Abstract
Aim
The aim of this study was to evaluate the effect of ethanol and hyaluronic acid (HA) on cell survival and apoptosis in cultured human skin fibroblasts. Regarding the mechanism of ethanol action on human skin fibroblasts, we investigated cell viability and apoptosis, expression of focal adhesion kinase (FAK), and the influence of HA on those processes.
Materials and methods
Studies were conducted in confluent human skin fibroblast cultures that were treated with 25 mM, 50 mM, and 100 mM ethanol or with ethanol and 500 µg/mL HA. Cell viability was examined using methyl thiazolyl tetrazolium (MTT) assay and NC-300 Nucleo-Counter. Imaging of the cells using a fluorescence microscope Pathway 855 was performed to measure FAK expression.
Results
Depending on the dosage, ethanol decreased cell viability and activated the process of apoptosis in human skin fibroblasts. HA prevented the negative influence of ethanol on cell viability and prevented apoptosis. The analysis of fluorescence imaging using BD Pathway 855 High-Content Bioimager showed the inhibition of FAK migration to the cell nucleus, depending on the increasing concentration of ethanol.
Conclusion
This study proves that downregulation of signaling pathway of FAK is involved in ethanol-induced apoptosis in human skin fibroblasts. The work also indicates a protective influence of HA on FAK activity in human skin fibroblasts exposed to ethanol.
Keywords: apoptosis, skin fibroblast, focal adhesion kinase, hyaluronic acid, ethanol
Introduction
Chronic alcohol abuse contributes to liver diseases, hypertension, immune system dysfunction, and accelerated skin aging. Alcohol intoxication leads to impairment of fibroblast proliferation and affects the amount of extracellular matrix (ECM) elements produced by fibroblasts, eg, hyaluronic acid (HA), and collagen.1,2 The studies by Neuman et al3,4 performed on A431 epidermal cell cultures and primary skin cell cultures in infants (2002) indicated an increase in cytotoxicity that was directly dependent on alcohol concentration. The cytotoxic effect of ethanol on fibroblasts is caused by the release of TNFα and TNFβ pro-inflammatory cytokines by the cells, which consequently leads to apoptosis. Cells exposed to ethanol were characterized by condensed chromatin, changes within organelles (endoplasmic reticulum and cell membrane) and, in further stages, the formation of apoptotic bodies.5 The cytotoxic activity of ethanol also causes a decrease in the amount of glutathione, which is an antioxidant protecting the cells from damage caused by oxidative stress. Ethanol causes the formation of DNA and protein adducts.6,7 The hydroxyl group in the alcohol molecule is a highly reactive oxidant and thus possesses a high oxidation–reduction potential. The raised reactive oxygen species (ROS) level results in the activation of apoptosis in cells.1,2,8 However, exposing MCF-7 cancer cells to ethanol leads to opposing processes.9 The level of apoptotic markers is decreased; however, ketone bodies are produced in larger amounts, and the transporters and enzymes involved in the metabolism of ketone bodies are expressed, thus feeding the tumor. The exposition of MCF-10A cells (with overexpres-sion of cytochrome CYP2E1) to ethanol leads to the increase in oxidative stress and the activation of the signaling pathway from epidermal growth factor receptor (EGFR).10 The main effect of these regulative changes is the impairment of the process of cell proliferation and viability.
HA is an equally relevant component of the ECM. HA acts through CD44, its principal receptor, and RHAMM (receptor for HA motility) to regulate cell proliferation and movement.11,12 Medicines that contain this substance are commonly used in treating illnesses accompanied by disorders in the metabolism of ECM components, eg, collagen. Presumably, because of its physical properties, HA binds and inactivates many water-soluble substances, including pro-inflammatory cytokines.4,13 Half of the HA in the human body is located in the epidermis and dermis, where it is synthesized by keratinocytes and fibroblasts. It is thus responsible for the skin’s moisture, tension, and resistance to mechanic stress, thereby binding water in ECM.14 The biological properties of HA strongly depend on its molecular weight. Low molecular weight HA (LMW HA) has different cellular receptor binding affinities and biological effects, which is a target for anticancer drugs.11,12,15 There is dependence between HA-stimulated tyrosine phosphorylation and the resulting cell locomotion and cytoskeletal reorganization.16 The focal adhesion kinase (FAK) is rapidly phosphorylated and dephosphorylated after HA stimulation,16 which suggests that HA stimulates locomotion via a rapid and transient protein tyrosine kinase signaling event mediated by RHAMM.
