Abstract
Different degrees of threat predictability are thought to induce either phasic fear or sustained anxiety. Maladaptive, sustained anxious apprehension is thought to result in overgeneralization of anxiety and thereby to contribute to the development of anxiety disorders. Therefore, differences in threat predictability have been associated with pathological states of anxiety with specific phobia (SP) representing phasic fear as heightened response to predictable threat, while panic disorder (PD) is characterized by sustained anxiety (unpredictable threat) and, as a consequence, overgeneralization of fear. The present study aimed to delineate commonalities and differences in the neural substrates of the impact of threat predictability on affective processing in these two anxiety disorders.
Twenty PD patients, 20 SP patients and 20 non-anxious control subjects were investigated with an adapted NPU-design (no, predictable, unpredictable threat) using whole-head magnetoencephalography (MEG).
Group independent neural activity in the right dlPFC increased with decreasing threat predictability. PD patients showed a sustained hyperactivation of the vmPFC under threat and safety conditions. The magnitude of hyperactivation was inversely correlated with PDs subjective arousal and anxiety sensitivity. Both PD and SP patients revealed decreased parietal processing of affective stimuli. Findings indicate overgeneralization between threat and safety conditions and increased need for emotion regulation via the vmPFC in PD, but not SP patients. Both anxiety disorders showed decreased activation in parietal networks possibly indicating attentional avoidance of affective stimuli. Present results complement findings from fear conditioning studies and underline overgeneralization of fear, particularly in PD.
Keywords: Anxiety, Panic disorder, MEG, Ventromedial prefrontal cortex, Dorsolateral prefrontal cortex
Highlights
-
•
Threat predictability influences prefrontal cortical face processing.
-
•
Unpredictability of threat leads to increased emotion regulation via the right dlPFC.
-
•
Panic patients showed hyperactivation of the vmPFC under threat and safety conditions.
-
•
vmPFC overgeneralization correlated with panic patients' anxiety sensitivity.
-
•
Both, panic and phobic patients revealed decreased parietal affective processing.
1. Introduction
The predictability of threat is thought to modulate the discrimination between potential threat, imminent threat and safety conditions (Grillon et al., 2006). According to the concept of phasic fear and sustained anxiety, phasic fear is the response to an explicit, predictable threat, while sustained anxiety is defined as anxious apprehension anticipating an unpredictable, distant threat (Grillon et al., 2004). The NPU (no threat, predictable, and unpredictable threat) threat test has been used as common experimental method to investigate the impact of threat predictability (Grillon et al., 2004, Schmitz and Grillon, 2012). On a clinical level, phasic fear and sustained anxiety have been linked to different anxiety phenotypes, e.g. specific phobia (SP) as a model disorder of phasic fear and panic disorder (PD) as a model for sustained anxiety (McNaughton and Corr, 2004). The specific neural signature of threat predictability in these anxiety disorders possibly representing two extrema on the fear and anxiety continuum are, however, poorly understood.
There is growing evidence that phasic fear and sustained anxiety evoke activity in overlapping but also different neurofunctional systems (Alvarez et al., 2011, Davis et al., 1997, McNaughton and Corr, 2004). While phasic fear has been linked to activity of the central amygdala, sustained anxiety predominately activated the bed nucleus of the stria terminalis (BNST), anterior cingulate cortex (ACC), and insula (Herrmann et al., 2016, Muensterkoetter et al., 2015). Using a novel paradigm based on the NPU design (Klahn et al., 2016, Klinkenberg et al., 2016) in an MEG study focusing on cortical activation patterns, we recently showed that the dorsolateral prefrontal cortex (dlPFC) modulated threat predictability while parietal cortex activation dissociated between threat and safety conditions. Individuals with specific phobia were characterized by reduced overall parietal processing compared to non-anxious controls (Klahn et al., 2016).
Anxiety disorders have been linked to heightened threat sensitivity and dysfunctional prefrontal emotion regulation mechanisms resulting in exaggerated fearful defensive responses and prolonged anxiety (Grillon, 2008, Shankman et al., 2013). As one example for dysfunctional emotion regulation (Bouton et al., 2001, Lissek et al., 2010), PD patients showed deficient safety signal processing during fear conditioning resulting in overgeneralization of fear and, as a putative consequence, sustained anxiety (Lissek et al., 2009, Lissek et al., 2010). The inability to differentiate between threatening and safe environments and to inhibit aversive responding under safety conditions which in turn results in a failure to relax under safety conditions is considered as a core dysfunction in PD (Gorka et al., 2014, Lieberman et al., 2015, Lissek, 2012). Regarding the regulation of defensive and negative affective responding, neural circuitry models propose the ventromedial (vm) PFC to down-regulate negative affect and fearful arousal by inhibiting the amygdala and other brain regions involved in the processing of negative emotions (Myers-Schulz and Koenigs, 2012, Schiller and Delgado, 2010). In fact, pathological anxiety is thought to partly result from such deficient vmPFC emotion regulation ability (Ball et al., 2013, Banks et al., 2007, Motzkin et al., 2016). In PD patients, increased activity in an anterior cingulate cortex (ACC)-medial prefrontal-limbic network during safety signal processing has been associated with enhanced defensive responding under safety conditions (Tuescher et al., 2011). Furthermore, activity in this network predicted the response to exposure-based cognitive behavioral therapy, potentially by enhanced emotion regulation capacities via fear extinction (Lueken et al., 2013). While passively viewing emotional faces, reduced vmPFC activity along with greater amygdala responsiveness was reported in PD as well as in SP compared to controls (Killgore et al., 2014). In SP, anticipating phobia-relevant stimuli led to greater vmPFC activity under controllable compared to uncontrollable conditions (Kerr et al., 2012). Although the role of this vmPFC-limbic circuit has been investigated in other forms of emotion regulation, only little is known in relation to threat predictability in anxiety disorders.
The aim of this study was to compare the neural signature of threat predictability and overgeneralization between two anxiety phenotypes as model disorders for phasic fear (SP) and sustained anxiety (PD). As PD has been associated with prefrontal emotion regulation deficits and based on previous evidence on safety signal processing, we assumed PD patients to show altered vmPFC activity during both threat and safety conditions, but predominately during unpredictable threat. We expected them to fail at discriminating these conditions due to the phenomenon of overgeneralization. Considering SP as phasic fear related disorder, altered vmPFC activity in SP should particularly occur under conditions of predictable threat. On a subjective level, we expected PD patients to report higher subjective distress and arousal than SP and non-anxious controls. Symptom severity and subjective arousal as reaction to a threat should be related to mid-latent to late neural activity in emotion-regulating circuits e.g. the vmPFC. Based on previous findings in SP (Klahn et al., 2016), we additionally hypothesized to find a disorder unspecific effect of decreased mid-latency parietal processing.
