Abstract
Melanoma remains the leading cause of skin cancer–related deaths. Surgical resection and adjuvant therapies can result in disease-free intervals for stage III and stage IV disease; however, recurrence is common. Understanding microRNA (miR) dynamics following surgical resection of melanomas is critical to accurately interpret miR changes suggestive of melanoma recurrence. Plasma of 6 patients with stage III (n = 2) and stage IV (n = 4) melanoma was evaluated using the NanoString platform to determine pre- and postsurgical miR expression profiles, enabling analysis of more than 800 miRs simultaneously in 12 samples. Principal component analysis detected underlying patterns of miR expression between pre- vs postsurgical patients. Group A contained 3 of 4 patients with stage IV disease (pre- and postsurgical samples) and 2 patients with stage III disease (postsurgical samples only). The corresponding preoperative samples to both individuals with stage III disease were contained in group B along with 1 individual with stage IV disease (pre- and postsurgical samples). Group A was distinguished from group B by statistically significant analysis of variance changes in miR expression (P < .0001). This analysis revealed that group A vs group B had downregulation of let-7b-5p, miR-520f, miR-720, miR-4454, miR-21-5p, miR-22-3p, miR-151a-3p, miR-378e, and miR-1283 and upregulation of miR-126-3p, miR-223-3p, miR-451a, let-7a-5p, let-7g-5p, miR-15b-5p, miR-16-5p, miR-20a-5p, miR-20b-5p, miR-23a-3p, miR-26a-5p, miR-106a-5p, miR-17-5p, miR-130a-3p, miR-142-3p, miR-150-5p, miR-191-5p, miR-199a-3p, miR-199b-3p, and miR-1976. Changes in miR expression were not readily evident in individuals with distant metastatic disease (stage IV) as these individuals may have prolonged inflammatory responses. Thus, inflammatory-driven miRs coinciding with tumor-derived miRs can blunt anticipated changes in expression profiles following surgical resection.
Keywords: Melanoma, microRNA, surgical resection, principal component analysis
Introduction
Melanoma remains the leading cause of skin cancer–related deaths, and an estimated 76 380 individuals will be diagnosed in the United States in 2016.1 Early-stage disease has a favorable prognosis with surgical intervention as the mainstay of treatment. The survival rate of individuals with stage I and stage II disease is 53% to 97%, whereas involvement of regional lymph nodes (stage III) results in a 5-year survival of 40% to 78%.2 Not surprisingly, distant metastatic disease has the worst prognosis with a survival rate of just 33% to 62%.2 Adjuvant therapies with targeted molecular and immunotherapies have been investigated in individuals with locally advanced disease; however, recurrence rates are high.3 Eggermont et al4 found that treatment of stage III melanoma with the anti-Cytotoxic T-Lymphocyte Associated Protein 4 (CTLA-4) drug, ipilimumab, resulted in a recurrence-free survival of 46.5% at 3 years. Unfortunately, in the setting of metastatic disease, many individuals with a primary therapeutic response to immune-based agents will often develop recurrence despite initial disappearance of most or all of their disease.5
MicroRNAs (miRs) consist of 19 to 22 nucleotides, noncoding RNA molecules present within all cells. MicroRNAs target complementary segments on the 3′ untranslated region of messenger RNA and result in silencing of translation. More than 800 different miRs are present within cells, and the expression of miRs differs across tissues and disease processes. Dysregulated miRs are involved in several key cellular pathways responsible for malignant progression including cellular invasion, migration, proliferation, angiogenesis, replicative immortality, immune evasion, and avoidance of senescence and apoptosis.6 Consequently, miRs have been investigated in various malignancies including melanoma.7 MicroRNA dysregulation has been found across different stages of melanoma and has been implicated as a biomarker with potential diagnostic and prognostic usefulness.8 Investigation into its usefulness for prediction of recurrence has also been studied.9
Understanding miR dynamics following surgical resection of melanoma is incompletely understood yet is necessary to accurately interpret miR patterns suggestive of recurrence. MiR-210 has been suggested as a marker of early metastatic recurrence in melanoma.10 However, evaluation of a single miR carries the possibility of overlooking potentially meaningful candidates; thus, global investigation of the miR transcriptome is more useful. Ferracin et al11 have used microarray and small RNA sequencing techniques for postoperative analysis of serum and plasma melanoma patient samples. They found dysregulation of miR-181a-5p and miR-320a following surgical resection. However, staging of melanomas in the aforementioned study was not specified. Modern approaches such as NanoString allow for analysis of the miR transcriptome yet require lower starting amounts of RNA and have lower associated costs compared with microarray.12 Thus, NanoString is well suited for noninvasive detection of miRs using plasma blood samples where RNA quantities are often miniscule. Herein, we perform an exploratory study to evaluate the potential usefulness of miRs in plasma samples of metastatic melanoma following surgical resection. This work highlights the similarities of neoplastic and inflammatory-related miRs, which may act as a confounding variable in the interpretation of plasma miR studies of metastatic melanoma. Thus, future studies will need to be carefully constructed to potentially minimize this possibility.