Despite the long-standing implementation of HA in the therapy of diseases accompanied by the decrease in production of ECM elements, the mechanism of the protective influence of HA on impaired cell metabolism has not yet been fully learnt.
Materials and methods
Cell culture
Dermal Fibroblast (Primary): Human, Adult, Normal ATCC® PCS-201-012™ was sourced from the American Type Culture Collection (ATCC, Manassas, VA, USA), and HA sodium salt (H5542) was sourced from Sigma-Aldrich (St Louis, MO, USA; macromolecular HA sodium salt). The cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM) with the addition of 10% fetal bovine serum (FBS; HyClone Laboratories, Logan, UT, USA), 2 mM glutamine, 50 U/mL penicillin, and 50 µg/mL streptomycin at 37°C in a 5% CO2 incubator. Experiments were performed on cells between passages 10 and 14 for all experiments. Ethical Commission of Medical University of Bialystok permitted the use of the human cell lines.
Methyl thiazolyl tetrazolium (MTT) assay
The viability of the cells was assayed by using the MTT assay. This is based on the conversion of the yellow tetrazolium bromide, MTT, (Sigma-Aldrich) to the purple formazan derivative, in viable cells, by the mitochondrial enzyme succinate dehydrogenase. After the incubation, the medium was transferred to 96-well trays for the determination of cytokine. The cells were washed with 0.2 mL of phosphate-buffered saline (PBS) and assayed for the cytotoxicity in the same tray. Toxicity was expressed as a percentage.
Cell imaging with the Pathway 855 fluorescence microscope
Contact-inhibited cells were transferred into 96-well black plates having transparent bottom – 200 µL per well. Cells were cultured with the examined substances for 24 hours and then washed twice with PBS. For fixation, cells were incubated for 10 minutes with 3.7% formaldehyde at 37°C. Formaldehyde was removed after that, and the cells were washed twice with PBS. After that, cell permeabilization was performed. Cultured cells were incubated with 0.1% Triton X-100 for 5 minutes and then (after 0.1% Triton X-100 removal) washed twice with PBS. Then, incubation with 3% FBS for 30 minutes was performed to block unspecific sites. After the removal of 3% FBS, cells were incubated for 1 hour with the primary mouse antibody against FAK, in blocking solution. Then, the cells were washed with PBS three times, and the secondary antibody against mouse IgG labeled with fluorescein isocyanate (FITC; Sigma-Aldrich; in blocking solution) was added. Cells were incubated for 1 hour in the dark, and then they were washed three times with PBS. Subsequently, PBS including Hoechst 33342 (Sigma-Aldrich) was added to label cell nuclei. Autofluorescence of the cells was visualized with the use of fluorescence microscope using confocal imaging system (BD Pathway 855 High-Content Bioimager; BD Biosciences, San Jose, CA, USA).
Cell viability test using Nucleo-Counter® NC-300™ apparatus
In this test, it is possible to detect intracellular changes of thiol groups taking place in the cells when exposed to stress. The method was used to determine the fluorescence when solution 5 (VB-48 PI-AO; ChemoMetec, Allerod, Denmark) was added to label thiol groups. Human skin fibroblasts were incubated for 24 hours with different concentrations of ethanol when HA was added, and then washed three times with PBS. Then, the cells were detached from the plate by adding 2 mL of trypsin solution. The enzyme activity was stopped by adding FBS after 5 minutes. Thereafter, cells transferred to the tubes were centrifuged for 10 minutes at 2000× g. To 190 µL of the suspension of human skin fibroblasts including 1×106 of cells, 10 µL of solution 5 was added and stirred with the pipette. After that, 10 µL samples of the stained cells of each probe were transferred immediately to 8-chamber Slide A8TM and placed in Nucleo-Counter® NC-3000™ (ChemoMetec), and fluorescence measurement was performed. Results are illustrated as a histogram showing the intensity of fluorescence.