2. Methods and materials
2.1.1. Participants
We included 22 patients diagnosed with PD (of which 2 dropped out due to anxiety before scanning), 20 patients diagnosed with SP (both according to DSM-IV-TR-criteria), and 20 non-anxious controls (for detailed characteristics of the sample, see Table 1). Parts of this sample have been published addressing a different research question (Klahn et al., 2016); the sample was enriched by the PD group for the present analysis. All participants were right-handed and fulfilled general MEG-related requirements. Exclusion criteria were any current or lifetime psychosis, bipolar disorder, severe Major Depression, Posttraumatic Stress Disorder (PTSD), Obsessive Compulsive Disorder (OCD), any severe somatic or neurological illness, or any complex psycho-pharmacological treatment. A stable treatment with SSRIs as well as psychotherapeutic treatment within the past 2 years was only tolerated if current symptoms were still clinically significant. All participants were diagnosed using the structured interview (SCID-I) for DSM-IV-TR (American Psychiatric Association, 2000, Wittchen et al., 1997) and completed the Beck Depression Inventory (BDI (Beck et al., 1996)), Beck Anxiety Inventory (BAI (Beck and Steer, 1993)), Anxiety Sensitivity Index (ASI (Taylor et al., 2007)), trait version of the State-Trait Anxiety Inventory (STAI-T (Spielberger, 1983)), Anxiety Cognitions Questionnaire (ACQ (Chambless et al., 1984)), Panic and Agoraphobia Scale (PAS (Bandelow, 1995)), Spider Phobia Questionnaire (SPQ (Watts and Sharrock, 1984)), and Fear of Spider Questionnaire (FSQ (Szymanski and O'Donohue, 1995)).
Table 1.
Mean differences for patients with panic disorder (PD), spider phobia (SP) and healthy controls (HC) concerning age, depression (BDI), anxiety levels (BAI), anxiety sensitivity (ASI), trait anxiety (STAI-T), spider phobia (SPQ/FSQ), and panic and agoraphobic symptoms (ACQ, PAS).
| Panic disorder (PD) M ± SD |
Specific phobia (SP) M ± SD |
Healthy controls (HC) M ± SD |
||
|---|---|---|---|---|
| Sex (m/f) | 5/15 | 3/17 | 3/17 | χ2 = 4.45, p = 0.108 |
| Age | 32.05 ± 11.26 | 25.50 ± 5.26 | 25.20 ± 4.84 | F(1,2) = 5.07, p = 0.009 |
| Medication | ||||
| SSRI | 3 | 1 | 0 | χ2 = 3.69, p = 0.158 |
| SNRI | 2 | 0 | 0 | χ2 = 4.07, p = 0.131 |
| BDI | 15.75 ± 8.06 | 3.84 ± 3.11 | 2.55 ± 3.16 |
F(1,2) = 32.56, p < 0.001 (PD > SP, HC) |
| BAI | 23.10 ± 11.03 | 7.35 ± 4.44 | 3.05 ± 4.02 |
F(1,2) = 40.14, p < 0.001 (PD > SP, HC) |
| ASI | 32.65 ± 11.24 | 15.15 ± 9.32 | 10.50 ± 7.44 |
F(1,2) = 29.16, p < 0.001 (PD > SP, HC) |
| STAI-T | 54.15 ± 9.40 | 38.20 ± 9.89 | 33.44 ± 9.36 |
F(1,2) = 24.91, p < 0.001 (PD > SP, HC) |
| ACQ | 28.72 ± 6.80 | 19.75 ± 9.23 | 18.61 ± 3.44 |
F(1,2) = 12.19, p < 0.001 (PD > SP, HC) |
| PAS | 23.20 ± 13.26 | 1.25 ± 2.86 | 0.50 ± 2.12 |
F(1,2) = 50.18, p < 0.001 (PD > SP, HC) |
| SPQ | 11.40 ± 7.43 | 22.10 ± 6.22 | 9.77 ± 4.53 |
F(1,2) = 22.53, p < 0.001 (SP > PD, HC) |
| FSQ | 13.05 ± 20.02 | 62.55 ± 18.65 | 9.50 ± 16.22 |
F(1,2) = 50.64, p < 0.001 (SP > PD, HC) |
M = Mean; SD = standard deviation. BDI, Beck Depression Inventory; BAI, Beck Anxiety Inventory; ASI, Anxiety Sensitivity Index; STAI-T, State-Trait Anxiety Inventory, Trait version; ACQ, Anxiety Cognitions Questionnaire; PAS, Panic and Agoraphobia Scale; SPQ, Spider Phobia Questionnaire; FSQ, Fear of Spider Questionnaire.
2.1.2. Ethics statement
All procedures were approved by the Ethics Committee of the Medical Faculty of the University of Muenster. The ethical standards of the Declaration of Helsinki were met. All participants provided written informed consent after the study procedure was fully explained and received financial compensation for their participation.
2.2. Material and procedure
The modified NPU paradigm (Klinkenberg et al., 2016) consisted of three consecutive runs presented in randomized order across subjects. In each run, a different set of 56 greyscaled male and female faces with fearful or neutral expressions (i.e. 28 faces per face expression), randomly chosen from a total compilation of 236 facial stimuli (see Klinkenberg et al., 2016 for more details regarding stimulus choice), was presented four times resulting in a total presentation of 112 facial stimuli per run and experimental condition (stimulus duration: 500 ms; jittered ITI: 825-1325 ms). In the predictable (P) and unpredictable (U) runs, a video (760 ms duration) of a rapidly appearing monster paired with an aversive scream served as threat stimulus and was presented four times per run. The threat stimulus could appear at any time in the unpredictable condition, but was cued by a warning signal in the predictable condition. No threat (N) runs were regarded as safety conditions with only facial stimuli being presented. Participants were informed about the respective threat or safety conditions before run onsets. Four additional filler faces presented between the warning signal and the aversive stimulus, as well as one filler presented after the threat stimulus were excluded from the main analysis to correct for movement artifacts. After each run, participants completed the scales agitation and mood of the multidimensional mood state questionnaire (MDSQ, German version (Steyer et al., 1997)) and were asked to rate the threat stimulus regarding perceived valence (unpleasant to pleasant) and arousal (calm to arousing) on a 9-point Likert SAM-rating scale (Bradley and Lang, 1994). Prior to and after MEG-measurement, participants completed a SAM-rating regarding valence and arousal for all fearful and neutral faces, respectively. For more details on the experimental paradigm see (Klinkenberg et al., 2016).
