Abstract
Pharmacies have been endorsed as alternative vaccine delivery sites to improve vaccination rates through increased access to services. Our objective was to identify challenges and facilitators to adolescent and adult vaccination provision in pharmacy settings in the United States. We recruited 40 licensed pharmacists in states with different pharmacy vaccination laws. Eligible pharmacists previously administered or were currently administering human papillomavirus (HPV); tetanus, diphtheria, and pertussis (TDAP); or meningitis (meningococcal conjugate vaccine [MCV4]) vaccines to adolescents aged 9 to 17 years. Pharmacists participated in a semistructured survey on in-pharmacy vaccine provision. Pharmacists commonly administered vaccinations to age-eligible adolescents and adults: influenza (100%, 100%), pneumococcal (35%, 98%), TDAP (80%, 98%), MCV4 (60%, 78%), and HPV (45%, 53%). Common challenges included reimbursement/insurance coverage (28%, 78%), education of patients/parents (30%, 40%), and pharmacists’ time constraints (28%, 35%). Three-quarters of pharmacists reported that vaccination rates could be increased. National efforts should expand insurance coverage for vaccine administration reimbursement and improve data information systems to optimize provision within pharmacies.
Keywords: pharmacies, vaccinations, adolescents, adults
Introduction
Underuse of immunization for vaccine-preventable diseases is a significant public health concern in the United States.1 The Advisory Committee on Immunization Practices (ACIP) recommends routine provision of several vaccines for adolescents and adults to reduce and control the burden of vaccine-preventable diseases in the United States.2 Maximizing uptake of these vaccines is critical to reduce disease-associated outcomes, including meningitis (meningococcal conjugate vaccine or MCV4 vaccine), tetanus, diphtheria, whooping cough (tetanus, diphtheria, and pertussis or TDAP vaccine), human papillomavirus (HPV)-associated cancers (HPV vaccine), and influenza (influenza vaccine). Although overall adolescent vaccination coverage for those aged 9 to 17 years has increased in recent years, TDAP and MCV4 initiation rates have been consistently higher than HPV3 initiation, indicating a missed opportunity for HPV vacation nationwide.4–6 Adult vaccination coverage is even lower.7 For example, in 2010, the vaccination rate for herpes zoster was 14% of adults aged 60 years or older, and the hepatitis B vaccination rate (3-dose completion) was 42% among high-risk adults aged 19 to 49 years.7 Given the ACIP’s recommendations, these rates highlight the need to increase the access and availability of adolescent and adult vaccination.7
Due to substantial challenges to improving vaccination uptake in the United States, public health leaders have endorsed alternative vaccination delivery sites, including pharmacies, as a means to potentially improve vaccination rates.8–10 These organizations include the Centers for Disease Control and Prevention (CDC), the National Vaccine Advisory Committee, the American Cancer Society, and the President’s Cancer Panel. Pharmacists are able to administer vaccines to populations that may have limited access to traditional physicians’ offices for financial, geographical, or other reasons. Furthermore, pharmacists’ convenient locations, flexibility, extended work hours, and ease of access could allow for greater vaccine coverage.11 Therefore, vaccination of adolescents and adults through pharmacies has the potential to improve national vaccine rates. However, allowing pharmacists to provide immunizations does not remove all barriers of the vaccine delivery process. In previous studies, pharmacists from Washington cited challenges of lack of time, concerns for legal liability, lack of reimbursement, and issues with tracking/recall of vaccinations with multiple doses.12 The CDC recommends improving immunization information systems to better track vaccinations with multiple doses or to increase pharmacy-located vaccination.13
Further research to determine the facilitators and challenges related to pharmacists administering adolescent and adult vaccines is needed to inform vaccine delivery strategies within pharmacies whose presence continues to expand nationwide. Such studies will provide critical insights not yet available concerning the feasibility of initiating or completing adolescent and adult vaccines within pharmacy settings and completing recommended vaccination doses. Our study sought to fill this gap by providing data from pharmacists on challenges and facilitators of in-pharmacy vaccine administration to adolescents and adults.