Statistical methods
At least three assays ± standard deviation (±SD) values were considered in all appropriate experiments to calculate the mean value. The statistical analysis was performed using double-sided, unpaired Student’s t-test and analysis of variance. The IBM SPSS 22.0 Statistics Genericom (IBM Corporation, Armonk, NY, USA) program was applied. P=0.05 was acknowledged as statistically significant.
Results
Figure 1 shows that ethanol reduces the viability of human skin fibroblasts in cell culture when measured with MTT assay.
Figure 1.
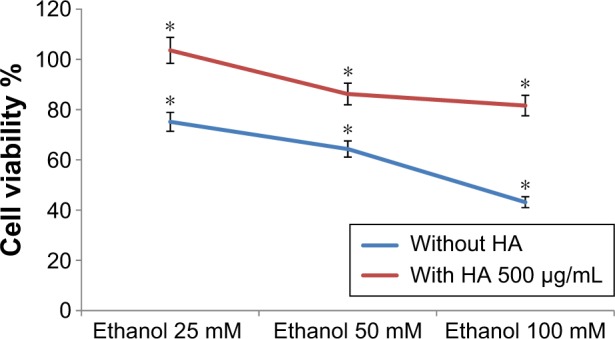
Viability of the cells in MTT assay; cultured human skin fibroblasts were exposed for 24 hours to 25 mM, 50 mM, or 100 mM ethanol or to 25 mM, 50 mM, or 100 mM ethanol +500 µg/mL HA. *P<0.05.
Abbreviations: HA, hyaluronic acid; MTT, methyl thiazolyl tetrazolium.
The viability of cells decreased according to increasing concentrations of ethanol. The viability of the cells was 75.15%, 64.32%, and 43.15% when incubated with 25 mM, 50 mM, and 100 mM of ethanol, respectively. Results also indicated the presence of 500 µg of HA in the well constitutes cell viability. Cell viability was 103.62%, 86.22%, and 81.60% when incubated with 25 mM of ethanol +500 µg/mL HA, 50 mM of ethanol +500 µg/mL HA, and 100 mM of ethanol +500 µg/mL HA, respectively.