2.3. Apparatus and data analysis
MEG volume conductor modeling was based on head surface detection using polhemus 3Space® Fasttrack. For later spatial coregistration of anatomy and function, landmark coils (MEG) were attached to the two auditory canals and the nasion. Visually evoked magnetic fields were acquired using a 275 MEG whole-head sensor system (VSM Medtech Ltd.) with first-order axial SQUID gradiometers. Continuous recorded MEG data were down-sampled offline to 300 Hz and filtered between 0.01 Hz and 148 Hz. Data were aligned to stimulus onset, with an averaging epoch ranging from 200 ms before to 600 ms after stimulus, and baseline-adjusted using a 150 ms pre-stimulus interval. The method for statistical control of artifacts in high density EEG/MEG data was used for single trial data editing and artifact rejection (Junghöfer et al., 2000). On average, 97.5 trials per experimental condition remained after artifact handling. The number of rejected trials did not significantly differ across experimental conditions as tested by a Group (PD, SP, HC) by Threat Condition (N, P, U) by Face Expression (fearful, neutral) ANOVA. After averaging, cortical sources of the event-related fields were estimated using the L2-Minimum-Norm-Estimates method (Hämäläinen and Ilmoniemi, 1994), an inverse modeling technique for reconstructing the topography of the primary current underlying the magnetic field distribution allowing the estimation of distributed neural network activity without a-priori assumptions regarding the location and number of current sources (Hauk, 2004). A spherical shell with a radius of 87% of the individually fitted head radius was used as source model. Topographies of source direction independent neural activities were calculated for each individual participant, condition and time point. Across all participants and conditions, a Tikhonov regularization parameter k of 0.1 was used.
2.4. Statistical analysis
Exploring group differences in the clinical data, one-way analyses of variance (ANOVA) and subsequent pairwise comparisons were applied. MDSQ scores and SAM-ratings (valence, arousal) of the facial stimuli were analyzed using repeated-measures ANOVAs with the factors Group (PD, SP, HC), Threat Condition (N,P,U) and Face Expression (fearful, neutral), whereas repeated-measures ANOVAs with the factors Group (PD, SP, HC) and Threat Condition (P,U) were applied for SAM-ratings (valence, arousal) of the threat stimulus.
For the MEG data, repeated measures ANOVAs with the factors Group (PD, SP, HC), Threat Condition (N,P,U) and Face Expression (fearful, neutral) were performed for each estimated neural source and time point.
To correct for multiple comparisons and consider potential deviations from normal distribution, nonparametric cluster level statistics as suggested by Maris and Oostenveld (Maris and Oostenveld, 2007) have been applied. Analyses were performed on 1000 random permutations of the original participant data set. Cluster permutation analyses based on the first level (sensor level) statistics p < 0.05 were conducted. Effects were considered meaningful only when emerging in a spatio-temporal cluster with a minimal extent of five neighboring sources and five successive time points and achieving a cluster level criterion of p < 0.05. Based on the differentiation of early (< 100 ms), early to mid-latent (100–300 ms) and late (> 300 ms) visual affective processing stages (Schupp et al., 2006, Steinberg et al., 2013), cluster based permutation tests were performed within the respective intervals. For interval overlapping clusters, follow-up cluster permutation tests for the respective merged intervals were performed. When appropriate, significant effects were followed up by post-hoc t-tests based on the neural activity averaged within the respective spatio-temporal cluster as result of the cluster permutation analyses. MEG data analysis was conducted with the Matlab (The MathWorks Inc., MA, USA) based EMEGS software (Peyk et al., 2011). Statistical analyses were carried out using SPSS 22 (IBM, Armonk, N.Y.).
Linear correlations of estimated cortical activity with symptom severity (ASI) as well as with arousal threat ratings (within the two patient groups) were performed for each spatio-temporal cluster (mean cortical activity) with significant main effects of Group or significant interaction effects of Group and Threat Condition.
3. Results
3.1. Behavioral data: self-reported measures
Face ratings revealed main effects of Face Expression on both scales (valence: F(1,1) = 78.26, p < 0.001; arousal: F(1,1) = 126.41, p < 0.001; fearful > neutral). No group difference was found in valence and arousal ratings of the facial stimuli (main effect Group: valence: F(1,2) = 0.54, p = 0.587; arousal: F(1,2) = 1.91, p = 0.157).
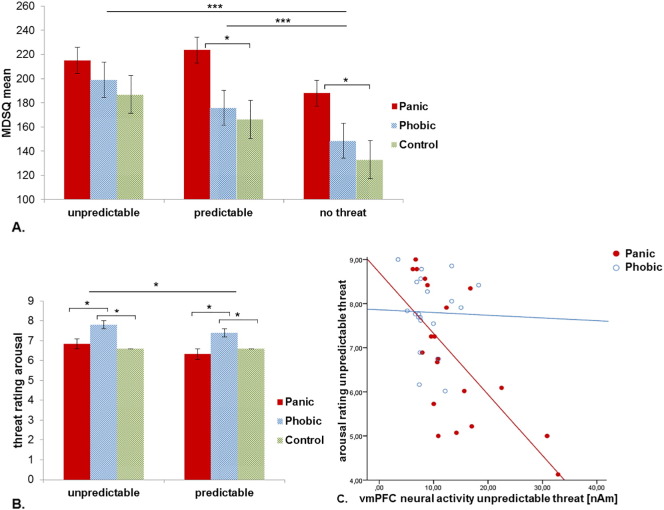
MDSQ scores revealed a main effect for Threat Condition with higher threat perception within both predictable and unpredictable threat compared to the no threat condition (F(1,2) = 19.35, p < 0.001). While no main effect of Group was observed (F(1,2) = 2.20, p = 0.120) and although the Group by Threat Condition interaction did not reach significance (F(1,4) = 1.76, p = 0.178), PD patients reported higher discomfort under predictable (t(38) = 2.47, p = 0.018) and no threat (t(38) = 2.49, p = 0.017) conditions compared to controls (see Fig. 1A).
Fig. 1.
A) MDSQ mean score of the scales mood and agitation for panic patients, phobic individuals and controls subjects. Bar plots show the participants' subjective mood state in response to the three threat conditions unpredictable, predictable and no threat. B) Subjective arousal in response to the predictable or unpredictable threat. Higher values (SAM rating scale 0–9) indicate stronger agitation (arousal). Error bars depict standard error of the mean. C) Correlation of panic and phobic patients' vmPFC neural activity within 270–470 ms under unpredictable threat conditions with the subjective arousal rating of the unpredictable threat stimulus.
Valence and arousal ratings of the threat stimuli confirmed effects of threat predictability with unpredictable threat being rated as more aversive and more arousing compared to predictable threat (main effect Threat Condition: valence: F(1,2) = 5.98, p = 0.018; arousal: F(1,2) = 4.30, p = 0.043). While groups did not differ with regard to valence ratings (F(1,2) = 1.87, p = 0.163), perceived arousal was disparate (F(1,2) = 4.08, p = 0.022): SP patients reported higher arousal compared to both PD patients and controls under both predictable and unpredictable conditions (see Fig. 1B). No Group by Threat Condition interaction was observed (valence: F(1,1) = 1.02, p = 0.367; arousal: F(1,1) = 1.14, p = 0.326).
