Abstract
Introduction
Symptomatic chronic subdural hematomas (CSDH) remain one of the most frequent diagnoses in current neurosurgical practice. Burr-hole craniostomy with irrigation and placement of close-system drainage is the current recommended surgery for symptomatic CSDH. The aim of this study is to perform a direct comparison between two surgical techniques in the treatment of symptomatic CSDH, which have been proven in previous studies to be efficient. Our main objective was to compare the efficacy of placement of a subperiosteal drain (SPD) and a subdural drain (SDD) following single burr-hole craniostomy and irrigation, and to demonstrate any significant differences in terms of overall surgical complications, functional outcome at three months and mortality rate.
Materials and Methods
The study was carried out in two local neurosurgical centres. The SPD group was performed in Hospital Umum Sarawak (HUS) and the SDD group was performed in Hospital Sultanah Aminah Johor Bahru (HSAJB), from 1 January 2012 till 30 January 2014 with a total of 30 patients in both treatment groups.
Results
Overall, there were no statistically significant difference in terms of patient general characteristics, pre-operative and post-operative symptoms, Markwalder grades, post-operative hematoma volume and recurrence, mortality and functional outcome at discharge and at three month follow-up between both groups. Albeit not achieving statistical significance, we observed a lower rate of surgical complication especially for post-operative intracranial hematoma with placement of the SPD system.
Conclusions
Our study concludes that both treatment methods proved to be highly effective in the treatment of CSDH. However, with a lower overall surgical complication rate, treatment with single burr-hole craniostomy, irrigation and placement of the SPD system can be considered a treatment of choice for the management of symptomatic CSDH.
Keywords: subperiosteal drain, subdural drain, burr-hole drainage, chronic subdural hematoma, Markwalder grade, Glasgow Outcome Scale
Introduction
Recent years have seen a steady rise in the incidence of patients presenting with symptomatic chronic subdural hematoma (CSDH) due to prolonged life expectancy, especially in developing countries (1, 6). To date, there are only a few class (II) evidence publications in the literature on the treatment of CSDH. The standard surgical method of choice for symptomatic CSDH is burr-hole craniostomy combined with irrigation and placement of a closed-drainage system (10, 12). A randomised controlled trial by Santarius et al. in 2009 concluded that placement of a subdural drain (SDD) after burr-hole evacuation of CSDH was associated with reduced recurrence and mortality.
More recent studies have reported on a considerably less invasive method involving placement of a subperiosteal drain (SPD) instead of the conventional SDD (3, 5, 14). This was investigated because placement of an SDD on the cortical brain surface can potentially lead to complications such as hematoma, seizures, and surgical-site infections such as empyema. With a clear trend toward reduced mortality and complications, SPD placement has been recommended as the treatment of choice for patients with a predictable high risk of complications, especially those over 80 years of age (3). A single-centre prospective randomised study by Kaliaperumal et al. in 2012, comparing the outcomes of SDD and SPD, showed statistically significant modified Rankin Score (mRS) measurements, with better outcomes in the SPD group at three and six months of follow-up. The present study performed a direct comparison between SDD and SPD placement for the treatment of CSDH to further analyse the recurrence rates and overall outcomes in terms of surgical complications, functional outcomes, and mortality in both groups.
Materials and Methods
In this prospective interventional study, we aimed to perform a direct comparison between patients receiving SDP versus SDD drain placement for the treatment of symptomatic CSDH. The main objective was to compare efficacy and to identify differences in overall surgical complications, functional outcomes, and mortality between the two groups. From 1 January 2012 to 1 January 2014, we recruited a total of 60 patients (30 per group) who fulfilled the inclusion and exclusion criteria, and we performed the surgical procedures based on the standardised techniques outlined below. The patients in the SDD group were recruited from Hospital Sultanah Aminah Johor Bahru and those in the SPD group were from Hospital Umum Sarawak, based on the institutions’ respective standards of practice for the treatment of CSDH.
General patient characteristics, including age, sex, associated medical conditions, medications, and other risk factors were assessed prior to surgery. Pre-operative symptoms, admission Glasgow Coma Scale (GCS) and Markwalder scores, and radiological findings (hematoma volume) of pre-operative CT scans were analysed. Estimations of hematoma size and volume were calculated based on the XYZ/2 formula (7). Post-operative symptoms, neuroradiological findings on post-operative CT at 24 hours, rates of surgical complications and repeat surgeries, Glasgow Outcome Score (GOS), Markwalder score, and mortality during hospitalisation were noted. At three months of follow-up, GOS scores, repeat CT findings, and mortality rates were analysed. A good outcome was defined as a reduction in pre-operative symptoms, a Markwalder grade of 0 or 1, low surgical morbidity and mortality, and a GOS score of 4 or higher. Recurrent hematoma was defined as a thickness of > 10 mm in a symptomatic patient. Any patient who presented within three months prior to the designated follow-up appointment with a recurrent hematoma was noted and treated accordingly.