Methods
Participants
Study participants were 40 licensed pharmacists, 5 in each of 8 states: Alabama, California, Indiana, Kentucky, Maine, Tennessee, Texas, and Washington. A broad geographic distribution of states was included to obtain lessons learned from pharmacists under varied state vaccination laws. These vaccination laws varied based on the authority that states granted pharmacists to administer vaccines.14 In Alabama and Indiana, pharmacists were allowed to administer vaccination to all age groups with a prescription from a primary care physician. Similar policies applied in Kentucky and Texas for vaccinations administered to those 9 and 12 years of age. However, pharmacists from Kentucky and Texas were able to administer vaccines to adults (19 years and above) upon the signing of a supervision agreement with a prescriber,14 which allowed pharmacists to administer the vaccine to patients regardless of their primary care doctor. In California, Tennessee, and Washington, pharmacists were able to administer vaccines to any age group based on a similar supervision agreement.14 State policies on vaccination authority in Maine did not permit pharmacists to administer the HPV or MCV4 vaccines to adolescents above the age of 12 years and adults14; however, they were authorized to provide TDAP with a prescription.14 Pharmacists were eligible to participate if they previously administered or were currently administering (1) any type of vaccine to adults (⩾18 years of age) and (2) HPV, TDAP, or TD (tetanus and diphtheria) or MCV4 vaccines to adolescents (⩾9 and <18 years of age) within the pharmacy in all states except Maine. In Maine, pharmacists were eligible to participate if they provided any vaccines to adolescents and adults within a pharmacy.
We identified pharmacies using HealthMap Vaccine Finder (vaccinefinder.org), a searchable, opt-in database of pharmacies providing vaccines across the United States.15 We obtained all pharmacy data from the HealthMap research investigators in June 2014 for pharmacies providing HPV, TDAP/TD, or MCV4 vaccinations in each of the 8 aforementioned states. By state, we randomly sampled pharmacies to contact. Using a script, we identified pharmacists available at the pharmacy location who met our inclusion criteria and were willing to participate in our survey.
Procedures and measures
Enrolled pharmacists completed a semistructured interview administered by telephone. The survey included 52 close-ended questions and 26 open-ended questions related to potential challenges or facilitators to administering adolescent and adult vaccines within pharmacies. Questions covered pharmacy contextual information, demographics of clients served, pharmacist education and training, pharmacy operational issues related to vaccination provision, vaccination provision within pharmacies (ie, whether a vaccine is offered at the pharmacy for administration), insurance reimbursement, health care communication, and minor consent procedures. Study staff were asked to follow the survey questions as a guide, but allowed the interview to include supplemental open responses from pharmacists when appropriate. We included open-ended questions and trained study staff to allow for additional information elicited from participants. Interviews lasted 30 to 60 minutes.
Pharmacists received an incentive of $50 for completing the interview. The University of North Carolina Institutional Review Board reviewed the study protocol and deemed the study to be a nonhuman subjects research.
Data analyses
We calculated the percent and Wald 95% confidence interval for proportions of vaccines administered, promotion methods used, and most common challenges and facilitators experienced by pharmacists. We categorized challenges or facilitators as common if at least 20% of pharmacists reported the factor for either adolescent or adult immunization. The Fisher exact test was conducted to evaluate statistical differences in challenges and facilitators between adolescent and adult vaccinations. We used SAS version 9.1 (SAS Institute, Cary, NC, USA) to perform statistical analyses.
Results
Survey participants and pharmacies
Most pharmacists enrolled were white (75%) and held a doctorate in Pharmacy (PharmD) degree (70%) (Table 1). Most pharmacists worked in chain pharmacies (78%) and provided services to an English-speaking population (93%). Over half (68%) of the pharmacists reported providing vaccines to adolescents and adults for at least 4 years. Although nearly half (49%) of pharmacists administered more than 1000 injections in the past year, most pharmacists (75%) reported that their pharmacy had the capacity to vaccinate more people.
Table 1.
Characteristics of pharmacists and pharmacies providing vaccines to adolescents and adults in 8 states in the United States (n = 40).
