejection, and improvement in surgical technique has decreased the rate of post-operative complications after transplant surgery, the risk of graft loss due to opportunistic infections has become an important cause of graft loss [10]. Infection with BK polyomavirus resulting in BK virus nephropathy was initially described in 1971 and is now known to affect approximately 15% of kidney transplant patients within the first year post-transplant [4]. Several risk factors for BK virus infection have been identified, including male gender [5], older age [5], donor positive BK virus serostatus [6], pre-transplant recipient positive BK virus serostatus [3, 6, 7], and acute rejection [3]. Modifiable risk factors for BK virus infection include use of ATG induction therapy [8], tacrolimus and/or mycophenolate mofetil maintenance therapy [5, 8], corticosteroid therapy [3, 5], and UrSt placement at time of transplant surgery [2, 9, 10, 13, 16].
In this single center, retrospective case-control study exploring risk factors for BK viremia in patients receiving kidney transplant, we confirmed that UrSt placement is associated with increased risk of BK viremia in the first year after kidney transplant. In addition, multivariate analysis revealed that immediate graft function is associated with increased rate of BK viremia. These findings support results from previous studies performed on smaller populations [5, 13] and affirm the need for greater vigilance for BK related kidney disease post-transplant in patients receiving ureteral stent placement.
The use of ureteral stents has been proven to reduce the post-operative complications of kidney transplant surgery, especially complications related to the vesicoureteric anastomosis [17–19]. A recent Cochrane review of seven randomized controlled trials demonstrated that routine use of prophylactic stenting at the time of graft implantation decreases the incidence of major urologic complications, specifically urine leaks and ureteric stenosis [14]. The authors described that longer stent length (>20 cm) and stent placement for extended duration (>6 weeks) were associated with increased risk of encrustation and migration. They also described increased risk of urinary tract infections among patients who received stent placement. Of note, the authors did not specifically address the incidence of BK viremia or BK virus nephropathy so the advantage of stent placement in decreasing the incidence of major urologic complications was described without considering the effect on the risk of BKVN.
Multiple single-center studies comparing outcomes of patients receiving kidney transplant surgery with and without stent placement have investigated the incidence of BK viremia as well as BK virus nephropathy (Table 4). The study by Brennan, et al. from our center published in 2005 used a prospective study design with a population of 200 patients and identified the use of ureteral stents as a risk factor for sustained BK viremia (defined as positive blood samples spanning 3 or more weeks) with a significant hazard ratio of 4.3 (P = 0.044) [2]. The study by Thomas, et al. in 2007 used a retrospective case-control study design with 20 cases of polyoma virus nephropathy (PVN) matched to 46 controls and reported that the placement of ureteral stent carried an odds ratio of 5.63 (P = 0.004) for the development of PVN in univariate analysis and remained an independent risk factor after multivariate analysis (adjusted OR, 4.71; P = 0.03) [9]. The study by Siparsky, et al. in 2011 used a retrospective study design with a study population of 186 patients and reported that ureteral stent placement conferred an increased risk of BK viremia with an odds ratio of 3.17 (P = 0.02). No additional risk factors were identified in the study. However, aside from use of ureteral stent, only demographic data and allograft type (donated after cardiac death vs donation after brain death vs living unrelated donation), diabetes status, and re-transplant status were compared [12]. The study by Hashim, et al. in 2014, which included one of the present study authors but at a separate center, used a retrospective study design with a larger study population of 621 patients and reported an increased adjusted risk of both BK viruria (adjusted OR, 1.67; P = 0.04) and BK viremia (adjusted OR, 1.55; P = 0.04) [13].
Table 4.
