Abstract
Background
The neuroprotective role of minocycline in the treatment of brachial plexus injury is controversial.
Objective
To study the neuroprotective effect of minocycline via different routes in adult Sprague Dawley rats with brachial plexus injury.
Methods
The C7 nerve roots of the animals were avulsed via an anterior extravertebral approach. Traction force was used to transect the ventral motor nerve roots at the preganglionic level. Intraperitoneal and intrathecal minocycline (50 mg/kg for the first week and 25 mg/kg for the second week) were administered to promote motor healing. The spinal cord was harvested six weeks after the injury, and structural changes following the avulsion injury and pharmacological intervention were analysed.
Results
Motor neuron death and microglial proliferation were observed after the administration of minocycline via two different routes (intraperitoneal and intrathecal) following traumatic avulsion injury of the ventral nerve root. The administration of intraperitoneal minocycline reduced the microglia count but increased the motor neuron count. Intrathecal minocycline also reduced the microglial count, with a greater reduction than in the intraperitoneal group, but it decreased the motor neuron count.
Conclusions
Intraperitoneal minocycline increased motor neuron survival by inhibiting microglial proliferation following traumatic avulsion injury of the nerve root. The inhibitory effect was augmented by the use of intrathecal minocycline, in which the targeted drug delivery method increased the bioavailability of the therapeutic agent. However, motor neuron survival was impaired at a higher concentration of minocycline via the intrathecal route due to the more efficient method of drug delivery. Microglial suppression via minocycline can have both beneficial and damaging effects, with a moderate dose being beneficial as regards motor neuron survival but a higher dose proving neurotoxic due to impairment of the glial response and Wallerian degeneration, which is a pre-requisite for regeneration.
Keywords: minocycline, avulsion injury, histological study, microglia
Introduction
Brachial plexus injuries are common in those with polytrauma, such as young men of reproductive age (10). Previous research reported that ventral root avulsion injury in experimental animals resulted in a cascade of the glial response, together with motor neuron loss. The role of glial cells is axonal regeneration is controversial, with reactive microglia affecting axonal regeneration in a variety of ways. For example, they not only produce pro-regenerative factors, such as insulin-like growth factor-1 (1), but also neurotoxic compounds, such as free radicals and glutamate (7).
Minocycline hydrochloride is a semi-synthetic derivative of tetracycline and a potent neuroprotective agent, which acts by inhibiting microglial activation (13). Previous studied showed that minocycline alleviated the degree of neuropathology in animal models of stroke, spinal cord injury, multiple sclerosis, and neurodegenerative diseases (Parkinson’s disease and amyotrophic lateral sclerosis) (3, 15). Using a lumbosacral ventral root avulsion model of cauda equina injury, Hoang et al. (8) demonstrated that minocycline conferred significant protection against retrograde degeneration and death of axotomised motor neurons (8).
Although the mechanism underlying the activity of minocycline is not fully understood, the drug is known to readily cross the blood-brain barrier. Minocycline has also been shown to have a strong anti-inflammatory effect and a good safety record. The FDA has approved minocycline for the suppression of apoptosis (13). A previous study demonstrated that minocycline reduced the activation of microglia after peripheral nerve injury, partly by inhibiting the expression of p38 mitogen-activated protein kinases (MAPKs) and reducing that of pro-nerve growth factors in microglia, thereby decreasing the death of oligodendrocytes after traumatic spinal cord injury (16).
Materials and Methods
This was a randomised laboratory-based experimental study carried out at the Universiti Sains Malaysia from March 2013 until July 2015. The study was approved by the Animal Ethics Committee of the Universiti Sains Malaysia [reference number: 2011/(73) (346)].
Animals
Adult female Sprague Dawley rats (N = 21), with a weight of 200 g–300 g were used in the experiment. Rats outside this weight range were excluded.
Sampling design
The sampling design used was a randomised controlled trial. The animals were randomised into three groups, with seven animals in each group: two treatment groups administered minocycline via intraperitoneal and intrathecal routes and a control group given normal saline.