| n (%) | |
|---|---|
| Pharmacist | |
| Age, y | |
| 25-34 | 16 (40) |
| 35-44 | 13 (33) |
| 45-65 | 11 (28) |
| Sex | |
| Female | 22 (55) |
| Male | 18 (45) |
| Race | |
| White | 30 (75) |
| Asian | 6 (15) |
| Black | 2 (5) |
| Othera | 4 (10) |
| Highest degree earned | |
| PharmD | 28 (70) |
| BSPharm or BS | 11 (28) |
| MS—Pharmacy Administration | 1 (3) |
| Pharmacy type and population served | |
| Type | |
| Chain | 31 (78) |
| Independent | 5 (13) |
| Grocery | 2 (5) |
| Big box retailer | 2 (5) |
| English-speaking | |
| <80% | 3 (8) |
| 80-94 | 8 (20) |
| ⩾95 | 29 (73) |
| Pharmacy vaccinations | |
| Designated vaccination area | |
| Yes | 36 (90) |
| No | 4 (10) |
| No. of pharmacists currently administering vaccines | |
| 2-3 | 31 (78) |
| 4-5 | 7 (18) |
| ⩾6 | 2 (5) |
| Alternative health care providers administering vaccines | |
| No other types | 37 (93) |
| Interns | 2 (5) |
| Nurses | 1 (3) |
| Training required of pharmacists to administer immunizationsa | |
| APhA pharmacy-based immunization program | 39 (98) |
| BLS Certification | 22 (55) |
| OSHA/blood-borne pathogen | 7 (18) |
| CPE-approved certificate program | 4 (10) |
| Class/elective in pharmacy school | 4 (10) |
| Internal staff trainings | 4 (10) |
| Methods used to administer vaccinesb | |
| Walk-in | 40 (100) |
| Off-sitec | 31 (78) |
| Appointments during business hours | 21 (53) |
| Mass clinics | 15 (38) |
| Appointments during advertised date | 12 (30) |
| No. of years vaccines given to adolescents or adults | |
| 1-3 | 8 (20) |
| 4-6 | 18 (45) |
| ⩾7 | 9 (23) |
| Don’t know | 5 (13) |
| Estimated number of injections administered in the past year | |
| <500 | 8 (20) |
| 500-999 | 12 (30) |
| ⩾1000 | 19 (49) |
| Don’t know | 1 (3) |
| Self-reported pharmacy performance | |
| Could vaccinate more people | 30 (75) |
| Pharmacy at capacity | 8 (20) |
| Pharmacy turning away people | 2 (5) |
Abbreviations: APhA, American Pharmacists Association; BLS, Basic Life Support; BS, Bachelor of Science; BSPharm, Bachelor of Science in Pharmacy; CPE, Continuing Pharmacy Education; PharmD, Doctor of Pharmacy; OSHA, Occupational Safety and Health Administration.
Pacific Islander/Native Hawaiian (n = 1), American Indian/Alaska Native (n = 1), and multiracial (n = 2).
Totals may exceed 100% due to multiple answers possible.
Off-site: church, business, nursing homes.
Most pharmacies had a designated vaccination area (90%) and had 2 to 3 pharmacists who administered vaccines (78%). Pharmacies administered vaccines in several ways, including by different types of appointments: walk-ins (100%), appointments during business hours (53%), mass clinics (38%), appointments during advertised dates (30%), and by off-site locations (78%), eg, churches, businesses, and nursing homes.
Immunization practices
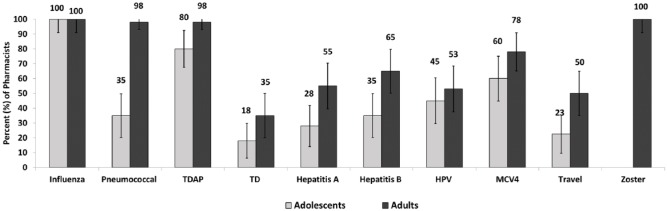
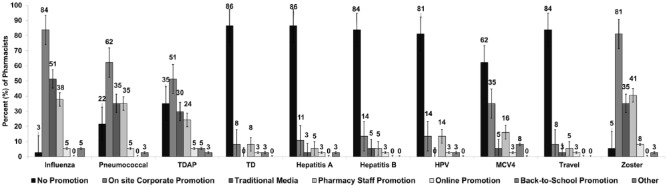
In total, 80% of pharmacists provided Tdap booster, 60% provided MCV4 vaccine, and 45% provided HPV vaccine to adolescents (Figure 1). In addition, all 40 pharmacists reported administering the herpes zoster and influenza vaccines to adults. Most pharmacists reported not having promotion efforts for several vaccines, including TD (86%), hepatitis A (86%), hepatitis B (84%), HPV (84%), MCV4 (62%), and travel vaccines (84%). However, on-site corporate promotion was used for influenza (84%), herpes zoster (81%), pneumococcal (62%), and TDAP (62%) vaccines (Figure 2).
Figure 1.
Vaccines currently provided by pharmacists to adults and adolescents in 8 states of the United States (n = 40; percent and 95% confidence interval). HPV indicates human papillomavirus; MCV4, meningococcal conjugate vaccine; TD, tetanus and diphtheria; TDAP, tetanus, diphtheria, and pertussis.
Figure 2.
Vaccine promotion methods used by pharmacists in 8 states in the United States (n = 37; percent and 95% confidence interval). HPV indicates human papillomavirus; MCV4, meningococcal conjugate vaccine; TD, tetanus and diphtheria; TDAP, tetanus, diphtheria, and pertussis. Total respondents are 37 as 3 pharmacists did not provide responses to this question.