Studies describing association between stent placement and BK virus
| Study | Year | Study Design | Number of patients (n) | Outcome Associated with Stent Placement | Significant Results |
|---|---|---|---|---|---|
| Brennan et al.[2] | 2005 | Prospective cohort | 200 | BK viremia | Hazard ratio 0.44 (P = 0.044) |
| Thomas et al.[9] | 2007 | Retrospective case-control | 66 | Polyoma virus nephropathy | Univariate OR 5.63 (P = 0.004) Multivariate Adj. OR 4.71 (P = 0.03) |
| Dharnidharka et al.[10] | 2008 | Retrospective case-control | 93 | BK nephropathy | Multivariate adj. hazard ratio 4.07 (P = 0.24) |
| Siparsky et al.[16] | 2011 | Retrospective case-control | 186 | BK viremia | OR 3.17 (P = 0.02) |
| Hashim et al.[13] | 2014 | Retrospective case-control | 621 | BK viruria and BK viremia | Viruria Adj. OR 1.67 (P = 0.04) Viremia Adj. OR 1.55 (P = 0.04) |
Our study confirmed the previously described association between use of ureteral stent and increased risk for BK viremia. The results clearly support the findings in the previous studies that identified use of ureteral stent as a risk factor for BK viremia or BK virus nephropathy.
Interestingly, we also observed that among patients with IGF, there was a statistically significant association with BK viremia, which reproduces the results presented in the study by Hashim et al. in 2014 [13]. We found that there was no statistical significance between rate of DGF among patients with and without stent placement, which suggests that the effect of stent use to avoid major urologic complication is not a confounding variable. A literature review of risk factors for BK viremia provided at least four points to explain the observed association between IGF and BK viremia. First, IGF may be associated with preservation of uroepithelial cell function and nucleic acid replication machinery which could facilitate viral replication. Although DGF has been shown to be associated with BK virus nephropathy in the renal parenchyma [9], similar studies have failed to show an association between DGF and replication of the BK virus in blood and urine [13]. These findings suggest that intact uroepithelial cell function and nucleic acid replication machinery as well as intact urine volume may be required for viral replication and transfer to the blood, but different risk factors potentially contribute to progression to nephropathy. In this regard, studies have shown that BK virus infection of uroepithelium is significantly different than BK infection of renal tubular epithelium [20]. A study by Priftakis, et al. compared BK viruria rates between living donor and deceased donor kidney transplant recipients concluded that longer cold ischemia time does not increase the risk of BK viruria [21]. In addition, BK virus replication requires the functionality of the host cell for entry [22], transport to host cell nucleus [23], and replication within host cell [24]. A second reason why IGF may be associated with BK viremia is that patients with IGF are more likely to have increased urine output in the immediate post-operative phase which may facilitate the development of BK viruria, a known precursor to the development of BK viremia [3]. A third possible contributing factor is that patients with IGF are more likely to have shorter hospitalizations and are thereby are at higher risk for community acquired infection with BK virus given that the prevalence of BK virus is as high as >80% in the adult population [25]. Fourth, some mouse models have suggested that uroepithelial cell injury, which may occur with prolonged cold ischemia and DGF, might increase the risk for BKV nephropathy, however these hypotheses are related to nephropathy, not precursor viral replication [12, 26, 27]. One study in humans by Thomas et al. identified DGF as a risk factor for BK nephropathy in univariate analysis (P = 0.02), however this association was not confirmed in multivariate analysis (P = 0.22) [9]. The study concluded that DGF is not an independent risk factor for BK nephropathy and confirmed that among all the variables included in multivariate analysis, only stent placement was an independent risk factor for BK nephropathy (P = 0.03). Our results, in conjunction with those of Hashim et al., from two independent populations found that DGF was associated with decreased risk for BK viremia.