Methods
Blood samples
Six patients undergoing surgical resection for malignant melanoma with metastatic progression to regional lymph node basins or distant metastatic sites were selected. These patients were tested between 2013 and 2015 at The Ohio State University Wexner Medical Center under the auspices of institutional review board protocol No. 1999C0348. Peripheral venous blood collection was performed in EDTA collection tubes prior to surgical therapy and on postoperative follow-up to 3 weeks after surgery. Peripheral blood samples were centrifuged at 1700 rpm for 11 minutes. The plasma layer was isolated and stored at −80°C.
Isolation of RNA
RNA isolation was carried out using 200 µL of plasma samples with the miRNeasy mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s recommendations. The addition of 3 miR spike-ins with known concentrations was used as loading controls. Additional RNA purification was performed using Amicon (Millipore, Billerica, Massachusetts) 0.5-mL 3-kDa column as per the manufacturer’s recommendations. Isolated RNA was stored at −80°C.
NanoString
Three microliters of isolated RNA was loaded onto NanoString nCounter (NanoString Technologies, Seattle, Washington) platform, and miR quantification was carried out as previously described.13 Briefly, miRs were ligated with DNA tags to normalize melting temperatures and provide unique identification of each miR. Excess tags were removed, and a panel of capture and reporter probes containing unique fluorescent signals corresponding to individual miRs were hybridized at 64°C for 18 hours. Immobilization of hybridized probes onto a streptavidin-coated cartridge was performed using nCounter Prep Station (NanoString Technologies). The fluorescence of each hybridized miR was measured by an nCounter Digital analyzer (NanoString Technologies) with a high-density scan containing 600 fields of view. The investigation included 5 negative and 5 positive controls as well as 5 housekeeping genes.
Data analysis
To control for technology-based experimental error, technical normalization was applied to the raw expression data of 800 miRs before log transformation. Technical normalization controls for performance-based experimental error, and it is applied to the raw data by the NanoString company. We cannot independently verify the algorithm used in the technical normalization. However, it is standard practice and advised by the company to utilize technical normalized data rather than the raw expression. No miR filtering was applied. Using JMP 10 software, variables with highest variance were determined using the principal component analysis (PCA).
Results
Plasma miR expression of 6 patients undergoing surgical resection for melanoma was measured preoperatively and postoperatively using a NanoString platform. Two patients were diagnosed with stage III disease (patients 2, 3), whereas the remaining 4 patients had stage IV disease (patients 1, 4, 5, 6; Table 1). Notably, standard analysis (t test) of pre- and postsurgical miR expression data did not reveal any statistically significant changes in miR expression. Consequently, PCA was used in an exploratory manner to identify potentially meaningful associations. This approach mathematically transforms a number of possibly correlated variables into a smaller number of uncorrelated variables termed principal components. PCA reduces a complex data set to a limited quantity of new variables (components) according to variation within the data set. The variable with the greatest variation is deemed the principle component. PCA is an exploratory analysis method that emphasizes variation to bring out strong patterns in complex data sets by reducing their dimensionality. Accordingly, using NanoString miR expression data from pre- and postsurgical melanoma samples, PCA allows for categorization of correlated patterns of miR expression variation (ie, principal components) and identification of miRs exhibiting variation across the identified components.