Insurance coverage for vaccination
All pharmacies accepted Medicare, out-of-pocket/self-pay, and private insurance. In total, 98% of pharmacies accepted Medicaid, 65% accepted Tricare, and 60% accepted Children’s Health Insurance Program (Table 2). Over a quarter (28%) of the surveyed pharmacies reported being in-network providers for the Vaccines for Children Program. Over half of the pharmacists considered the level of reimbursement available to clients as a challenge for in-pharmacy vaccine program success (55%). In total, 57% of surveyed pharmacists did not consider insurance reimbursement adequate to cover vaccine administration costs for all vaccines and insurance plans.
Table 2.
Insurance concerns in pharmacies in 8 states in the United States (n = 40).
| n (%) | |
|---|---|
| Type of payment accepted by pharmacy a | |
| Medicare | 40 (100) |
| Out of pocket/Self Pay | 40 (100) |
| Private Insurance | 40 (100) |
| Medicaid | 39 (98) |
| Tricare | 26 (65) |
| Children’s Health Insurance Program (CHIP) | 24 (60) |
| Pharmacy categorized as network provider for Vaccines for Children Program | |
| Yes | 11 (28) |
| No | 15 (38) |
| Don’t know | 13 (33) |
| Level of reimbursement considered challenge for pharmacy | |
| Yes | 22 (55) |
| No | 14 (35) |
| Don’t know | 4 (10) |
| Insurance reimbursement adequate to cover vaccine administration costs | |
| Yes, true for all vaccines and insurance plans | 17 (43) |
| Yes, true for some vaccines only | 6 (15) |
| Yes, true for some insurance plans only | 3 (8) |
| No | 2 (5) |
| Don’t know | 12 (30) |
| Administrative costs not always reimbursed by insurance a,b | |
| Pharmacists’ time | 9 (23) |
| Vaccine stocking | 4 (10) |
| Refrigerator space | 2 (5) |
| Medicare vaccine coverage insufficient | 2 (5) |
| Don’t know | 1 (3) |
| Pharmacy experience difficulty determining whether patient’s insurance covers vaccine | |
| Yes | 21 (53) |
| No | 19 (48) |
| Issues faced by pharmacists due to insurance/billing of patient a,c | |
| Insurance does not cover pharmacy vaccination | 21 (53) |
| Coverage unclear at time of vaccination | 6 (15) |
| Pharmacist cannot administer vaccine/refers to physician | 5 (13) |
| Patient misinformed by insurance company/vaccine only covered in doctor’s office | 4 (10) |
| Insurance only covers some vaccinations | 3 (8) |
| Insurance rejected, required out-of-pocket payment | 2 (5) |
| Other | 1 (3) |
Totals may exceed 100% due to multiple answers possible.
Sample limited to those who responded “Yes, true for some vaccines,” “Yes, true for some insurance plans,” or “No” to “Is insurance reimbursement adequate to cover vaccine administrative costs?”
Sample limited to those who experience issues determining whether patient’s insurance covers vaccine.
Pharmacists’ time was the most commonly reported administrative cost not always reimbursed by insurance. Half of the pharmacists reported previously experiencing difficulty determining whether their patient’s insurance covered pharmacy-administered vaccination (53%). In fact, 13% of pharmacists in our study reported being unable to administer the requested vaccines and referred their clients to a physician. The most reported issue by pharmacists regarding insurance and billing was simply that the insurance provider did not provide coverage for vaccination administered in pharmacies (53%) (Table 2).
Challenges and facilitators to vaccine administration
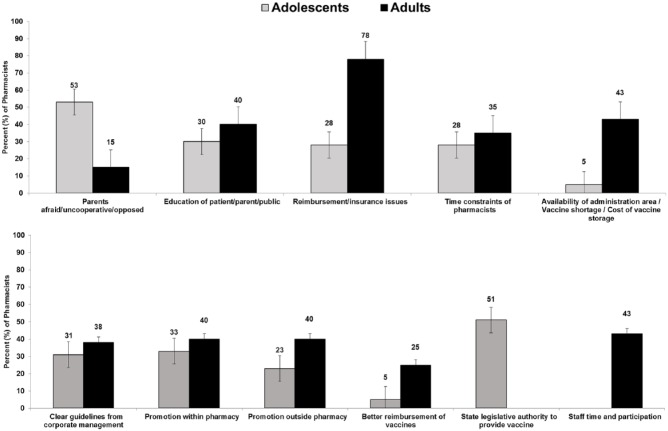
Adolescents
The challenges to adolescent vaccination most commonly cited by pharmacists included “patient’s fear, lack of cooperation, and opposition” (53%); education of patient/parent/public (30%); reimbursement/insurance issues (28%); and time constraints of the pharmacists (28%) (Table 3). Other challenges were less frequent, including parental consent issues (18%) and pharmacists being uncomfortable or resistant to vaccinating adolescents (10%). Pharmacists reported that potential adverse drug reactions (20%) were the primary concern of their patient population regarding adolescent vaccine administration within a pharmacy. Other key concerns from patients about adolescent vaccination included misconceptions and opposition to vaccination (18%) and cost to clients or concerns that insurance might not cover costs (15%).
