Abstract
Current United States regulatory policies allow for the addition of pharmacologically active substances in dietary supplements if derived from a botanical source. The inclusion of certain nootropic drugs, such as vinpocetine, in dietary supplements has recently come under scrutiny due to the lack of defined dosage parameters and yet unproven short- and long-term benefits and risks to human health. This study quantified the concentration of vinpocetine in several commercially available dietary supplements and found that a highly variable range of 0.6–5.1 mg/serving was present across the tested products, with most products providing no specification of vinpocetine concentrations.
Keywords: Vinpocetine, vincamine, dietary supplements, nootropic, cognitive enhancement, high-performance liquid chromatography
Vinpocetine is a semisynthetic derivative of vincamine, an alkaloid extracted from the Vinca minor lesser periwinkle plant. In the United States, vinpocetine is not regulated in the neutraceutical industry, and it is often included as an active ingredient in dietary supplements. This chemical substance acts as a vasodilator, leading to manufacturer claims of cognitive enhancement and neuronal protection due to increased cerebral blood-flow. It is also added to many bodybuilding supplements to increase blood flow to muscles, facilitating the delivery of oxygen and nutrients, and removal of deleterious catalytic waste products. Currently in Europe, vinpocetine is only available as a prescription medication for treatment of dysfunctional cognitive abilities and cerebrovascular diseases. Vinpocetine is banned from Australia, New Zealand, and Canada due to potential harmful nootropic characteristics as a cognitive enhancing supplement [1–3]. Although the health benefits of vinpocetine have been proven in scientific studies, more research is needed to define the dosage parameters with respect to benefits and risks of both short- and long-term usage of vinpocetine as a dietary or pharmaceutical supplement [4]. Of primary concern are the negative consequences of long-term usage of vinpocetine and the potential for addiction to this and other nootropic drugs [4–6].
There are currently not enough scientific or clinical studies to support or denounce the claims of vinpocetine as a cognitive enhancer. Vinpocetine has not yet been approved by the U.S. Food and Drug Administration for medical treatments, but it can be sold as a dietary supplement because it can be isolated from a botanical source. Recent legislative measures in the United States called for the FDA to review and revoke alleged nootropic compounds, including vinpocetine and picamilon, from dietary supplements that claim to boost cognition. Of growing concern is uniformed product consumption of dietary supplements stating the amount of vinpocetine per dosage within a given time period to be within safe usage parameters. The range of vinpocetine concentrations in dietary supplements are highly variable, and this inconsistency between supplements is dangerous to consumers [7]. There is a movement in the U.S. to pass legislation that bans the inclusion of vinpocetine from dietary supplements.
Vincamine is the primary Vinca alkaloid present in the periwinkle plant, which can be used directly as a medication or chemically modified to produce vinpocetine (Figure 1). Vinca alkaloids are characterized by their anti-mitoic and anti-microtuble chemical properties, which have allowed for these types of alkaloids to be utilized in the treatments of tumors and cancerous tissue [8,9]. Vincamine has previously been used for circulatory disorders, especially cerebral circulatory impairment by boosting metabolism in the brain and improving oxygen supply. Along with vinpocetine, it is currently prescribed in the European Union as a cerebral vasodilator to assist with the treatment of stroke patients and those suffering from general cognitive impairments.
Figure 1.
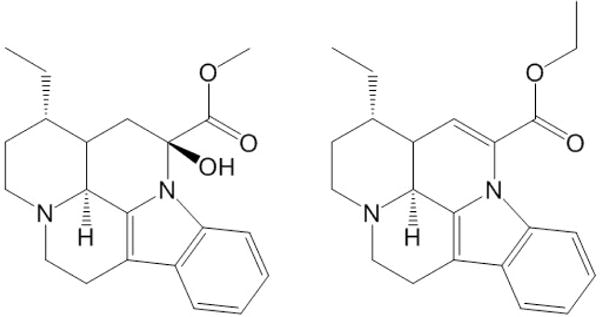
Chemical structures of vincamine (left) and the semi-synthetic derivative vinpocetine (right).