Study inclusion and exclusion criteria
The inclusion criteria for this study were an age of ≤ 80 years, the presence of symptomatic CSDH depicted on plain CT scan, and an admission Glasgow Coma Scale (GCS) of 6 or higher (with a motor score of at least 4). The exclusion criteria were terminal illness, pregnancy, history of previous surgery for CSDH, or an admission GCS of < 6.
Peri-, intra-, and post-operative management outline
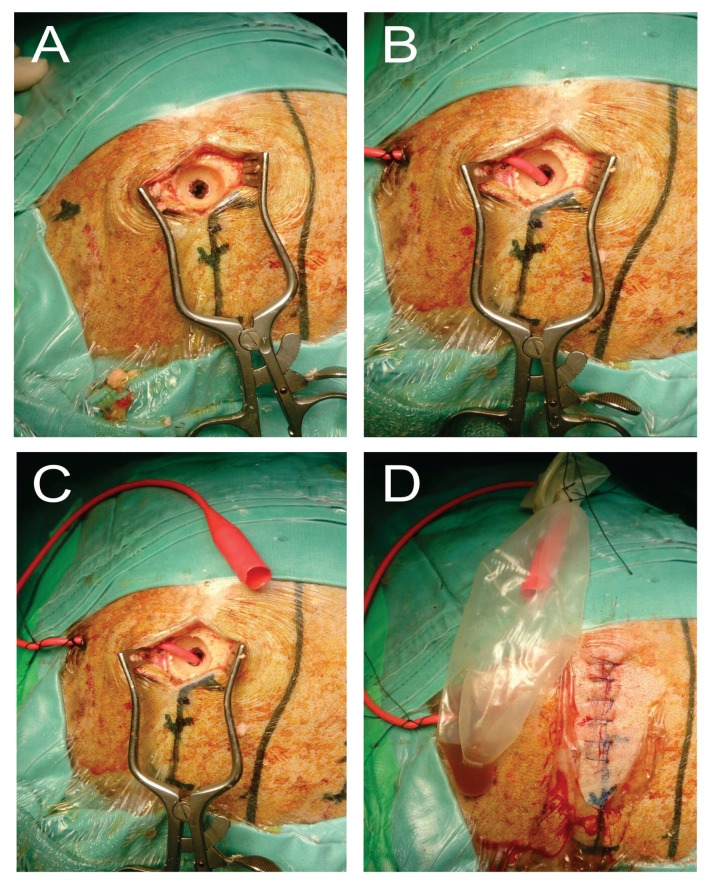
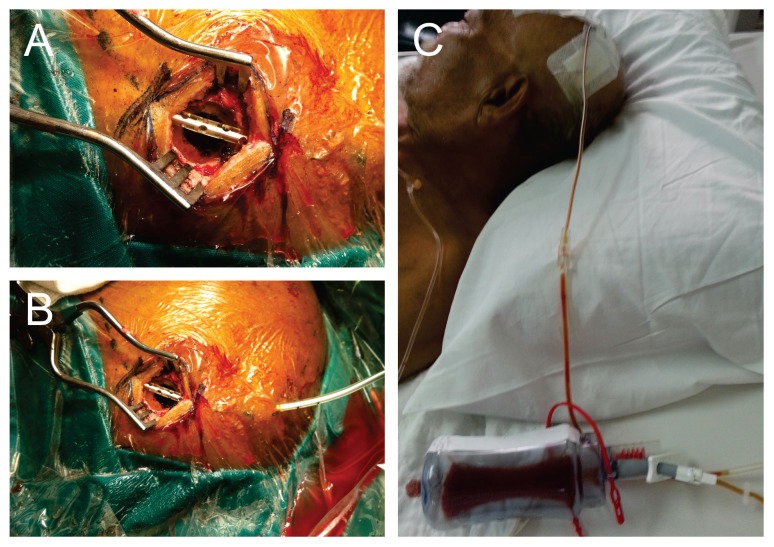
Prior to surgery, informed consent was obtained from each patient or their immediate family members or caregivers. Perioperatively, anticoagulants and antiplatelet medications were withheld, and fresh frozen plasma with intravenous (IV) vitamin K was administered to establish normal clotting parameters. Antiepileptic medications were given to patients who presented with seizures upon admission. These perioperative measures were standardised for both groups of patients at both study centres. In all cases, the patient was positioned supine and given general anesthesia, with the head stabilised on a rubber horseshoe ring. The incision area was marked and cleaned with povidone iodine and covered with sterile surgical drapes. A single injection of antibiotic prophylaxis with 1.5 g of IV cefuroxime (Zinacef; GlaxoSmithKline MY) and local anesthetic (IV marcaine + adrenaline) was administered to each patient immediately before the skin incision. A single burr-hole was made at the point of maximal clot thickness, with the craniostomy measuring at least 10 mm × 10 mm in diameter. The dura mater was coagulated and opened widely to the size of the burr-hole. Intraoperative subdural irrigation was performed with body-temperature normal saline until the effluent was fairly clear and not totally removed. Placement of the closed drainage system was done according to the standard practice at each centre. For SPD systems, a passive corrugated Redivac catheter was placed across the burr-hole beneath the galea. For SDD systems, a Jacques catheter was negotiated through the burr-hole and gently placed in the subdural space (Figures 1 and 2). Each drain was pulled through a small skin incision posterolateral to the burr-hole and connected to a passive collecting system, with no suction force applied. The subdural space was then filled with body-temperature saline before closing the skin incision to minimise intracranial air collection. The drainage system was placed below the level of the head. Drain removal was performed after a repeat brain CT within 24 hours.
Figure 1.
The burr-hole craniostomy and subdural drain (SDD) placement technique which was performed in HSAJB
