Abstract
The complexity of cancer chemotherapy requires pharmacists be familiar with the complicated regimens and highly toxic agents used. This column reviews various issues related to preparation, dispensing, and administration of antineoplastic therapy, and the agents, both commercially available and investigational, used to treat malignant diseases. Questions or suggestions for topics should be addressed to Dominic A. Solimando, Jr, President, Oncology Pharmacy Services, Inc., 4201 Wilson Blvd #110-545, Arlington, VA 22203, e-mail: OncRxSvc@comcast.net; or J. Aubrey Waddell, Professor, University of Tennessee College of Pharmacy; Oncology Pharmacist, Pharmacy Department, Blount Memorial Hospital, 907 E. Lamar Alexander Parkway, Maryville, TN 37804, e-mail: waddfour@charter.net. The information presented in this review is based on published data and clinical expertise and includes information not included in the product labeling. Incorporation of such published data provides a more robust assessment of the drugs and assists pharmacists in evaluation of orders for off-label use of these agents.

COMMENTS
Two versions, a standard dose1–20 and an enhanced dose,21–25 of the PCV regimen have been widely studied. This review will focus on the standard dose regimen (Table 1).
Table 1.
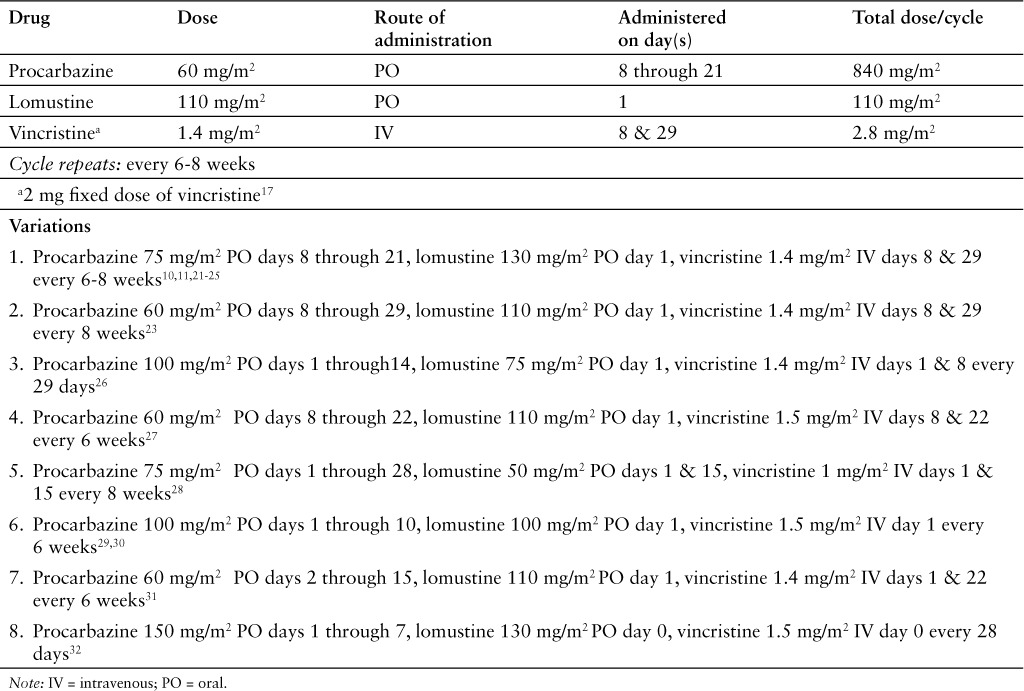
INDICATIONS
The PCV regimen has been studied for treatment of primary brain tumors, particularly gliomas and astrocytomas.1–32 Current guidelines recommend PCV as adjuvant therapy or treatment for recurrent low-grade astrocytoma, oligodendroglioma, anaplastic gliomas, and glioblastoma.33
DRUG PREPARATION
Follow institutional policies for the preparation of hazardous medications when preparing vincristine and dispensing procarbazine and lomustine.