3.2. MEG data
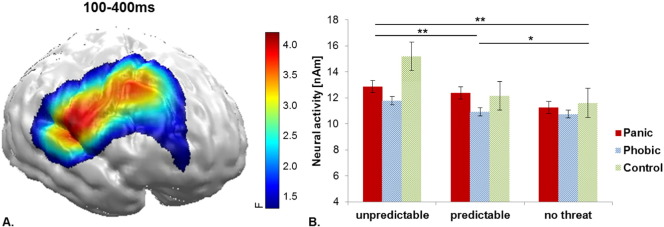
The three-way Group by Face Expression by Threat Condition repeated-measures ANOVA revealed a main effect for Threat Condition within an extended mid-latency (100-400 ms) time interval at right dlPFC areas (F(2114) = 5.86, p = 0.004; see Fig. 2) driven by an increase of neural activity with increasing threat unpredictability (U > P: t(59) = 1.91, p = 0.061; U > N: t(59) = 2.85, p = 0.006; P > N: t(59) = 2.90, p = 0.005). No significant interaction effect of Group by Threat Condition was revealed in this cluster.
Fig. 2.
A) Significant spatio-temporal cluster of neural activity for the main effect of Threat Condition between 100 and 400 ms in the right DLPFC. B) Bar plots depict the mean neural activity within this cluster for both groups and each threat condition with error bars representing standard errors of the mean.
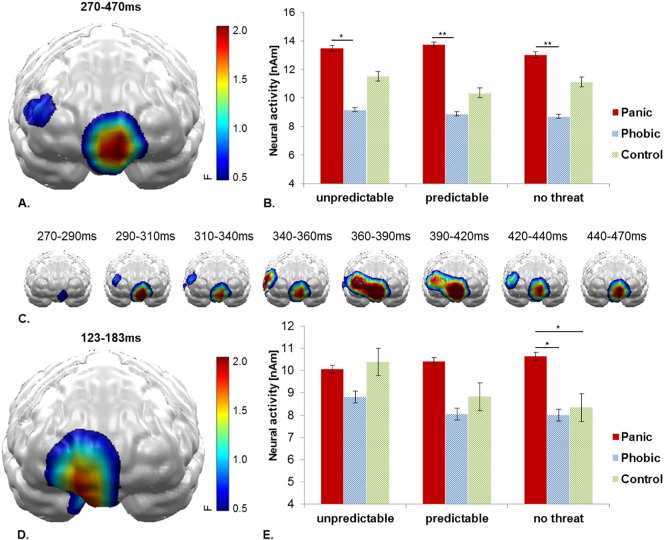
A main effect of Group was observed in a vmPFC cluster within a time interval of 270–470 ms (F(2,57) = 3.90, p = 0.026). PD patients showed overall higher activation compared to SP subjects across all threat conditions (U: t(38) = 2.92, p = 0.028; P: t(38) = 2.70, p = 0.010; N: t(38) = 2.89, p = 0.006; see Fig. 3A–C).
Fig. 3.
A) Significant spatio-temporal cluster of neural activity for the main effect Group within 270–470 ms in the vmPFC. B) Bar plots depict the mean neural activity within this cluster for both groups and each threat condition with error bars representing standard errors of the mean. C) Time course of spatio-temporal cluster with significant effect of Group. D) Significant spatio-temporal cluster of neural activity for Threat Condition × Group interaction effect during 123–183 ms. E) Bar plots depict the mean neural activity within this cluster for both groups and each threat condition with error bars representing standard errors of the mean.
In a preceding 123-183 ms time interval, a spatially overlapping vmPFC cluster revealed an interaction of Group by Threat Condition (F(4114) = 3.07, p = 0.019; see Fig. 3D–E). While SP patients and controls revealed decreasing neural activation with increasing threat predictability, PD patients maintained a heightened neural activity across all three threat conditions with significantly higher neural activation also in the no threat condition compared to SP patients (t(38) = 2.19, p = 0.034) and controls (t(38) = 2.05, p = 0.047).
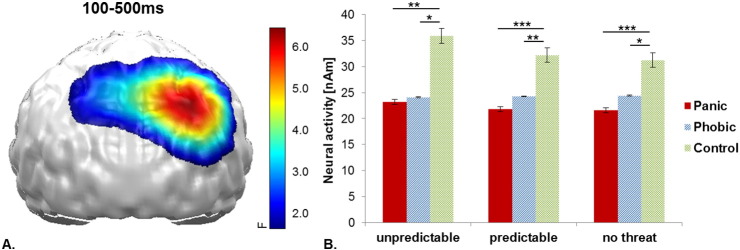
A further main effect of Group was observed between 100 and 500 ms in a right parietal cluster (F(2,57) = 9.48, p < 0.001) depicting that PD patients (U: t(38) = − 2.76, p = 0.009; P: t(38) = − 4.02, p < 0.001; N: t(38) = − 3.48, p = 0.001) and SP patients (U: t(38) = − 2.46, p = 0.019; P: t(38) = − 2.76, p = 0.009; N: t(38) = − 2.26, p = 0.030) both revealed relatively decreased parietal neural activity compared to controls (see Supplementary Fig. A1). No clusters for interaction effects with the factor Face Expression were revealed.
Supplementary Fig. A1.
A) Significant spatio-temporal cluster of neural activity for the main effect Group in the left and right inferior parietal cortex (back view). B) Bar plots depict the mean neural activity for both groups and each threat condition with error bars representing standard errors of the mean.
Visual inspection of the statistical parametric maps of the dlPFC effect of Threat Condition and the parietal Group effect indicated a lateralization toward the right hemisphere. To test for a potential lateralization effect, these spatio-temporal clusters were mirrored to the left hemisphere and a factor Hemisphere was included in the ANOVAs described above. However, for the reported effects, no interactions with Hemisphere were revealed.
3.3. Correlation analysis
Linear correlations of mean estimated cortical activity within spatio-temporal clusters with main or interaction effects of the factor Group (early and mid-latency vmPFC) with subjective arousal and symptom severity (ASI) showed that PD patients' subjective arousal toward the unpredictable threat stimulus was inversely correlated with neural activity of the vmPFC in the unpredictable condition within the later 270–470 ms interval (r = − 0.68, p = 0.001, see Fig. 1C). Furthermore, the mean neural activity in the same later vmPFC cluster in PD patients correlated inversely with their ASI score (r = − 0.61, p = 0.004). As PD patients had significantly higher depression scores, we corrected this correlation for BDI scores using partial correlations revealing, still, a significant effect (r = − 0.53, p = 0.020). In SP patients and controls, no correlations of the vmPFC activity with arousal or ASI scores were found (see Supplementary Fig. A2).
Supplementary Fig. A2.
