Abstract
Cancer metastasis is the major cause of cancer mortality. Despite extensive research efforts, effective treatment for cancer metastasis is still lacking. Cancer metastasis involves 4 essential steps: cell detachment, migration, invasion, and adhesion. Detachment is the first and required step for metastasis. Glutathione disulfide (GSSG) is derived from the oxidation of glutathione (GSH), which is present in biological systems in millimolar concentration. Although GSSG is commercially available, the impact of GSSG on cell functions/dysfunctions has not been fully explored due to the fact that GSSG is not cell membrane permeable and a lack of method to specifically increase GSSG in cells. We have developed GSSG liposomes that effectively deliver GSSG to cells. Unexpectedly, cells treated with GSSG liposomes were resistant to detachment by trypsinization. This observation led to the investigation of the antimetastatic effect of GSSG liposomes. Our data demonstrate that GSSG liposomes at 1 mg/mL completely blocked cell detachment and migration, and significantly inhibited cancer cell invasion. Aqueous GSSG showed no such effect, confirming that the effects on cell detachment, migration, and invasion were caused by the intracellular delivery of GSSG. An in vivo experiment with a murine melanoma experimental metastasis model showed that GSSG liposomes prevented melanoma lung metastasis. The unique antimetastatic mechanism through the effects on detachment and migration, and effective in vitro and in vivo metastasis inhibition, warrants further investigation of the GSSG liposomes as a potential treatment for cancer metastasis.
Keywords: Glutathione disulfide liposomes, antidetachment, antimigration, antimetastasis
Introduction
Cancer metastasis contributes to more than 90% of cancer mortality due to its systemic nature and resistance to the existing treatments.1–3 Most cancer patients die from metastatic disease, not from the primary tumor. Metastasis is, therefore, considered a terminal disease for most types of cancers.
Despite extensive research efforts, an effective clinical treatment for cancer metastasis is still lacking. Current treatments for metastatic cancer are similar to those for primary cancers, mainly chemotherapy and radiation therapy with the latter as a mainstay for treatment.4 Both therapies are primarily for cancer killing and growth control rather than metastasis inhibition.4–7 More recently, targeted biological/molecular therapies through targeting cancer-related pathways have been major components of antimetastasis therapy.4–6 Most of these therapies involve the use of monoclonal antibodies.4–6
Extensive efforts have been made to develop effective treatments for metastasis by targeting the various steps involved in metastasis.4,5 Compared with chemotherapy and radiation therapy, treatments derived from targeting metastatic steps have the advantages of being more selective against metastatic cells. Clinical treatments derived from targeting metastatic steps include angiogenesis inhibitors, growth factor pathway blockers, and matrix metalloproteinase (MMP) inhibitors.5,6 Additional approaches targeting metastatic steps in development include integrin inhibitors, Focal Adhesion Kinase (FAK) inhibitors,8–10 chemokine inhibitors that inhibit cell migration, transforming growth factor β inhibitors, bisphosphonates, and others.4–7,11 Nevertheless, a novel and effective treatment for metastatic cancer is desirable in our battle against this deadly disease.
Metastasis is a process of dissemination of tumor cells from a primary tumor mass to a different site through blood vessels and lymphatic vessels. It is a complex succession of a series of cell-biological events termed the invasion-metastasis cascade. The cascade involves the development of new blood vessels (angiogenesis), detachment and migration of metastatic cells from the primary tumor, invasion through the basement membrane (BM) and extracellular matrix (ECM) surrounding the tumor, invasion of BM supporting the endothelium of local blood and lymphatic vessels, intravasation of the metastatic cells into the blood and/or lymphatic vessels, adhesion of the metastatic cells to the endothelium of capillaries of the target organ site, invasion of the cells through the endothelial cell layer and the surrounding BM (extravasation), and finally adhesion and growth of the metastatic cells at the site.12–14 Overall, metastatic cell dissemination requires 4 essential steps: detachment, migration, invasion, and adhesion.5,14–16 Under normal circumstances, epithelial cells will undergo apoptosis (programmed cell death) when detached, a phenomenon termed as anoikis. This is a mechanism designed to protect multicellular organisms from rogue cells establishing themselves outside their anatomical location. Metastatic cells are resistant to anoikis during dissemination.5 The resistance to anoikis, together with other property changes of tumor cells (cell-to-cell or cell-to-matrix adhesion, cell polarity, and cell migratory property), is collectively known as the epithelial-mesenchymal transition (EMT), a characteristic feature all metastatic cells must have. It is known that not all tumor cells are metastatic nor are all cells within metastatic tumors capable of metastasizing.5 Primary tumors that are not metastatic need to undergo EMT to acquire the ability to metastasize.5
Glutathione disulfide (GSSG) is the oxidized form of glutathione (GSH), which is the major endogenous antioxidant.17 Glutathione protects biological systems from oxidizing factors such as reactive oxygen species through terminating them although GSH itself is oxidized to GSSG. Glutathione disulfide is then reduced back to GSH by glutathione reductase (GR). Under normal conditions, the biological system maintains a high ratio of GSH:GSSG (>100:1) through effective reduction of GSSG back to GSH by GR. An increase in GSSG is considered as a state of oxidative stress.9 Although GSSG is commercially available, the impact of an increase in intracellular GSSG on cellular functions/dysfunctions has not been fully studied due to the inability of GSSG to pass through the cell membrane and a lack of method to specifically increase the intracellular GSSG concentration.
We have developed a cell membrane permeable GSSG formulation through the use of cationic liposomes.18 The GSSG cationic liposome formulation effectively delivers GSSG into cells.18 Unexpectedly, we found that GSSG liposomes completely blocked cell detachment by trypsinization, suggesting that GSSG liposomes might be able to inhibit cancer metastasis. This investigation was aimed to explore the effects of GSSG liposomes on the 4 essential steps in cancer metastasis in vitro and the antimetastatic effect in vivo. Our results show that GSSG liposomes completely prevented cancer cells from detachment and migration, and significantly inhibited cancer cells from invasion. An in vivo experiment with a murine experimental melanoma pulmonary metastasis model demonstrated that GSSG liposomes completely prevented melanoma metastasis in lungs.
Materials and Methods
Animals
Female C57BL/6 mice were obtained from the National Cancer Institute (NCI). All mice were used at approximately 7 to 10 weeks of age and given at least a 1-week break after arrival. Animals were housed in a cage with 4 mice/cage and provided free access to food and water. The animal experimental protocols were approved by the South Dakota State University Institutional Animal Care and Use Committee.