As a nootropic alkaloid, vinpocetine has been shown to facilitate learning and memory, specifically to prevent cognitive deficits commonly coupled with dementias [10,11]. Vinpocetine acts as a neuroprotective agent that reduces damage to the brain from ischemia, stroke, and trauma [12]. Physiologically, vinpocetine acts as a phosphodiesterase type 1 (PDE1) inhibitor, leading to enhanced levels of second messengers, cAMP/cGMP, and ultimately expression of neuronal plasticity-related genes, neurotrophic factors, and neuroprotective molecules [13]. These neuronal plasticity enhancement properties make PDE1 inhibitors good candidates for development into therapeutic agents for many neurological conditions. In addition to its potential as a plasticity enhancer, vinpocetine was recently proven to have a strong anti-inflammatory effect [14,15]. Vinpocetine inhibits I kappa B kinase, a key protein kinase involved in inflammatory response [14]. Surprisingly, this mechanism is independent of vinpocetine action on PDE1 [16–18]. Furthermore, the use of vinpocetine in animal models suggests that vinpocetine has a role to play in restoring neuronal plasticity (learning and memory in particular) in different conditions [19]. Although the scientific studies show that vinpocetine possesses promising therapeutic properties, clinical trials remain controversial and inconclusive for unsupervised human health applications.
The objective of this study was to measure vinpocetine concentrations in a range of dietary supplements while creating an efficient and reliable methodology for extraction and quantitative determination. In the event the United States congress passes legislation to regulate vinpocetine in dietary supplements, all suppliers of vinpocetine-containing products will require compliance testing. This investigation identifies high inconsistency in vinpocetine concentrations between dietary supplement products.
Chromatograms of product extracts yielded well-resolved peaks representing both vinpocetine and the internal standard, DL-propranolol, with retention times of approximately 11.9 and 9.9 minutes, respectively (Figure 2). Integration of vinpocetine peaks and comparison to calibration curves from calibration standards allowed for quantitative determination of vinpocetine concentrations, ranging from 0.118–11.55 mg/g product with standard deviations from triplicate trials on the order of 0.002 to 0.287 mg/g (Table 1). HPLC chromatograms obtained for each product tested are provided in the Supplementary Data.
Figure 2.
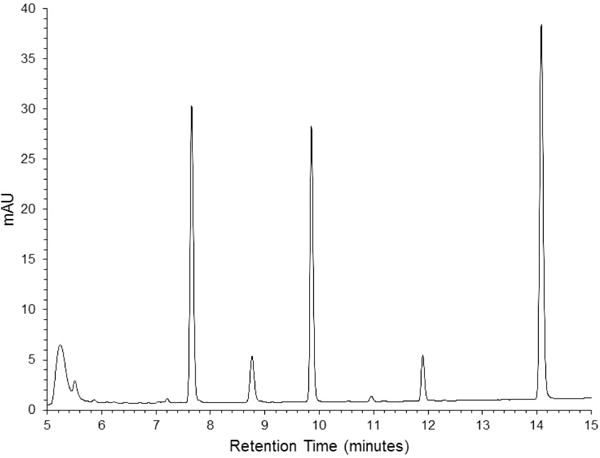
HPLC-UV chromatogram obtained for product B. Retention times for vinpocetine and DL-propranolol are at 11.9 and 9.9 minutes, respectively.
Table 1.
Quantity of vinpocetine in eight commercially available dietary supplements.
| Product | mg/g supplement | mg/serving |
|---|---|---|
| A | 0.165 ± 0.008 | 1.487 ± 0.067 |
| B | 0.199 ± 0.014 | 2.437 ± 0.172 |
| C | 0.118 ± 0.002 | 0.623 ± 0.012 |
| D | 1.356 ± 0.031 | 3.163 ± 0.070 |
| E | 11.55 ± 0.287 | 5.073 ± 0.251 |
| F | 0.857 ± 0.007 | 1.070 ± 0.017 |
| G | 1.372 ± 0.022 | 1.060 ± 0.052 |
| H | 7.318 ± 0.095 | 5.087 ± 0.151 |
The concentration of vinpocetine present in the products varied considerably, ranging on average from 0.632 to 5.087 mg per serving. Two of the sample extracts, products E and H, required additional dilution prior to analysis due to concentrations of vinpocetine that were in excess of the upper limits of the calibration curve. The extraction method was validated by low variation in vinpocetine concentration over triplicate trials for each of the products tested. The extraction efficiency for vinpocetine was estimated by comparison to the surrogate standard (DL-propranolol) that yielded >95% return on known amount. The extraction method demonstrated highly reproducible results for powder, capsules, and tablet samples.