Outcome variables
The outcome variables were the numbers of microglia and motor neuron cells. The numbers of microglia and motor neuron cells in a lesioned site and normal site were calculated. The cell count was expressed as a ratio of the number of cells on the avulsed side divided by the number of cells on the contralateral, nonlesioned side. The mean was obtained by averaging the ratio obtained from 10 cross-section measurements. A staff member blinded to the study was appointed the cell-counting task.
Surgical methods
The Sprague Dawley rats were anesthetised with an intramuscular injection of ketamine (80 mg/kg) and xylazine (8 mg/kg). The seventh cervical segment (C7) of the spinal roots of the rats was avulsed using the following extravertebral extraction procedure: With a microhemostat forceps, extravertebral avulsion was carried out by applying steady, moderate traction away from the intervertebral foramen. The avulsed ventral and dorsal roots, as well as the dorsal root ganglia, were cut from the peripheral nerve and then examined using a dissecting microscope to confirm the success of the avulsion. Skin closure was done with non-absorbable polyamide monofilament sutures. The rats received intramuscular meloxicam (1 mg/kg) and amoxicillin (15 mg/kg) for three days after the surgery. The Otago animal welfare score sheet was used to observe the rats’ well-being (21).
Treatment methods
Intraperitoneal minocycline
Minocycline (50 mg/kg) or saline injections were administered intraperitoneally daily for seven days postoperatively. The first dose was given on the day of the surgery at the time of the root injury procedure. Half the original minocycline dose (25 mg/kg intraperitoneally daily) was administered on days 8–14 post-operatively, providing a total of two weeks of treatment.
Intrathecal minocycline
A dorsal midline incision was made at the neck. Subcutaneous dissection was performed at the skin incision site for placement of a microinfusion pump. C6/7 hemilaminectomy was performed using a microrounger. An intrathecal catheter was then inserted at the root avulsion site. The microinfusion pump was filled with minocycline and was programmed to deliver the drug at a rate of 50 mg/kg/day for seven days, followed by 25 mg/kg/day for another seven days. The paraspinal muscles were closed with polyglactin synthetic absorbable sutures. Skin closure was done with nonabsorbable polyamide monofilament sutures. There is no information in the literature on the optimal intrathecal dosage of minocycline that can prevent motor neuron death. In the present study, the dosage was intended to potentiate the inhibition of microglia activation and promote motor neuron survival.
Staining methods
Four weeks after the surgical avulsion of brachial nerve root, under deep anesthesia, the rats were intravascularly perfused with 100 ml of phosphate buffer, followed by 500 ml of 4% paraformaldehyde (4 °C). The cervical spinal cord segments were removed and placed in perfusion fixative (4 °C) for 6 h. The cross-sections were cryoprotected in 30% sucrose in phosphate-buffered saline (PBS), frozen overnight, and serially sectioned (40 μm thick), using a cryostat microtome in the transverse plane.
Motor neuron staining
The spinal segments of each animal were incubated at 37 °C for 1 h in 10 ml of 0.1 M Tris-HCL (pH 8.0) containing 0.2% Triton X100, 10 mg of nicotinamide adenine dinucleotide phosphate (NADPH), and 2.5 mg of nitroblue tetrazolium, followed by washing three times with 0.1M PBS. The stained sections were then mounted onto slides and counterstained with 1% neutral red. The surviving motor neurons were stained red, with neutral red stained the Nissl substance.
Microglial staining
The spinal cord cross-sections were placed on slides for immunocytochemical detection using rabbit IBA1. The sections were rinsed in PBS and incubated overnight in a well with rabbit IBA1 in 0.3% Triton X-100/PBS at room temperature. This primary antibody for IBA1 detection was left to cross-react with anti-rabbit Alexa 594 rhodamine (red) conjugated secondary antibody. The sections were rinsed the next day, incubated in solution containing the secondary antibody for 1 h, rinsed, and placed on slides. Antifading solution was applied over the section and cover-slided.
Motor neuron and microglia counting
The physical disector method was used for obtaining stereological counts of motor neurons and IBA1-labeled microglial cells in 10 sections from the C7 spinal cord segment in each animal. The cell count was expressed as the ratio of the number of neurons on the avulsed side divided by the number of neurons on the contralateral, nonlesioned side.