Table 1.
Patient characteristics.
| Patient | Age | Sex | Preoperative PCA group | Postoperative PCA group | Primary tumor location | Primary resected | Stage | Active disease (at preoperative blood draw) | Procedure | Largest tumor foci of specimen | Recurrence | Survival |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 52 | M | A | A | Right arm | Yes (2 mo prior) T3a—2.8 mm 0/3 SLNB—right axilla |
IV | Left axilla | Left ALND 9/55 nodes positive |
3 cm | Yes 5 mo |
Dead 35 mo |
| 2 | 81 | M | B | A | Left shoulder | Yes (1 y prior) T4a—4.6 mm deep 2/2 SLNB—left axilla |
IIIc | Left axilla | Left ALND 5/47 nodes positive |
1.6 cm | Yes 5 mo |
Dead 11 mo |
| 3 | 71 | F | B | A | Unknown primary | NA | IIIc | Left axilla | Left ALND 5/31 nodes positive |
9.9 cm | Yes 5 mo |
Dead 22 mo |
| 4 | 71 | F | B | B | Right heel | Yes (5 y prior) T2b—10 mm deep 0/4 SLNB—right inguinal |
IV | Metastatic cutaneous lesion of left groin | WLE of metastatic cutaneous lesion | 3.4 cm | Yes 5 mo |
Dead 24 mo |
| 5 | 30 | M | A | A | Unknown primary | NA | IV | Abdominal wall | Wide excision of abdominal wall | 4.7 cm | No (18 mo) |
Alive |
| 6 | 56 | M | A | A | Right calf | Yes (3 y prior) T4b—4.6 mm 0/2 SLNB—right axilla |
IV | Malignant enterocolonic fistula Liver Lung |
Small bowel resection Left hemicolectomy Hepatic wedge resection (Pulmonary metastasis not resected) |
5.5 cm | Remaining distant metastatic disease at time of resection | Dead 30 mo |
Abbreviations: ALND, axillary lymph node dissection; PCA, principal component analysis; SLNB, sentinel lymph node biopsy, expressed as ratio of number of lymph nodes containing microscopic disease relative to the total number of lymph nodes biopsied; WLE, wide local excision; NA, Not applicable (unknown primary).
Six patients undergoing surgical resection of melanoma lesions were classified according to pathologic stage and grouping on PCA before and after surgical resection.
Two categories (groups A and B) became evident when analyzed through PCA (Figure 1). Two individuals (patients 2 and 3) did have preoperative and postoperative samples in separate groups as defined by PCA. Both of these patients had stage IIIc disease. However, this pattern was not observed in the remaining individuals with stage IV disease. Three of 4 individuals with stage IV disease had both preoperative and postoperative samples in group A, whereas the remaining patient had both samples in group B. Overall, individuals with high variability in miR patterns following surgical resection corresponded to individuals with IIIc disease, whereas others with distant metastatic disease (stage IV) had less variability.
Figure 1.