Chemicals
Glutathione disulfide liposomes were prepared as described previously.18 Glutathione disulfide liposomes at 1 mg/mL represent the liposome formulation containing 1 mg GSSG and 1 mg lipid. The 10 mg/mL GSSG liposomes were prepared as a stock solution for all the experiments. Type I collagen, type IV collagen, laminin, and fibronectin were purchased from Fisher Scientific (Fair Lawn, NJ).
Cell lines, cell culture, and antibodies
McCoy’s 5A medium was obtained from Gibco (Grand Island, NY). Roswell Park Memorial Institute (RPMI) 1640 growth medium, penicillin/streptomycin, phosphate-buffered saline (PBS), and Dulbecco’s Phosphate-Buffered Saline (DPBS) were obtained from Mediatech (Herndon, VA). Fetal bovine serum (FBS) was purchased from Atlanta Biologicals (Lawrenceville, GA). Trypsin/EDTA (0.05%) was purchased from Invitrogen (Carlsbad, CA). NCI-H226 (human lung cancer), HCT 116 (human colon cancer), and PC-3 (human prostate cancer) cells were obtained from the NCI. B16-F10 cells (murine melanoma) were obtained as a gift from Dr Hemachand Tummala. NCI-H226, B16-F10, and PC-3 cells were maintained as monolayers and passaged as necessary in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin. HCT 116 cells were maintained in McCoy’s 5A medium. Antibodies against integrins β1, β3, α4, α5, E-cadherin, N-cadherin, β-actin, and β-catenin were purchased from Cell Signaling Technology (Danvers, MA).
Cell detachment assay
The effect of GSSG liposomes on cancer cell detachment was investigated by a controlled trypsinization procedure with minor modification19 for NCI-H226 and B16-F10 cells. Cells (100 000 cells/well) plated in RPMI medium with 10% FBS and 1% penicillin/streptomycin in a 12-well plate were allowed to attach for 24 hours before being treated with GSSG liposomes, blank liposomes, or aqueous GSSG for 24 hours in a CO2 incubator at 37°C. After treatment, cells were washed with DPBS without calcium and magnesium salts (1 mL 3×). Dulbecco’s Phosphate-Buffered Saline was removed completely before addition of a diluted trypsin solution (0.005% trypsin/5 mM EDTA, 0.5 mL). The plates were shaken slowly. At the end of the experiment, 2 mL of FBS-containing medium was added to stop the proteolytic action of trypsin. The supernatant was transferred to a conical tube, and cells that remained attached were photographed under a Fisher Micromaster Microscope (Waltham, MA). The supernatant was centrifuged and resuspended in 0.1 mL of the RPMI medium before being counted by a Cellometer Auto T4 Plus Cell Counter (Nexcelom Bioscience, Lawrence, MA) for any cells present in the supernatant.
Cell migration assay
The effects of GSSG liposomes on cell migration were investigated by following a literature procedure with minor modification.20 Cells (2.5 × 104 cells/well for NCI-H226 and 2 × 104 cells/well for B16-F10) were seeded into a 12-well plate in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin overnight for attachment. A “wound” was created by scraping the confluent portion of cells with a sterile 200 µL plastic pipette tip. The monolayers were washed twice and added with fresh growth medium containing GSSG liposomes, blank liposomes, or aqueous GSSG. The wound gap was photographed under a Fisher Micromaster Microscope at time 0 and 24 hours.
Cell adhesion assay
The effects of GSSG liposomes on cell adhesion were investigated by following a literature procedure with minor modification21 using 4 ECM proteins (type I collagen, type IV collagen, laminin, and fibronectin). A stock solution of an ECM protein was prepared as 30 µg/mL in PBS. A 24-well plate was first coated (1 mL/well) with one of the four ECM proteins at 10 µg/mL in PBS with 4 wells per protein for 4 hours at 37°C. Phosphate-buffered saline in the well was removed, and the residual liquid was air-dried in a biological safety cabinet. Cells were serum starved for 24 hours followed by a treatment (medium containing aqueous GSSG in PBS [1 mg/mL], medium containing blank liposomes [1 mg/mL], and a medium containing GSSG liposomes [1 mg/mL]) in a serum-free medium in a culture tube at 37°C for an additional 24 hours in a CO2 incubator. Cells were seeded at 2 × 104 cells/well in the ECM-coated 24-well plate and allowed for adhesion for 1 hour in a CO2 incubator followed by a wash with ice-cold PBS twice. The attached cells were fixed with 10% paraformaldehyde in PBS for 10 minutes and washed 3 times with PBS before addition of 100 µL of Hoechst solution (2 µg/mL in PBS) for staining for 30 minutes. Images were obtained on an inverted fluorescence microscope (Observer A1, AX-10 Zeiss) connected to digital microscopes & digital camera (Zeiss AxioCam MRc5, Jena, Germany). The number of stained nuclei in a well was enumerated using ImageJ software22,23 from an average of 6 different locations with a minimum of 80 cells/location.
Cell invasion experiment
The effects of GSSG liposomes on the invasion property were investigated by using a cell invasion kit from Trevigen (Gaithersburg, MD).24 The manufacturer protocol was followed. The cell invasion assay kit consists of an invasion chamber (8 µm pore) and an assay chamber. Briefly, cells were subjected to serum starvation for 24 hours, and then pretreated with a drug treatment for 24 hours in suspension before being transferred to the invasion chamber (50 000 cells/chamber) at 37°C in a CO2 incubator. At the end of incubation, cells reaching to the assay chamber were quantified through a fluorescence microplate reader by using Calcein-AM as a cell number detecting agent.
Western blot analysis
Cell lysates and Western blot analysis were performed as described by Dachineni and coworkers.25 Briefly, cells (2 × 106 cells in 10 mL in a Petri dish) were treated with various treatments for 24 hours, followed by washing with PBS and scraping in lysis buffer (10 mM Tris-HCl pH 7.4, 150 mM NaCl, 15% glycerol, 1% Triton X-100 with protease inhibitors). Samples containing 50 µg of proteins were separated on 6% or 10% polyacrylamide gel electrophoresis and immunoblotted with respective antibodies. The intensities of bands were quantified using ImageJ software.