The purpose for this study was to identify whether vinpocetine could be quantitatively analyzed across a range of consumer products. In the analysis of eight dietary supplements, only one of them had a quantity of vinpocetine per serving specified on the nutritional label. The measured concentration of 1.070 mg/serving (product F) was well within a suitable range for dietary supplement formulation parameters, in this case the manufacturer specifying 1 mg/serving. The remaining supplements listed vinpocetine as an ingredient, although not always obviously apparent, and without a specified quantity. Some products stated the addition of vinpocetine directly, while others had the ingredient listed indirectly as a component of a natural extract. The difference in labeling practices most likely reflects the form in which vinpocetine was added to the dietary supplement formulation; either as a synthetic compound, purified extract, or as a component of a plant extract. Regardless of identification details on product label, the amount of vinpocetine present in the supplements tested was found to vary by as much as 10 times between products.
From consumer and physiological standpoints, not having the proper labeling of the absolute amounts of vinpocetine per serving has the potential of putting people at risk for adverse effects of consumption. Furthermore, due to contradictory research reports stating the possible benefits and risks of vinpocetine as a dietary supplement, the safe dosage parameters are not well established. Around the world, countries that have approved vinpocetine as a therapeutic drug for treatment of patients recovering from cognitive disabilities permit prescribed doses ranging from 5 mg – 40 mg. Some of the dietary supplements tested have vinpocetine concentrations within this range, however these are being consumed without the direction or supervision of a physician.
The results presented in this study show that dietary supplements available in the United States have inconsistent dosage quantities of active ingredients, like vinpocetine. With bioactive ingredients that have diverse applications and availabilities worldwide due to unknown short- and long-term health effects, concerns are often raised about the safety and regulation of the dietary supplement industry. Although current United States economic conditions allow for the initial sale and consumption of natural products outside of the Food and Drug Administration’s regulatory reach, research must be expedited in this area to ensure the safety of these natural products, or otherwise produce credible research that supports the discontinuation of certain supplement additives. At the least, it would be to the consumer’s advantage to be informed as to the compounds and the quantity of which they are consuming.
The results obtained in this study showed that dietary supplements contain a considerable range of vinpocetine from 0.6 to 5 mg per serving. With the benefits and damaging effects of vinpocetine usage being disputed in the medical community, and the concentrations of vinpocetine not identified in dietary supplements, some lawmakers in the United States have called for the reexamination of whether or not vinpocetine should be approved for consumption without the supervision of a physician. This study shows that the concentrations of vinpocetine in readily obtainable dietary supplements are highly variable, and that this important information is not always disclosed by the manufacturer.
Experimental
Commercially available dietary supplements which stated the presence of vinpocetine on the product label were used in this study. Eight supplements, identified as samples A–H, were obtained in powder, capsule, and tablet form, and analyzed for the presence of vinpocetine. Products A–D were powder/granule, products E and F were in capsule form containing powdered supplement, and products G and H were in tablets. Products F, G, and H were in single-dose pill packs consisting of 9–10 individual capsules and tablets, with the vinpocetine ingredient contained within a single tablet or capsule for each pill pack. For extraction and analysis, only the vinpocetine-containing portion of the supplement was used. HPLC-grade acetonitrile (Fisher A998) and methanol (Fisher A452), DL-propranolol hydrochloride (Acros 207320050, 99%), and trifluoroacetic acid (Fisher O4901) were purchased from Fisher Scientific, and vinpocetine (Sigma V6383, ≥ 98%) was purchased from Sigma-Aldrich.
