Abstract
It has been reported that tuberculosis (TB) worsens after cessation of tumor necrosis factor-α inhibitors and starting anti-TB treatment. Little is known about the immunological pathogenesis of this paradoxical response (PR). We report the first case of a TB patient in whom PR occurred concurrently with elevation of circulating tumor necrosis factor-α (TNFα) levels. A 75-year-old woman, who had been treated with adalimumab for SAPHO syndrome, developed disseminated TB. Soon after administration of anti-TB treatment (isoniazid, rifampicin, pyrazinamide, and ethambutol), and after discontinuation of adalimumab, a PR occurred. Serial testing of serum cytokine levels revealed a marked increase in TNFα, and a decline in interferon-γ levels. Despite intensive treatment with antibiotics, prednisolone, noradrenaline, and mechanical ventilation, acute respiratory distress syndrome developed and she died. Thus, overproduction of TNFα after cessation of TNFα inhibitors may partially account for the pathogenesis of a PR. This supports preventative or therapeutic reinitiation of TNFα inhibitors when PR occurs. Serial monitoring of circulating inflammatory cytokine levels could lead to earlier identification of a PR.
Keywords: Adalimumab, Immune reconstitution inflammatory syndrome, Paradoxical response, Tuberculosis, Tumor necrosis factor alpha, TNF-α inhibitor
Abbreviations: ELISA, enzyme-linked immunosorbent assay; HRCT, high resolution computer tomography; IGRA, Interferon gamma release assay; IRIS, immune reconstruction syndrome; PR, Paradoxical response; TB, tuberculosis; TNFα, Tumor necrosis factor alpha
1. Introduction
Tumor necrosis factor-α (TNFα) inhibitors, including adalimumab, have been widely used for patients with auto-immune disease. Patients who develop tuberculosis (TB) during TNFα blockade usually discontinue TNFα inhibitors prior to starting treatment with anti-TB chemotherapy; however, this sometimes causes progressive worsening after initial improvement of TB, known as the paradoxical response (PR) or immune reconstruction syndrome (IRIS) [1].
Although distinct immunological profiles may characterize TB-associated IRIS patients [2], no data are available for those patients with inflammatory disease who develop a PR. We here report the disease course, serum levels of TNFα, and results of interferon-γ (IFNγ) release assays (IGRAs) for a patient who developed a PR after discontinuation of adalimumab.
2. Case presentation
A 75-year-old woman with SAPHO syndrome was started on bi-weekly adalimumab, which improved her joint pain. She had a brother with a history of TB. A chest radiograph showed calcification of mediastinum lymph nodes, suggestive of past TB infection. She started preventative isoniazid therapy, but discontinued after 3 weeks at her physician's discretion. Five months after starting adalimumab therapy, she presented with fever and pain in the right upper jaw and was admitted to the previous hospital, where adalimumab was discontinued. A high-resolution computer tomography (HRCT) scan of the thorax at 1-mm slice thickness revealed multiple small nodules, ranging from 2 to 5 mm in diameter, and diffuse ground glass opacities in the lung, which was suggestive of disseminated TB (Fig. 1). The presence of Mycobacterium tuberculosis was confirmed by polymerase chain reaction on gastric lavage obtained on admission to the previous hospital (day -1). Growth of M. tuberculosis in solid culture was detected in 3 weeks and susceptibility testing using the culture isolate showed no resistance to anti-TB agents.
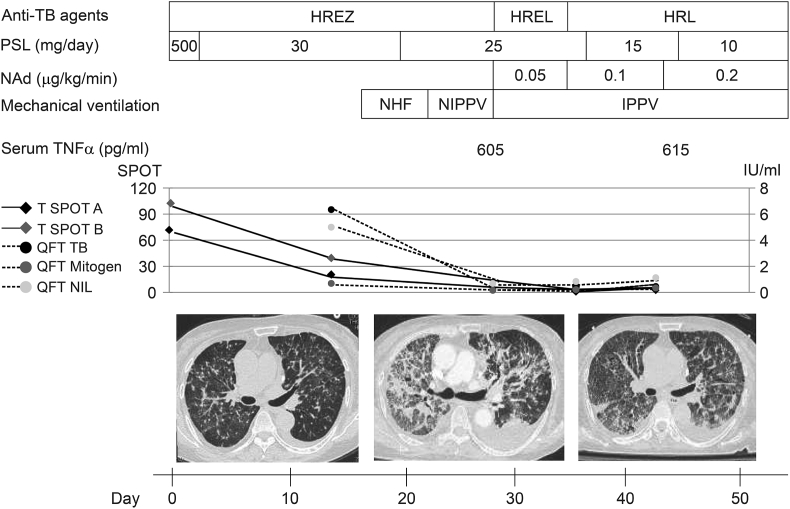
Fig. 1.