Murine lung metastasis assay
A procedure for murine lung metastasis assays reported previously was followed with minor modification.26–28 Mice were divided into 7 mice per group for treatment. B16-F10 cells were grown and maintained as monolayers in RPMI 1640 medium supplemented with 10% FBS and 1% penicillin/streptomycin in 5% CO2 at 37°C. The cells were maintained in RPMI 1640 medium without FBS for 24 hours before being harvested by trypsinization, and adjusted to a density of 950 000 cells/mL with serum-free medium. Cells were pretreated with different treatments as indicated in Table 1 prior to being injected into mice. Each mouse received a 0.2-mL aliquot (875 000 cells/mL) of cells (175 000/mouse) through tail vein injection. Treatments, as described in Table 1, started 24 hours after introduction of the cells and continued daily for 5 days. The weight of the mice was recorded daily. Mice were euthanized by cervical dislocation under isoflurane on day 21, and the lungs were removed, washed in PBS, and fixed with buffered formalin solution (Fisher Scientific, Fair Lawn, NJ) for 24 hours before being photographed. Tumor nodules on the lung surface were counted under a magnifier.
Table 1.
Mouse treatment protocol.
| Groups | Treatment |
|||||
|---|---|---|---|---|---|---|
| Control 1 | Control 2 | Control 3 | Treatment 1 | Treatment 2 | Positive control | |
| B16-F10 cells pretreated for 24 h | PBS | Blank liposomes | Aqueous GSSG (1 mg/mL) | GSSG liposomes (1 mg/mL) | PBS | PBS |
| B16-F10 cells were injected to mice through a tail vein | ||||||
| Treatment | PBS by IV daily for 5 d | Blank liposomes by IV daily for 5 d | Aqueous GSSG 0.48 g/kg by IV daily for 5 d | GSSG liposomes 0.48 g/kg by IV daily for 5 d | GSSG liposomes 0.48 g/kg by IV daily for 5 d | Dacarbazine 50 mg/kg by IP on every fourth day on days 1 and 429 |
Abbreviations: GSSG, Glutathione disulfide; IP, intraperitoneal; IV, intravenous; PBS, phosphate-buffered saline.
Statistical analysis
Comparison of data from different treatments was evaluated with the Student t test and analysis of variance.
Results
Effects on cell detachment
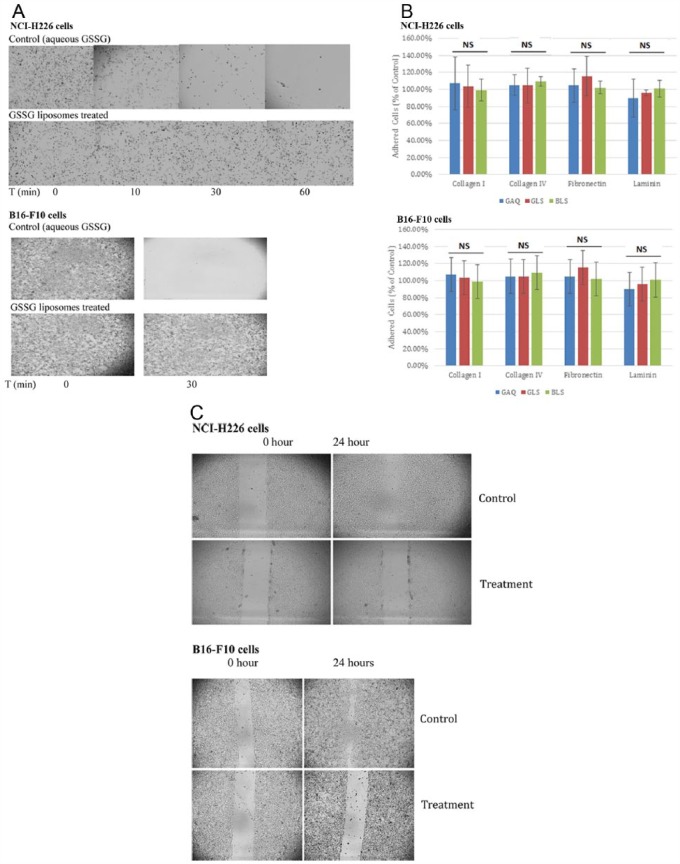
The effects of GSSG liposomes on cancer cell detachment were investigated with NCI-H226 cells and B16-F10 cells through controlled trypsinization.19 After being treated with GSSG liposomes (1 mg GSSG/mL) for 24 hours, cells that remained attached were photographed under a Fisher Micromaster Microscope, whereas cells that detached into the supernatant were counted by a Cellometer Auto T4 Plus Cell Counter. No cell detachment was observed for both cell lines treated with GSSG liposomes. Figure 1A provides representative images derived from these 2 cell lines treated with GSSG liposomes (treatment) and aqueous GSSG (control). As demonstrated in Figure 1A, both NCI-H226 cells and B16-F10 cells in controls were completely detached in 1 hour and 30 minutes, respectively, by a diluted trypsin solution, whereas GSSG liposomes–treated cells showed no detachment. Cells treated with blank liposomes did not show any effect on cell detachment either (data not shown). The complete prevention of cell detachment was further confirmed by a cell count in the supernatant (Table 2). No cells were detected for both cell lines in the supernatant of GSSG liposomes–treated samples confirming that GSSG liposomes completely blocked cell detachment. We also treated cells with GSSG liposomes for 4 and 8 hours; no effect on cell detachment was observed indicating that the effect on cell detachment by GSSG liposomes required longer than 8 hours. This observation excludes the possibility that the antidetachment effect might be due to the inhibition of trypsin. The possibility was further ruled out by a nonenzymatic dissociation assay (10 mM EDTA in PBS). The nonenzymatic dissociation solution readily detached both cell lines (B16-F10 and NCI-H226) but could not detach cells treated with GSSG liposomes. Prevention of detachment was also observed with other cell lines treated with normal trypsinization. These cell lines include HCT 116, PC-3, MDA-MB-231 (human breast cancer cells), and H9C2 cells (rat cardiomyocyte cells). When treated with 1 mg/mL GSSG liposome, these cells could not be detached by normal trypsinization.
Figure 1.