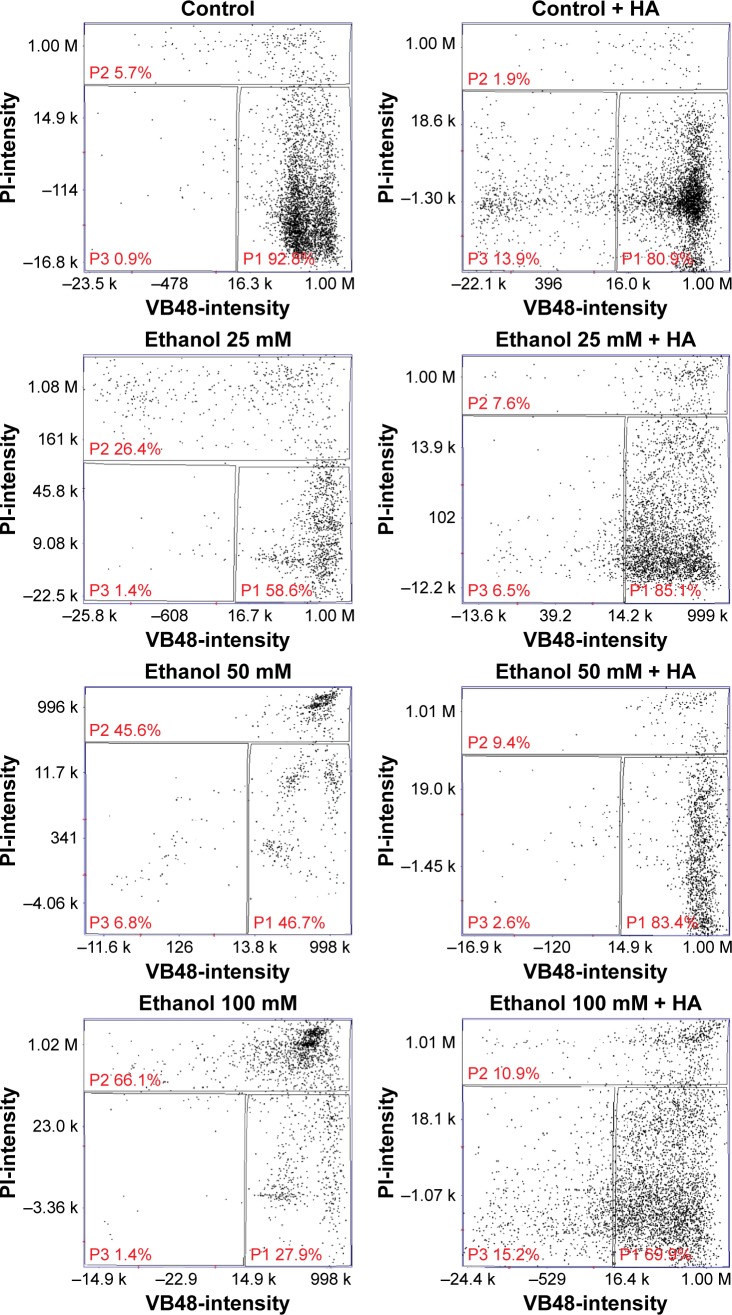
The analysis of fluorescence imaging with BD Pathway 855 High-Content Bioimager showed the downregulation of FAK migration to the cell nucleus, depending on the increasing concentration of ethanol (Figures 2 and 3). Cell viability test using Nucleo-Counter® NC-300 apparatus showed that in control cultures, apoptotic cells constituted 5.7% of the population, whereas in cultures incubated for 24 hours with 25 mM, 50 mM, and 100 mM ethanol, the amount of apoptotic cells was at the levels of 26.4% (1.12% ± SD; n=3), 45.6% (2.06% ± SD; n=3), and 66.1% (1.58% ± SD; n=3), respectively, with proportional decrease in live cell percentage (Figure 4). The adjunctive implementation of HA and the tested substances in incubated cultures revealed protective influence on the impairments caused by them, with the percentage of apoptotic cells at levels 7.6% (0.34% ± SD; n=3), 9.4% (0.5% ± SD; n=3), and 10.9% (0.65%; ± SD; n=3) compared to fibroblasts incubated with 25 mM, 50 mM, and 100 mM ethanol. The presented results indicate the protective influence of HA on the decrease in cell survivability caused by different concentrations of ethanol.
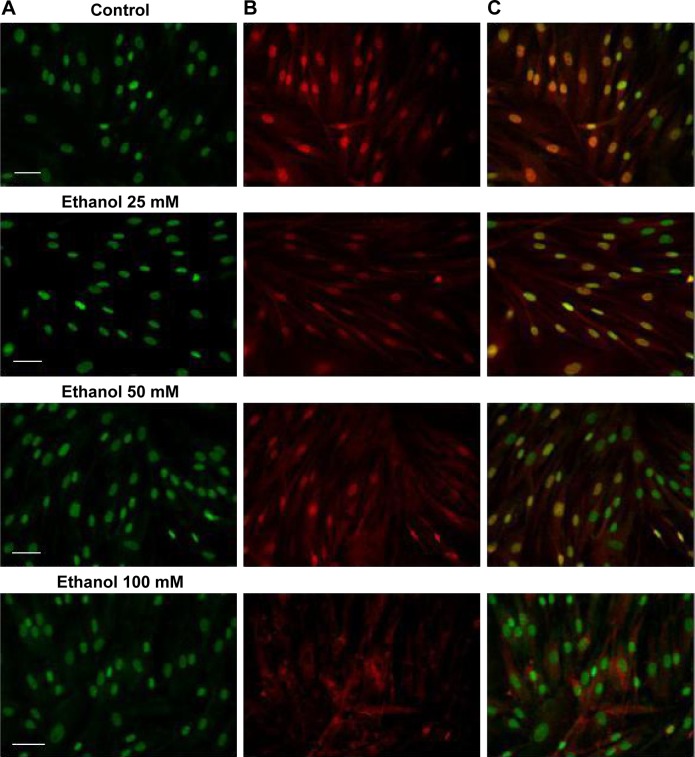
Figure 2.