In addition, our study identified African American race as an independent risk factor for BK viremia among patients without DGF. All the previously mentioned studies describing risk factors for BK viremia compared the incidence of BK viremia between races, including the studies by Brennan et al., Thomas et al., Siparsky et al. and Hashim et al. [2, 9, 12, 13]. None of these studies identified race as a risk factor after multivariate analysis. Given the number of patients in the study, the increased statistical power likely contributed to our ability to identify a significant difference not detected in other studies. African American patients were exposed to increased immunosuppression because the transplant protocol used at our center recommended routinely using either thymoglobulin or basiliximab during for induction therapy unless the transplant case involved an HLA identical Caucasian living donor, in which case neither thymoglobulin nor basiliximab was used for induction. However, given that use of anti-thymocyte globulin was not a risk factor for BK viremia, there is no evidence to suggest that selection of induction medication based on race was associated with increased risk for BK viremia. African American patients may be at risk for increased immunosuppression given that higher doses of tacrolimus are often required to achieve goal trough levels. However, at our center goal tacrolimus levels were not different for African Americans.
Despite the multiple clinical reports that suggest that the use of ureteral stents is associated with increased risk of BK viremia and subsequently BK virus nephropathy, the mechanism behind this increased risk is not well understood [28]. The concept of the two hit hypothesis is described by Thomas, et al. which suggests that patients who undergo kidney transplant surgery requiring immunosuppression are at risk for opportunistic infection and that the mild ureteral irritation induced by stent placement causes the second hit resulting in increased translocation of the BK virions from the urinary space into the blood space and subsequently into the renal parenchyma [9]. Also, a study by Funk, et al. used mathematical modeling of BK virus replication dynamics in order to demonstrate that viral replication likely begins in the kidney, enters the urinary space, and then flows bi-directionally between the kidney and urinary tract [29]. While the exact mechanism by which stent placement results in infection with BK virus remains elusive, these studies suggests that a causal relationship is plausible.
The limitations of this study include its single center study population and retrospective study design. We were unable to collect pre-transplant BK viremia or viruria levels in either recipient or donor because they are not routinely performed at our center per current clinical practice guidelines [30]. We did not collect data on timing of stent removal as this risk factor for viremia was studied by Kayler et al. and was not found to be associated with BK viremia [31]. We did not study peak BK viremia level, incidence of biopsy proven BK virus nephropathy or graft survival. We did not collect data describing etiology of end stage renal disease, HLA matching, timing of stent removal, tacrolimus levels, other maintenance immunosuppression data, ureteral complications, duration of Foley catheter placement or incidence of urinary tract infection. To our knowledge, no published data suggest that the aforementioned clinical characteristics place patients at risk for BK virus nephropathy. Plasma BK virus testing was performed at a maximum frequency of every month (during the first 6 months) and minimum frequency of once every 3 months (at 9 and 12 months), so interval censoring prevented detection of BK viremia that developed between testing time points. In addition, the decision for stent placement was based on surgeon preference so stent placement was not random and therefore contributes to bias in patient selection. The criteria used by each surgeon for stent placement varied based on multiple factors including training experience and complexity of anastomosis and these variables were not controlled in the data analysis. Finally, IGF is the desired outcome in all patients receiving kidney biopsy, so even if prospective studies confirmed an association between IGF and increased risk of BK viremia awareness of this association is unlikely to change standard practice for reducing DGF, but may impact stent placement practices.
Although the findings in this study confirm the results of previous studies that explored the association between use of ureteral stents and BK viremia, more efforts are needed to further characterize this association. A prospective randomized controlled study is needed to confirm the increased risk of ureteral stents on the development of BK nephropathy. Once an association is established, additional studies will be needed to determine whether stent placement results in any measureable morbidity, (i.e. worsening renal function, graft rejection or graft loss). Recognition of the impact of stent placement would help lead to strategies for improved transplant survival. For example, calculation of the rate of stent avoidance (number needed to treat) in order to prevent a single episode of BKVN or graft loss would identify specific opportunities to improve these outcomes. Also, subsequent investigation would be needed to determine the trade-offs between the positive impact of stents preventing post-operative complications and the negative impact of increased risk of BK nephropathy.