A: The burr-hole craniostomy size of at least 10 mm diameter.
B: Following intra-operative irrigation with body tempered normal saline solution; the Jack’s catheter was placed inside the burr-hole within the subdural compartment.
C: The catheter was pulled through from underneath the galea postero-laterally from the skin incision.
D: The drain was connected to the collecting system which was placed under non-active suction pressure (passive drain).
Figure 2.
The burr-hole craniostomy and subperiosteal drain placement technique was performed in HUS
A: Burr-hole craniostomy followed with SPD placement of the corrugated Radivac catheter over the burr-hole of at least 10 mm diameter.
B: The drain was then pulled through from underneath the galea postero-laterally away from the skin incision.
C: The drain was connected to the collecting system which was placed under non-active suction pressure (passive drain) below the level of the head.
Statistical Analysis
The data were analysed with SPSS software for Windows version 21.0. All variables were expressed as mean ± standard deviation (X ± SD). The investigated parameters were analysed with the t-test and the chi-square test. The calculated sample size was 30 patients per group (power of 80%) to demonstrate statistical differences in overall surgical complications, functional outcomes, and mortality. Significance was assumed at a level of P < 0.05.
Results
General demographics and patient characteristics
A total of 30 subjects per group were eligible for analysis. The SPD group included 21 males (70%) and nine females (30%), while the SDD group contained 20 males (66.7%) and 10 females (33.3%). The mean age was 63 years in the SPD group and 68 years in the SDD group. As depicted in Table 1, there were no significant differences in mean age or gender between the groups (P > 0.05). In the SPD group, the CSDHs included 16 right-sided, 13 left-sided, and one bilateral. The SDD group included 17 right-sided and 13 left-sided CSDHs. Two patients (6.7%) in the SPD group and three (10%) in the SDD group were on chronic oral antiplatelet therapy. Only one subject (3.3%) in the SPD group was on anticoagulation. The most frequent associated comorbid conditions in the SPD versus SDD groups, respectively, were hypertension (63% versus 66.7%), type II diabetes mellitus (26.7% versus 26.7%), ischemic heart disease (16.7% versus 20%), chronic kidney disease (6.7% versus 6.7%), chronic liver disease (6.7% versus 6.7%), epilepsy (6.7% versus 0%), and chronic alcoholism (3.3% versus 6.7%). There were no significant differences regarding these clinical parameters between the SPD and SDD groups (P > 0.05).
Table 1.