Results
Comparison of the neuroprotective strength (motor neuron survival) of minocycline administered via different routes
The number of motor neuron cells was significantly higher in the intraperitoneal group (median, 19: IQR: 2) as compared to the control (median, 14; IQR: 4) (P = 0.006) and intrathecal groups (median, 12: IQR: 2) (P = 0.006). There was no difference in the motor neuron count of the intrathecal and control groups (P = 0.279; Tables 1 and 2).
Table 1.
Motor neuron survival in three experimental groups (n = 21)
| Experimental subject | Motor Neuron Counts | ||
|---|---|---|---|
|
| |||
| In sham Group (n = 7) | After intraperitoneal administration of Minocycline (n = 7) | After intrathecal administration of Minocycline (n = 7) | |
| 1 | 16 | 20 | 11 |
| 2 | 14 | 18 | 10 |
| 3 | 15 | 21 | 13 |
| 4 | 14 | 19 | 12 |
| 5 | 12 | 17 | 14 |
| 6 | 10 | 18 | 12 |
| 7 | 17 | 19 | 13 |
| Mean | 14 | 18.6 | 12.1 |
Table 2.
Comparison of motor neuron survival in three experimental groups by Kruskal-Wallis test (n = 21). Intraperitoneal minocycline group has the highest motor neuron survival as compared to the sham and intrathecal minocycline group.
| Variables | Experimental groups | Chi-square (df) | P-value* | ||
|---|---|---|---|---|---|
|
| |||||
| Sham group (n = 7) Median (IQR) | Intraperitoneal group (n = 7) Median (IQR) | Intrathecal group (n = 7) Median (IQR) | |||
| Motor Neuron Counts | 14 (4) | 19 (2) | 12 (2) | 14.40(2) | 0.001 |
Kruskall-Wallis tests
Post hoc test with Bonferonni corrections by Mann-Whitney test:
Sham group versus Intraperitoneal group, P = 0.002 × 3 = 0.006 (< 0.05);
Sham group versus Intrathecal group, P = 0.093 × 3 = 0.279 (> 0.05);
Intraperitoneal group versus Intrathecal group, P = 0.002 × 3 = 0.006 (< 0.05)
Comparison of the neuroprotective strength (microglial inhibition) of minocycline administered via different routes
The numbers of microglia cells in the intrathecal group were significantly lower (median, 10; IQR: 1) than those in the control group (median, 15; IQR: 1) (P = 0.006) and intraperitoneal group (median, 13; IQR: 2) (P = 0.018). However, there was no difference in the microglia count of the intraperitoneal and control groups (P = 0.198; Tables 3 and 4).
Table 3.
Microglial activation in three experimental groups (n = 21)
| Experimental subject | Microglia Counts | ||
|---|---|---|---|
|
| |||
| In sham Group (n = 7) | After intraperitoneal administration of Minocycline (n = 7) | After intrathecal administration of Minocycline (n = 7) | |
| 1 | 15 | 10 | 12 |
| 2 | 14 | 12 | 10 |
| 3 | 15 | 13 | 10 |
| 4 | 14 | 12 | 9 |
| 5 | 12 | 14 | 10 |
| 6 | 16 | 15 | 9 |
| 7 | 15 | 14 | 8 |
| Mean | 14.4 | 12.9 | 9.7 |
Table 4.