- A. Procarbazine
- Procarbazine is available as 50 mg capsules.
- Store at controlled room temperature (15°C to 30°C [59°F to 86°F]).
- B. Lomustine
- Use 10 mg, 40 mg, or 100 mg capsules.
- Store at controlled room temperature (15°C to 30°C [59°F to 86°F]).
- Lomustine is given as a single dose.
- All the capsules may be dispensed in a single container to reduce patient confusion.
- The container should be clearly labeled to take all the capsules at one time.
- C. Vincristine
- Use vincristine sulfate injection 1 mg/mL.
- Vincristine should be diluted in 25 mL or 50 mL of 0.9% sodium chloride injection in polyvinyl chloride bags.
- Dispensing diluted vincristine in minibags, rather than undiluted in syringes, has been recommended to prevent inadvertent intrathecal injection.
- Brief (less than 24 hours) exposure to temperatures up to 30°C (86°F) is acceptable.
DRUG ADMINISTRATION
A. Procarbazine is given orally as a single daily dose or in 3 or 4 divided doses.
- B. Lomustine
- Lomustine is given orally as a single dose.
- The drug should be taken on an empty stomach (1 hour before or 2 hours after eating).
- Take at bedtime 30 to 60 minutes after taking an antiemetic.
- Although not well documented in the literature, some clinicians believe taking lomustine at bedtime reduces the incidence and/or severity of nausea.
C. Vincristine is administered as a short (10 to 15 minute) intravenous (IV) infusion.
SUPPORTIVE CARE
- A. Acute and Delayed Emesis Prophylaxis: The PCV regimen is predicted to cause acute emesis in 30% to more than 90% of patients.34–38 The studies reviewed reported nausea or vomiting in 5% to 80% of patients.3–5,13,15,20 Prophylactic antiemetic therapy with a serotonin antagonist is recommended.35–38 One of the following regimens given 30 minutes prior to therapy is recommended:
- Ondansetron 8 mg to 16 mg orally (PO), ±dexamethasone 12 mg PO, given 30 minutes before lomustine on day 1.
- Granisetron 1 mg to 2 mg PO, ±dexamethasone 12 mg PO, given 30 minutes before lomustine on day 1.
- Dolasetron 100 mg PO, ±dexamethasone 12 mg PO, given 30 minutes before lomustine on day 1.
- Palonosetron 0.25 mg IV and dexamethasone 12 mg PO, given 30 minutes before lomustine on day 1 only.
The antiemetic therapy should continue for at least 3 days, then resume on days 8 through 21 when procarbazine is given. A meta-analysis of several trials of serotonin antagonists recommends against prolonged (greater than 24 hours) use of these agents, making a steroid, or steroid and dopamine antagonist combination, most appropriate with the procarbazine.39 One of the following regimens is recommended:
Metoclopramide 0.5 to 2 mg/kg PO every 4 to 6 hours, ±dexamethasone 4 mg PO twice a day for 3 days, ±diphenhydramine 25 to 50 mg PO every 6 hours if needed, starting on day 2 of PCV.
Prochlorperazine 10 mg PO every 4 to 6 hours, ±dexamethasone 4 mg PO twice a day for three days, ±diphenhydramine 25 to 50 mg PO every 6 hours if needed, starting on day 2 of PCV.
Promethazine 25 to 50 mg PO every 4 to 6 hours, ±dexamethasone 4 mg PO twice a day for 3 days, ±diphenhydramine 25 to 50 mg PO every 6 hours if needed, starting on day 2 of PCV.
Patients who experience significant nausea or vomiting with one of these regimens should receive an agent from a different pharmacologic category.34–38 There is no evidence that substituting granisetron for ondansetron in subsequent treatment cycles or increasing the dose, even to very high doses, is effective. This approach is not recommended.40–44
- B. Breakthrough Nausea and Vomiting34–38: Patients should receive a prescription for an antiemetic to treat breakthrough nausea. One of the following regimens is recommended:
- Metoclopramide 0.5 to 2 mg/kg PO every 4 to 6 hours if needed, ±diphenhydramine 25 to 50 mg PO every 6 hours if needed.