In conclusion, our era of modern immunosuppression has brought an increased incidence of infection as the etiology of renal allograft nephropathy and the new challenge of identifying modifiable risk factors for graft loss due to BK virus nephropathy. Our study, with the largest sample size to date, confirms studies performed at other centers demonstrating that ureteral stent placement increases the risk of BK viremia within the first year post-transplant. The study identified an increased risk of BK viremia among patients with immediate graft function as previously reported by Hashim et al. We also identified an increased risk of BK viremia among African American patients with immediate graft function. We recommend routine viral PCR monitoring to guide immunosuppression dosing, as recommended in KDIGO guidelines with increased emphasis when stent is placed [32]. In addition, a multicenter prospective study is needed to understand whether these findings can be confirmed in the general kidney transplant population nationwide. Finally, we recommend that the risk of developing BK virus nephropathy post-transplant be weighed against the risk of developing urologic complications when planning for placement of ureteral stent during kidney transplantation.
Acknowledgments
Funding: This study was partially supported through Washington University School of Medicine Just in Time Grant Number UL1 TR000448 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research.
The authors thank Craig Cole and Paul Chung for assistance with data collection. This publication was made possible by Grant Number UL1 TR000448 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NCRR or NIH. The study was presented in part at the World Transplant Congress in July 2014 in San Francisco, USA.
Abbreviations
- ATG
Anti-thymocyte globulin
- BKV
BK virus
- BKVN
BK virus nephropathy
- DGF
Delayed graft function
- HR
Hazard ratio
- IGF
Immediate graft function
- MMF
Mycophenolate mofetil
- OR
Odds ratio
- PCR
Polymerase chain reaction
- RTx
Renal transplant
- UrSt
Ureteral stent
Footnotes
Daniel C. Brennan: Participated in an advisory role and in research design and writing of paper; no conflict of interest. DBrennan@dom.wustl.edu.
Howard Chen: Participated in data collection and data analysis; no conflict of interest. Howard.Chen@wustl.edu.
Dennis K. Fong: Participated in data collection and data analysis; no conflict of interest. DKFong@wustl.edu.
Nikhil Agarwal: Participated in data collection; no conflict of interest. Nikhil93@berkley.edu.
Joseph G. Maliakkal: Participated in research design, data collection, data analysis, and writing of paper; supported by Washington University School of Medicine Just in Time Grant Number UL1 TR000448 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research; no conflict of interest. Joseph.Maliakkal@bcm.edu.
Charles Goss: Participated in data analysis and writing of paper; supported by Washington University in St. Louis; no conflict of interest. cwgoss@wustl.edu
Timothy A. Horwedel: Participated in data collection; supported by Washington University in St. Louis Department of Internal Medicine, Division of Renal Diseases; no conflict of interest. TXH3253@bjc.org.
Jie Zheng: Participated in data analysis and writing of paper; supported by Washington University in St. Louis; no conflict of interest. jiezheng_xjtu@163.com.
Kenneth B. Schechtman: Participated in data analysis and writing of paper; supported by Washington University in St. Louis; no conflict of interest. ken@wubios.wustl.edu
Vikas R. Dharnidharka: Participated in research design, data collection, data analysis, and writing of paper; supported by Washington University School of Medicine Just in Time Grant Number UL1 TR000448 from the National Center for Research Resources (NCRR), a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research and Washington University in St. Louis School of Medicine; no conflict of interest. VikasD@wustl.edu.
Disclosure: The authors declare no conflicts of interest.