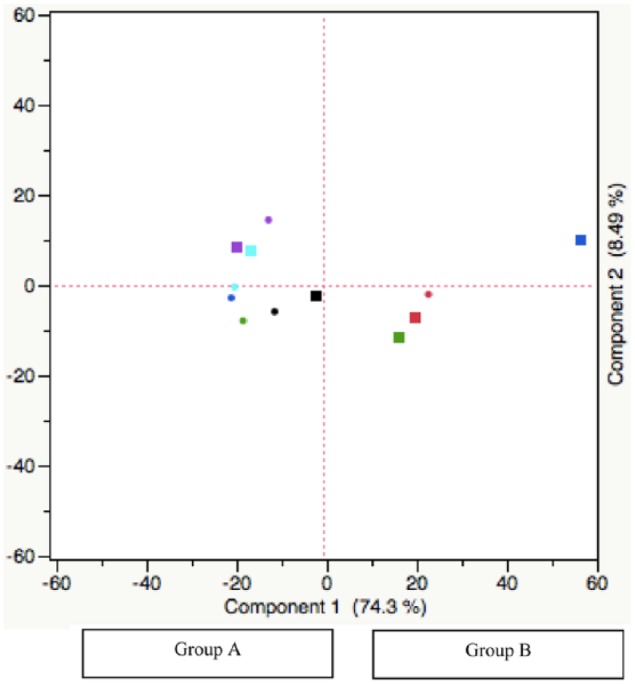
Principal component analysis of plasma miR expression following resection of melanoma.
MicroRNA (miR) expression was measured in 6 patients preoperatively (■) and postoperatively (●) using the NanoString platform. Relationship of plasma miR expression before and after surgical resection was investigated using principal component analysis according to components that could explain the largest variation within the data set. Components 1 and 2 account for the largest variation and were used for generation of the score plot.
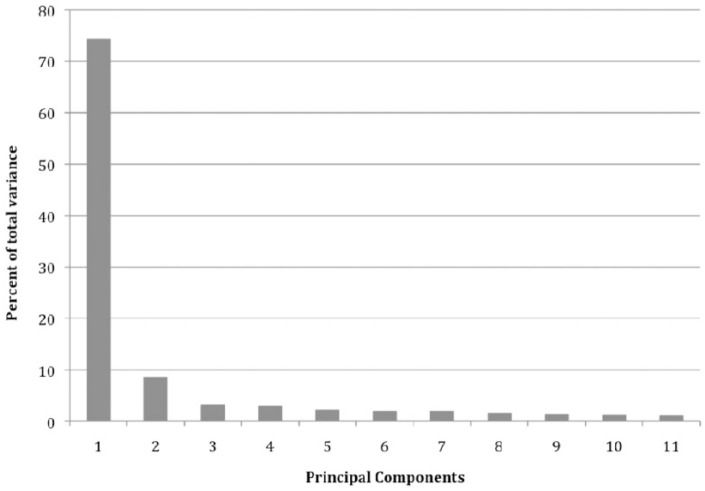
Principal component analysis was performed using multiple stratifications. The dominant stratification (component 1) contained the most variability among the samples (74.3%). Additional groupings accounted for some variation but to a lesser extent (Figure 2). Component 2 contained the second highest variability among the sample set followed by the remaining components in a sequentially lower degree. Overall, the majority of variability within the data set was explained by component 1.
Figure 2.
Components contributing to total variation in principal component analysis.
Principal component analysis of microRNA expression in pre- and postsurgical melanoma samples revealed that several components contribute to overall variation within the data set. Component 1 contributes to the largest variation.
Individual miR expression between groups A and B was analyzed using analysis of variance (ANOVA), and several miRs were differentially expressed to a significant degree (P < .0001). This analysis revealed that group A vs group B had downregulation of let-7b-5p, miR-520f, miR-720, miR-4454, miR-21-5p, miR-22-3p, miR-151a-3p, miR-378e, and miR-1283 and upregulation of miR-126-3p, miR-223-3p, miR-451a, let-7a-5p, let-7g-5p miR-15b-5p, miR-16-5p, miR-20a-5p, miR-20b-5p, miR-23a-3p, miR-26a-5p, miR-106a-5p, miR-17-5p, miR-130a-3p, miR-142-3p, miR-150-5p, miR-191-5p, miR-199a-3p, miR-199b-3p, and miR-1976 (Table 2).
Table 2.