- Prochlorperazine 10 mg PO every 4 to 6 hours if needed, ±diphenhydramine 25 to 50 mg PO every 6 hours if needed.
- Prochlorperazine 25 mg rectally every 4 to 6 hours if needed, ±diphenhydramine 25 to 50 mg PO every 4 to 6 hours if needed.
- Promethazine 25 to 50 mg PO every 4 to 6 hours if needed, ±diphenhydramine 25 to 50 mg PO every 4 to 6 hours if needed.
C. Hematopoietic Growth Factors: Accepted practice guidelines and pharmaco economic analysis suggest that an antineoplastic regimen have a greater than 20% incidence of febrile neutropenia before prophylactic use of colony stimulating factors (CSFs) is warranted. For regimens with an incidence of febrile neutropenia between 10% and 20%, use of CSFs should be considered. For regimens with an incidence of febrile neutropenia less than 10%, routine prophylactic use of CSFs is not recommended.45,46 The incidence of febrile neutropenia reported in the PCV trials reviewed was only 1%20; prophylactic use of CSFs is not recommended.45,46 CSFs should be considered if a patient experiences febrile neutropenia or grade 4 neutropenia in a prior cycle of PCV.
D. Extravasation: Vincristine is a moderate vesicant; extravasation should be avoided. If extravasation occurs, stop the infusion immediately and aspirate as much of the extravasated solution as possible before withdrawing the needle. The limb should be elevated and cooled intermittently (ice packs for 15–20 minutes 4 times a day for 3 days) 46,47 Although Larson reported applying ice to all extravasations,47,48 most other groups suggest dry heat for 30 minutes 4 times a day for 3 days.49 Hyaluronidase 150 units/1 mL injected intradermally at the extravasation site also has been recommended for treatment of vinca alkaloid extravasations.49
MAJOR TOXICITIES
Most of the toxicities listed below are presented according to their degree of severity. Higher grades represent more severe toxicities. Although there are several grading systems for cancer chemotherapy toxicities, all are similar. One of the frequently used systems is the National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events (http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_8.5x11.pdf). Oncologists generally do not adjust doses or change therapy for grade 1 or 2 toxicities but make, or consider, dosage reductions or therapy changes for grade 3 or 4 toxicities. Incidence values are rounded to the nearest whole percent unless incidence was less than or equal to 0.5%.
B. Constitutional: Fatigue (grade 1) 37%,20 (grade 2) 20%,20 (grade 3) 8%,20 (grade 4) 1%20; weight loss (grade 1) 11%,20 (grade 2) 8%,20 (grade 3) 3%.20
C. Dermatologic: Rash 15% to 17%,4,9 urticaria 10%,5 unspecified skin toxicity (grade 3) 10%.13
D. Gastrointestinal: Anorexia (grade 1) 18%,20 (grade 2) 6%,20 (grade 3) 1%20; constipation 33%,4 (grade 1) 13%,20 (grade 2) 9%20; nausea “severe” 33%4; nausea (grade 1) 21% to 37%,3,20 (grade 2) 23%,20 (grade 3) 3% to 6%15,20; nausea/vomiting 80%,5 (grade 2) 15%,3 (grade 3) 5% to 11%,3,13 (grade 4) 1%13; vomiting (grade 1) 18%,20 (grade 2) 12%,20 (grade 3) 3%,20 (grade 3) 6%.15