References
- 1.Kuppachi S, Thomas B, Kokko KE. BK virus in the kidney transplant patient. Am J Med Sci. 2013;345:482–8. doi: 10.1097/MAJ.0b013e31826c64ef. [DOI] [PubMed] [Google Scholar]
- 2.Brennan DC, Agha I, Bohl DL, et al. Incidence of BK with tacrolimus versus cyclosporine and impact of preemptive immunosuppression reduction. Am J Transplant. 2005;5:582–94. doi: 10.1111/j.1600-6143.2005.00742.x. [DOI] [PubMed] [Google Scholar]
- 3.Hirsch HH, Knowles W, Dickenmann M, et al. Prospective study of polyomavirus type BK replication and nephropathy in renal-transplant recipients. N Engl J Med. 2002;347:488–96. doi: 10.1056/NEJMoa020439. [DOI] [PubMed] [Google Scholar]
- 4.Sawinski D, Goral S. BK virus infection: an update on diagnosis and treatment. Nephrol Dial Transplant. 2015;30:209–17. doi: 10.1093/ndt/gfu023. [DOI] [PubMed] [Google Scholar]
- 5.Hirsch HH, Vincenti F, Friman S, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 2013;13:136–45. doi: 10.1111/j.1600-6143.2012.04320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bohl DL, Storch GA, Ryschkewitsch C, et al. Donor origin of BK virus in renal transplantation and role of HLA C7 in susceptibility to sustained BK viremia. Am J Transplant. 2005;5:2213–21. doi: 10.1111/j.1600-6143.2005.01000.x. [DOI] [PubMed] [Google Scholar]
- 7.Mitterhofer AP, Tinti F, Pietropaolo V, et al. Role of BK virus infection in end-stage renal disease patients waiting for kidney transplantation--viral replication dynamics from pre- to post-transplant. Clin Transplant. 2014;28:299–306. doi: 10.1111/ctr.12312. [DOI] [PubMed] [Google Scholar]
- 8.Dharnidharka VR, Cherikh WS, Abbott KC. An OPTN analysis of national registry data on treatment of BK virus allograft nephropathy in the United States. Transplantation. 2009;87:1019–26. doi: 10.1097/TP.0b013e31819cc383. [DOI] [PubMed] [Google Scholar]
- 9.Thomas A, Dropulic LK, Rahman MH, Geetha D. Ureteral stents: a novel risk factor for polyomavirus nephropathy. Transplantation. 2007;84:433–6. doi: 10.1097/01.tp.0000269616.21698.10. [DOI] [PubMed] [Google Scholar]
- 10.Dharnidharka VR, Araya CE, Wadsworth CS, McKinney MC, Howard RJ. Assessing the value of ureteral stent placement in pediatric kidney transplant recipients. Transplantation. 2008;85:986–91. doi: 10.1097/TP.0b013e318169bf11. [DOI] [PubMed] [Google Scholar]
- 11.Kayler L, Zendejas I, Schain D, Magliocca J. Ureteral stent placement and BK viremia in kidney transplant recipients. Transpl Infect Dis. 2013;15:202–7. doi: 10.1111/tid.12051. [DOI] [PubMed] [Google Scholar]
- 12.Siparsky NF, Kushnir LF, Gallichio MH, Conti DJ. Ureteral stents: a risk factor for polyomavirus BK viremia in kidney transplant recipients undergoing protocol screening. Transplant Proc. 2011;43:2641–4. doi: 10.1016/j.transproceed.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 13.Hashim F, Rehman S, Gregg JA, Dharnidharka VR. Ureteral Stent Placement Increases the Risk for Developing BK Viremia after Kidney Transplantation. J Transplant. 2014;2014:459747. doi: 10.1155/2014/459747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wilson CH, Rix DA, Manas DM. Routine intraoperative ureteric stenting for kidney transplant recipients. Cochrane Database Syst Rev. 2013;6:CD004925. doi: 10.1002/14651858.CD004925.pub3. [DOI] [PubMed] [Google Scholar]
- 15.Solez K, Colvin RB, Racusen LC, et al. Banff 07 classification of renal allograft pathology: updates and future directions. Am J Transplant. 2008;8:753–60. doi: 10.1111/j.1600-6143.2008.02159.x. [DOI] [PubMed] [Google Scholar]
- 16.Siparsky NF, Kushnir LF, Gallichio MH, Conti DJ. Ureteral stents: a risk factor for polyomavirus BK viremia in kidney transplant recipients undergoing protocol screening. Transplant Proc. 43:2641–4. doi: 10.1016/j.transproceed.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 17.Bassiri A, Amiransari B, Yazdani M, Sesavar Y, Gol S. Renal transplantation using ureteral stents. Transplant Proc. 1995;27:2593–4. [PubMed] [Google Scholar]