Table 3.
Challenges and facilitators to vaccine implementation to adolescents and adults for pharmacists in 8 states in the United States (n = 40).
| Adolescent, n (%) | Adult, n (%) | P. valuea | |
|---|---|---|---|
| Challenges to vaccine implementation | |||
| Challenges to providing immunizationsb | |||
| Patient afraid/uncooperative/opposed | 21 (53) | 6 (15) | .001 |
| Education of patient/parent/public | 12 (30) | 16 (40) | .482 |
| Reimbursement/insurance issues | 11 (28) | 31 (78) | <.001 |
| Time constraints of pharmacists | 11 (28) | 14 (35) | .630 |
| Parent consent issues | 7 (18) | — | — |
| Pharmacist uncomfortable or resistant to vaccinate adolescents | 4 (10) | — | — |
| Patient needs vaccine records | 3 (8) | 7 (18) | .311 |
| Staff support and staff training | 3 (8) | 6 (16) | .481 |
| Availability of administration area/vaccine shortage/cost of vaccine storage | 2 (5) | 11 (43) | .013 |
| Patient needs prescription | 2 (5) | — | — |
| Language barriers | — | 2 (5) | — |
| Legal liability | 1 (3) | — | — |
| Adverse reaction/allergy | 1 (3) | 1 (3) | 1.000 |
| State laws that prevent it | 1 (3) | — | — |
| Screening questions | 1 (3) | — | — |
| Adolescent access to pharmacy | 1 (3) | — | — |
| Changing/confusing vaccine recommendations | — | 1 (3) | — |
| Don’t know | 1 (3) | — | — |
| None | 2 (5) | — | — |
| Concerns pharmacists heard from patients of vaccine administration at pharmaciesb | |||
| Adverse drug reactions | 8 (20) | 8 (20) | 1.000 |
| Cost to patient/insurance might not cover costs | 6 (15) | 10 (25) | .402 |
| Misconceptions/oppositions to vaccines | 7 (18) | 7 (18) | 1.000 |
| Do not know or trust pharmacist | 3 (8) | 2 (5) | 1.000 |
| Fear of needles | 3 (8) | 2 (5) | 1.000 |
| Inadequate staff training | — | 1 (3) | — |
| Otherc | 1 (3) | 1 (3) | 1.000 |
| Don’t know | 1 (3) | 1 (3) | 1.000 |
| No concerns | 14 (35) | 14 (35) | 1.000 |
| Facilitators to vaccine implementation | |||
| Requirements of a successful vaccination program in pharmaciesb | |||
| Education/promotion to public and patients | 25 (63) | 24 (60) | 1.000 |
| Staff time and participation | — | 11 (28) | — |
| Insurance coverage of pharmacy adolescent vaccinations | 7 (18) | — | — |
| Communication/support from doctors | 7 (18) | 2 (5) | .154 |
| Improved pharmacy logistics | 4 (10) | 9 (23) | .225 |
| Time and dedication to adolescents | 4 (10) | — | — |
| Staff training | 4 (10) | 5 (13) | 1.000 |
| Private/comfortable vaccination space | 3 (8) | 5 (13) | .712 |
| Do not know | — | 1(3) | — |
| Factors contributing to vaccine program success at included pharmacies (n = 39)b | |||
| State legislative authority to provide vaccine | 20 (51) | — | — |
| Staff time and participation | — | 17 (43) | — |
| Clear guidelines from corporate management/protocol | 12 (31) | 15 (38) | .482 |
| Patient education/promotion within pharmacy | 13 (33) | 16 (40) | .642 |
| Patient education/promotion outside pharmacy | 9 (23) | 16 (40) | .147 |
| Adolescent vaccine program unsuccessful | 4 (10) | — | — |
| School vaccination requirements | 6 (15) | — | — |
| Better reimbursement of vaccines | 2 (5) | 10 (25) | .025 |
| Communication/support by doctors | — | 2 (5) | — |
| Pharmacy convenience/preferred by patients | — | 3 (8) | — |
| Trust of community/word of mouth | — | 2 (5) | — |
| Otherd | 2 (5) | — | — |
| Don’t know | 2 (5) | — | — |
Fisher exact P values presented.
Totals may exceed 100% due to multiple answers possible.
Adolescents: time constraint to meet school start dates (n = 1); adults: scheduling (n = 1).
Adolescents: parent education and better time management (n = 2).