General demographics and patient characteristics between both groups (chi-square test)
| Subperiosteal Drain | Subdural Drain | P-value | |
|---|---|---|---|
| No. of Patients | 30 | 30 | |
|
| |||
| Age (mean) | 68 | 70 | 0.072 |
|
| |||
| Sex | |||
| Male | 21 (70%) | 20 (66.7%) | 0.781 |
| Female | 9 (30%) | 10 (33.3%) | |
|
| |||
| Right SDH | 16 (53.3%) | 14 (46.7%) | 0.606 |
| Left SDH | 13 (43.3%) | 16 (53.3%) | 0.438 |
| Bilateral SDH | 1 (3.3%) | 0 | 0.313 |
|
| |||
| Patients on anti-platelet | 2 (6.7%) | 3 (10%) | 0.389 |
| Patients on anti-coagulation | 1 (3.3%) | 0 | 0.313 |
|
| |||
| Co-morbidities | |||
| HPT | 19 (63%) | 20 (66.7%) | 0.787 |
| IHD | 5 (16.7%) | 6 (20%) | 0.542 |
| DM | 8 (26.7%) | 8 (26.7%) | 0.739 |
| CKD | 2 (6.7%) | 2 (6.7%) | > 0.95 |
| CLD | 2 6.7%) | 2 (6.7%) | > 0.95 |
| Previous Stroke | 2 (6.7%) | 4 (13.3%) | 0.389 |
| Chronic Alcoholic | 1 (3.3%) | 2 (6.7%) | 0.554 |
Pre- and post-operative symptoms
All patients in both study groups were assessed for presenting symptoms and signs on admission, and subsequently reassessed and documented to ascertain post-operative outcomes upon discharge. As shown in Table 2, a majority of patients presented with GCS scores of 13–15, including 26 patients (86.7%) in the SPD group and 28 (93.3%) in the SDD group. Three patients (10%) in the SDP group and one (3.3%) in the SDD group presented with a GCS of 9–12. One patient (3.3%) from each group presented with a GCS of 8.
Table 2.
Pre-operative and post-operative symptoms and signs between both groups (chi-square test)
| Subperiosteal Drain | Subdural Drain | P-value (between groups) | |
|---|---|---|---|
| Pre-op Symptoms | |||
| GCS 13–15 | 26 (86.7%) | 28 (93.3%) | |
| GCS 9–12 | 3 (10%) | 1 (3.3%) | |
| GCS 3–8 | 1 (3.3%) | 1 (3.3%) | |
|
| |||
| Symptoms & Signs on Admission | |||
| Hemiparesis | 20 (66.7%) | 18 (60%) | 0.592 |
| Hemiplegia | 1 (3.3%) | 1 (3.3%) | > 0.95 |
| Aphasia | 10 (33.3%) | 12 (40%) | 0.592 |
| Headache | 26 (86.7%) | 28 (93.3%) | 0.389 |
| Seizures | 1 (3.3%) | 2 (6.7%) | 0.554 |
| Altered Sensorium | 28 (93.3%) | 26 (86.7%) | 0.389 |
| Reflex Asymmetry | 24 (80%) | 26 (86.7%) | 0.739 |
|
| |||
| Symptoms and Signs at Discharge | |||
| Hemiparesis | 4 (13.3%) | 1 (3.3%) | 0.161 |
| Hemiplegia | 0 | 2 (6.7%) | 0.472 |
| Aphasia | 0 | 0 | – |
| Headache | 10 (33.3%) | 8 (26.7%) | 0.573 |
| Seizures | 1 (3.3%) | 2 (6.7%) | 0.554 |
| Altered Sensorium | 0 | 0 | – |
| Reflex Asymmetry | 0 | 0 | – |
The most common symptoms at admission in the SPD versus SDD groups, respectively, included headache (86.7% versus 93.3%), altered sensorium (93.3% versus 83.3%), asymmetrical reflexes (80% versus 86.7%), hemiparesis (66.7% versus 60%), aphasia (33.3% versus 40%), hemiplegia (3.3% versus 3.3%), and seizures (3.3% versus 6.7%). Overall, there were no significant differences in pre-operative symptoms and signs between the two groups (P > 0.05).
All patients were re-examined post-operatively with regard to presenting symptoms. As shown in Table 2, all patients in both groups fully recovered post-operatively from the initial symptoms and signs of aphasia, altered sensorium, and asymmetrical reflexes. Both groups showed significant improvements in headache (from 86.7% to 33.3% in the SPD group and from 93.3% to 26.7% in the SDD group) and hemiparesis (from 66.7% to 13.3% in the SPD group and from 60% to 3.3% in the SDD group) (P < 0.05). No patients in the SPD group and two patients (6.7%) in the SDD group developed persistent hemiplegia post-operatively. There were no detectable differences in seizure control post-operatively between the groups (3.3% versus 6.7%); all patients who presented with pre-operative seizures had residual focal seizures post-operatively, which were controlled by optimisation of antiepileptic drugs. Overall, no statistically significant differences were demonstrated in post-operative symptoms between the groups (P > 0.05).