Comparison of microglial activation in three experimental groups by Kruskal-Wallis test (n = 21). Subjects in intrathecal group have significantly lower microglia cells as compared to sham group and intraperitoneal group
| Variables | Experimental groups | Chi-square (df) | P-value* | ||
|---|---|---|---|---|---|
|
| |||||
| Sham group (n = 7) Median (IQR) | Intraperitoneal group (n = 7) Median (IQR) | Intrathecal group (n = 7) Median (IQR) | |||
| Microglia cell counts | 15 (1) | 13 (2) | 10 (1) | 13.05 (2) | 0.002 |
Kruskall-Wallis tests
Post hoc test with Bonferonni corrections by Mann-Whitney test:
Sham group versus Intraperitoneal group, P = 0.066 × 3 = 0.198 (> 0.05);
Sham group versus Intrathecal group, P = 0.002 × 3 = 0.006 (< 0.05);
Intraperitoneal group versus Intrathecal group, P = 0.006 × 3 = 0.018 (< 0.05)
Discussion
Cellular changes following traumatic avulsion injury and primary injury secondary to traumatic avulsion injury
In the present study, we created an animal preganglionic avulsion injury model by separating the motor neurons from the anterior horn of the spinal cord grey matter. This type of avulsion injury involves both the peripheral nerves and the spinal cord. The segment proximal to the lesion (i.e., the primary injury) is primarily responsible for the mechanical trauma created by the avulsion force, with subsequent extensive changes in the spinal cord and anterior horn cells. During the mechanical injury, local motor neurons at the affected level are sacrificed. In addition, axons of the ascending and descending tracts that traverse the lesioned side are transected. In some cases, local micro-hemorrhages may be observed under the microscope immediately after the injury. These hematomas are created by local disruption of the vascular endothelia of the epidural (Batson’s) plexus and occasionally radiculo-medullary vessels on the spinal cord. They can interrupt the delivery of oxygen and nutrients to the injury site, leading to hemorrhagic necrosis. If the hemorrhage spreads and affects adjacent spinal segments, this leads to a local mass effect and compression of the cord and its nerve roots. As neurons have a critically high demand for glucose and oxygen for metabolism, the ischemic state produced by the injury can cause defective metabolism, culminating in cell death (9).
Secondary injury after traumatic avulsion injury
The present study revealed drastic consequences of the secondary injury, including atrophic and degenerative changes within the ventral horn of the spinal cord. These histological changes involved a complex cascade of secondary inflammatory responses. Six weeks after the injury, neuronal loss was as high as 85%. This phenomenon was related to traumatic lysis, ischemia, hemorrhage, excitotoxicity, and a marked inflammatory response, including activation of the complement system. The latter resulted in granulocyte, lymphocyte, and macrophage infiltration of the lesioned site, in addition to the release of chemotactic agents, such as cytokines, interleukins, and tumor necrosis factor-α, which triggered a complex cascade of inflammatory responses. The disruption of the spinal cord blood barrier facilitated this process. Furthermore, the injured neurons underwent morphological changes, which included swelling of the soma, decentralisation of perikarya, and dispersal of Nissl substances. Furthermore, a number of structural changes were observed in the immediate vicinity of the affected motor neurons. These included a glial cell response (i.e., gliosis). Six weeks after the injury, microscopic inspections of histological specimens revealed an increase of IBA1 staining (14.4%). IBA1 is an ionising calcium-binding adaptor molecule, which is specifically expressed in the presence of microglia activation, especially in pathological conditions. As reported elsewhere (4, 20), the ultimate outcome of the inflammatory process following the avulsion injury was apoptosis of the motor neurons as a result of the detrimental effects of free radicals and oxidative stress, mediated by cytokines.
The neuroprotective therapeutic effect of minocycline on the nervous system
In the present study, the number of motor neuron cells in the experimental group treated with intraperitoneal minocycline was significantly higher (median, 19; IQR: 2) than in the control group (median, 14; IQR: 4) (P = 0.006). The hypothesized pathway through which minocycline exerts its biochemical effects plays an important role in neuroprotection. The pharmacological pathway of minocycline involves the inhibition of p38-MAPK phosphorylation and microglial reactivity, in addition to the blockade of caspase and metallomatrix proteins and endocannabinoid production. This pathway plays a crucial anti-inflammatory role in reducing microglia activation and motility and hence glial scar formation, which serves as both a microanatomical and functional barrier to nerve regeneration (2, 12). Hoang et al. (8) reported that minocycline helped to combat motor neuron degeneration and exerted an antiapoptotic effect by interfering with the caspase pathway and microglial inhibition (8). In their study, minocycline was administered to rats via a peritoneal injection. The peritoneal cavity worked as a slow release depot, maintaining the drug in a steady state in the circulation for 8 h. They reported that the therapeutic effect of minocycline was concentration and time dependent. As regards its therapeutic potential, administering minocycline via an intraperitoneal route is relatively effective and convenient. In theory, it has lower bioavailability than when administered via an intrathecal route, which is a more costly and technically demanding method of targeted drug delivery. A previous study reported no systemic side effects of minocycline in rodents when the drug was administered intraperitoneally (19). This positive result prompted us to attempt a more potent mode of drug delivery (i.e., the intrathecal route). Although the motor neuron count of the intrathecal group was slightly lower than that of the control group (12 versus 14), the difference was not statistically significant. Thus, intrathecal minocycline seemed to have a beneficial effect. However, some technical issues need to be considered. The insertion of an intrathecal pump in rodents requires a second surgery, which can be technically challenging. The operative time for the electronic implant insertion is also longer as compared to the time taken for avulsion surgery.