Eighteen percent of pharmacists stated that insurance coverage of adolescent vaccination in pharmacy and communication/support from doctors were requirements for program success of adolescent vaccination in pharmacies. Surveyed pharmacists reported several facilitators contributing to vaccine program success, including state legislative authority to provide vaccines (51%), patient education/promotion within the pharmacy (33%), and clear guidelines from corporate management/protocol (31%).
Adults
For adult vaccination, most pharmacists (78%) found reimbursement and insurance issues to be the greatest challenge (Figure 3). The availability of administration area/vaccine shortage/cost of vaccine storage (43%) and education of patient/parent/public (40%) were also commonly cited challenges. Pharmacists reported “cost to patient or concerns that insurance might not cover costs” (35%) as the most commonly expressed concern of adult patients regarding vaccine administration in pharmacies (Table 3).
Figure 3.
Common challenges and facilitators of vaccine provision to adolescents and adults in pharmacies in 8 states in the United States (n = 40; percent and 95% confidence intervals).
Pharmacists reported “education/promotion to public and patients” as the most important requirement for a successful vaccination program in pharmacies among both adolescents (63%) and adults (60%) (Table 3). For adult populations, staff time and participation (28%) and improved pharmacy logistics (23%) were additional requirements for a successful vaccination program. Just under half of the participating pharmacists reported staff time, participation (43%), and patient education/promotion both within (40%) and outside (40%) the pharmacy as key facilitators of a successful vaccination program.
Discussion
In this survey of pharmacists providing vaccination in 8 states, reimbursement and insurance coverage issues were the greatest challenges to providing adolescent and adult vaccinations in pharmacies as reported by pharmacists. Nearly half of pharmacists cited availability of administration area, vaccine shortage, and cost of vaccine storage as challenges to providing immunizations to adults. Parental opposition was another significant challenge that pharmacists cited, specifically for vaccine administration to adolescents. Most surveyed pharmacists cited that education/promotion of vaccination to the public is necessary for a successful vaccination program in pharmacies for both adolescent and adult provision.
Few studies have previously assessed the type and proportion of vaccines offered in pharmacies in the United States. A survey of 148 pharmacists in North Carolina published in 2008 showed that inactivated influenza vaccine was the most commonly offered vaccine (96%) and that other vaccines (including hepatitis A, hepatitis B, tetanus-containing vaccines (TDAP/TD), HPV, and herpes zoster) were offered less frequently (7%).16 Similar findings were reported in 2010 from community pharmacists in Washington, whereby the influenza vaccine was offered by all participating pharmacists, whereas other vaccines, including pneumococcal, MMR, and MCV4 vaccine, were offered less often.17 Similar to previous studies, 100% of pharmacists surveyed in our study reported providing the influenza vaccine to both adolescents and adults. In addition, in this survey, almost all pharmacists offered the pneumococcal vaccine to adults (98%). The TDAP was commonly offered to both adults (98%) and adolescents (80%). However, unlike previous studies, all pharmacists in this study offered the herpes zoster vaccine to adults and also offered other vaccines to both adolescents and adults, respectively, including hepatitis A, hepatitis B, HPV, and MCV4 vaccines. This observation may be due to recent changes in state laws to permit nonphysician health professionals, including pharmacists, to administer vaccines18 or as a result of our study inclusion criteria.
Pharmacists in our survey reported that administrative costs are not adequately covered by insurers for on-site pharmacy provision. Over half of pharmacists reported that the level of reimbursement of vaccines by insurance providers was a significant challenge. Pharmacists’ time was the most commonly cited administrative not reimbursed by insurers. Reimbursement for pharmacy vaccinations has been previously documented as a barrier to vaccination.19 Despite the national expansion of pharmacy-based vaccination services, the inability of clients to use medical insurance to fully pay for immunization services at the pharmacy remains a barrier to programmatic success of in-pharmacy vaccination and has been notable for individuals with private insurance.19 Common administrative costs generally not covered by such insurance plans are pharmacists’ time and vaccine stocking costs. To improve coverage among private insurance holders, previous studies have highlighted the key role employers play in requesting changes in insurance benefits to include pharmacy-based vaccinations.20
Interestingly, pharmacists identified state legislative authority to provide vaccines as an important factor to vaccine program success for adolescent provision, however, not for adults. Although pharmacists are able to administer vaccines in all states across the country, few states (Maine, Missouri, New Jersey, North Carolina, and Texas) provide pharmacists with the legal authority to assess patient vaccination status through immunization information systems.18 Prior to 2004, national data showed higher rates in influenza vaccination among states where legislation allowed pharmacies to immunize compared with states where such legislation did not exist.21
The potential benefits of in-pharmacy vaccine administration include expanded hours of operation (eg, evenings and weekends), flexibility of scheduling (no appointment needed with walk-ins welcome), easy access to multiple locations, rapidity, and potential for high patient traffic. Despite these benefits, we found that three-quarters of surveyed pharmacists reported that they could vaccinate more people at their respective pharmacy location. This indicates a potential underuse of pharmacies as an alternative vaccination site in the United States. Several factors contribute to the underuse of on-site pharmacy provision, including challenges identified in our present survey, lack of public awareness of vaccines available at pharmacies, and lack of trust in pharmacists to administer vaccines.22
Although previous studies have shown that parents consider pharmacists as acceptable immunization providers for their children,23 adults frequently hesitate to use pharmacies as an immunization site and often do not perceive pharmacists as regular providers of immunizations.24 Pharmacists included in our study emphasized the importance of public education through vaccination promotion and advertisements to overcome the educational barriers to vaccine uptake. One pharmacist (Tennessee) noted success by visiting local schools to promote vaccinations and educate parents about in-pharmacy resources available for vaccine administration. Another pharmacist (Indiana) described the importance of calling local school districts to ensure that the leaders were aware that pharmacists were allowed to administer vaccines to children even without a prescription from a primary physician when vaccinations were necessary for school entry.