Markwalder grade
Markwalder grades at admission and upon discharge were used to assess the clinical courses of patients in both groups. As shown in Table 3, the majority of patients presented with Markwalder grade 2 on admission, including 20 patients (66.7%) in the SPD group and 18 (60%) in the SDD group. Nine subjects (30%) in the SPD group and 11 (36.7%) in the SDD group had Markwalder grade 1 on admission. One patient (3.3%) in each group had Markwalder grade 3 on admission.
Table 3.
Markwalder grades on admission and upon discharge comparing mean grade within and between both groups (independent t-test)
| Markwalder Grade | Subperiosteal Drain (SPD) | Subdural Drain (SDD) | P-value (between groups) |
|---|---|---|---|
| On admission | |||
| MW 0 | 0 | 0 | |
| MW 1 | 9 (30%) | 11 (36.7%) | |
| MW 2 | 20 (66.7%) | 18 (60%) | |
| MW 3 | 1 (3.3%) | 1 (3.3%) | |
| MW 4 | 0 | 0 | |
| Mean Markwalder Grade | 1.73 | 1.67 | 0.195 |
|
| |||
| On discharge | |||
| MW 0 | 17 (56.7%) | 19 (63.3%) | |
| MW 1 | 9 (30%) | 8 (26.7%) | |
| MW 2 | 4 (13.3%) | 1 (3.3%) | |
| MW 3 | 0 | 2 (6.7%) | |
| MW 4 | 0 | 0 | |
| Mean Markwalder Grade | 0.50 | 0.53 | 0.872 |
|
| |||
| P-value (within groups) | 0.000 | 0.000 | |
Post-operatively, the patients in both groups were examined and reassigned post-operative Markwalder grades. All patients in each group with admission Markwalder grade 1 improved to Markwalder grade 0 by the time of discharge. Therefore, the majority of patients demonstrated good post-operative Markwalder scores (grade 0 or 1) at discharge, for a total of 26 patients (86.7%) in the SPD group and 27 (90%) in the SDD group.
Table 5 shows that the calculated mean admission Markwalder score was 1.73 for the SPD group and 1.67 for the SDD group. Upon discharge, the mean Markwalder scores in both groups had improved significantly (from 1.73 to 0.50 in the SPD group and from 1.67 to 0.53 in the SDD group). These changes were statistically significant within each group, respectively (P < 0.05). However, there were no significant differences in mean Markwalder scores between the SPD and SDD groups upon admission and at discharge (P > 0.05).
Table 5.
Overall surgical complications and mortality between both groups (independent t-test)
| Subperiosteal Drain (SPD) | Subdural Drain (SDD) | P-value (between groups) | |
|---|---|---|---|
| Repeat surgeries while hospitalised | |||
| Re-evacuation | 0 | 0 | – |
| Craniotomies | 0 | 2 (6.7%) | 0.150 |
| Repeat surgeries after discharge | |||
| Re-evacuation | 2 (6.7%) | 1 (3.3%) | 0.554 |
| Craniotomies | 0 | 0 | – |
|
| |||
| Intracerebral Hematoma | 0 | 2 (6.7%) | 0.150 |
| Recurrent Hematoma | 2 (6.7%) | 1 (3.3%) | 0.554 |
| Surgical Site Infection | 0 | 0 | – |
| Tension Pneumocephalus | 0 | 0 | – |
| Seizures | 0 | 0 | – |
|
| |||
| Overall Surgical Complications | 6.7% | 10.0% | 0.071 |
Hematoma volume
The mean hematoma volume was estimated pre-operatively, within 24 hours post-operatively, and at three months of follow-up based on CT findings; Table 4 illustrates that there were no significant differences (P > 0.05). However, within the respective groups, hematoma volumes were significantly reduced compared to pre-operative imaging (P < 0.05). In the SPD group, the mean pre-operative hematoma volume decreased significantly from 119.83 × 103 mm3 to 9.13 × 103 mm3 during the post-operative period (P < 0.05). Significant changes were also noted in mean hematoma volume from the post-operative CT (9.13 × 103 mm3) to the three-month follow-up CT (4.67 × 103 mm3) (P < 0.05). Similar results were found for the SDD group, in which the mean pre-operative hematoma volume significantly decreased from 118.87 × 103 mm3 to 8.40 × 103 mm3 in the post-operative period, with a further drop to 4.63 × 103 mm3 on the three-month follow-up CT (P < 0.05).