In the present study, wound dehiscence secondary to infection occurred in up to 57% of the rats that received the implantable intrathecal pump. Infection and stress responses after a second surgical procedure could also impair the regeneration capability of the nervous system. It is possible that favorable outcomes could be obtained if improved microsurgical techniques were applied in the insertion of the intrathecal pump or if larger sized experimental animals were used.
The present study demonstrated that minocycline exerted a neuroprotective effect on motor neurons. This effect was dose dependent, and minocycline resulted in neurotoxicity at a higher concentration or potency. The primary mechanism implicated in the neuroprotection conferred by minocycline is the drug’s highly potent inhibition of microglial activation. According to the literature, other pathways implicated in minocycline-induced neuroprotection include inhibition of p38 phosphorylation and MAPK pathway signaling, resulting in the production of inflammatory cytokines. The latter was shown to lead to cell death and microglial activation, which prevented the clearance of debris. Minocycline also induced changes in the regulation and transportation of neurofilaments and was shown to have lipophilic properties, which allowed it to easily penetrate the nervous system when introduced via a peritoneal route. Apart from its neuroprotective potential, additional advantages of minocycline include antimicrobial and anti-inflammatory properties, which reduce graft rejection-related problems, especially in brachial plexus surgery involving nerve transfer and implantation. Furthermore, minocycline is widely available, affordable and generally tolerable, without serious side effects.
The effect of high-dose, concentrated minocycline through targeted drug delivery
In the present study, minocycline strongly suppressed microglia activation when it was introduced intrathecally, with the rodents in the intrathecal group having significantly lower numbers of microglia cells (median, 10; IQR: 1) as compared to those in the control group (median 15, IQR: 1) (P = 0.006) and intraperitoneal group (median, 13; IQR: 2) (P = 0.019). The motor neuron count in the intrathecal group was also lower than that in the control group, although this finding was not statistically significant. As shown by the present study, minocycline administered via the intrathecal route, which is deemed to result in higher bioavailability, might have adverse effects on motor neuron survival. These adverse effects could be a direct result of microglial inhibition or an indirect result of other mechanisms.
As reported in many studies, minocycline also hindered scar formation, which is a natural, beneficial response to injury. For example Cui et al. (2001) reported that glial cell removal led to a reduction of glutamate transporters, which helped to evacuate glutamate out of synapses. In that study, low levels of glutamate transporters led to an accumulation of glutamate and subsequently excitotoxic neuronal cell death. The same study reported that reactive microglia phagocytised the debris, which contained neuronal growth inhibitory molecules, such as myelin-associated glycoproteins and a myelin component, Nogo-A (11). Other research demonstrated that minocycline-induced inhibition of microglia exposed motor neurons to damage from nitric oxide and reactive oxygen species. Minocycline was also shown to reduce microglial motility, thus hampering glial scar formation. A previous study described a neuroprotective effect of glial scar formation via a glutathione-dependent mechanism. It also inhibited astroglial motility, migration, and coupling, conferring a neurotrophic effect. Astrocytes were shown to play a crucial role in repair of the blood-brain barrier, regulation of the immune response, and promotion of neuronal survival and axonal outgrowth. Therefore, minocycline prevents the creation of a microenvironment at the site of injury.