Primary care physicians can play an integral role in improving this perception, given that physicians’ recommendation is the strongest predictors of vaccine uptake.25 A quarter of the pharmacists in our study believed that communication and support from doctors was a requirement of a successful vaccination program, especially among adolescents. In a study conducted in 2003 of family practice physicians in North Carolina, three-quarters of physicians were not aware that pharmacists could administer vaccinations in the state.24 Although half of the physicians considered that it was appropriate for pharmacists to administer the influenza vaccine, other vaccines received less support.24 A more recent national study found that physicians believed that it is helpful for pharmacists to share a role in vaccine administration efforts but found it problematic because both physicians and patients do not routinely receive documentation of in-pharmacy vaccination.26 Thus, collaborative efforts to establish electronic data management systems between pharmacists and primary care physicians will be necessary to document vaccinations provided for the successful implementation of vaccination programs among target populations, especially among those with limited access to primary health care services.
Strengths of our study include that it was conducted in 8 US states to provide a sample of pharmacist views toward on-site vaccination from a range of policy environments. In addition, our survey interviews included open-ended questions to elicit open-ended and in-depth responses on barriers and facilitators to vaccine administration within pharmacies. These interviews lasted up to 1 hour. Due to the qualitative nature of this data collection, the target sample size for this assessment was 40 pharmacists in total. The main limitation of this study was this relatively small sample size, with inclusion of 5 pharmacists per state to estimate statewide practices. Nonetheless, study findings provide new and significant insights into the role of pharmacists in vaccination and the potential means to increase adolescent and adult uptake.
Opportunities exist for improvements in vaccine access within pharmacies to improve uptake in both adolescent and adult populations to prevent vaccine-preventable diseases in the general population. Information collected in this survey could be used as a framework for collaborative efforts between pharmacists, primary care providers, and policymakers to expand pharmacists’ role in providing vaccinations.
Acknowledgments
The authors thank the organization HealthMap Vaccine Finder for generously providing their database of pharmacy providers for this research.
Footnotes
Peer review:Four peer reviewers contributed to the peer review report. Reviewers’ reports totaled 457 words, excluding any confidential comments to the academic editor.
Funding:The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding for this research was provided by Merck (VT ID #50873).
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: JSS, JKG, SBS, and NTB conceived and designed the experiments. JYI and AL analyzed the data. JYI wrote the first draft of the manuscript. JYI, JFG, AL, MK, SW, SBS, NTB, and JSS contributed to the writing of the manuscript. All authors agree with manuscript results and conclusions. All authors jointly developed the structure and arguments for the paper. All authors reviewed and approved the final manuscript.
References
- 1. 2010 National Vaccine Plan. Washington, DC: U.S. Department of Health and Human Services; 2010. [Google Scholar]
- 2. Centers for Disease Control and Prevention. General recommendations on immunization: recommendations of the Advisory Committee on Immunization Practices (ACIP). Recomm Rep: Morb Mortal Wkly Rep. 2011;60:1–64. [PubMed] [Google Scholar]
- 3.Accelerating HPV Vaccine Uptake: Urgency for Action to Prevent Cancer. Washington, DC: National Cancer Institute, National Institute for Health, U.S. Department of Health and Human Services; 2012. [Google Scholar]
- 4. Centers for Disease Control and Prevention. National and state vaccination coverage among adolescents aged 13 through 17 years: United States, 2010. Morb Mortal Wkly Rep. 2011;60:1117–1123. [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention. Final state-level influenza vaccination coverage estimates for the 2010–11 season–United States, National Immunization Survey and Behavioral Risk Factor Surveillance System, August 2010 through May 2011. http://www.cdc.gov/flu/fluvaxview/coverage_1011estimates.htm. Published 2011.