Fluorescence labeling of FAK in human skin fibroblasts exposed for 24 hours to 25 mM, 50 mM, or 100 mM ethanol.
Notes: Pictures of fluorescing cells from microscope Nicon Eclipse Ti/C Plus. (A) Cellular nuclei stained with Hoechst dye (green). (B) Immunodetection of FAK using antibodies to FAK labeled with FITC (red). (C) Overlapped pictures with labeled cellular nuclei and with labeled FAK. Scale bar =20 µm.
Abbreviations: FAK, focal adhesion kinase; FITC, fluorescein isocyanate.
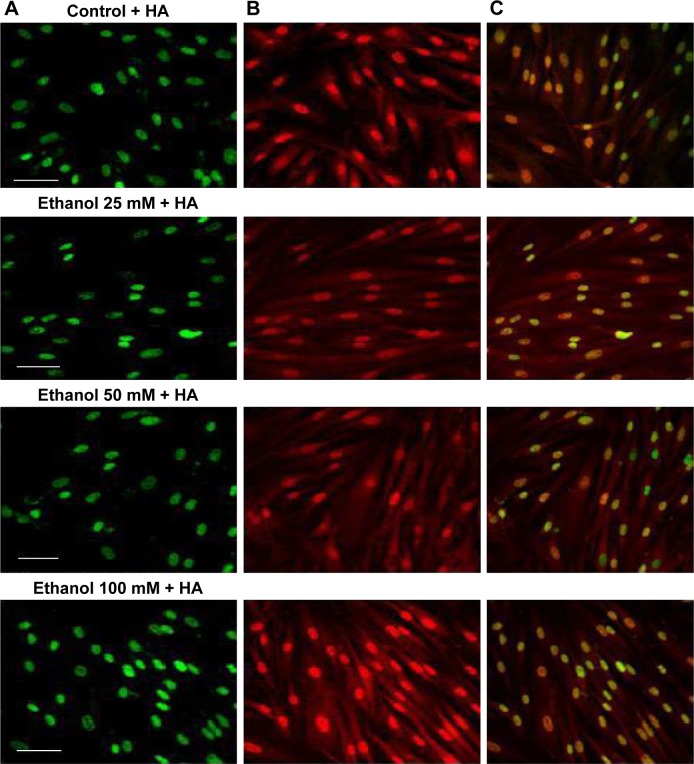
Figure 3.
Fluorescence labeling of FAK in human skin fibroblasts exposed to 25 mM, 50 mM, or 100 mM ethanol +500 µg/mL HA.
Notes: Pictures of fluorescing cells from microscope Nicon Eclipse Ti/C Plus. (A) Cellular nuclei stained with Hoechst dye (green). (B) Immunodetection of FAK using antibodies to FAK labeled with FITC (red). (C) Overlapped pictures with labeled cellular nuclei and with labeled FAK. Scale bar =20 µm.
Abbreviations: FAK, focal adhesion kinase; FITC, fluorescein isocyanate; HA, hyaluronic acid.
Figure 4.
Percent of apoptotic cells in contact-inhibited human skin fibroblast cultures exposed for 24 hours to 25 mM, 50 mM, or 100 mM ethanol or to 25 mM, 50 mM, or 100 mM ethanol +500 µg/mL HA.
Note: Measurement was performed in Nucleo-Counter® NC-300™ apparatus.
Abbreviation: HA, hyaluronic acid.
Discussion
The present study proves that ethanol may have degrading influence on cellular metabolism in the fibroblasts of human skin, leading to a significant decrease in metabolism and pushing the cells toward apoptosis. Ethanol reduces the viability of human skin fibroblasts and induces apoptosis,17 whereas the implementation of HA exerts positive influence on metabolism disturbance and cell proliferation caused by ethanol. In our previous study,17,18 we described an impairment of collagen synthesis in fibroblasts, which may involve dysregulation of cell surface receptors, signaling pathways, and prolidase activity. It seems that ethanol impairs survival as well as stimulates the apoptotic process in human skin fibroblasts. It is possible to reverse the impairing influence of ethanol when we add 500 µg/mL of HA.