Differential microRNA expression between groups A and B in principal component analysis.
| MicroRNA | Group A average | Group B average | Fold change |
|---|---|---|---|
| hsa-miR-1976 | 1.509 | 3.242 | 2.148 |
| hsa-miR-191-5p | 1.639 | 2.867 | 1.750 |
| hsa-miR-150-5p | 1.498 | 2.560 | 1.709 |
| hsa-let-7a-5p | 1.832 | 3.112 | 1.699 |
| hsa-miR-126-3p | 2.147 | 3.422 | 1.593 |
| hsa-miR-16-5p | 1.924 | 3.002 | 1.561 |
| hsa-miR-20a-5p + hsa-miR-20b-5p | 2.059 | 2.942 | 1.429 |
| hsa-miR-451a | 2.827 | 3.941 | 1.394 |
| hsa-miR-106a-5p + hsa-miR-17-5p | 2.130 | 2.940 | 1.380 |
| hsa-miR-142-3p | 2.386 | 3.286 | 1.377 |
| hsa-miR-15b-5p | 2.327 | 3.039 | 1.306 |
| hsa-let-7g-5p | 2.532 | 3.223 | 1.273 |
| hsa-miR-199a-5p | 2.048 | 2.535 | 1.238 |
| hsa-miR-223-3p | 3.536 | 4.146 | 1.173 |
| hsa-miR-15a-5p | 2.139 | 2.461 | 1.150 |
| hsa-miR-23a-3p | 2.830 | 3.112 | 1.100 |
| hsa-miR-130a-3p | 2.774 | 2.959 | 1.067 |
| hsa-miR-4454 | 4.273 | 4.049 | 0.948 |
| hsa-miR-21-5p | 3.198 | 2.968 | 0.928 |
| hsa-let-7b-5p | 3.477 | 3.085 | 0.887 |
| hsa-let-7c | 2.216 | 1.781 | 0.803 |
| hsa-miR-199b-5p | 1.237 | 0.961 | 0.777 |
| hsa-miR-378e | 3.383 | 2.613 | 0.772 |
| hsa-miR-720 | 3.668 | 2.644 | 0.721 |
| hsa-miR-1283 | 3.557 | 2.503 | 0.704 |
Differences in individual microRNA expression between groups A and B based on principal component analysis.
Discussion
Exploratory evaluation of miR profiles in plasma of melanoma patients before and after surgery did not yield any significant miRs (data not shown). However, PCA was able to distinguish the 2 groups across all plasma samples, group A and group B, as shown in Figure 1. Through the analysis of the miRs that comprise component 1 of the PCA analysis, we determined that group A vs group B had downregulation of let-7b-5p, miR-520f, miR-720, miR-4454, miR-21-5p, miR-22-3p, miR-151a-3p, miR-378e, and miR-1283 and upregulation of miR-126-3p, miR-223-3p, miR-451a, let-7a-5p, let-7g-5p miR-15b-5p, miR-16-5p, miR-20a-5p, miR-20b-5p, miR-23a-3p, miR-26a-5p, miR-106a-5p, miR-17-5p, miR-130a-3p, miR-142-3p, miR-150-5p, miR-191-5p, miR-199a-3p, miR-199b-3p, and miR-1976 (P < .0001 using ANOVA). Given the absence of technical replicates and normalized controls, this form of data driven cohort discovery seemed a sensible approach to elucidate potential underlying relationships.
Several of the 5 most differentially expressed miRs in this study have been previously reported in the setting of melanoma. Both miR-150 overexpression and downregulation have been described in malignant melanoma and may exert effects on several pathways such as cellular proliferation through regulation of v-myb avian myeloblastosis viral oncogene homolog.14 MiR-126 is downregulated in malignant melanoma relative to primary melanoma cells and leads to increased chemotaxis and cellular proliferation in A375M melanoma cells transfected with anti-miR-126.15 This effect is mediated in part through reduced inhibition of miR-126–targeted metalloproteases: a disintegrin and metalloprotease domain 9 and matrix metalloprotease-7.15 Let-7a is downregulated in malignant melanoma relative to primary melanoma cell lines, which results in induction of integrin β3 and increased invasive capacity as assessed by Boyden Chamber invasion assays.16 MiR-191 downregulation has been described in association with melanoma patients containing BRAF mutations.17 Notably, miR-1976 was the most differentially expressed miR; however, it has not been previously described in the setting of malignant melanoma.