Ironically, minocycline has also been shown to have deleterious effects, which are largely dose dependent. For example, it enhanced 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced damage in dopaminergic neurons in mice. Under conditions of oxygen glucose deprivation and ischemic injury, a high concentration of minocycline increased neuronal and astrocyte cell death. In the peripheral nervous system, Wallerian degeneration, a prerequisite for regeneration, was significantly impaired. Suppression of microglia exposed motor neurons to oxidative stress-induced damage (14). A previous study reported deleterious effects of minocycline on neurons following the administration of a high dose (90 mg/kg) to mice (13). Another study described reduced motor neuron survival following the administration of a higher concentration (100 μM) of minocycline as compared to a lower concentration (10 μM) (9).
Conclusions
Following traumatic avulsion injury of the ventral nerve root, motor neuron death and microglial proliferation were observed. The findings showed that the administration of minocycline in a rat model of avulsion injury suppressed microglia proliferation. The administration of minocycline via the intraperitoneal route conferred beneficial effects, prolonging motor neuron survival by inhibiting microglia activation and proliferation, thus hampering apoptosis of motor neurons. However, motor neuron survival was compromised when minocycline was administered via the intrathecal route to increase the bioavailability of the therapeutic agent. Therefore, we concluded that the suppression of microglia by minocycline has both beneficial and damaging effects. A moderate dosage of minocycline may be beneficial as regards the survival of motor neurons. However, a high concentration of minocycline may be neurotoxic, resulting in impairment of the glial response and Wallerian degeneration, which is a prerequisite for regeneration.
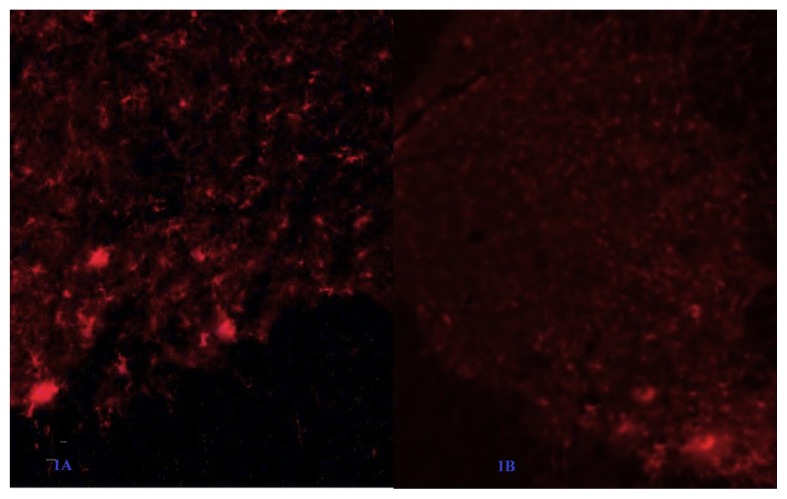
Figure 1.
Reduction of microglia count in the intrathecal minocycline group (B) is higher as compared to saline group (A)
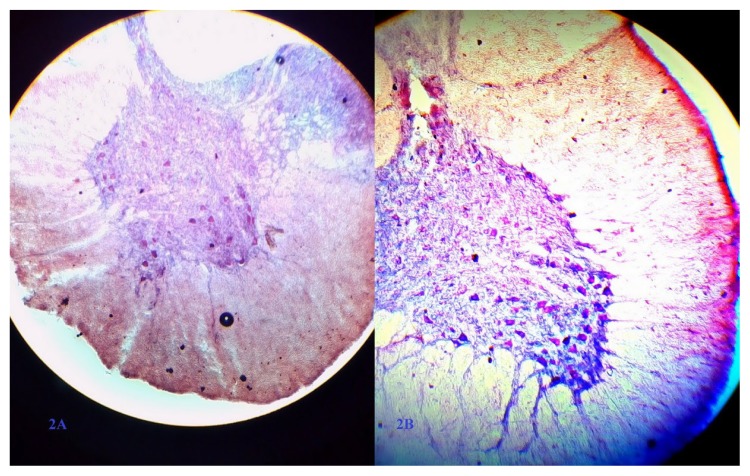
Figure 2.