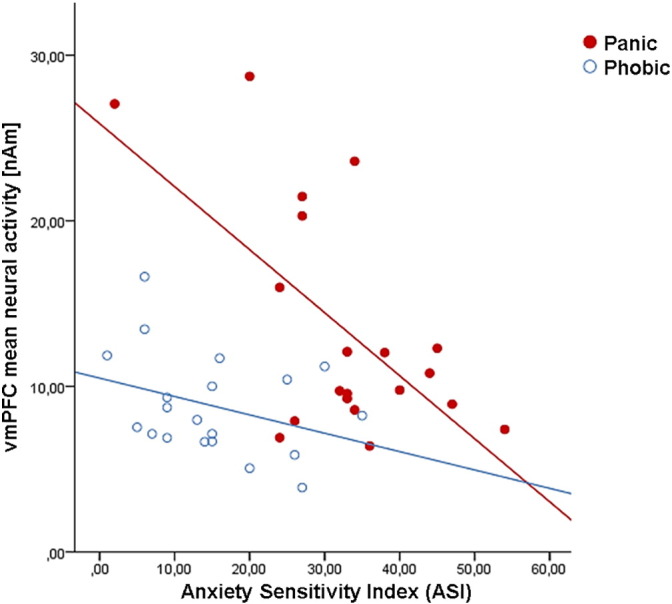
Correlation of mean vmPFC neural activity within 270–470 ms with the Anxiety Sensitivity Index for panic patients and phobic individuals.
4. Discussion
The present study aimed at exploring the neural correlates and temporal dynamics of affective processing under different conditions of threat predictability in anxiety disorders. Using a comparative study design, threat predictability was investigated in two anxiety phenotypes: specific phobia (SP) as a model disorder of phasic fear and panic disorder (PD) as a model for sustained anxiety. The following main findings were observed: a) increasing threat unpredictability led to increasing dlPFC activation across all groups which could reflect greater need for attentional monitoring in general; b) both patient groups showed decreased parietal processing of all stimuli regardless of threat predictability, and c) PD patients showed stronger recruitment of vmPFC activation both under threat and safety conditions with the magnitude of activation being inversely correlated with subjective arousal and anxiety sensitivity. Findings support the hypothesis that PD is specifically characterized by deficits in differentiating between threat and safety conditions both on a behavioral and neural level. This overgeneralization may increase the need for emotion regulation activities conferred by the vmPFC and may contribute to new perspectives for novel treatment approaches directly targeting dysfunctional emotion regulation circuits in PD.
In line with our hypotheses, we were able to replicate effects of threat predictability in the right dlPFC. Increasing threat unpredictability led to increasing dlPFC activity during visual processing. Although parts of the present sample have already been reported previously, the effect of threat predictability on the right dlPFC appears to be consistent over different samples including two clinical groups (Klahn et al., 2016, Klinkenberg et al., 2016). The more unpredictable a threat, the more frontal capacities are engaged into cognitive, e.g. attentional functions conferred by a dlPFC network aiming to support homeostasis by enhanced vigilance and selective attention toward potential threats (Peers et al., 2013, Ray and Zald, 2013). However, the absence of a consequential increasing attention driven sensory processing in occipito-temporal areas suggests a simultaneous down-regulation recruiting overlapping dlPFC regions. The rather early onset around 100 ms supports some stimulus driven automatic attentional tuning processes within this area.
Group-specific alterations in threat processing were observed in PD patients. In a later time interval (> 270 ms), PD patients revealed increased vmPFC activity compared to SP patients and controls, irrespective of the presence or absence of a threat or its predictability. In a preceding interval (120–180 ms), PD patients not only showed consistently higher vmPFC activation, but additionally failed to discriminate between threat and safety conditions. In fact, while SP patients and control subjects revealed increased stimulus processing in the most stressful (U) condition compared to safety, PD patients showed a vmPFC hyperactivation across all threat and safety conditions. Even during the absence of threat, PD patients still showed a similar level of neural activation that went along with throughout higher subjective distress levels in PD patients. We may thus conclude that PD patients lack the ability to discriminate between threat and safety conditions, thereby possibly increasing the need for emotion regulation activities that are conferred by a fronto-limbic network centered at the vmPFC (Killgore et al., 2014, Reinecke et al., 2015). The vmPFC is thought to modulate emotional anticipation as well as emotional and motivational aspects of decision making (Ray and Zald, 2013, Schiller and Delgado, 2010). It has strong direct connections to the amygdala and functionally inhibits responding to aversive stimuli, e.g. during extinction processes (Milad et al., 2007). Present findings are in line with deficient safety signal processing in PD (Lueken et al., 2013, Tuescher et al., 2011), altered ACC/vmPFC-amygdala connectivity (Lueken et al., 2016) and consequently disinhibition of the amygdala (Lissek et al., 2010, Lueken et al., 2014, Pillay et al., 2006).
While results do not favor the notion of exaggerated neural processing in SP patients under predictable threat, they show that SP also is not characterized by aberrant neural processing under sustained anxiety. We conclude that in terms of threat predictability, SP is rather comparable to neural functioning of healthy controls. Future studies are needed to explore whether SP show specific alterations solely toward phobia-relevant stimuli or if these generalize to other stimulus domains.
Present findings of increased vmPFC processes, potentially reflecting increased need for emotion regulatory, in PD patients are supported by behavioral data: the subjective arousal in response to the threat stimuli was inversely associated with the vmPFC neural activity in PD patients only. The more vmPFC activity was shown, the less threat driven arousal was reported, an effect which has previously been shown in healthy subjects (Simpson et al., 2001). PD patients reported higher discomfort under all, threat and safety, conditions. Beyond these observed effects, we may speculate whether, in PD, emotion regulation capacities conferred via the vmPFC are limited and therefore only capable to inhibit disorder related anxiety symptoms (i.e. chronically hyperreactive amygdalae), but not the overall level of subjective discomfort during the experiment. Furthermore, PD patients' vmPFC activity also correlated inversely with anxiety sensitivity. Heightened anxiety sensitivity in PD has been associated with decreased dmPFC activity as well as with increased activation of a cortico-limbic network including ACC, mPFC and insula (Ball et al., 2013, Poletti et al., 2015).
Finally, we were able to replicate previous findings of reduced parietal cortical processing of emotional information in SP (Klahn et al., 2016) for a second group of anxiety disorders. Both PD and SP patients showed reduced neural activity in bilateral but right hemispheric dominant parietal areas compared to non-anxious controls. This differential effect started around 100 ms and lasted for almost the complete time of stimulus presentation, being independent from the affective valence of the faces as well as from the threat condition. Parietal neural activity has been linked to emotional attention and attention allocation, as well as with sensory encoding of motivationally relevant information, especially as being part of the dorsal visual processing stream and strongly connected to limbic and frontal systems (Bayle and Taylor, 2010, Bradley, 2009, Morecraft et al., 1993). A general parietal hypoactivation toward affective stimuli as shown in the present anxiety samples has already been shown for major depression (Domschke et al., 2015, Kayser et al., 2000). Although PD patients were significantly more depressed than SP patients, no differential effect between the two anxiety groups was found nor was this parietal effect associated with depression sores. Therefore, we assume that this specific pattern of parietal hypoactivation might hint at a general level of dysfunctional emotional information processing and limited integration of motivationally relevant information at higher cortical processing stages in both anxiety disorders. Future studies should address the important question whether this neural substrate is associated with attentional avoidance as a clinical feature of the anxiety disorders.