Effect of GSSG liposomes on cell detachment (A), adhesion (B), and migration (C). (A) Cells (100 000 cells/well) in a 12-well plate were treated with GSSG liposomes (1 mg GSSG/mL) or aqueous GSSG (1 mg/mL) for 24 hours followed by a controlled trypsinization as described in the “Materials and Methods” section. Cells that remained attached were photographed under a Fisher Micromaster Microscope. Images presented were from 1 of the 3 representative experiments. (B) Cells were treated with a treatment (GAQ: GSSG aqueous solution [1 mg/mL], GLS: GSSG liposomes [1 mg/mL], or BLS: blank liposomes) for 24 hours in a serum-free medium before seeded at 2 × 104 cells/well in an ECM protein–coated 24-well plate and allowed for adhesion for 1 hour. Cells were fixed with 10% paraformaldehyde before addition of 100 µL of Hoechst solution (2 µg/mL in PBS) for staining. Images were obtained on an inverted fluorescence microscope (Observer A1, AX-10 Zeiss) connected to digital microscopes & digital camera (Zeiss AxioCam MRc5). The number of stained nuclei in a well was enumerated using ImageJ software from an average of 6 different locations with a minimum of 80 cells/location. The results are expressed as the percentage of the control and presented as the mean ± SD of 3 independent experiments. Statistical analysis was conducted by 1-way ANOVA. No significant difference was found among 4 groups (labeled as NS in the figure). (C) Cells (2.5 × 104 cells/well for NCI-H226 and 2 × 104 cells/well for B16-F10) were seeded into each well of a 12-well plate for 24 hours for attachment, followed by a “wound” creation before being treated by a drug treatment (0.3 mg/mL GSSG liposomes as treatment, blank liposomes or aqueous GSSG [0.3 mg/mL] as control). The wound gap was photographed under a Fisher Micromaster Microscope. Images presented were from 1 of the 3 representative experiments. ANOVA indicates analysis of variance; ECM, extracellular matrix; GSSG, glutathione disulfide; NS, not significant; PBS, phosphate-buffered saline; GAQ, aqueous GSSG; GLS, GSSG liposomes; BLS, blank liposomes.
Table 2.
Cells detached into supernatant after controlled trypsinization.
| T, min | 10 | 30 | 60 |
|---|---|---|---|
| NCI-H226 Cells | |||
| Control (number of cells in supernatant) | 116 950 | 195 616 | 245 266 |
| Treatment (number of cells in supernatant) | Not detected | Not detected | Not detected |
| B16-F10 cells | |||
| Control (number of cells in supernatant) | 479 400 | 537 050 | |
| Treatment (number of cells in supernatant) | Not detected | Not detected | |
Abbreviation: RPMI, Roswell Park Memorial Institute.
Cells were treated as described in Figure 1A. At each time point, the supernatant in the well was collected into 0.5 mL of RPMI 1640 medium, centrifuged, resuspended in 0.1 mL of RPMI medium, and counted by a Cellometer Auto T4 Plus Cell Counter. Data presented were from 1 of the 3 representative experiments.
Effect on cell adhesion
The effects of GSSG liposomes on cell adhesion were investigated with NCI-H226 cells and B16-F10 cells. Type 1 and type 2 collagens, laminin, and fibronectin were used as ECM proteins. Cells were treated with a treatment for 24 hours in a serum-free medium before being seeded in an ECM protein–coated 24-well plate to check for adhesion. Figure 1B presents the results derived from the experiments with NCI-H226 and B16-F10 cells. As shown in Figure 1B, the percentages of cell adhesion to all 4 ECM proteins were not statistically different for cells treated with GSSG liposomes vs controls. These data demonstrate that GSSG liposomes exhibit no effect on cell adhesion.
Effects on cell migration
The effects of GSSG liposomes on cell migration were investigated by following a literature procedure with minor modification.20 The procedure checks the effect on cell migration by observing cells to fill a “wound” (wound healing). Figure 1C presents representative images derived from the experiments with NCI-H226 cells and B16-F-10 cells. As shown in Figure 1C, no significant migration was observed in both cell lines treated with GSSG liposomes while the wound was almost completely filled in the control groups. Aqueous GSSG and blank liposomes showed no effect on cell migration (data not shown). In a separate work, GSSG liposomes were also found to induce cell apoptosis at 1 mg/mL.30 To rule out the possibility that the “wound healing” effect was due to cell apoptosis, we conducted “wound healing” experiments at lower concentrations to identify the minimum concentration that produced the antimigration effect. Significant antimigration effect was observed for both cell lines at 0.125 and 0.25 mg/mL. However, when cells were treated with 0.25 mg/mL GSSG liposomes, no apoptosis was observed for both cell lines (data not shown). Therefore, it is concluded that the effect on wound healing was due to a loss of the cell migration ability and not due to cell apoptosis.
Effects on cell invasion
The effects of GSSG liposomes on the invasion property were investigated with 3 human cancer cell lines (NCI-H226, PC-3, and HCT 116) and 1 murine melanoma cancer cell line (B16-F10). A cell invasion kit using Matrigel Invasion Chambers (8 µm pore) from BD Biosciences (San Jose, CA) was employed for the investigation.24 As presented in Figure 2, when cancer cells were incubated with GSSG liposomes, invasion of these cells through the BM matrix was inhibited. At 1 and 0.3 mg/mL, GSSG liposomes produced an invasion inhibition of about 80% and 50% for both NCI-H226 and B16-F10, 80% and 55% for PC-3, and 75% and 35% for HCT 116, respectively. Incubation of the cancer cells with aqueous GSSG or blank liposomes produced no invasion inhibition (Figure 2). Doxycycline at the concentration that completely inhibits MMP was used as a positive control (Figure 2).27 Glutathione disulfide liposomes produced more invasion inhibition than Doxycycline with NCI-H226, PC-3, and B16-F10 cells, and produced a similar extent of invasion inhibition with HCT 116 cells. The trypan blue assay was used to check cell viabilities. The assay revealed no cell viability difference between cells treated with GSSG liposomes (1 mg/mL) and controls (no treatment); cell viability for both were >95%. Blank liposomes and aqueous GSSG showed no effects on invasion (Figure 2).
Figure 2.
Effects of GSSG liposomes on the invasion property of cancer cell lines. Cells (50 000/chamber) were treated with a treatment and incubated in the culture chamber for 24 hours. At the end of incubation, cells reaching to the assay chamber were quantified through a fluorescence microplate reader by using Calcein-AM as a cell number detecting agent. The results are expressed as the percentage of the control. GSSG: GSSG aqueous solution; Treatment: GSSG liposomes. GSSG indicates glutathione disulfide.