We speculate that the activated cellular receptor sends a signal to the cell nucleus with a cascade of signaling proteins. One of the kinases capable of not only signaling pathway transduction but also traveling to the cell nucleus is FAK.19 FAK is one of the several components of signal transduction of type 1 insulin-like growth factor receptor (IGF-1R) as well as beta-integrin surface receptor. Phosphorylated FAK can influence further signal pathway proteins. However, under specific conditions, it can dissociate from a sub-membrane complex to be directly transferred to cell nucleus. Recently, a question of mechanisms and circumstances under which FAK dissociates from the complex and transfers into the cell nucleus is a matter of intensive investigations. Activating of FAK play a role in suppressing apoptosis.20 FAK is strictly connected with the β1 integrin receptor.19,21 The analysis of fluorescence imaging showed the downregulation of FAK migration to the cell nucleus, depending on the increasing concentration of ethanol (Figures 2 and 3). The observed change in the amount of phosphorylated FAK in the cell nucleus in trials incubated with ethanol may be the result of impairing the expression of the β1 integrin receptor and thereby the lack of signaling pathway induction. The addition of HA exerts protective influence on the impairments induced by ethanol.
The activation of the β1 integrin receptor by an extracellular ligand, eg, type I collagen, leads to the formation of an adhesive complex, the main element of which is FAK. Phosphorylated kinase may activate further signaling pathways, including the ERK1/2 MAP-kinase signaling pathway.22,23 Non-receptor FAK may also disconnect from the area of focal adhesion and move toward the cell nucleus. The performed analyses indicate that increased concentrations of ethanol cause impairment of the β1 integrin receptor and the IGF-1R proportional to the used concentrations. The result of the inhibited expression of receptors may be the impairment of FAK expression. The analysis of FAK protein expression carried out using a confocal microscope indicates decreased protein expression as well as inhibition of its movement toward the cell nucleus. As a result of the impairment of cell surface receptor, the intracellular signaling pathway is not activated.
The implementation of HA exerted protective influence on fibroblasts incubated with different concentrations of alcohol, which led to the increase in viability and thus also in the number of live cells.
Survival pathways of cells are connected with apoptosis. Apoptosis is a process during which damaged cells activate programmed death mechanisms without triggering an inflammatory reaction. The present study indicates that increasing concentrations of alcohol induce apoptosis in human skin fibroblasts. Depending on the concentration, ethanol induces an increase in the amount of apoptotic cells, which reflects the observed higher expression of caspase 9 and the NFκB transcription factor.24 This factor is considered as both pro- and anti-apoptotic.23,24 In another experimental system, exposition of fibroblasts to the toxic substances leads to inhibition of collagen synthesis and activation of apoptotic pathways, which indicates the pro-apoptotic character of NFκB.25 Caspase 9 is a factor that initiates apoptosis in the intrinsic signaling pathway, where it activates proteolysis of caspases 3, 6, and 7.26–28 Increasing concentrations of ethanol caused the activation of apoptosis in fibroblasts of the human skin, which was reflected in caspase 9 expression and increased the amount of apoptotic cells in the culture. HA used with the tested substances protected the cells from apoptosis.
Previous studies showed that HA counteracts the detrimental effect of ethanol on epidermal cells and skin fibroblasts.3,4 Hyaluronate regulates the excretion of cytokine and inflammatory substances to the ECM, thus preventing chemotaxis and inhibiting the inflammatory process.29–31
Conclusion
This study proves that downregulation of the signaling pathway of FAK is involved in ethanol-induced apoptosis in human skin fibroblasts. The work also indicates a protective influence of HA on FAK activity in human skin fibroblasts exposed to ethanol.