E. Hematologic: Anemia (grade 1) 26%,20 (grade 2) 9%,20 (grade 3) 5% to 6%,15,20 (grade 3 or 4) 19%,10 (grade 4) 1%15,20; febrile neutropenia (grade 4) 1%20; leukopenia 36%,6 (grade 1) 14% to 17%,3,6,9 (grade 2) 11% to 43%,3,6,9 (grade 3) 1% to 27%,3,6,9,15 (grade 3 or 4) 81%,10 (grade 4) 3% to 8%3,15; lymphopenia (grade 2) 2%,20 (grade 3) 1%20; “myelosuppression” 66%,4 (grade 2) 37%,12 (grade 3) 63%12; neutropenia (grade 1) 6%,20 (grade 2) 9%,20 (grade 3) 30% to 35%,13,20 (grade 4) 9% to 15%13,20; thrombocytopenia (grade 1) 7% to 16%,3,6,9,20 (grade 2) 3% to 17%,3,6,9,20 (grade 3) 1% to 24%,3,6,9,13,15,20 (grade 3 or 4) 44%,10 (grade 4) 1% to 11%,3,6,13,15 unspecified hematologic toxicity (grade 2 or 3) 10%.17
F. Hepatic: Increased alanine aminotransferase (ALT) (grade 1) 9%,20 (grade 2) 1%,20 (grade 3) 1%,20 (grade 4) 1%20; increased aspartate aminotransferase (AST) (grade 1) 9%,20 (grade 2) 1%,20 (grade 4) 1%20; increased liver enzymes (grade 2) 5,6 (grade 2 or 3) 10%.17
G. Hypersensitivity: Allergic reactions (grade 2 or 3) 10%,17 allergic skin reactions (grade 3) 1%,15 generalized erythema 4%.12
H. Infection: Herpes zoster (grade 2 or 3) 1%17; pneumonia 4%,12 (grade 2 or 3) 1%,17 unspecified (grade 1) 9%,20 (grade 2) 12%,20 (grade 3) 10%.20
I. Neurologic: Motor paresthesias 11%12; neuropathy, unspecified 8%,9 (grade 1) 15%,3 (grade 2) 15%,3 (grade 3) 8%3; paresthesias 17% to 24%4,6; polyneuropathy (grade 3) 2%,15 (grade 2 or 3) 7%17; reduced/loss of tendon reflexes/reduced vibration sense 100%12; sensory loss 22%.12
J. Pain: Abdominal 33%.4
PRETREATMENT LABORATORY STUDIES NEEDED
- A. Baseline
- AST/ALT
- Total bilirubin
- Serum creatinine
- Complete blood count (CBC) with differential
- B. Prior to Each Treatment
- AST/ALT
- Total bilirubin
- Serum creatinine
- Complete blood count (CBC) with differential
- C. Recommended Pretreatment Values: The minimally acceptable pretreatment CBC values required to begin a cycle with full-dose therapy in the protocols reviewed were:
- ALT: Less than or equal to 2 times the ULN.8
In clinical practice, a pretreatment ANC of 1,000 cells/mcL and platelets of 75,000 cells/mcL are usually considered acceptable.
DOSAGE MODIFICATIONS
- A. Renal Function
- Procarbazine: Dose reduction may be required; no formal guidelines available.50
- Lomustine – creatinine clearance:
- No information available.51
- Less than 60 mL/min, reduce dose 25%.52
- Less than 45 mL/min, reduce dose 30%.52
- Less than 30 mL/min, do not give the drug.52
- Greater than 50 mL/min, no adjustment required.53
- Less than or equal to 50 mL/min and greater than or equal to 10 mL/min, reduce dose 25%.53
- Less than 10 mL/min, reduce dose 50% to 75%.53
- B. Liver Function
- ALT or AST greater than 3 times the ULN20:
- Hold all drugs until ALT and AST are less than or equal to 2 times the ULN, and
- Then reduce all drug doses 25%.
- Procarbazine:
- Dose reduction may be required; no formal guidelines available.50
- Bilirubin greater than 5 mg/dL or ALT/AST greater than 180 international units, do not give the drug.55
- Bilirubin greater than 5 mg/dL or ALT/AST greater than 3 times the ULN, do not give the drug.56
- ALT/AST 1.6 to greater than 6 times the ULN, reduce dose 25%.56
- ALT/AST greater than 6 times the ULN, use clinical judgement.56
- Lomustine: No information available.51
- C. Myelosuppression
- WBC:
- Greater than or equal to 3,000 cells/mcL and less than or equal to 3,900 cells/mcL, reduce lomustine and procarbazine dose 25%.6
- Less than 3,000 cells/mcL6:
- (1) Do not give lomustine or procarbazine.