4.1. Limitations
Considering the higher depression and anxiety scores in PD, we cannot exclude that PD specific effects in the vmPFC might, at least in part, reflect effects of covarying symptom severity. To control for this factor, clinical samples with equally severe anxiety and comorbid depression (such as GAD patients) could be included in a future study. We did not control for gender effects in our analysis. Anxiety disorders predominate in female participants (Frederikson et al., 1996, McLean and Anderson, 2009) leading to an unequal distribution of gender in the present sample.
While pictures of emotional scenes such as attacking animals represent imminent danger, a fearful face in another person is only cueing a potential environmental threat (e.g. Whalen et al., 2009, Wieser and Keil, 2014). Although other affective picture categories - such as mutilations - are also indirectly representing potential threat, we cannot provide inferences about a potential differential impact of threat predictability on direct threat. Here we opted for emotional expressions as these are plausibly connected to the threat stimulus, because they evoke quite reliable magnetoencephalographic correlates of affective processing even in very early time intervals (< 100 ms; e.g. Klahn et al., 2016) and because - due to its weaker electrophysiological reactions compared to direct threat stimuli (e.g. Sabatinelli et al., 2011) - might better allow detection of enhancing threat effects on affective processing (i.e. avoid ceiling effects).
With regard to the limited depth resolution of an MEG gradiometer-system and with particular interest toward cortical responses, we chose an inverse method that excluded subcortical structures from the modeling. Our analysis can thus not provide any inferences about subcortical structures - such as the amygdala or BNST - that should also be susceptible to disordered emotional regulation. A combination of high temporally and high spatially resolving neuroimaging techniques appear suited to explore cortico-subcortical interactions between the vmPFC and the amygdala/BNST under threat processing in anxiety disorders.
4.2. Conclusion
The present study showed that PD can serve as a clinical model of sustained anxiety revealing behavioral and neural indices of overgeneralization toward threat under different conditions of threat predictability. In contrast, no phobia-specific alterations were detected under phasic fear which might be partly explained by the absence of phobic stimuli in the present paradigm. Increased dlPFC activation appeared to be related to the unpredictability of threat irrespective of the presence of pathological anxiety. These findings may indicate adaptive coping strategies of an organism by tuning selective attention toward potential threat. In contrast, previously demonstrated parietal hypoactivation, which is in line with findings of reduced parietal visual attention under threat conditions in SP, could be generalized also to PD and may constitute a cross-cutting pathological feature of anxiety disorders. Finally, exaggerated vmPFC activation was exclusively observed in PD. It may reflect compensation of inefficient emotion regulation capacities in this disorder that may contribute to symptoms of sustained anxiety and hyperarousal observed on a clinical level. Based on the inability to discriminate between threat and safety and given the well-known decelerated acquisition of discriminative conditioning in PD, novel behavioral techniques such as discriminatory trainings or extended amounts of treatment sessions could counteract pathological overgeneralization of fear (Lissek et al., 2010). Present findings on the specificity of the neural substrate located within the vmPFC may contribute to novel treatment approaches that more directly target this neural circuit via neurostimulatory or -feedback techniques (Lueken et al., 2013, Nakamura-Palacios et al., 2016, Peña et al., 2014), thus supporting more tailored and thus more effective treatment approaches.
The following are the supplementary data related to this article.
Funding
This study was supported by grants of the SFB-TRR-58 subproject C01 to CP, MJ and PZ. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Acknowledgements
We are very grateful to K. Berning, H. Deitermann, U. Trompeter, J. Maitzen and C. Winker for their valuable support.
References
- Alvarez R.P., Chen G., Bodurka J., Kaplan R., Grillon C. Phasic and sustained fear in humans elicits distinct patterns of brain activity. NeuroImage. 2011;55(1):389–400. doi: 10.1016/j.neuroimage.2010.11.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association . American Psychiatric Association; Washington (DC): 2000. Diagnostic and Statistical Manual of Mental Disorders (4th ed. Text rev.) [Google Scholar]
- Ball T., Ramsawh H., Campbell-Sills L., Paulus M., Stein M. Prefrontal dysfunction during emotion regulation in generalized anxiety and panic disorder. Psychol. Med. 2013;43(7):1475–1486. doi: 10.1017/S0033291712002383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandelow B. Assessing the efficacy of treatments for panic disorder and agoraphobia. II. The panic and agoraphobia scale. Int. Clin. Psychopharmacol. 1995;10(2):73–81. doi: 10.1097/00004850-199506000-00003. [DOI] [PubMed] [Google Scholar]
- Banks S.J., Eddy K.T., Angstadt M., Nathan P.J., Phan K.L. Amygdala-frontal connectivity during emotion regulation. Soc. Cogn. Affect. Neurosci. 2007;2(4):303–312. doi: 10.1093/scan/nsm029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayle D.J., Taylor M.J. Attention inhibition of early cortical activation to fearful faces. Brain Res. 2010;1313:113–123. doi: 10.1016/j.brainres.2009.11.060. [DOI] [PubMed] [Google Scholar]
- Beck A.T., Steer R.A. Harcourt Brace and Company; San Antonio: 1993. Beck Anxiety Inventory Manual. [Google Scholar]
- Beck A.T., Steer R.A., Brown G.K. Psychological Corporation; San Antonio: 1996. Manual for the BDI-II. [Google Scholar]
- Bouton M., Mineka S., Barlow D. A modern learning theory perspective on the etiology of panic disorder. Psychol. Rev. 2001;108:4–32. doi: 10.1037/0033-295x.108.1.4. [DOI] [PubMed] [Google Scholar]
- Bradley M.M. Natural selective attention: orienting and emotion. Psychophysiology. 2009;46:1–11. doi: 10.1111/j.1469-8986.2008.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. Measuring emotion: the self-assessment manikin and the semantic differential. J. Behav. Ther. Exp. Psychiatry. 1994;25:45–59. doi: 10.1016/0005-7916(94)90063-9. [DOI] [PubMed] [Google Scholar]
- Chambless D., Caputo G., Bright P., Gallagher R. Assessment of fear of fear in agoraphobics: the body sensations questionnaire and the agoraphobic cognitions questionnaire. J. Consult. Clin. Psychol. 1984;52(6):1090–1097. doi: 10.1037//0022-006x.52.6.1090. [DOI] [PubMed] [Google Scholar]