- 6. Reagan-Steiner S, Yankey D, Jeyarajah J, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years—United States, 2014. Morb Mortal Wkly Rep. 2015;64:784–792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Centers for Disease Control and Prevention. Adult vaccination coverage—United States, 2010. Morb Mortal Wkly Rep. 2012;61:66–72. [PubMed] [Google Scholar]
- 8. McIntosh J, Sturpe DA, Khanna N. Human papillomavirus vaccine and cervical cancer prevention: practice and policy implications for pharmacists. J Am Pharm Assoc. 2008;48:e1–13; quiz e14-17. [DOI] [PubMed] [Google Scholar]
- 9. Saslow D, Castle PE, Cox JT, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7–28. [DOI] [PubMed] [Google Scholar]
- 10. Smith JS, Brewer NT, Saslow D, et al. Recommendations for a national agenda to substantially reduce cervical cancer. Cancer Causes Control. 2013;24:1583–1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Crawford ND, Blaney S, Amesty S, et al. Individual- and neighborhood-level characteristics associated with support of in-pharmacy vaccination among ESAP-registered pharmacies: pharmacists’ role in reducing racial/ethnic disparities in influenza vaccinations in New York City. J Urban Health. 2011;88:176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madhavan SS, Rosenbluth SA, Amonkar M, Borker RD, Richards T. Pharmacists and immunizations: a national survey. J Am Pharm Assoc (Wash). 2001;41:32–45. [DOI] [PubMed] [Google Scholar]
- 13. Centers for Disease Control and Prevention. Progress in immunization information systems—United States, 2012. Morb Mortal Wkly Rep. 2013;62:1005–1008. [PMC free article] [PubMed] [Google Scholar]
- 14. Brewer NT, Chung JK, Baker HM, Rothholz MC, Smith JS. Pharmacist authority to provide HPV vaccine: novel partners in cervical cancer prevention. Gynecol Oncol. 2014;132:S3–S8. [DOI] [PubMed] [Google Scholar]
- 15. HealthMap Vaccine Finder. http://flushot.healthmap.org/ Published 2012.
- 16. Kummer GL, Foushee LL. Description of the characteristics of pharmacist-based immunization services in North Carolina: results of a pharmacist survey. J Am Pharm Assoc. 2008;48:744–751. [DOI] [PubMed] [Google Scholar]
- 17. Westrick SC. Pharmacy characteristics, vaccination service characteristics, and service expansion: an analysis of sustainers and new adopters. J Am Pharm Assoc. 2010;50:52–61. [DOI] [PubMed] [Google Scholar]
- 18. Stewart AM, Lindley MC, Cox MA. State law and standing orders for immunization services. Am J Prev Med. 2016;50:e133–e142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Pilisuk T, Goad J, Backer H. Vaccination delivery by chain pharmacies in California: results of a 2007 survey. J Am Pharm Assoc. 2010;50:134–139. [DOI] [PubMed] [Google Scholar]
- 20. Ko KJ, Wade RL, Yu HT, Miller RM, Sherman B, Goad J. Implementation of a pharmacy-based adult vaccine benefit: recommendations for a commercial health plan benefit. J Manag Care Spec Pharm. 2014;20:273–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Steyer TE, Ragucci KR, Pearson WS, Mainous AG., III The role of pharmacists in the delivery of influenza vaccinations. Vaccine. 2004;22:1001–1006. [DOI] [PubMed] [Google Scholar]
- 22. Hogue MD, Meador AE. Vaccines and immunization practice. Nurs Clin North Am. 2016;51:121–136. [DOI] [PubMed] [Google Scholar]
- 23. Deshpande M, Schauer J, Mott DA, Young HN, Cory P. Parents’ perceptions of pharmacists as providers of influenza vaccine to children. J Am Pharm Assoc. 2013;53:488–495. [DOI] [PubMed] [Google Scholar]
- 24. Blake EW, Blair MM, Couchenour RL. Perceptions of pharmacists as providers of immunizations for adult patients. Pharmacotherapy. 2003;23:248–254. [DOI] [PubMed] [Google Scholar]
- 25. Vadaparampil ST, Kahn JA, Salmon D, et al. Missed clinical opportunities: provider recommendations for HPV vaccination for 11–12 year old girls are limited. Vaccine. 2011;29:8634–8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hurley LP, Bridges CB, Harpaz R, et al. U.S. physicians’ perspective of adult vaccine delivery. Ann Intern Med. 2014;160:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]