Extraction of Vinpocetine
Triplicate analysis was used for each supplement. Powdered supplement (250 mg) was placed into 15 mL Falcon tubes and an exact mass obtained to 0.1 mg precision. To each sample tube was added 10 mL of methanol followed by vortexing and sonicating (30 min). Each sample was spiked with a surrogate standard, DL-propranolol, at a concentration of 10 μg/mL. Samples were centrifuged at 1000 rpm for 2 minutes. The supernatant was filtered (0.45 μm) and used directly for HPLC analysis. An additional 1:5 dilution with methanol was performed for products E and H due to high vinpocetine concentrations.
Standard Solutions
A vinpocetine stock solution in methanol was prepared at a concentration of 1 mg/mL in methanol. Calibration standards were prepared at concentrations of 1, 5, 10, 25, 50, and 100 μg/mL. DL-Propranolol hydrochloride was used as an internal standard at 10 μg/mL. The stock solution and calibration standards for DL-propranolol hydrochloride were prepared in the same manner.
High Performance Liquid Chromatography
Identification and quantification of vinpocetine was performed on a Thermo Scientific Ultimate 3000 UHPLC Plus equipped with a diode array UV/Vis detector, and an Acclaim 120 C18 column (3 μm, 120 Å, 2.1 × 150 mm). Solvent A was 0.1% TFA in 18.2 MΩ H2O and solvent B was 0.1% TFA in ACN. The flow rate was 0.400 mL/minute and each run was 20 minutes. The gradient began at 90% A:10% B, with a linear gradient to 30% A:70% B by 15 minutes. At 15.100 minutes the gradient was returned to 90% A:10% solvent B by 20 minutes. The column oven was maintained at a temperature of 40°C. The injection volume of 1 μL was used for each sample. UV-vis detection wavelengths were monitored at 205 and 269 nm for the detection of vinpocetine and propranolol, respectively. Data acquisition and quantitative analysis were performed using Chromeleon software (v 7.2).
Supplementary Material
Acknowledgments
We wish to thank Jason Hendrickson for material and consultation support of this project. The Idaho Global Entrepreneurial Mission grant program funded the HPLC-UV instrument that made this work possible. This project was supported by NIH #P20GM109095, NSF #0619793 and #0923535, and the M.J. Murdock Charitable Trust.
Footnotes
Supplementary data: Supplementary material for this article includes the HPLC chromatograms for all eight dietary supplements tested in this study, as well as calibration curves for vinpocetine and DL-propranolol used in quantification of vinpocetine concentrations.
References
- 1.Chemical Information Review Document for Vinpocetine [CAS No. 42971-09-5] U.S. Department of Health and Human Services. National Toxicology Program. 2013 http://ntp.niehs.nih.gov/ntp/htdocs/chem_background/exsumpdf/vinpocetine091613_508.pdf Accessed January 8, 2016.