Clinical course of the patient. Top: Treatments and interventions. H, isoniazid; R, rifampicin; E, ethambutol; Z, pyrazinamide; L, levofloxacin; PSL, prednisolone; NAd, noradrenaline; NHF, nasal high flow; NIPPV, non-invasive positive pressure ventilation; IPPV, invasive positive pressure ventilation. Middle: Serum level of TNFα and whole-blood level of IFNγ. IFNγ-release assays were performed with the T-SPOT. TB and QuantiFERON TB Gold–In–Tube kits. T-SPOT panel A, the number of ESAT-6-specific spot-forming cells; T-SPOT panel B, the number of CFP-10-specific spot-forming cells. QFT TB, amount of IFNγ produced in response to TB antigen; QFT Mitogen, amount of IFNγ produced in response to stimulator; QFT Nil, non-specific baseline IFNγ level. Bottom: High-resolution computed tomography of the thorax on days 0, 25, and 37, showing progressive worsening of disseminated TB, followed by fibrosis.
To assist in the diagnosis of TB and monitor the efficacy of anti-TB agents, successive testing of whole-blood IFNγ levels in vitro was started on day 0, using two types of IGRAs. The first was the T-SPOT. TB, which enumerates the effector T cells that respond to the TB antigens ESAT-6 and CFP-10 by capturing IFNγ around the T-cells from which it was secreted, based on an enzyme-linked immunospot method (Oxford Immunotec Ltd., Marlborough, MA, USA). The second is the QuantiFERON TB Gold-In-Tube, which measures the amount of serum IFNγ produced by T cells responding to ESAT6, CFP-10, and TB7.7 peptide antigens in an enzyme-linked immunosorbent assay (ELISA) (Cellestis Ltd., Carnegie, Australia).
Two weeks after discontinuation of adalimumab (day 0), anti-TB treatment using isoniazid (200 mg), rifampicin (450 mg), pyrazinamide (1.2 g), and ethambutol (750 mg) was initiated in the previous hospital, with high dose of methylprednisolone (500 mg) for severe disseminated TB [3]. On day 3, she was transferred to Jikei Daisan Hospital with oxygen saturation of 92% while receiving 4 L/min of oxygen. Her respiratory failure improved, and the high dose of methylprednisolone was replaced by 30 mg of prednisolone (representing an effective dose of approximately 15 mg, due to the drug interaction between the steroid and rifampicin). Three weeks after initiation of anti-TB treatment, the patient's condition deteriorated into acute respiratory distress syndrome, disseminated intravascular coagulopathy, and shock. On day 25, the complication of a PR was confirmed, as an HRCT scan revealed consolidation, extensive bilateral ground-glass opacities, and pleural effusion. Automated liquid cultures of sputum, pleural fluid, and urine on day 25 yielded a positive result only for M. tuberculosis in 2 weeks. To reconfirm the absence of resistance to anti-TB agents, susceptibility tests were performed with those isolates, but no resistance to anti-TB drugs were found. On day 26, noradrenaline was initiated at an infusion rate of 0.05–0.2 μg·kg-1·min-1. She was intubated and mechanically ventilated with 100% oxygen and at 20-cm positive end-expiratory pressure. Liver injury led to replacement of pyrazinamide with levofloxacin (250 mg). As hepatotoxicity was sustained, ethambutol was discontinued, which resulted in the improvement of liver damage [4]. Since the efficacy of adjunctive corticosteroid therapy was unclear, the dose of prednisolone was tapered to 10 mg.
Serial IGRAs were performed on days 0, 14, 28, 35, and 42. The T-SPOT panel A and B (normalized to negative control, normal range for each: ≤ 5) were 71 and 102 on day 0; 19 and 38 on day 14; 0, and 2 on day 35; and 2 and 5 on day 42, respectively. The results of T-SPOT. TB were positive on day 0 and 14, and borderline on day 35 and 42. The QFT TB (amount of IFNγ [IU/ml] produced in response to TB antigen), QFT Mitogen (amount of IFNγ produced in response to stimulator), and QFT Nil (non-specific baseline IFNγ level) were 6.25, 0.60, and 4.91, respectively, on day 14; 0.21, 0.18, and 0.69, respectively, on day 28; 0.40, 0.15, and 0.77, respectively, on day 35; and 0.44, 0.32, and 1.11, respectively, on day 42. The results of QuantiFERON TB Gold–In–Tube kits were positive on day 14, 35 and 42, and indeterminate on day 28. The results of both IGRAs were obtained using manufacturer's diagram for interpretation of the value.