Acknowledgments
This work was supported by grants from the Medical University in Białystok.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Ranzer MJ, Chen L, DiPietro LA. Fibroblast function and wound breaking strength is impaired by acute ethanol intoxication. Alcohol Clin Exp Res. 2011;35(1):83–90. doi: 10.1111/j.1530-0277.2010.01324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seth D, D’Souza El-Guindy NB, Apte M, et al. Alcohol, signaling, and ECM turnover. Alcohol Clin Exp Res. 2010;34(1):4–18. doi: 10.1111/j.1530-0277.2009.01060.x. [DOI] [PubMed] [Google Scholar]
- 3.Neuman MG, Haber JA, Malkiewicz IM, Cameron RG, Katz GG, Shear NH. Ethanol signals for apoptosis in cultured skin cells. Alcohol. 2002;26(3):179–190. doi: 10.1016/s0741-8329(02)00198-2. [DOI] [PubMed] [Google Scholar]
- 4.Neuman MG, Nanau RM, Oruña L, Coto G. In vitro anti-inflammatory effects of hyaluronic acid in ethanol-induced damage in skin cells. J Pharm Pharm Sci. 2011;14(3):425–437. doi: 10.18433/j3qs3j. [DOI] [PubMed] [Google Scholar]
- 5.Mello T, Ceni E, Surrenti C, Galli A. Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol Aspects Med. 2008;29:17–21. doi: 10.1016/j.mam.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 6.McKillop IH, Schrum LW. Role of alcohol in liver carcinogenesis. Semin Liver Dis. 2009;29(2):222–232. doi: 10.1055/s-0029-1214377. [DOI] [PubMed] [Google Scholar]
- 7.Page A, Paoli PP, Hill SJ, et al. Alcohol directly stimulates epigenetic modifications in hepatic stellate cells. J Hepatol. 2015;62(2):388–397. doi: 10.1016/j.jhep.2014.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Alvarez R, Martinez-Outschoorn UE, Lin Z, et al. Ethanol exposure induces the cancer-associated fibroblast phenotype and lethal tumor metabolism. Implications for breast cancer prevention. Cell Cycle. 2013;12(2):289–301. doi: 10.4161/cc.23109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Przylipiak A, Rabe T, Hafner J, Przylipiak M, Runnebaum B. Influence of ethanol on in vitro growth of human mammary carcinoma cell line MCF-7. Arch Gynecol Obstet. 1996;258(3):137–140. doi: 10.1007/s004040050114. [DOI] [PubMed] [Google Scholar]
- 10.León-Buitimea A, Rodríguez-Fragoso L, Lauer FT, Bowles H, Thompson TA, Burchiel SW. Ethanol-induced oxidative stress is associated with EGF receptor phosphorylation in MCF-10A cells overexpressing CYP2E1. Toxicol Lett. 2012;209(2):161–165. doi: 10.1016/j.toxlet.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qhattal HS, Hye T, Alali A, Liu X. Hyaluronan polymer length, grafting density, and surface poly(ethylene glycol) coating influence in vivo circulation and tumor targeting of hyaluronan-grafted liposomes. ACS Nano. 2014;8(6):5423–5440. doi: 10.1021/nn405839n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oommen OP, Duehrkop C, Nilsson B, Hilborn J, Varghese OP. Multifunctional hyaluronic acid and chondroitin sulfate nanoparticles: impact of glycosaminoglycan presentation on receptor mediated cellular uptake and immune activation. ACS Appl Mater Interfaces. 2016;8(32):20614–20624. doi: 10.1021/acsami.6b06823. [DOI] [PubMed] [Google Scholar]
- 13.Sueblinvong V, Kerchberger VE, Saghafi R, Mills ST, Fan X, Guidot DM. Chronic alcohol ingestion primes the lung for bleomycin-induced fibrosis in mice. Alcohol Clin Exp Res. 2014;38(2):336–343. doi: 10.1111/acer.12232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Maquart FX, Monboisse JC. Extracellular matrix and wound healing. Pathol Biol. 2014;62:91–95. doi: 10.1016/j.patbio.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Platt VM, Szoka FC., Jr Anticancer therapeutics: targeting macromolecules and nanocarriers to hyaluronan or CD44, a hyaluronan receptor. Mol Pharm. 