- 2.National Center for Biotechnology Information. PubChem BioAssay Database; AID=2299. Scripps Research Institute Molecular Screening Center; Source. https://pubchem.ncbi.nlm.nih.gov/compound/vincamine#section=Absorption-Distribution-and-Excretion Accessed January 13, 2016. [Google Scholar]
- 3.Avula B, Chittiboyina AG, Sagi S, Wang Y-H, Wang M, Khan IA, Cohen PA. Identification and quantification of vinpocetine and picamilon in dietary supplements sold in the United States. Drug Testing and Analysis. 2015 doi: 10.1002/dta.1853. [DOI] [PubMed] [Google Scholar]
- 4.Goldman P. Herbal medicines today and the roots of modern pharmacology. Annals of Internal Medicine. 2001;135:594–600. doi: 10.7326/0003-4819-135-8_part_1-200110160-00010. [DOI] [PubMed] [Google Scholar]
- 5.Shaw DM. Neuroenhancers, addiction and research ethics. Journal of Medical Ethics. 2012;38:605–608. doi: 10.1136/medethics-2012-100616. [DOI] [PubMed] [Google Scholar]
- 6.Noble KA. Brain Gain: Adolescent Use of Stimulants for Achievement. Journal of PeriAnesthesia Nursing. 2012;27:415–419. doi: 10.1016/j.jopan.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 7.Bhadra S, Das SC, Roy S, Arefeen S, Rouf ASS. Development and Validation of RP-HPLC Method for Quantitative Estimation of Vinpocetine in Pure and Pharmaceutical Dosage Forms. Chromatography Research International. 2011;2011:801656. [Google Scholar]
- 8.Jordan MA, Thrower D, Wilson L. Mechanism of inhibition of cell proliferation by Vinca alkaloids. Cancer Research. 1991;51:2212–2222. [PubMed] [Google Scholar]
- 9.Kruczynski A, Barret J-M, Etievant C, Colpaert F, Fahy J, Hill BT. Antimitotic and tubulin-interacting properties of vinflunine, a novel fluorinated Vinca alkaloid. Biochemical Pharmacology. 1998;55:635–648. doi: 10.1016/s0006-2952(97)00505-4. [DOI] [PubMed] [Google Scholar]
- 10.Szatmari S, Whitehouse P. Vinpocetine for cognitive impairment and dementia. Cochrane Database of Systematic Reviews. 2003;1:CD003119. doi: 10.1002/14651858.CD003119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nicholson CD. Pharmacology of nootropics and metabolically active compounds in relation to their use in dementia. Psychopharmacology. 1990;101:147–159. doi: 10.1007/BF02244119. [DOI] [PubMed] [Google Scholar]
- 12.Umar T, Hoda N. Selective Inhibitors of Phosphodiesterases: therapeutic promise for neurodegenerative disorders. MedChemComm. 2015;16:2063–2080. [Google Scholar]
- 13.Deshmukh R, Sharma V, Mehan S, Sharma N, Bedi KL. Amelioration of intracerebroventricular streptozotocin induced cognitive dysfunction and oxidative stress by vinpocetine – a PDE1 inhibitor. European Journal of Pharmacology. 2009;620:49–56. doi: 10.1016/j.ejphar.2009.08.027. [DOI] [PubMed] [Google Scholar]
- 14.Medina AE. Vinpocetine as a potent anti-inflammatory agent. Proceedings of the National Academy of Science. 2010;107:9921–9922. doi: 10.1073/pnas.1005138107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruiz-Miyazawa KW, Pinho-Ribeiro FA, Zarpelon AC, Staurengo-Ferrari L, Silva RL, Alves-Filho JC, Cunha TM, Cunha FQ, Casagrande R, Verri WA., Jr Vinpocetine reduces lipopolysaccharide-induced inflammatory pain and neutrophil recruitment in mice by targeting oxidative stress, cytokines and NF-κB. Chemico-Biological Interactions. 2015;25:9–17. doi: 10.1016/j.cbi.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 16.Medina AE, Krahe TE, Ramoa AS. Restoration of neuronal plasticity by a phosphodiesterase type I inhibitor in a model of fetal alcohol exposure. Journal of Neuroscience. 2006;26:1057–1060. doi: 10.1523/JNEUROSCI.4177-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nunes F, Ferreira-Rosa K, Pereira MS, Kubrusly RC, Manhaes AC, Abreu-Villaca Y, Filgueiras CC. Acute administration of vinpocetine, a phosphodiesterase type 1 inhibitor, ameliorates hyperactivity in a mice model of fetal alcohol spectrum disorder. Drug and Alcohol Dependence. 2011;19:81–87. doi: 10.1016/j.drugalcdep.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh R, Sawant O, Ganpathy P, Pitre S, Kadam VJ. Phosphodiesterase inhibitors: their role and implications. International Journal of PharmTech Research. 2009;1:1148–1160. [Google Scholar]
- 19.Filgueiras CC, Krahe TE, Medina AE. Phosphodiesterase type I inhibition improves learning in rats exposed to alcohol during the third trimester equivalent of human gestation. Neuroscience Letters. 2010;473:202–207. doi: 10.1016/j.neulet.2010.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