Among the downregulated miRs, several turned out to be known oncomiRs, including miR-21. MiR-21 upregulation in malignant melanoma relative to benign nevi has previously been shown.7 MiR-21 upregulation in melanoma leads to downregulation of tissue inhibitor of metalloprotease-3, which in turn leads to increased cellular invasion, thereby suggesting a mechanism of miR-21–mediated tumor progression.8 Furthermore, miR-720 has previously been shown to be upregulated in cutaneous melanoma compare with benign melanocytic nevi.18
Conversely, there was upregulation of several important miRs between group A vs group B. Several of these miRs are tumor suppressors that are downregulated in malignant melanoma and might be expected to increase following surgical excision. For instance, miR-26a-5p downregulation has been reported in primary cutaneous melanoma tissue relative to nevi and is responsible for inhibition of alpha-type platelet-derived growth factor receptor that can lead to increased cellular proliferation.19,20
Unlike patients with stage IIIc disease, clear differences in miR expression were not observed in plasma samples of pre- and postsurgical patients with distant metastatic (stage IV) disease. Greater degrees of inflammation have been associated with later stages of melanoma.21 Bernardes et al21 revealed that increased proinflammatory markers such as C-reactive protein, γ-glutamyl transpeptidase and malondialdehyde (MDA) were present in later stages of melanoma. In addition, high levels of MDA persist following surgical resection and suggest a sustained inflammatory response.21 As such, inflammatory miRs associated with postoperative wound healing and remodeling may blunt large differences across the pre- and postsurgical samples in melanoma patients. Indeed, the oncomiR miR-21 can be induced by interleukin 6, an important mediator in wound healing.22,23 Furthermore, angiogenesis is an important component of wound healing, and miRs can regulate several proteins involved with both angiogenesis and tumor progression, including vascular endothelial growth factor (VEGF) (miR-20a) and Sprouty-related EVH1 domain-containing protein 1 (SPRED1) (miR-126).24,25 Thus, miRs implicated in oncogenesis may also participate in the postoperative inflammatory response, thereby blunting anticipated changes following surgical excision for distant metastatic lesions.
Fitting with this presumption, 1 patient with stage IV disease who underwent a lymphadenectomy had cellulitis postoperatively (patient 1) and did not have a major reduction in global miR expression. Tili et al26 report upregulation of miR-155 not only in solid and hematologic malignancies but also during the inflammatory response, particularly during proliferation of lymphocytes where miR-155 can in turn regulate expansion of granulocytes and monocytes, T-cell differentiation, and B-cell maturation. Igglezou et al27 similarly found transient upregulation of miR-155 in postoperative blood samples following mastectomy. Our results suggest postoperative blood draws within weeks of surgical resection for miR analysis may not be suitable for proper evaluation of tumor-driven miR changes in stage IV patients, given the overlap of miRs involved with inflammation and tumor progression.
There are some notable limitations to this study. First, a limited patient sample of pre- and postsurgical patients was studied due to the difficulty in obtaining consent from patients who were embarking on surgery with the hopes of achieving no evidence of disease. This was an exploratory study, and ultimately, greater numbers of patients will be needed in future studies to adequately evaluate these findings as well as any potential differences. Second, nonsurgical controls were absent; however, this was acceptable given the intent to address changes within miR profiles between patients before and after surgery. These factors limit the power of this analysis and strength of our conclusions. The PCA analysis used herein identified miRs with a high degree of variance. Additional work is warranted to better understand changes in miR expression after surgery and validate the significance of the miRs herein.