2008;5(4):474–486. doi: 10.1021/mp800024g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hall CL, Wang C, Lange LA, Turley EA. Hyaluronan and the hyaluronan receptor RHAMM promote focal adhesion turnover and transient tyrosine kinase activity. J Cell Biol. 1994;126(2):575–588. doi: 10.1083/jcb.126.2.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donejko M, Przylipiak A, Rysiak E, et al. Hyaluronic acid abrogates ethanol-dependent inhibition of collagen biosynthesis in cultured human fibroblasts. Drug Des Devel Ther. 2015;9:6225–6233. doi: 10.2147/DDDT.S91968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donejko M, Przylipiak A, Rysiak E, Głuszuk K, Surażyński A. Influence of caffeine and hyaluronic acid on collagen biosynthesis in human skin fibroblasts. Drug Des Devel Ther. 2014;8:1923–1928. doi: 10.2147/DDDT.S69791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lim ST, Chen XL, Lim Y, et al. Nuclear FAK promotes cell proliferation and survival through FERM-enhanced p53 degradation. Mol Cell. 2008;29(1):9–22. doi: 10.1016/j.molcel.2007.11.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kurenova E, Xu LH, Yang X, et al. Focal adhesion kinase suppresses apoptosis by binding to the death domain of receptor-interacting protein. Mol Cell Biol. 2004;24(10):4361–4371. doi: 10.1128/MCB.24.10.4361-4371.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lim ST. Nuclear FAK: a new mode of gene regulation from cellular adhesions. Mol Cells. 2013;36(1):1–6. doi: 10.1007/s10059-013-0139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersson S, D’Arcy P, Larsson O, Sehat B. Focal adhesion kinase (FAK) activates and stabilizes IGF-1 receptor. Biochem Biophys Res Commun. 2009;387(1):36–41. doi: 10.1016/j.bbrc.2009.06.088. [DOI] [PubMed] [Google Scholar]
- 23.Surazynski A, Miltyk W, Wolczynski S, Palka J. The effect of prolactin and estrogen cross-talk on prolidase- dependent signaling in MCF-7 cells. Neoplasma. 2013;60(4):355–363. doi: 10.4149/neo_2013_047. [DOI] [PubMed] [Google Scholar]
- 24.Liang M, Lv J, Chu H, et al. Vertical inhibition of PI3K/Akt/mTOR signaling demonstrates in vitro and in vivo anti-fibrotic activity. J Dermatol Sci. 2014;76(2):104–111. doi: 10.1016/j.jdermsci.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Lavrik IN. Systems biology of death receptor networks: live and let die. Cell Death Dis. 2014;5:e1259. doi: 10.1038/cddis.2014.160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JJ, Accili D. Signaling through IGF-I and insulin receptors: where is the specificity? Growth Horm IGF Res. 2002;12:84–90. doi: 10.1054/ghir.2002.0265. [DOI] [PubMed] [Google Scholar]
- 27.Chowdhury I, Tharakan B, Bhat GK. Caspases – an update. Comp Biochem Physiol B Biochem Mol Biol. 2008;151(1):10–27. doi: 10.1016/j.cbpb.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 28.Chen WS, Xu PZ, Gottlob K, et al. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 2001;15(17):2203–2208. doi: 10.1101/gad.913901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nawrat P, Surazyński A, Karna E, Pałka JA. The effect of hyaluronic acid on interleukin-1-induced deregulation of collagen metabolism in cultured human skin fibroblasts. Pharmacol Res. 2005;51(5):473–477. doi: 10.1016/j.phrs.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 30.Karna E, Surazynski A, Palka J. Collagen metabolism disturbances are accompanied by an increase in prolidase activity in lung carcinoma planoepitheliale. Int J Exp Pathol. 2000;81(5):341–347. doi: 10.1111/j.1365-2613.2000.00168.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girish KS, Kemparaju K. The magic glue hyaluronan and its eraser hyaluronidase: a biological overview. Life Sci. 2007;80(21):1921–1943. doi: 10.1016/j.lfs.2007.02.037. [DOI] [PubMed] [Google Scholar]