Table 4.
Mean hematoma volume in both groups pre-operatively, post-operatively and at 3 months follow-up (independent t-test)
| Mean Hematoma volume (*103) | Subperiosteal Drain (SPD) | Subdural Drain (SDD) | P-value (between groups) |
|---|---|---|---|
| Pre-op | 119.83 | 118.87 | 0.152 |
| Post-op (24 hours) | 9.13 | 8.40 | 0.194 |
| Follow-up (3 months) | 4.67 | 4.63 | 0.873 |
| P-value (within groups) | 0.000 | 0.000 |
Overall surgical complications
The overall surgical complications were categorised into intracerebral hematoma (ICH), recurrent hematoma, surgical-site infection, and tension pneumocephalus. Table 5 shows that the overall complication rate in the SPD group was 6.7%, with two patients who developed recurrent hematoma during follow-up, requiring re-evacuation surgery via burr-hole craniostomy and SPD drainage. None of the patients developed ICH as a post-operative complication in this group. The two recurrent hematomas were a direct result of restarting anticoagulant treatment by the cardiology team within two weeks of surgery.
In the SDD group, the overall complication rate was 10%, with two patients (6.7%) developing ICH while hospitalised, and one (3.3%) with recurrent hematoma during follow-up. Both patients with ICH underwent craniotomy and clot evacuation while hospitalised, and a repeat burr-hole craniostomy was performed for the other patient with recurrent CSDH. Similarly, the single recurrent hematoma in this group was also a result of recommencing anticoagulation medications within two weeks post-operatively.
A higher rate of post-operative ICH in the SDD group (6.7%) was clearly illustrated in comparison to the SPD group, which experienced none. However, as shown in Table 5, this did not translate into a significant statistical difference (P > 0.05). There was also no detectable difference between the groups in post-operative recurrent hematomas (P > 0.05). Additionally, in terms of the overall surgical complication rate, there was no significant difference between the SPD and SDD groups (P > 0.05). None of the patients in either group developed post-operative seizures, surgical-site infections, or tension pneumocephalus.
Functional outcomes and mortality
Functional outcomes were measured with the Glasgow Outcome Score and compared between the two groups. A good functional outcome was defined as a GOS of 4 or higher (Table 6). In the SPD group at discharge, a total of 26 patients (86.7%) had a GOS of 5, and four patients (13.3%) had a GOS of 4. The mean GOS at discharge was 4.9 in the SPD group. In the SDD group, 28 subjects (93.3%) had a GOS of 5, one (3.3%) had a GOS of 4, and two (6.67%) had a GOS of 3 at discharge. The mean GOS at discharge was calculated at 4.8 for the SDD group. There was no significant difference between the two groups in GOS scores at discharge (P > 0.05).
Table 6.
Functional outcome assessment between both groups
| Glasgow Outcome Score | Subperiosteal Drain (SPD) | Subdural Drain (SDD) | P-value (between groups) |
|---|---|---|---|
| Outcome at discharge | |||
| GOS 5 | 26 (86.7%) | 27 (90.0%) | |
| GOS 4 | 4 (13.3%) | 1 (3.3%) | |
| GOS 3 | 0 | 2 (6.7%) | |
| GOS 2 | 0 | 0 | |
| GOS 1 | 0 | 0 | |
|
| |||
| Mean GOS (at discharge) | 4.9 | 4.8 | 0.781 |
|
| |||
| Outcome at 3 months follow-up | |||
| GOS 5 | 29 (96.7%) | 28 (93.3%) | |
| GOS 4 | 1 (3.3%) | 2 (6.7%) | |
| GOS 3 | 0 | 0 | |
| GOS 2 | 0 | 0 | |
| GOS 1 | 0 | 0 | |
|
| |||
| Mean GOS (at 3 months follow-up) | 4.9 | 4.9 | 0.297 |
|
| |||
| Mortality | 0 | 0 | – |
At three months of follow-up in the SPD group, 29 (96.7%) patients had a GOS of 5, while one (3.3%) had a GOS of 4. In the SDD group, 28 patients (93.3%) had a GOS of 5 and two (6.7%) had a GOS of 4. No significant difference was noted in mean GOS scores at three months. The mean GOS was 5 in the SPD group and 4.9 in the SDD group (P > 0.05). There was no mortality throughout the entire study duration in either group.