Conclusions
We suggest miR analysis of plasma in melanoma patients is feasible to analyze miR dynamics following surgical excision. However, care should be exercised with respect to the design and timing of future postoperative plasma studies as analyses in close temporal proximity to surgical excision may obscure differences in miR trends. Ideally, future studies will incorporate additional postsurgical blood draws at different time points to more comprehensively analyze these trends. Likewise, analysis of distant metastatic samples does not appear to have large variations in miR patterns following surgical resection, which may be attributed, in part, to a sustained inflammatory response postoperatively. Future studies may specifically tailor their design to include individuals limited to locoregional disease when performing plasma-based approaches and consider the use of tissue-based approaches for those with distant metastatic disease to potentially avoid this discrepancy. Ultimately, further characterization of the impact of staging and temporal response on postsurgical inflammation is necessary to accurately understand plasma miR dynamics in melanoma.
Footnotes
Peer review:Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 890 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by National Institutes of Health (NIH) T32 CA090223 (to W.E.C.), and K24 CA093670 (to W.E.C.), and NLM grant T15LM011270 (to Z.B.A).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: NL, JHH, AT, DA, and WEC conceived and designed the experiments. NL, ZBA, KR, NJ, PF, and PP analyzed the data. NL, ZBA, KR, and WEC wrote the first draft of the manuscript. NL, ZBA, KR, and WEC contributed to the writing of the manuscript. All authors agree with manuscript results and conclusions. NL, ZBA, KR, JM, PP, and WEC jointly developed the structure and arguments for the manuscript. NL, ZBA, KR, and WEC made critical revisions and approved the final version. All authors reviewed and approved the final manuscript.
References
- 1. Miller KD, Siegel RL, Lin CC, et al. Cancer treatment and survivorship statistics, 2016. CA Cancer J Clin. 2016;66:271–289. [DOI] [PubMed] [Google Scholar]
- 2. Balch CM, Gershenwald JE, Soong SJ, et al. Final version of 2009 AJCC melanoma staging and classification. J Clin Oncol. 2009;27:6199–6206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Thalanayar PM, Agarwala SS, Tarhini AA. Melanoma adjuvant therapy. Chin Clin Oncol. 2014;3:26. [DOI] [PubMed] [Google Scholar]
- 4. Eggermont AM, Chiarion-Sileni V, Grob JJ, et al. Adjuvant ipilimumab versus placebo after complete resection of high-risk stage III melanoma (EORTC 18071): a randomised, double-blind, phase 3 trial. Lancet Oncol. 2015;16:522–530. [DOI] [PubMed] [Google Scholar]
- 5. Sullivan R, LoRusso P, Boerner S, Dummer R. Achievements and challenges of molecular targeted therapy in melanoma. Am Soc Clin Oncol Educ Book. 2015;35:177–186. [DOI] [PubMed] [Google Scholar]
- 6. Bennett PE, Bemis L, Norris DA, Shellman YG. MiR in melanoma development: miRNAs and acquired hallmarks of cancer in melanoma. Physiol Genomics. 2013;45:1049–1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Grignol V, Fairchild ET, Zimmerer JM, et al. MiR-21 and miR-155 are associated with mitotic activity and lesion depth of borderline melanocytic lesions. Br J Cancer. 2011;105:1023–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Martin del Campo SE, Latchana N, Levine KM, et al. MiR-21 enhances melanoma invasiveness via inhibition of tissue inhibitor of metalloproteinases 3 expression: in vivo effects of MiR-21 inhibitor. PLoS ONE. 2015;10:e0115919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Friedman EB, Shang S, de Miera EV, et al. Serum microRNAs as biomarkers for recurrence in melanoma. J Transl Med. 2012;10:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Ono S, Oyama T, Lam S, Chong K, Foshag LJ, Hoon DS. A direct plasma assay of circulating microRNA-210 of hypoxia can identify early systemic metastasis recurrence in melanoma patients. Oncotarget. 