Serum samples on days 28 and 45 were analyzed for TNFα using an ELISA assay (Human TNF-α Quantikine HS ELISA Kit, R&D) to assess the severity of the immunological response. The value of TNFα was 605 pg/ml on day 28, and 615 pg/ml on day 45 (normal range: 0.6–2.8 pg/ml). TNFα level was not measured on day 0 and 14 owing to insufficient amount of serum samples to be tested.
Eight weeks after the diagnosis of TB, the patient's condition progressively deteriorated, resulting in death.
3. Discussion
TNFα functions in host immunity to TB infection, but it plays a proinflammatory role that can potentially cause host-damaging effects. Cytokine analyses performed in IRIS patients with human-immunodeficiency virus (HIV) and TB co-infection have shown characteristic immunological profiles with greater increases in inflammatory biomarkers, which might lead to the development of IRIS [2].
Of a variety of cytokines related to the PR, we assessed the levels of two pivotal cytokines: IFNγ and TNFα. Though IGRAs can assess TB-specific IFNγ production, a predictive role of IGRAs for treatment outcome remains controversy [5]. However, the results of IGRAs have been also reported to reflect the mycobacterial antigen load and decline during successful anti-TB therapy [6]. In the present case, since IGRAs are more easily accessed and more rapidly show the results than testing of other inflammatory cytokines in clinical practice, we employed IGRAs to monitor TB activity. Considering a lack of resistance to anti-TB agents shown in the susceptibility test of cultures, the decreased level of IGRAs might show the efficacy of anti-TB treatment. By contrast, serum TNFα has not been fully examined in TB patients receiving anti-TNFα agents. Wallis et al. reported that a TB patient in whom a PR occurred after the cessation of adalimumab was successfully retreated with adalimumab, which has raised the question as to how TNFα itself was involved in the underlying mechanism of a PR [1]. In our case, it was shown for the first time that a marked increase in serum TNFα levels can occur concurrently with a PR in patients who had been treated with TNFα blockade. The concentration of circulating TNFα seemed to be high, as compared with that reported in HIV-infected TB patients (50–60 pg/ml) [7], in those who developed IRIS (38.2 pg/ml) [2] or in acute respiratory distress syndrome non-TB patients (71 pg/ml) [8]. The increase in serum TNFα could be explained by multiple immunological steps. Prior administration of adalimumab neutralizes the TNF pathway in TB-infected sites and causes granular breakdown and dissemination of TB antigen, increasing sensitivity to anti TB treatment [9]. Anti-TB agents killing the released TB antigen could facilitate a rapid and excessive production of TNFα by macrophages and lymphocytes. Concurrent discontinuation of adalimumab, which binds specifically to TNFα and inhibits T-cell activation [10], could lead to aberrant immune reconstruction and further increase in TNFα levels. Another reason for TNFα elevation might be the relatively low dose of prednisolone used. A phase 2 trial investigating immunoadjuvant steroid therapy for HIV-related TB patients has shown that a 50% reduction in TNFα levels was achieved by daily administration of 2.75 mg/kg prednisolone [7], which is nearly 10 times higher than that used in our case.
Prevention of a PR in patients in whom anti-TNFα agents have been discontinued remains a controversial issue. Guidelines have suggested that resumption of those agents along with effective anti-TB treatment has the potential of averting a PR [11]. Kim et al. showed that resuming TNFα inhibitors was safe in patients who received anti-TB treatment [12]; however, the optimal time for restarting anti-TNFα treatment has not yet been determined. Our case demonstrated that a flare of circulating TNFα could occur shortly after initiation of anti-TB therapy and discontinuation of TNFα-blocking agents. Serial monitoring of circulating cytokine levels may play a key role in detecting the development of a PR at an earlier stage, which would indicate the urgent need for patients to be retreated with TNFα inhibitors.
Optimal treatment for a PR has not been established. Our findings that elevation of serum TNFα levels might coincide with the development of a PR could support the therapeutic use of TNFα inhibitors against a PR. The efficacy of sufficient adjunctive steroid treatment for PR followed by discontinuation of TNFα blockade should be further investigated.
In summary, we here described a TB patient who developed PR with a marked increase in serum TNFα levels soon after starting anti-TB treatment and discontinuing adalimumab. Our case highlights that successive monitoring of inflammatory cytokines, including TNFα, with appropriate anti-TB treatment could help predict the occurrence of a PR and may provide immunological support for the therapeutic use of TNFα inhibitors against a PR. Further investigations and detailed analyses of cytokine dynamics are warranted to elucidate the underlying mechanism of PR.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written informed consent was obtained from the patient's family for publication of this Case Report and any accompanying images.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SW, HK, and TM were responsible for treatment of the patient. YB, IF, RK, TH, AK, and AS assisted in the patient's care. YK and KS supervised the study. KK helped to draft the manuscript. All authors have read the manuscript and approved the final version.