Effects on integrin, N-cadherin, β-actin, andβ-catenin
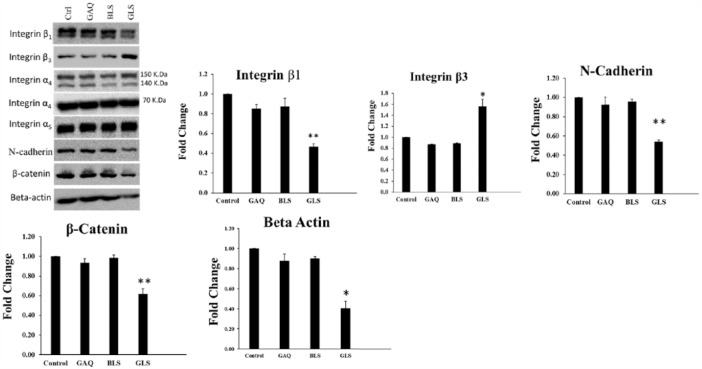
Using the NCI-H226 cell line, Western blot analysis was performed to determine the effects of GSSG liposomes on integrin, E-cadherin, N-cadherin, β-actin, and β-catenin. The results are presented in Figure 3. We observed that treatment of cells with GSSG liposomes exerted differential effects on integrin. Protein levels of integrin β3 were upregulated. Similar results were observed in B16-F10 (data not shown). In contrast, protein levels of integrin β1 were downregulated, whereas no change in protein levels was observed with integrins α4 and α5. Interestingly, protein levels of N-cadherin, β-actin, and β-catenin were downregulated. Downregulation of β-catenin was also observed in B16-F10 cells (data not shown). We failed to detect integrins β1, α4, α5, β-actin, and N-cadherin from the B16-F10 cell line and E-cadherin from both NCI-H226 and B16-F10 cells due to the poor cross-reactivity of the antibodies.
Figure 3.
Effects of various treatments on integrin, N-cadherin, β-actin, and β-catenin in NCI-H226 cells. Cells were treated with various treatments for 24 hours, followed by washing with PBS. Cells were collected using a sterile cell scraper in lysis buffer. Samples containing 50 µg of proteins were separated on 6% or 10% PAGE and immunoblotted with respective antibodies. The intensities of bands were quantified using ImageJ software. Student t test was used to compare the treatment of GLS with other groups (Control: PBS control; GAQ: aqueous GSSG [1 mg/mL], BLS: blank liposomes; GLS: GSSG liposomes [1 mg/mL]). GSSG indicates glutathione disulfide; PAGE, polyacrylamide gel electrophoresis; PBS, phosphate-buffered saline.
In vivo effects on lung metastasis
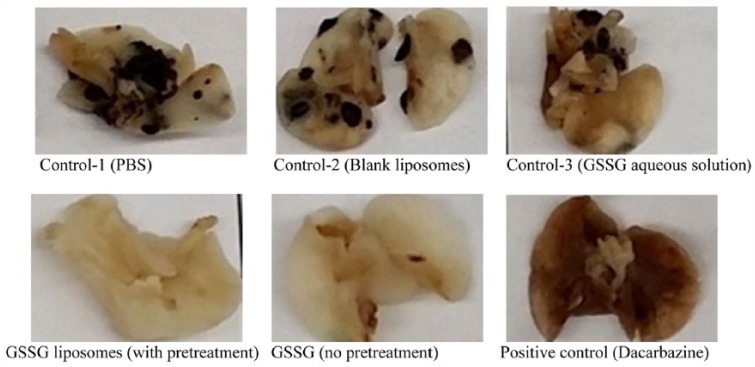
The in vivo effect on lung metastasis was investigated through the use of a murine B16-F10 melanoma lung colonization assay.27–29 The assay involves the injection of B16-F10 cells through a tail vein to female C57BL/6 mice and determined the effect of dacarbazine on the melanoma tumors settled in lungs.28,29 Dacarbazine is a drug used clinically for the treatment of melanoma. For comparison, female mice were also used in this study and Dacarbazine was employed as a positive control. Each mouse received 175 000 cells through tail vein injection and was treated daily for 5 days. The dosage of GSSG liposomes was based on the in vitro concentration that produced complete blockage of cell detachment. The mice were maintained for 21 days before lung collection. During the treatment course, no significant weight loss was observed in all groups (data not shown). Figure 4 presents representative photographs of the lungs dissected from mice with different treatments. As shown in the figure, no lung metastasis was observed in mice treated with GSSG liposomes (in both pretreatment and without pretreatment groups), whereas significant lung metastasis was observed in control 1 (PBS), control 2 (blank liposomes), and control 3 (aqueous GSSG). The rationale to pretreat the cancer cells before being injected to mice was based on the in vitro results that cells lost the abilities to detach and migrate after 24-hour treatment. We would like to check whether a pretreatment would be required for the in vivo antimetastatic effect. Our data did not show a significant difference in the antimetastatic effects between the pretreatment group and no pretreatment group. No lung metastasis was visually observed with the positive control (dacarbazine).29 The effects were further confirmed by the metastatic tumors counted under a magnifier (Table 3). As shown in Table 3, blank liposomes and aqueous GSSG showed no inhibitory effects on lung metastasis. The lungs of the mice in the GSSG liposomes pretreatment group were completely free of metastasis. Metastatic tumors were spotted in 1 mouse in the GSSG liposomes without pretreatment group and 1 mouse in the dacarbazine treatment group (Table 3).
Figure 4.
Representative photographs of lungs dissected from mice from different treatments. B16-F10 cells were pretreated as indicated in Table 1 before being injected to 9-week old female C57BL/6 mice at 175 000 cells/mouse through a tail vein. Treatments with 7 mice/treatment (control 1: PBS; control 2: blank liposomes; control 3: aqueous GSSG [0.48 g/kg]; GSSG liposomes [with pretreatment, 0.48 g/kg]; GSSG liposomes [no pretreatment, 0.48 g/kg]; positive control: dacarbazine [50 mg/kg]) started 24 hours after introduction of the cells and continued daily for 5 days except dacarbazine, which was given every fourth day on days 1 and 4.29 Mice were euthanized by cervical dislocation under isoflurane on day 21. The lungs were dissected, washed in PBS, and fixed with a buffered formalin solution for 24 hours before being photographed. Tumor nodules were counted under a magnifier and presented in Table 3. GSSG indicates glutathione disulfide; PBS, phosphate-buffered saline.
Table 3.
Number of metastatic tumors in murine lungs.
| Mouse | 1 | 2 | 3 | 4 | 5 | 6 | 7 | Average |
|---|---|---|---|---|---|---|---|---|
| Tumor nodules in lungs | ||||||||
| PBS (control 1) | 60 | 51 | 65 | 44 | 39 | 63 | 47 | 52.7 ± 10.1 |
| BLS (control 2) | 65 | 27 | 57 | 45 | 28 | 16 | 48 | 40.9 ± 17.7 |
| GSSG aqueous solution (0.48 g/kg; control 3) | 8 | 17 | 94 | 36 | 67 | 74 | 5 | 42.9 ± 35.5 |
| GSSG liposomes (0.48 g/kg, no pretreatment) | 0 | 7 | 0 | 0 | 0 | 0 | 0 | 1 ± 2.6 |
| GSSG liposomes (0.48 g/kg, with pretreatment) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 ± 0 |
| Dacarbazine (50 mg/kg) | 0 | 0 | 0 | 5 | 0 | 0 | dead | 0.8 ± 2.0 |
Mice were treated as described in Figure 4. Tumor nodules on the lung surface were counted under a magnifier. One mouse died in the course of treatment with dacarbazine. Glutathione disulfide liposomes group (both no pretreatment group and with pretreatment group) shows significantly less metastatic tumor when compared with each of the 3 control groups (P < .001, Student t test).