- Davis M., Walker D.L., Lee Y. Amygdala and bed nucleus of the stria terminalis: differential roles in fear and anxiety measured with the acoustic startle reflex. Philos. Trans. R Soc. Lond. B. 1997;352:1675–1687. doi: 10.1098/rstb.1997.0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke K., Zwanzger P., Rehbein M.A., Steinberg C., Knoke K., Dobel C. Magnetoencephalographic correlates of emotional processing in major depression before and after pharmacological treatment. Int. J. Neuropsychopharmacol. 2015;19(2) doi: 10.1093/ijnp/pyv093. (pyv093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frederikson M., Annas P., Fischer H., Wik G. Gender and age differences in the prevalence of specific fears and phobias. Behav. Res. Ther. 1996;34(1):33–39. doi: 10.1016/0005-7967(95)00048-3. [DOI] [PubMed] [Google Scholar]
- Gorka S.M., Lieberman L., Nelson B.D., Sarapas C., Stewart A. Moderating role of intolerance of uncertainty. J Anxiety Disord. 2014;28(7):731–736. doi: 10.1016/j.janxdis.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C. Models and mechanisms of anxiety: evidence from startle studies. 2008;199(3):421–437. doi: 10.1007/s00213-007-1019-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C., Baas J.M.P., Lissek S., Smith K., Milstein J. Anxious responses to predictable and unpredictable aversive events. Behav. Neurosci. 2004;118(5):916–924. doi: 10.1037/0735-7044.118.5.916. [DOI] [PubMed] [Google Scholar]
- Grillon C., Bass J.M., Cornwell B., Johnson L. Context conditioning and behavioral avoidance in a virtual reality environment: effect of predictability. Biol. Psychiatry. 2006;60:752–759. doi: 10.1016/j.biopsych.2006.03.072. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M., Ilmoniemi R. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput. 1994;32:35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hauk O. Keep it simple: a case for using classical minimum norm estimation in the analysis of EEG and MEG data. NeuroImage. 2004;21:1612–1621. doi: 10.1016/j.neuroimage.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Herrmann M., Boehme S., Becker M.P.I., Tupak S.V., Guhn A., Schmidt B., Brinkmann L. Phasic and sustained brain responses in the amygdala and the bed nucleus of the stria terminalis during threat anticipation. Hum. Brain Mapp. 2016;37(3):1091–1102. doi: 10.1002/hbm.23088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junghöfer M., Elbert T.R., Tucker D., Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37:523–532. [PubMed] [Google Scholar]
- Kayser J., Bruder G.E., Tenke C.E., Stewart J.W., Quitkin F.M. Event-related potentials (ERPs) to hemifield presentations of emotional stimuli: differences between depressed patients and healthy adults in P3 amplitude and asymmetry. Int. J. Psychophysiol. 2000;36:211–236. doi: 10.1016/s0167-8760(00)00078-7. [DOI] [PubMed] [Google Scholar]
- Kerr D.L., McLaren D.G., Mathy R.M., Nitschke J.B. Controllability modulates the anticipatory response in the human ventromedial prefrontal cortex. Front. Psychol. 2012;3:557. doi: 10.3389/fpsyg.2012.00557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Killgore W., Britton J., Schwab Z., Price L., Weiner M., Gold A.L. Cortico-limbic responses to masked affective faces across ptsd, panic disorder, and specific phobia. Depress. Anxiety. 2014;31(2):150–159. doi: 10.1002/da.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klahn A.L., Klinkenberg I.A.G., Notzon S., Arolt V., Pantev C., Zwanzger P., Junghöfer M. Prepare for scare-impact of threat predictability on affective visual processing in spider phobia. Behav. Brain Res. 2016;307:84–91. doi: 10.1016/j.bbr.2016.03.045. [DOI] [PubMed] [Google Scholar]
- Klinkenberg I., Rehbein M., Steinberg C., Klahn A., Zwanzger P., Zwitserlood P., Junghöfer M. Healthy individuals maintain adaptive stimulus evaluation under predictable and unpredictable threat. NeuroImage. 2016;136:174–185. doi: 10.1016/j.neuroimage.2016.05.041. [DOI] [PubMed] [Google Scholar]
- Lieberman L., Gorka S.M., Sarapas C., Shankman S.a. Cognitive flexibility mediates the relation between intolerance of uncertainty and safety signal responding in those with panic disorder. Cognit. Emot. 2015;9931(April 2016):1–9. doi: 10.1080/02699931.2015.1067189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depress. Anxiety. 2012;29(4):257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S., Rabin S., McDowell D., Dvir S., Bradford D., Geraci M., Pine D. Impaired discriminative fear-conditioning resulting from elevated fear-responding to learned safety cues among individuals with panic disorder. Behav. Res. Ther. 2009;47(2):111–118. doi: 10.1016/j.brat.2008.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S., Rabin S., Heller R.E., Lukenbaugh D., Geraci M., Pine D.S., Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am. J. Psychiatry. 2010;167(1):47–55. doi: 10.1176/appi.ajp.2009.09030410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lueken U., Straube B., Konrad C., Wittchen H., Ströhle A., Wittmann A. Neural substrates of treatment response to cognitive-behavioral therapy in panic disorder with agoraphobia. Am. J. Psychiatry. 2013;170:1345–1355. doi: 10.1176/appi.ajp.2013.12111484. [DOI] [PubMed] [Google Scholar]
- Lueken U., Straube B., Reinhardt I., Maslowski N.I., Wittchen H.-U., Ströhle A., Wittmann A. Altered top-down and bottom-up processing of fear conditioning in panic disorder with agoraphobia. Psychol. Med. 2014;44:381–394. doi: 10.1017/S0033291713000792. [DOI] [PubMed] [Google Scholar]
- Lueken U., Zierhut K.C., Hahn T., Straube B., Kircher T., Reif A. Neurobiological markers predicting treatment response in anxiety disorders: a systematic review and implications for clinical application. Neurosci. Biobehav. Rev. 2016;66:143–162. doi: 10.1016/j.neubiorev.2016.04.005. [DOI] [PubMed] [Google Scholar]
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164:177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- McLean C.P., Anderson E.R. Brave men and timid women? A review of the gender differences in fear and anxiety. Clin. Psychol. Rev. 2009;29(6):496–505. doi: 10.1016/j.cpr.2009.05.003. [DOI] [PubMed] [Google Scholar]
- McNaughton N., Corr P.J. A two-dimensional neuropsychology of defense: fear/anxiety and defensive distance. Neurosci. Biobehav. Rev. 2004;28(3):285–305. doi: 10.1016/j.neubiorev.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Milad M.R., Wright C.I., Orr S.P., Pitman R.K., Quirk G.J., Rauch S.L. Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol. Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- Morecraft R., Geula C., Mesulam M. Architecture of connectivity within a cingulo-fronto-parietal neurocognitive network for directed attention. Arch. Neurol. 1993;50:279–284. doi: 10.1001/archneur.1993.00540030045013. [DOI] [PubMed] [Google Scholar]