Discussion
In recent years, there have been increasing numbers of studies published on the efficacy of placement of SPD systems following burr-hole craniostomy for the treatment of symptomatic CSDH. SPD placement has been deemed a technically easy option that is safer and equally effective compared to conventional SDD placement. Our study was designed to perform a prospective direct comparison between these two surgical techniques, with a realistic goal of evaluating their efficacy and identifying any differences with regard to overall surgical complications, functional outcomes, and mortality.
We found no significant differences in patient characteristics, mean hematoma size, comorbid conditions, or pre-operative symptoms. We found that SPD placement was equally effective and tended to result in a lower rate of surgical complications compared to the conventional SDD technique. This was demonstrated by the significant reduction of post-operative symptoms and improved Markwalder scores within each respective group. Due to the minor invasiveness of the SPD procedure, which involves no contact with the brain parenchyma, we found a lower rate of post-operative ICH, which can be caused by inadvertent placement of the SDD into the brain. This difference, however, did not reach statistical significance. We also detected no significant differences in the reduction of post-operative seizures or recurrent hematomas in the SPD group, as reported in previous studies (3, 14). These findings were largely limited by the relatively small sample size in our study. We also noted that the recurrent hematomas were directly related to the recommencement of anticoagulant treatment during in the post-operative period. In three separate studies yielding class (III) recommendations, the respective authors found that reinstating oral anticoagulants 72 hours after surgery was safe and did not lead to a higher risk of post-operative intracranial bleeding (4, 9, 13). Therefore, further studies need to be undertaken to ascertain better recommendations on restarting anticoagulant treatment during the post-operative period.
Favorable functional outcomes were determined by a GOS score of 4 or higher at three months of follow-up. Other studies with similar follow-up duration reported comparable good outcomes at three months post-operatively (3, 14). In keeping with these findings, our study also showed good outcomes at three months, but there was no detectable difference between the two groups. However, Kaliaperumal et al. in 2012 found significantly better modified Rankin scores after six months in patients treated with the SPD system. Therefore, a longer follow-up duration would be needed to demonstrate any significant differences in the overall functional outcomes of the patients in our study. Despite a standardised outline of peri-, intra-, and post-operative measures, another inherent limitation of our study was the lack of performance-bias elimination via a single surgeon, which was not possible due to the logistic constraints and timeframe of the study.
Conclusion
Our research concluded that the SPD and SDD systems both appear to be safe, technically easy, and highly effective in the treatment of CSDH, with no statistically significant differences in terms of overall surgical complications, functional outcomes, or mortality. The patients in the SPD group demonstrated a reduced tendency toward post-operative ICH secondary to inadvertent subdural drain placement in the brain parenchyma, but the difference was not statistically significant. Our study was based on a relatively small sample size and a short follow-up duration. Therefore, we recommend a prospective, randomised, multicenter study in the future, with a larger sample size and a longer follow-up period (at least six months) to further substantiate our results.
Footnotes
Authors’ Contributions
Conception and design: ANWC, JMA, AWSH, NAAR
Analysis and interpretation of the data: ANWC
Drafting of the article: ANWC, JMA
Critical revision of the article for important intellectual content: ANWC, JMA, AWSH, NAAR
Final approval of the article: JMA
Provision of study materials or patients: AWSH, NAAR
Statistical expertise: ANWC, JMA
Administrative, technical, or logistic support: ANWC, AWSH, NAAR