Abbreviations: GSSG, glutathione disulfide; PBS, phosphate-buffered saline; BLS, blank liposomes.
Discussion
In this investigation, we demonstrated that GSSG liposomes completely blocked the 2 key steps in cancer metastasis (detachment and migration) and significantly inhibited cancer cell invasion. Interestingly, GSSG liposomes exhibited no effect on cell adhesion. The antimetastatic effect of GSSG liposomes was further confirmed in vivo using B16-F10 cells in C57BL/6 mice, an experimental murine melanoma lung metastasis model. Glutathione disulfide liposomes completely prevented lung metastasis in this melanoma metastatic model. B16-F10 cells in C57BL/6 mice is a well-established and extensively employed model for the study of drug effects on malignant melanoma both in vitro and in vivo.31–35 Although the experimental metastasis model (introduction of tumor cells directly into the vasculature such as tail vein) has its limitation vs a spontaneous metastasis model (metastasis formed through inoculation of tumor cells into tissues),36 the number of pulmonary metastasis in mice that were tail vein injected with tumors cells, like melanoma, is known to directly correlate to the malignancy of a cell line and the effectiveness of an antineoplastic drug.36 Therefore, the results from this investigation are informative and provide the basis for further investigation.
Integrin and cadherin are 2 major adhesion proteins for cell attachment.14 Integrin, a heterodimer protein composed of α and β units, is responsible for cell attachment to ECM by connecting cytoskeleton protein β-actin to ECM proteins such as fibronectin, laminin, collagen, fibrinogen, and vitronectin. Cadherins are responsible for cell-to-cell attachment.14 Downregulation of integrin has been associated with cell detachment, whereas upregulation has been linked to metastasis.37 On the contrary, upregulation of N-cadherin (neural cadherin) and downregulation of E-cadherin (epithelial cadherin) have been reported to lead to an enhanced metastatic capability.14 E-cadherin and N-cadherin are also EMT markers.
We found a significant upregulation of integrin β3, downregulation of integrin β1, and no change on integrins α4 and α5 in NCI-H226 cells (Figure 3). Upregulation of integrin will increase cell resistance to detachment which is consistent with the antidetachment effect observed with cells treated with GSSG liposomes. It is not clear what is the relative contribution of upregulation of integrin β3 and downregulation of integrin β1 to the antimetastatic effects of GSSG liposomes.
Initially, β-actin was employed as a loading control for the Western blot analysis. Interestingly, after a few repeats, it was found that β-actin was significantly downregulated in NCI-H226 cells treated with GSSG liposomes (Figure 3). As β-actin is the major cytoskeleton protein for cell motility and invasion, it is possible that its downregulation may contribute to the decreased cell motility (antimigration and anti-invasion).
We were not able to detect E-cadherin from both cell lines due to the poor cross-reactivity of the antibodies. A striking observation in this study is the ability of the GSSG liposomes to downregulate N-cadherin and β-catenin in NCI-H226 cells (Figure 3). β-catenin, which acts as a linker protein complex with N-cadherin, has been shown to be important for cell-cell adhesion. Complexing of N-cadherin with β-catenin triggers downstream signal transduction, including Rho GTPases, PI3K, and MAPK, as well as other pathways that promote cell proliferation.14 The downregulation of these cell adhesion proteins would contribute to the antimetastatic effect.
In a separate study, STAT3 (signal transducer and activator of transcription 3) was found downregulated in both cell lines treated with GSSG liposomes.30 STAT3 plays a role in regulating the expression of transcriptional factors that drive EMT and modulating cytoskeleton dynamic during the initiation stage of the EMT.38 In addition, we also observed that treatment of cells with GSSG liposomes caused apoptosis in these cells.30
The downregulatory effects of GSSG liposomes on the protein levels of N-cadherin, β-catenin, and STAT3, and induction of apoptosis suggest a potential role for GSSG liposomes in the reversal of EMT.
The results of this study provide some insights of the molecular mechanism for the antimetastatic effects of GSSG liposomes. Nevertheless, much work remains to be done to identify other molecular factors that may contribute to the antimetastatic effects. Based on the in vitro data, the effects on cell detachment, migration, and invasion must be through an increase in intracellular GSSG because aqueous GSSG exhibited no such effect. We reported previously that an increase in intracellular GSSG leads to an increase in protein S-glutathionylation in NCI-H226 cells.18 It is likely that proteins involved in the key steps of detachment, migration, and invasion might be S-glutathionylated resulting in impacts on these steps. It is also most likely that the antimetastatic effect of GSSG liposomes is not a result from a single target, rather a result from multiple targets. Efforts in identifying S-glutathionylation of proteins involved in detachment, migration, and invasion are underway.
Consistently, Piskounova and colleagues reported recently that oxidative stress inhibited melanoma distant metastasis.39 Their study found that the ratio of GSH/GSSG was always significantly higher in a subcutaneous tumor as compared with metastatic nodules or circulating melanoma cells, and antioxidants promoted distant metastasis. In a separate work, we found that GSSG liposomes significantly decreased intracellular GSH in both cell lines.30 It is likely that a significant increase in oxidative stress, caused by a substantial increase in intracellular GSSG and decrease in intracellular GSH, contributed to the antimetastatic effect of GSSG liposomes.18
Although detachment, migration, invasion, and adhesion are 4 key steps in cancer metastasis, agents to effectively block these steps are limited. Therefore, it is unique, mechanistically, that GSSG liposomes produce the antimetastatic effect through affecting 3 of the 4 key steps: completely blocking detachment, migration, and significantly inhibiting invasion. It is recognized that the effective dosages for both in vitro and in vivo antimetastatic effects appear to be high. Efforts are underway to develop a more effective delivery system for GSSG to reduce the effective dosage. Nevertheless, no sign of toxicities was observed in a preliminary toxicity study with 4 CD-1 female mice at a dosage of 2.7 folds of the effective in vivo dosage for 5 days followed by a 2-day break for a total of 12 days before a pathological examination of liver, heart, kidney, brain, lung, intestine, and stomach was conducted (data not shown).