- (2) When toxicity resolves, reduce lomustine and procarbazine dose 25%.
- Less than 1,000 cells/mcL, reduce procarbazine dose 67%.12
- ANC: Less than or equal to 500 cells/mcL, reduce lomustine and procarbazine dose 25%.20
- Platelet count:
- Greater than or equal to 75,000 cells/mcL and less than or equal to 120,000 cells/mcL, reduce lomustine and procarbazine dose 25%.6
- Less than 75,000 cells/mcL6:
- (1) Do not give lomustine or procarbazine.
- (2) When toxicity resolves, reduce lomustine and procarbazine dose 25%.
- Less than 50,000 cells/mcL, reduce procarbazine dose 67%.12
- Less than or equal to 50,000 cells/mcL, reduce lomustine and procarbazine dose 25%.20
REFERENCES
- 1. Levin VA, Edwards MS, Wright DC, . et al. Modified procarbazine, CCNU and vincristine (PCV-3) combination chemotherapy in the treatment of malignant brain tumors. Cancer Treat Rep. 1980; 64( 2–3): 237– 241. [PubMed] [Google Scholar]
- 2. Levin VA, Silver P, Hannigan J, . et al. Superiority of post-radiotherapy adjuvant chemotherapy with CCNU, procarbazine, and vincristine (PCV) over BCNU for anaplastic gliomas: NCOG 6G61 final report. Int J Radiat Oncol Biol Phys. 1990; 18( 2): 321– 324. [DOI] [PubMed] [Google Scholar]
- 3. Levin VA, Wara WH, Davis RL, . et al. Phase III comparison of BCNU and the combination of procarbazine, CCNU and vincristine administered after radiotherapy with hydroxyurea for malignant glioma. J Neurosurg. 1985; 63( 2): 218– 223. [DOI] [PubMed] [Google Scholar]
- 4. Cairncross JG, Macdonald DR.. Successful chemotherapy for recurrent malignant oligodendrogliomas. Ann Neurol. 1988; 23( 4): 360– 364. [DOI] [PubMed] [Google Scholar]
- 5. Cairncross JG, Macdonald DR, Ramsay DA.. Aggressive oligodendroglioma: A chemosensitive tumor. Neurosurgery. 1992; 31( 1): 78– 82. [DOI] [PubMed] [Google Scholar]
- 6. Glass J, Hochberg FH, Gruber ML, . et al. The treatment of oligodendrogliomas and mixed oligodendroglioma-astrocytomas with PCV chemotherapy. J Neurosurg. 1992; 76( 5): 741– 745. [DOI] [PubMed] [Google Scholar]
- 7. Kyritsis AP, Yung WK, Bruner J, . et al. The treatment of anaplastic oligodendrogliomas and mixed gliomas. Neurosurgery. 1993; 32( 3): 365– 370. [DOI] [PubMed] [Google Scholar]
- 8. Levin VA, Prados MR, Wara WM, . et al. Radiation therapy and bromodeoxyuridine chemotherapy followed by procarbazine, lomustine, and vincristine for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 1995; 32( 1): 75– 83. [DOI] [PubMed] [Google Scholar]
- 9. Soffietti R, Ruda R, Bradac GB, . et al. PCV chemotherapy for recurrent oligodendrogliomas and oligoastrocytomas. Neurosurgery. 1998; 43( 5): 1066– 1073. [DOI] [PubMed] [Google Scholar]
- 10. van den Bent MJ, Kros JM, Heimans JJ, . et al. Response rate and prognostic factors of recurrent oligodendroglioma treated with PCV chemotherapy. Neurology. 1998; 51( 4): 1140– 1145. [DOI] [PubMed] [Google Scholar]
- 11. Paleologus NA, Macdonald DR, Vick NA, . et al. Neoadjuvant procarbazine, CCNU, and vincristine for anaplastic and aggressive oligodendroglioma. Neurology. 1999, 53( 5): 1141– 1143. [DOI] [PubMed] [Google Scholar]