- 18.Benoit G, Blanchet P, Eschwege P, Alexandre L, Bensadoun H, Charpentier B. Insertion of a double pigtail ureteral stent for the prevention of urological complications in renal transplantation: a prospective randomized study. J Urol. 1996;156:881–4. [PubMed] [Google Scholar]
- 19.Osman Y, Ali-El-Dein B, Shokeir AA, Kamal M, El-Din AB. Routine insertion of ureteral stent in live-donor renal transplantation: is it worthwhile? Urology. 2005;65:867–71. doi: 10.1016/j.urology.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 20.Li R, Sharma BN, Linder S, Gutteberg TJ, Hirsch HH, Rinaldo CH. Characteristics of polyomavirus BK (BKPyV) infection in primary human urothelial cells. Virology. 2013;440:41–50. doi: 10.1016/j.virol.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Priftakis P, Bogdanovic G, Tyden G, Dalianis T. Polyomaviruria in renal transplant patients is not correlated to the cold ischemia period or to rejection episodes. J Clin Microbiol. 2000;38:406–7. doi: 10.1128/jcm.38.1.406-407.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gilbert J, Dahl J, Riney C, et al. Ganglioside GD1a restores infectibility to mouse cells lacking functional receptors for polyomavirus. J Virol. 2005;79:615–8. doi: 10.1128/JVI.79.1.615-618.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eash S, Atwood WJ. Involvement of cytoskeletal components in BK virus infectious entry. J Virol. 2005;79:11734–41. doi: 10.1128/JVI.79.18.11734-11741.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Resnick J, Shenk T. Simian virus 40 agnoprotein facilitates normal nuclear location of the major capsid polypeptide and cell-to-cell spread of virus. J Virol. 1986;60:1098–106. doi: 10.1128/jvi.60.3.1098-1106.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stolt A, Sasnauskas K, Koskela P, Lehtinen M, Dillner J. Seroepidemiology of the human polyomaviruses. J Gen Virol. 2003;84:1499–504. doi: 10.1099/vir.0.18842-0. [DOI] [PubMed] [Google Scholar]
- 26.Atencio IA, Shadan FF, Zhou XJ, Vaziri ND, Villarreal LP. Adult mouse kidneys become permissive to acute polyomavirus infection and reactivate persistent infections in response to cellular damage and regeneration. J Virol. 1993;67:1424–32. doi: 10.1128/jvi.67.3.1424-1432.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Randhawa P, Brennan DC. BK virus infection in transplant recipients: an overview and update. Am J Transplant. 2006;6:2000–5. doi: 10.1111/j.1600-6143.2006.01403.x. [DOI] [PubMed] [Google Scholar]
- 28.Hirsch HH, Vincenti F, Friman S, et al. Polyomavirus BK replication in de novo kidney transplant patients receiving tacrolimus or cyclosporine: a prospective, randomized, multicenter study. Am J Transplant. 13:136–45. doi: 10.1111/j.1600-6143.2012.04320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Funk GA, Gosert R, Comoli P, Ginevri F, Hirsch HH. Polyomavirus BK replication dynamics in vivo and in silico to predict cytopathology and viral clearance in kidney transplants. Am J Transplant. 2008;8:2368–77. doi: 10.1111/j.1600-6143.2008.02402.x. [DOI] [PubMed] [Google Scholar]
- 30.Kidney Disease: Improving Global Outcomes Transplant Work G. KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]
- 31.Kayler L, Zendejas I, Schain D, Magliocca J. Ureteral stent placement and BK viremia in kidney transplant recipients. Transpl Infect Dis. 15:202–7. doi: 10.1111/tid.12051. [DOI] [PubMed] [Google Scholar]
- 32.KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant. 2009;9(Suppl 3):S1–155. doi: 10.1111/j.1600-6143.2009.02834.x. [DOI] [PubMed] [Google Scholar]