In addition to the antimetastatic effect, we reported that GSSG liposomes effectively inhibited cancer growth both in vitro and in vivo at the same dosage in a separate study.30
The unique antimetastatic mechanism, effective antimetastatic and anticancer growth activities, and low toxicity place GSSG liposomes in a perspective as a potential effective treatment for cancer.
NOV-002 is a proprietary product of a GSSG complex with cisplatin at a molar ratio of ~1000:1.8,40–43 NOV-002 in combination with chemotherapy has been found to increase efficacy and improve tolerance to chemotherapy,41,42 and has been in various phases of clinical trials for the treatment of advanced non–small cell lung cancer (NSCLC),41,42 ovarian cancer,41,42 and breast cancer.41–43 Furthermore, NOV-002 was reported to exhibit antimetastatic effects through inhibition of cancer cell invasive property.8
It needs to be noted that GSSG liposomes are different from NOV-002 in cancer inhibition. Gumireddy and coworkers demonstrated that NOV-002 produced the effects through interaction with cell surface receptor ErbB2 leading to reduced ErbB2 phosphorylation, PI3K activation, and their downstream targets AKT and RhoA, which regulate cell invasion and metastasis. However, their in vivo data show that NOV-002 exhibited no effect on primary tumor or lung metastasis in a mouse xenograft model.44 In addition, Townsend and colleagues suggested that the effect of NOV-002 was mediated through the interaction of NOV-002 with cell surface membrane protein thiols as GSSG could not penetrate the cell membrane.42,43 They also reported that quantitatively similar results were obtained after treatment of the cells with GSSG alone.42
Different from NOV-002, GSSG liposomes produced the antimetastatic effect through affecting detachment, migration, and invasion properties, and the effects were caused by intracellular delivery of GSSG not by GSSG alone. No effects on cell detachment and migration were reported for NOV-002. The observation on cell detachment would not have been missed in any cell culture experiments with NOV-002, had NOV-002 exhibited such an effect. Furthermore, NOV-002 was found to stimulate cell growth.41,42 In contrast, we found GSSG liposomes inhibited cell growth in a separate study.30 The anti-invasive spectra of GSSG liposomes and NOV-002 are also not the same. Glutathione disulfide liposomes significantly (80%) inhibited the invasion of a prostate cancer cell line (PC-3 cells), whereas NOV-002 was reported ineffective against this cell line.8 The failure to show any in vivo antimetastatic effect of NOV-002 provides an additional piece of evidence that GSSG liposomes are different from NOV-002.44 Therefore, all pieces of evidence support the conclusion that GSSG liposomes are different from NOV-002.
In summary, we have demonstrated GSSG liposomes effectively prevented cancer metastasis both in vitro and in vivo with a well-established murine melanoma metastasis model. This piece of work serves as a starting point to explore the potential of GSSG liposomes as a treatment for cancer and cancer metastasis.
Acknowledgments
This work was conducted with funds from grants from the National Institutes of Health (1R15GM093678-01; 1R15GM107197-01A1) awarded to Xiangming Guan.
Footnotes
Peer review:Five peer reviewers contributed to the peer review report. Reviewers’ reports totaled 1099 words, excluding any confidential comments to the academic editor.
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: XG played the major role in the experimental design, data analysis, and manuscript preparation. SSS was involved in the experimental design and manuscript preparation, and was the major participant in both in vitro and in vivo data collection and analysis. SW was the major participant in the in vitro data collection and both in vitro and in vivo data analysis. RKA, TS, JX, and HT were involved in the in vivo data collection. RD and GJB were involved in the data collection of the Western blot analysis.
References
- 1. Lorusso G, Ruegg C. New insights into the mechanisms of organ-specific breast cancer metastasis. Semin Cancer Biol. 2012;22:226–233. [DOI] [PubMed] [Google Scholar]
- 2. Valastyan S, Weinberg RA. Tumor metastasis: molecular insights and evolving paradigms. Cell. 2011;147:275–292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sleeman JP. Metastasis: understanding is the beginning of order in chaos. Semin Cancer Biol. 2012;22:173. [DOI] [PubMed] [Google Scholar]
- 4. Sleeman J, Steeg PS. Cancer metastasis as a therapeutic target. Eur J Cancer. 2010;46:1177–1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eccles SA, Welch DR. Metastasis: recent discoveries and novel treatment strategies. Lancet. 2007;369:1742–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sethi N, Kang Y. Unravelling the complexity of metastasis—molecular understanding and targeted therapies. Nat Rev Cancer. 2011;11:735–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weber GF. Why does cancer therapy lack effective anti-metastasis drugs? Cancer Lett. 2013;328:207–211. [DOI] [PubMed] [Google Scholar]
- 8. Pazoles C, Vulfson E, Huang Q. inventors. Treatment of metastatic tumors and other conditions. Patent US20110064828 A1, USA, 2011. [Google Scholar]
- 9. Zhao Y, Seefeldt T, Chen W, et al. Effects of glutathione reductase inhibition on cellular thiol redox state and related systems. Arch Biochem Biophys. 2009;485:56–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Schwock J, Dhani N, Hedley DW. Targeting focal adhesion kinase signaling in tumor growth and metastasis. Expert Opin Ther Targets. 2010;14:77–94. [DOI] [PubMed] [Google Scholar]
- 11. Orgaz JL, Sanz-Moreno V. Emerging molecular targets in melanoma invasion and metastasis. Pigment Cell Melanoma Res. 2013;26:39–57. [DOI] [PubMed] [Google Scholar]
- 12. Daenen LG, Roodhart JM, van Amersfoort M, et al. Chemotherapy enhances metastasis formation via VEGFR-1-expressing endothelial cells. Cancer Res. 2011;71:6976–6985. [DOI] [PubMed] [Google Scholar]
- 13. Hu YY, Zheng MH, Zhang R, Liang YM, Han H. Notch signaling pathway and cancer metastasis. Adv Exp Med Biol. 2012;727:186–198. [DOI] [PubMed] [Google Scholar]