- 12. Streffer J, Schabet M, Bamberg M, . et al. A role for preirradiation PCV chemotherapy for oligodendroglial brain tumors. J Neurol. 2000; 247( 4): 297– 302. [DOI] [PubMed] [Google Scholar]
- 13. Levin VA, Yung WK, Bruner J, . et al. Phase II study of accelerated fractionation radiation therapy with carboplatin followed by PCV chemotherapy for the treatment of anaplastic gliomas. Int J Radiat Oncol Biol Phys. 2002; 53( 1): 58– 66. [DOI] [PubMed] [Google Scholar]
- 14. Stege EM, Kros JM, de Bruin HG, . et al. Successful treatment of low-grade olgodendroglial tumors with a chemotherapy regimen of procarbazine, lomustine, and vincristine. Cancer. 2005; 103( 4): 802– 809. [DOI] [PubMed] [Google Scholar]
- 15. van den Bent MJ, Carpentier AF, Brandes AA, . et al: Adjuvant procarbazine, lomustine, and vincristine improves progression-free survival but not overall survival in newly diagnosed anaplastic oligodendrogliomas and oligoastrocytomas: A randomized European Organisation for Research and Treatment of Cancer phase III trial. J Clin Oncol. 2006; 24( 18): 2715– 2722. [DOI] [PubMed] [Google Scholar]
- 16. Lebrun C, Fontaine D, Bourg V, . et al. Treatment of newly diagnosed symptomatic pure low-grade oligodendrogliomas with PCV chemotherapy. Eur J Neurol. 2007; 14( 4): 391– 398. [DOI] [PubMed] [Google Scholar]
- 17. Wick W, Hartmann C, Engel C, . et al. NOA-04 Randomized phase III trial of sequential radiochemotherapy of anaplastic glioma with procarbazine, lomustine, and vincristine or temozolomide. J Clin Oncol. 2009; 27( 35): 5874– 5880. [DOI] [PubMed] [Google Scholar]
- 18. Shaw EG, Wang M, Coons SW, . et al. Randomized trial of radiation therapy plus procarbazine, lomustine, and vincristine chemotherapy for supratentorial adult low-grade glioma: Initial results of RTOG 9802. J Clin Oncol. 2012; 30( 25): 3065– 3070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. van den Bent MJ, Brandes AA, Taphoorn MJ, . et al. Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: Long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol. 2013; 31( 3): 344– 350. [DOI] [PubMed] [Google Scholar]
- 20. Buckner JC, Shaw EG, Pugh SL, . et al. Radiation plus procarbazine, CCNU, and vincristine in low-grade glioma. N Engl J Med. 2016; 374( 14): 1344– 1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cairncross G, Macdonald D, Ludwin S, . et al. Chemotherapy for anaplastic oligodendroglioma. National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1994; 12( 10): 2013– 2021. [DOI] [PubMed] [Google Scholar]
- 22. Kirby S, Macdonald D, Fisher B, . et al. Pre-radiation chemotherapy for malignant glioma in adults. Can J Neurol Sci. 1996; 23( 2): 123– 127. [DOI] [PubMed] [Google Scholar]
- 23. Mason WP, Krol GS, DeAngelis LM.. Low-grade oligodendroglioma responds to chemotherapy. Neurology. 1996; 46( 1): 203– 207. [DOI] [PubMed] [Google Scholar]
- 24. Buckner JC, Gesme D Jr, O'Fallon JR, . et al. Phase II trial of procarbazine, lomustine, and vincristine as initial therapy for patients with low-grade oligodendroglioma or oligoastrocytoma: Efficacy and associations with chromosomal abnormalities. J Clin Oncol. 2003; 21( 2): 251– 255. [DOI] [PubMed] [Google Scholar]