2015;6:7053–7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ferracin M, Lupini L, Salamon I, et al. Absolute quantification of cell-free microRNAs in cancer patients. Oncotarget. 2015;6:14545–14555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chatterjee A, Leichter AL, Fan V, et al. A cross comparison of technologies for the detection of microRNAs in clinical FFPE samples of hepatoblastoma patients. Sci Rep. 2015;5:10438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alder H, Taccioli C, Chen H, et al. Dysregulation of miR-31 and miR-21 induced by zinc deficiency promotes esophageal cancer. Carcinogenesis. 2012;33:1736–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Latchana N, Ganju A, Howard JH, Carson WE. MicroRNA dysregulation in melanoma. Surg Oncol. 2016;25:184–189. [DOI] [PubMed] [Google Scholar]
- 15. Felli N, Felicetti F, Lustri AM, et al. MiR-126&126* restored expressions play a tumor suppressor role by directly regulating ADAM9 and MMP7 in melanoma. PLoS ONE. 2013;8:e56824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Müller DW, Bosserhoff AK. Integrin beta 3 expression is regulated by let-7a miRNA in malignant melanoma. Oncogene. 2008;27:6698–6706. [DOI] [PubMed] [Google Scholar]
- 17. Pinto R, Strippoli S, De Summa S, et al. MicroRNA expression in BRAF-mutated and wild-type metastatic melanoma and its correlation with response duration to BRAF inhibitors. Expert Opin Ther Targets. 2015;19:1027–1035. [DOI] [PubMed] [Google Scholar]
- 18. Sand M, Skrygan M, Sand D, et al. Comparative microarray analysis of microRNA expression profiles in primary cutaneous malignant melanoma, cutaneous malignant melanoma metastases, and benign melanocytic nevi. Cell Tissue Res. 2013;351:85–98. [DOI] [PubMed] [Google Scholar]
- 19. Kozubek J, Ma Z, Fleming E, et al. In-depth characterization of microRNA transcriptome in melanoma. PLoS ONE. 2013;8:e72699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ishikawa O, LeRoy EC, Trojanowska M. Mitogenic effect of transforming growth factor beta 1 on human fibroblasts involves the induction of platelet-derived growth factor alpha receptors. J Cell Physiol. 1990;145:181–186. [DOI] [PubMed] [Google Scholar]
- 21. Bernardes SS, de Souza-Neto FP, Ramalho LN, et al. Systemic oxidative profile after tumor removal and the tumor microenvironment in melanoma patients. Cancer Lett. 2015;361:226–232. [DOI] [PubMed] [Google Scholar]
- 22. Banerjee J, Sen CK. MicroRNAs in skin and wound healing. Methods Mol Biol. 2013;936:343–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lin ZQ, Kondo T, Ishida Y, Takayasu T, Mukaida N. Essential involvement of IL-6 in the skin wound-healing process as evidenced by delayed wound healing in IL-6-deficient mice. J Leukoc Biol. 2003;73:713–721. [DOI] [PubMed] [Google Scholar]
- 24. Hua Z, Lv Q, Ye W, et al. MiRNA-directed regulation of VEGF and other angiogenic factors under hypoxia. PLoS ONE. 2006;1:e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Fish JE, Santoro MM, Morton SU, et al. MiR-126 regulates angiogenic signaling and vascular integrity. Dev Cell. 2008;15:272–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Tili E, Croce CM, Michaille JJ. MiR-155: on the crosstalk between inflammation and cancer. Int Rev Immunol. 2009;28:264–284. [DOI] [PubMed] [Google Scholar]
- 27. Igglezou M, Vareli K, Georgiou GK, Sainis I, Briasoulis E. Kinetics of circulating levels of miR-195, miR-155 and miR-21 in patients with breast cancer undergoing mastectomy. Anticancer Res. 2014;34:7443–7447. [PubMed] [Google Scholar]