- 14. Guan X. Cancer metastases: challenges and opportunities. Acta Pharm Sin B. 2015;5:402–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haraguchi M. The role of the transcriptional regulator snail in cell detachment, reattachment and migration. Cell Adh Migr. 2009;3:259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zanotti S, Gibertini S, Bragato C, Mantegazza R, Morandi L, Mora M. Fibroblasts from the muscles of Duchenne muscular dystrophy patients are resistant to cell detachment apoptosis. Exp Cell Res. 2011;317:2536–2547. [DOI] [PubMed] [Google Scholar]
- 17. Meister A, Anderson ME. Glutathione. Annu Rev Biochem. 1983;52:711–760. [DOI] [PubMed] [Google Scholar]
- 18. Sadhu SS, Xie J, Zhang HW, Perumal O, Guan X. Glutathione disulfide liposomes—a research tool for the study of thiol oxidative stress. Biochem Biophys Rep. 2016;7:225–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hong X, Jiang F, Kalkanis SN, et al. Intracellular free calcium mediates glioma cell detachment and cytotoxicity after photodynamic therapy. Lasers Med Sci. 2009;24:777–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu Y, Jovanovic B, Pins M, Lee C, Bergan RC. Over expression of endoglin in human prostate cancer suppresses cell detachment, migration and invasion. Oncogene. 2002;21:8272–8281. [DOI] [PubMed] [Google Scholar]
- 21. Hasan NM, Adams GE, Joiner MC, Marshall JF, Hart IR. Hypoxia facilitates tumour cell detachment by reducing expression of surface adhesion molecules and adhesion to extracellular matrices without loss of cell viability. Br J Cancer. 1998;77:1799–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Collins TJ. ImageJ for microscopy. Biotechniques. 2007;43:25–30. [DOI] [PubMed] [Google Scholar]
- 23. Gavet O, Pines J. Progressive activation of CyclinB1-Cdk1 coordinates entry to mitosis. Dev Cell. 2010;18:533–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Mitchell K, Svenson KB, Longmate WM, et al. Suppression of integrin alpha3beta1 in breast cancer cells reduces cyclooxygenase-2 gene expression and inhibits tumorigenesis, invasion, and cross-talk to endothelial cells. Cancer Res. 2010;70:6359–6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dachineni R, Ai G, Kumar DR, Sadhu SS, Tummala H, Bhat GJ. Cyclin A2 and CDK2 as novel targets of aspirin and salicylic acid: a potential role in cancer prevention. Mol Cancer Res. 2016;14:241–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bezault J, Bhimani R, Wiprovnick J, Furmanski P. Human lactoferrin inhibits growth of solid tumors and development of experimental metastases in mice. Cancer Res. 1994;54:2310–2312. [PubMed] [Google Scholar]
- 27. Kalland T. Effects of the immunomodulator LS 2616 on growth and metastasis of the murine B16-F10 melanoma. Cancer Res. 1986;46:3018–3022. [PubMed] [Google Scholar]
- 28. Schultz RM, Silberman S, Persky B, Bajkowski AS, Carmichael DF. Inhibition by human recombinant tissue inhibitor of metalloproteinases of human amnion invasion and lung colonization by murine B16-F10 melanoma cells. Cancer Res. 1988;48:5539–5545. [PubMed] [Google Scholar]
- 29. Wack C, Becker JC, Brocker EB, Lutz WK, Fischer WH. Chemoimmunotherapy for melanoma with dacarbazine and 2,4-dinitrochlorobenzene: results from a murine tumour model. Melanoma Res. 2001;11:247–253. [DOI] [PubMed] [Google Scholar]
- 30. Sadhu SS, Averineni RK, Yang Y, Wang S, Dachineni R, Guan X. In vitro and in vivo growth inhibition of B16-F10 murine melanoma by glutathione disulfide liposomes. Cancer Growth and Metastasis, 2017;10:1-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kim KM, Im AR, Kim SH, Hyun JW, Chae S. Timosaponin AIII inhibits melanoma cell migration by suppressing COX-2 and in vivo tumor metastasis. Cancer Sci. 2016;107:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu GS, Liu LF, Lin CJ, et al. Gene transfer of pro-opiomelanocortin prohormone suppressed the growth and metastasis of melanoma: involvement of alpha-melanocyte-stimulating hormone-mediated inhibition of the nuclear factor kappaB/cyclooxygenase-2 pathway. Mol Pharmacol. 2006;69:440–451. [DOI] [PubMed] [Google Scholar]
- 33. Serrano OK, Parrow NL, Violet PC, et al. Antitumor effect of pharmacologic ascorbate in the B16 murine melanoma model. Free Radic Biol Med. 2015;87:193–203. [DOI] [PubMed] [Google Scholar]
- 34. Huang CH, Lu SH, Chang CC, Thomas PA, Jayakumar T, Sheu JR. Hinokitiol, a tropolone derivative, inhibits mouse melanoma (B16-F10) cell migration and in vivo tumor formation. Eur J Pharmacol. 2015;746:148–157. [DOI] [PubMed] [Google Scholar]
- 35. Yu KF, Zhang WQ, Luo LM, et al. The antitumor activity of a doxorubicin loaded, iRGD-modified sterically-stabilized liposome on B16-F10 melanoma cells: in vitro and in vivo evaluation. Int J Nanomedicine. 2013;8:2473–2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Welch DR. Technical considerations for studying cancer metastasis in vivo. Clin Exp Metastasis. 1997;15:272–306. [DOI] [PubMed] [Google Scholar]
- 37. Jain M, Ratheesh A, Gude RP. Pentoxifylline inhibits integrin-mediated adherence of 12(S)-HETE and TNFalpha-activated B16F10 cells to fibronectin and endothelial cells. Chemotherapy. 2010;56:82–88. [DOI] [PubMed] [Google Scholar]
- 38. Santoni M, Conti A, Piva F, et al. Role of STAT3 pathway in genitourinary tumors. Future Sci OA. 2015;1:FSO15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Piskounova E, Agathocleous M, Murphy MM, et al. Oxidative stress inhibits distant metastasis by human melanoma cells. Nature. 2015;527:186–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Townsend DM, Tew KD. Pharmacology of a mimetic of glutathione disulfide, NOV-002. Biomed Pharmacother. 2009;63:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Townsend DM, Pazoles CJ, Tew KD. NOV-002, a mimetic of glutathione disulfide. Expert Opin Investig Drugs. 2008;17:1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Townsend DM, He L, Hutchens S, Garrett TE, Pazoles CJ, Tew KD. NOV-002, a glutathione disulfide mimetic, as a modulator of cellular redox balance. Cancer Res. 2008;68:2870–2877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Montero AJ, Diaz-Montero CM, Deutsch YE, et al. Phase 2 study of neoadjuvant treatment with NOV-002 in combination with doxorubicin and cyclophosphamide followed by docetaxel in patients with HER-2 negative clinical stage II-IIIc breast cancer. Breast Cancer Res Treat. 2012;132:215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gumireddy K, Li A, Cao L, et al. NOV-002, a glutathione disulfide mimetic, suppresses tumor cell invasion and metastasis. J Carcinog Mutagen. 2013;S7:002. [DOI] [PMC free article] [PubMed] [Google Scholar]