- 25. Cairncross G, Berkey B, Shaw E, . et al. Phase III trial of chemotherapy plus radiotherapy versus radiotherapy alone for pure and mixed anaplastic oligodendroglioma (RTOG 9402): Intergroup Radiation Therapy Oncology Group Trial 9402. J Clin Oncol. 2006; 24( 18): 2707– 2714. [DOI] [PubMed] [Google Scholar]
- 26. Gutin PH, Wilson CB, Kumar ARV, . et al. Phase II study of procarbazine, CCNU and vincristine combination chemotherapy in the treatment of malignant brain tumors. Cancer. 1975; 35( 5): 1398– 1404. [DOI] [PubMed] [Google Scholar]
- 27. Kim L, Hochberg FH, Thornton AF, . et al. Procarbazine, lomustine, and vincristine (PCV) chemotherapy for grade III and grade IV oligoastrocytomas. J Neurosurg. 1996; 85( 4): 602– 607. [DOI] [PubMed] [Google Scholar]
- 28. Källén K, Geijer B, Malmström P, . et al. Quantitative 201Tl SPET imaging in the follow-up of treatment for brain tumour: A sensitive tool for the early identification of response to chemotherapy? Nucl Med Commun. 2000; 21( 3): 259– 267. [DOI] [PubMed] [Google Scholar]
- 29. Sandberg-Wollheim M, Malmström P, Strömblad, . et al. A randomized study of chemotherapy with procarbazine, vincristine, and lomustine with and without radiation therapy for astrocytoma grades 3 and/or 4. Cancer. 1991; 68( 1): 22– 29. [DOI] [PubMed] [Google Scholar]
- 30. Medical Research Council Brain Tumor Working Party. . Randomized trial of procarbazine, lomustine, and vincristine in the adjuvant treatment of high-grade astrocytoma: A Medical Research Council trial. J Clin Oncol. 2001; 19( 2): 509– 518. [DOI] [PubMed] [Google Scholar]
- 31. Murphy C, Pickles T, Knowling M, . et al. Concurrent modified PCV chemotherapy and radiotherapy in newly diagnosed grade IV astrocytoma. J Neurooncol. 2002; 57( 3): 215– 220. [DOI] [PubMed] [Google Scholar]
- 32. Cornetta K, Croop J, Dropcho E, . et al. A pilot study of dose-intensified procarbazine, CCNU, vincristine for poor prognosis brain tumors utilizing fibronectin-assisted, retroviral-mediated modification of CD34+ peripheral blood cells with O6-methylguanine DNA methyltransferase. Cancer Gene Ther. 2006; 13( 9): 886– 895. [DOI] [PubMed] [Google Scholar]
- 33. National Comprehensive Cancer Network. . NCCN Clinical Practice Guidelines – Central Nervous System Cancers. V.1.2016. http://www.nccn.org/professionals/physician_gls/pdf/cns.pdf. Accessed November 10, 2016.
- 34. Hesketh PJ, Kris MG, Grunberg SM, . et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997; 15( 1): 103– 109. [DOI] [PubMed] [Google Scholar]
- 35. National Comprehensive Cancer Network. . NCCN Clinical Practice Guidelines – Antiemesis. V.2.2016. http://www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf. Accessed November 10, 2016.
- 36. Basch E, Prestrud AA, Hesketh PJ, . et al. Antiemetics: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2011; 29( 31): 4189– 4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hesketh PJ, Bohlke K, Lyman GH, . et al. Antiemetics: American Society of Clinical Oncology. Focused Guideline Update. J Clin Oncol. 2016; 34( 4): 381– 386. [DOI] [PubMed] [Google Scholar]
- 38. Multinational Association for Supportive Care in Cancer. . Antiemetic guidelines. 2013. http://www.mascc.org/assets/documents/mascc_guidelines_english_2013.pdf. Accessed November 10, 2016.