Acknowledgements
None.
References
- 1.Wallis R.A., van Vuuren C., Potgieter S. Adalimumab treatment of life-threatening tuberculosis. Clin. Infect. Dis. 2009;48:1429–1432. doi: 10.1086/598504. [DOI] [PubMed] [Google Scholar]
- 2.Ravimohan S., Tamuhla N., Steenhoff A.P., Letlhogile R., Nfanyana K., Bellamy S.L., MacGregor R.R., Gross R., Weissman D., Bisson G.P. Immunological profiling of tuberculosis-associated immune reconstitution inflammatory syndrome and non-immune reconstitution inflammatory syndrome death in HIV-infected adults with pulmonary tuberculosis starting antiretroviral therapy: a prospective observational cohort study. Lancet. Infect. Dis. 2015;15:429–438. doi: 10.1016/S1473-3099(15)70008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma S.K., Mohan A., Sharma A., Mitra D.K. Military tuberculosis: new insights into an old disease. Lancet Infect. Dis. 2005;5:415–430. doi: 10.1016/S1473-3099(05)70163-8. [DOI] [PubMed] [Google Scholar]
- 4.Joint Tuberculosis Committee of the British Thoracic Society Chemotherapy and management of tuberculosis in the United Kingdom: recommendations 1998. Thorax. 1998;53:536–548. [PMC free article] [PubMed] [Google Scholar]
- 5.Chee C.B.E., KhinMar K.W., Gan S.H., Barkham T.M., Koh C.K., Shen L., Wang Y.T. Tuberculosis treatment effect on T-cell interferon-γ responses to Mycobacterium tuberculosis-specific antigens. Eur. Respir. J. 2010;36:355–361. doi: 10.1183/09031936.00151309. [DOI] [PubMed] [Google Scholar]
- 6.Katiyar S.K., Sampath A., Bihari S., Mamtani M., Kulkarni H. Use of the QuantiFERON-TB Gold In-Tube test to monitor treatment efficacy in active pulmonary tuberculosis. Int. J. Tuberc. Lung Dis. 2008;12:1146–1152. [PubMed] [Google Scholar]
- 7.Mayanja-Kizza H., Jones-Lopez E., Okwera A., Wallis R.S., Ellner J.J., Mugerwa R.D. Immunoadjuvant prednisolone therapy for HIV-associated tuberculosis: a phase 2 clinical trial in Uganda. J. Infect. Dis. 2005;191:856–865. doi: 10.1086/427995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roten R., Markert M., Feihl F., Schaller M.D., Tagan M.C., Perret C. Plasma levels of tumor necrosis factor in the adult respiratory distress syndrome. Am. Rev. Respir. Dis. 1991;143:590–592. doi: 10.1164/ajrccm/143.3.590. [DOI] [PubMed] [Google Scholar]
- 9.Solovic I., Sester M., Gomez-Reino J.J., Rieder H.L., Ehlers S., Milburn H.J., Kampmann B., Hellmich B., Groves R., Schreiber S., Wallis R.S., Sotgiu G., Schölvinck E.H., Goletti D., Zellweger J.P., Diel R., Carmona L., Bartalesi F., Ravn P., Bossink A., Duarte R., Erkens C., Clark J., Migliori G.B., Lange C. The risk of tuberculosis related to tumor necrosis factor antagonist therapies: a TBNET consensus statement. Eur. Respir. J. 2010;36:1185–1206. doi: 10.1183/09031936.00028510. [DOI] [PubMed] [Google Scholar]
- 10.Wallis R.S. Tumour necrosis factor antagonists: structure, function, and tuberculosis risks. Lancet Infect. Dis. 2008;8:601–611. doi: 10.1016/S1473-3099(08)70227-5. [DOI] [PubMed] [Google Scholar]
- 11.British Thoracic Society Standards of Care C BTS recommendations for assessing risk and for managing Mycobacterium tuberculosis infection and disease in patients due to start anti-TNF-alpha treatment. Thorax. 2005;60:800–805. doi: 10.1136/thx.2005.046797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim Y.J., Kim Y.G., Shim T.S., Koo B.S., Hong S., Lee C.K., Yoo B. Safety of resuming tumour necrosis factor inhibitors in patients who developed tuberculosis as a complication of previous TNF inhibitors. Rheumatology. 2014;53:1477–1481. doi: 10.1093/rheumatology/keu041. [DOI] [PubMed] [Google Scholar]