Collection and assembly of data: ANWC
References
- 1.Baechli H, Nordmann A, Bucher HC, et al. Demographics and prevalent risk factors of chronic subdural haematoma: results of a large single-center cohort study. Neurosurg Rev. 2004;27(4):263–266. doi: 10.1007/s10143-004-0337-6. https://dx.doi.org/10.1007/s10143-004-0337-6. [DOI] [PubMed] [Google Scholar]
- 2.Belkhair S, Pickett G. One versus double burr holes for treating chronic subdural hematoma meta-analysis. Can J Neurol Sci. 2013;40(1):56–60. doi: 10.1017/s0317167100012956. https://dx.doi.org/10.1017/S0317167100012956. [DOI] [PubMed] [Google Scholar]
- 3.Bellut D, Woernle CM, Burkhardt JK, Kockro RA, et al. Subdural drainage versus subperiosteal drainage in burr-hole trepanation for symptomatic chronic subdural hematomas. World Neurosurg. 2012;77(1):111–8. doi: 10.1016/j.wneu.2011.05.036. https://dx.doi.org/10.1016/j.wneu.2011.05.036. [DOI] [PubMed] [Google Scholar]
- 4.Chari A, Clemente Morgado T, Rigamonti D. Recommencement of anticoagulation in chronic subdural haematoma: a systematic review and meta-analysis. Br J Neurosurg. 2014;28:2–7. doi: 10.3109/02688697.2013.812184. https://dx.doi.org/10.3109/02688697.2013.812184. [DOI] [PubMed] [Google Scholar]
- 5.Gazzeri R, Galarza M, Neroni M, Canova A, et al. Continuous subgaleal suction drainage for the treatment of chronic subdural haematoma. Acta Neurochir (Wien) 2007;149(5):487–493. doi: 10.1007/s00701-007-1139-8. discussion 93. https://dx.doi.org/10.1007/s00701-007-1139-8. [DOI] [PubMed] [Google Scholar]
- 6.Gelabert-Gonzalez M, Iglesias-Pais M, Garcia-Allut A, et al. Chronic subdural haematoma: surgical treatment and outcome in 1000 cases. Clin Neurol Neurosurg. 2005;107(3):223–229. doi: 10.1016/j.clineuro.2004.09.015. https://dx.doi.org/10.1016/j.clineuro.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 7.Hassan KS, Metin G, Fazil G. The vaule of XYZ/2 technique compared with computer-assisted volumetric analysis to estimate the volume of chronic subdural hematoma. Stroke. 2005;36:998–1000. doi: 10.1161/01.STR.0000162714.46038.0f. https://dx.doi.org/10.1161/01.STR.0000162714.46038.0f. [DOI] [PubMed] [Google Scholar]
- 8.Kaliaperumal C, Khalil A, Fenton E, et al. A prospective randomised study to compare the utility and outcomes of subdural and subperiosteal drains for the treatment of chronic subdural haematoma. Acta Neurochir (Wien) 2012;154(11):2083–2088. doi: 10.1007/s00701-012-1483-1. https://dx.doi.org/10.1007/s00701-012-1483-1. [DOI] [PubMed] [Google Scholar]
- 9.Kawamata T, Takeshita M, Kubo O, et al. Management of intracranial hemorrhage associated with anticoagulant therapy. Surg Neurol. 1995;44(5):438–442. doi: 10.1016/0090-3019(95)00249-9. https://dx.doi.org/10.1016/0090-3019(95)00249-9. [DOI] [PubMed] [Google Scholar]
- 10.Lee JK, Choi JH, Kik CH, Lee HK, et al. Chronic subdural hematomas: A comparative study of three types of operative procedures. J Korean Neurosurg Soc. 2009;46:210–214. doi: 10.3340/jkns.2009.46.3.210. https://dx.doi.org/10.3340/jkns.2009.46.3.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santarius T, Kirkpatrick PJ, Ganesan D, et al. Use of drains versus no drains after burr-hole evacuation of chronic subdural haematoma: a randomised controlled trial. Lancet. 2009 Sep 26;374(9695):1067–1073. doi: 10.1016/S0140-6736(09)61115-6. https://dx.doi.org/10.1016/S0140-6736(09)61115-6. [DOI] [PubMed] [Google Scholar]
- 12.Weigel R, Schmiedek P, Krauss JK. Outcome of contemporary surgery for chronic subdural haematoma: evidence based review. J Neurol Neurosurg Psychiatry. 2003;74(7):937–943. doi: 10.1136/jnnp.74.7.937. https://dx.doi.org/10.1136/jnnp.74.7.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yeon JY, Kong DS, Hong SC. Safety of early warfarin resumption following burr-hole drainage for warfarin-associated subacute or chronic subdural hemorrhage. J Neurotrauma. 2012;29(7):1334–1341. doi: 10.1089/neu.2011.2074. https://dx.doi.org/10.1089/neu.2011.2074. [DOI] [PubMed] [Google Scholar]
- 14.Zumofen D, Regli L, Levivier M, Krayenbuhl N. Chronic subdural hematomas treated by burr hole trepanation and a subperiosteal drainage system. Neurosurgery. 2009;64:1116–1121. doi: 10.1227/01.NEU.0000345633.45961.BB. https://dx.doi.org/10.1227/01.NEU.0000345633.45961.BB. [DOI] [PubMed] [Google Scholar]