- 39. Geling O, Eichler HG.. Should 5-hydroxytryptamine-3 receptor antagonists be administered beyond 24 hours after chemotherapy to prevent delayed emesis? Systematic re-evaluation of clinical evidence and drug cost implications. J Clin Oncol. 2005; 23( 6): 1289– 1294. [DOI] [PubMed] [Google Scholar]
- 40. Terrey JP, Aapro MS.. The activity of granisetron in patients who had previously failed ondansetron antiemetic therapy. Eur J Clin Res. 1996; 8: 281– 288. [Google Scholar]
- 41. Carmichael J, Keizer HJ, Cupissol D, Milliez J, Scheidel P, Schindler AE.. Use of granisetron in patients refractory to previous treatment with antiemetics. Anticancer Drugs. 1998; 9( 5): 381– 385. [DOI] [PubMed] [Google Scholar]
- 42. de Wit R, de Boer AC, vd Linden GH, Stoter G, Sparreboom A, Verweij J.. Effective cross-over to granisetron after failure to ondansetron, a randomized double blind study in patients failing ondansetron plus dexamethasone during the first 24 hours following highly emetogenic chemotherapy. Br J Cancer. 2001; 19; 85( 8): 1099– 1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Smith IE. A dose-finding study of granisetron, a novel antiemetic, in patients receiving cytostatic chemotherapy. The Granisetron Study Group. J Cancer Res Clin Oncol. 1993; 119( 6): 350– 354. [DOI] [PubMed] [Google Scholar]
- 44. Soukop M. A dose-finding study of granisetron, a novel antiemetic, in patients receiving high-dose cisplatin. Granisetron Study Group. Support Care Cancer. 1994; 2( 3): 177– 183. [DOI] [PubMed] [Google Scholar]
- 45. Smith TJ, Bohlke K, Lyman GH, . et al. Recommendations for the use of white blood cell growth factors: American Society of Clinical Oncology Clinical Practice Guideline Update. J Clin Oncol. 2015; 33( 28): 3199– 3212. [DOI] [PubMed] [Google Scholar]
- 46. NCCN Clinical Practice Guidelines in Oncology – Myeloid Growth Factors. V.1.2016. http://www.nccn.org/professionals/physician_gls/pdf/myeloid_growth.pdf. Accessed November, 10, 2016.
- 47. Larson DL. Treatment of tissue extravasation by antitumor agents. Cancer. 1982; 49( 9): 1796– 1799. [DOI] [PubMed] [Google Scholar]
- 48. Larson DL. What is the appropriate management of tissue extravasation by antitumor agents? Plast Reconstr Surg. 1985; 75( 3): 397– 402. [DOI] [PubMed] [Google Scholar]
- 49. Mullin S, Beckwith MC, Tyler LS.. Prevention and management of antineoplastic extravasation injury. Hosp Pharm. 2000; 35( 1): 57– 74. [Google Scholar]
- 50. Matulane [prescribing information]. Gaithersburg, MD: Sigma-Tau Pharmaceuticals, Inc.; 2008. [Google Scholar]
- 51. Kintzel PE, Dorr RT.. Anticancer drug renal toxicity and elimination: Dosing guidelines for altered renal function. Cancer Treatment Rev. 1995; 21( 1): 33– 64. [DOI] [PubMed] [Google Scholar]
- 52. Aronoff GR, Bennett WM, Berns JS, . et al. Smoyer WE. Drug Prescribing in Renal Failure. 5th ed. Philadelphia: American College of Physicians; 2007. [Google Scholar]
- 53. Vincristine [prescribing information]. Lake Forest, IL: Hospira, Inc.; 2013. [Google Scholar]
- 54. King PD., Perry MC. Hepatotoxicity of chemotherapy. Oncologist. 2001; 6( 2): 162– 176. [DOI] [PubMed] [Google Scholar]
- 55. Floyd J, Mirza I, Sachs B, Perry MC.. Hepatotoxicity of chemotherapy. Semin Oncol. 2006; 33( 1): 50– 67. [DOI] [PubMed] [Google Scholar]