- Motzkin J.C., Philippi C.L., Wolf R.C., Baskaya M.K., Koenigs M. Ventromedial prefrontal cortex is critical for the regulation of amygdala activity in humans. Biol. Psychiatry. 2016;77(3):276–284. doi: 10.1016/j.biopsych.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muensterkoetter A.L., Notzon S., Redlich R., Grotegerd D., Dohm K., Arolt V. Spider or no spider? Neural correlates of sustained and phasic fear in spider phobia. Depress. Anxiety. 2015;32(9):656–663. doi: 10.1002/da.22382. [DOI] [PubMed] [Google Scholar]
- Myers-Schulz B., Koenigs M. Functional anatomy of ventromedial prefrontal cortex: implications for mood and anxiety disorders. Mol. Psychiatry. 2012;17(2):132–141. doi: 10.1038/mp.2011.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Palacios E.M., Lopes I.B.C., Souza R.A., Klauss J., Batista E.K., Conti C.L. Ventral medial prefrontal cortex (vmPFC) as a target of the dorsolateral prefrontal modulation by transcranial direct current stimulation (tDCS) in drug addiction. J. Neural Transm. 2016 doi: 10.1007/s00702-016-1559-9. (epub ahead) [DOI] [PubMed] [Google Scholar]
- Peers P.V., Simons J.S., Lawrence A.D. Prefrontal control of attention to threat. Front. Hum. Neurosci. 2013;7:24. doi: 10.3389/fnhum.2013.00024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña D.F., Childs J.E., Willett S., Vital A., McIntyre C.K., Kroener S. Vagus nerve stimulation enhances extinction of conditioned fear and modulates plasticity in the pathway from the ventromedial prefrontal cortex to the amygdala. Front. Behav. Neurosci. 2014;8(327):1–8. doi: 10.3389/fnbeh.2014.00327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyk P., De Cesarei A., Junghöfer M. ElectroMagnetoEncephalography software: overview and integration with other EEG/MEG toolboxes. Comput. Intell. Neurosci. 2011;2011:86170. doi: 10.1155/2011/861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillay S.S., Gruber S.A., Rogowska J., Simpson N., Yurgelun-Todd D.A. fMRI of fearful facial affect recognition in panic disorder: the cingulate gyrus-amygdala connection. J. Affect. Disord. 2006;94(1–3):173–181. doi: 10.1016/j.jad.2006.04.007. [DOI] [PubMed] [Google Scholar]
- Poletti S., Radaelli D., Cucchi M., Ricci L., Vai B., Smeraldi E., Benedetti F. Neural correlates of anxiety sensitivity in panic disorder: a functional magnetic resonance imaging study. Psychiatry Res. 2015;233(2):95–101. doi: 10.1016/j.pscychresns.2015.05.013. [DOI] [PubMed] [Google Scholar]
- Ray R., Zald D.H. Anatomical insights into the interaction of emotion and cognition in the prefrontal cortex. Neurosci. Biobehav. Rev. 2013;36(1):479–501. doi: 10.1016/j.neubiorev.2011.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinecke A., Filippini N., Berna C., Western D.G., Hanson B., Cooper M.J. Effective emotion regulation strategies improve fMRI and ECG markers of psychopathology in panic disorder: implications for psychological treatment action. Transl. Psychiatry. 2015;5(11) doi: 10.1038/tp.2015.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatinelli D., Fortune E.E., Li Q., Siddiqui A., Krafft C., Oliver W.T., Beck S., Jeffries J. Emotional perception: meta-analyses of face and natural scene processing. NeuroImage. 2011;54(3):2524–2533. doi: 10.1016/j.neuroimage.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Schiller D., Delgado M.R. Overlapping neural systems mediating extinction, reversal and regulation of fear. Trends Cogn. Sci. 2010;14(6):268–276. doi: 10.1016/j.tics.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz A., Grillon C. Assessing fear and anxiety in humans using the threat of predictable and unpredictable aversive events (the NPU-threat test) Nat. Protoc. 2012;7(3):527–532. doi: 10.1038/nprot.2012.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Flaisch T., Stockburger J., Junghöfer M. Emotion and attention: event-related brain potential studies. Prog. Brain Res. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Shankman S.A., Nelson B.D., Sarapas C., Robison-Andrew E.J., Campbell M.L., Altman S.E. A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J. Abnorm. Psychol. 2013;122(2):322–338. doi: 10.1037/a0030747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson J.R., Drevets W.C., Snyder A.Z., Gusnard D.A., Raichle M.E. Emotion-induced changes in human medial prefrontal cortex: II. During anticipatory anxiety. PNAS. 2001;98(2):688–693. doi: 10.1073/pnas.98.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger C. Consulting Psychologists Press; Palo Alto (CA): 1983. Manual for the State-Trait Anxiety Inventory. [Google Scholar]
- Steinberg C., Bröckelmann A.-K., Rehbein M.A., Dobel C., Junghöfer M. Rapid and highly resolving associative affective learning: convergent electro- and magnetoencephalographic evidence from vision and audition. Biol. Psychol. 2013;92(3):526–540. doi: 10.1016/j.biopsycho.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Steyer R., Schwenkmetzger P., Notz P., Eid M. Hogrefe; Göttingen: 1997. Der Mehrdimensionale Befindlichkeitsfragebogen (MDBF) [Google Scholar]
- Szymanski J., O'Donohue W. Fear of spiders questionnaire. J. Behav. Ther. Exp. Psychiatry. 1995;26(1):31–34. doi: 10.1016/0005-7916(94)00072-t. [DOI] [PubMed] [Google Scholar]
- Taylor S., Zvolensky M., Cox B. Robust dimensions of anxiety sensitivty: development and initial validation of the anxiety sensitivity index-3. Psychol. Assess. 2007;19(2):176–188. doi: 10.1037/1040-3590.19.2.176. [DOI] [PubMed] [Google Scholar]
- Tuescher O., Protopopescu X., Pan H., Cloitre M., Butler T., Goldstein M. Differential activity of rostral cingulate and brainstem in panic disorder and PTSD. J Anxiety Disord. 2011;25(2):251–257. doi: 10.1016/j.janxdis.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watts F., Sharrock R. Questionnaire dimensions of spider phobia. Behav. Res. Ther. 1984;22(5):575–580. doi: 10.1016/0005-7967(84)90061-5. [DOI] [PubMed] [Google Scholar]
- Whalen P.J., Davis F.C., Oler J.A., Kim H., Kim J.M., Neta M. Human amygdala responses to facial expressions of emotion. In: Whalen P.J., Phelps E.A., editors. The Human Amygdala. The Guildford Press; New York: 2009. [Google Scholar]
- Wieser M.J., Keil A. Fearful faces heighten the cortical representation of contextual threat NeuroImage. NeuroImage. 2014;86(1):317–325. doi: 10.1016/j.neuroimage.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen H.-U., Wunderlich U., Gruschwitz S. Hogrefe; Göttingen: 1997. SKID-I, StrukturiertesKlinisches Interview für DSM-IV [SCID-I, Structured Clinical Interview for DSM-IV] [Google Scholar]