There is less motor neuron in saline group (A) versus intraperitoneal minocycline group (B)
Footnotes
Authors’ Contributions
Conception and design: TYC
Analysis and interpretation of the data: TYC, SSK
Drafting of the article: TYC
Critical revision of the article for important intellectual content: JMA
Final approval of the article: WWT, JMA
Provision of study materials or patients: WWT, JMA
Statistical expertise: WWT, JMA
Obtaining funding: TYC
Administrative, technical, or logistic support: HGF
Collection and assembly of data: HGF
References
- 1.Aldskogius H. Microglia in neuroregeneration. Microsc Res Tech. 2001;54:40–46. doi: 10.1002/jemt.1119. https://dx.doi.org/10.1002/jemt.1119. [DOI] [PubMed] [Google Scholar]
- 2.Bowes A, Yip PK. Modulating inflammatory cell responses to spinal cord injury: all in good time. J Neurotrauma. 2014;31(21):1753. doi: 10.1089/neu.2014.3429. https://dx.doi.org/10.1089/neu.2014.3429. [DOI] [PubMed] [Google Scholar]
- 3.Arvin KL, Han BH, Du Y, et al. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54–61. doi: 10.1002/ana.10242. https://dx.doi.org/10.1002/ana.10242. [DOI] [PubMed] [Google Scholar]
- 4.Barbizan R, Castro MV, Rodrigues AC, Barraviera B, Ferreira RS. Motor recovery and synaptic preservation after ventral root avulsion and repair with a fibrin sealant derived from snake venom. PLoS One. 2013;8:e63260. doi: 10.1371/journal.pone.0063260. https://dx.doi.org/10.1371/journal.pone.0063260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boje KM, Arora PK. Microglial-produced nitric oxide andreactive nitrogen oxides mediate neuronal cell death. Brain. 1992;587:250–256. doi: 10.1016/0006-8993(92)91004-x. https://dx.doi.org/10.1016/0006-8993(92)91004-X. [DOI] [PubMed] [Google Scholar]
- 6.Cui W, Allen ND, Skyner MJ, Gusterson B, Clark AJ. Inducible ablation of astrocytes shows that these cells are important for neurons survival. Glia. 2001;34:272–282. doi: 10.1002/glia.1061. https://dx.doi.org/10.1002/glia.1061. [DOI] [PubMed] [Google Scholar]
- 7.Giulian D. Reactive glia as rivals in regulating neuronal survival. Glia. 1993;7(1):102–110. doi: 10.1002/glia.440070116. https://dx.doi.org/10.1002/glia.440070116. [DOI] [PubMed] [Google Scholar]
- 8.Hoang TX, Akhavan M, Wu J, Havton LA. Minocycline protects motor but not autonomic neurons after cauda equina injury. Exp Brain Res. 2008;189:71–77. doi: 10.1007/s00221-008-1398-5. https://dx.doi.org/10.1007/s00221-008-1398-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pinkernelle J, Hisham F, Embayer U, Keilhoff G. Prolonged minocycline administration impairs motor neuron survival and glial function in organotypic rat spinal cord culture. Plos One. 2013;8(8):e73422. doi: 10.1371/journal.pone.0073422. https://dx.doi.org/10.1371/journal.pone.0073422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Juan MG, Karl JH, Lon MB, Joon YL. Traumatic brachial plexus root avulsion and cervical spine epidural hematoma in an 18-year-old male. The Spine Journal. 15(2):365–366. doi: 10.1016/j.spinee.2014.09.024. https://dx.doi.org/10.1016/j.spinee.2014.09.024. [DOI] [PubMed] [Google Scholar]
- 11.Kaiser R, Mencl L, Haninec P. Injuries associated with serious brachial plexus involvement in polytrauma among patients requiring surgical repair. Injury. 2014;45(1):223–226. doi: 10.1016/j.injury.2012.05.013. https://dx.doi.org/10.1016/j,injury.2015.05.13. [DOI] [PubMed] [Google Scholar]
- 12.Kiegerl KA, Gensel JC, Ankeny DP, et al. Identification of 2 distinct macrophage subset with divergent effect causing either neurotoxicity or regeneration in injured mouse’s spinal cord. J Neuroscience. 2009;29:13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. https://dx.doi.org/10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kobayashi K, Imagama S, Ohgomori T, Hirano K, Uchimura K, Sakamoto K, et al. Minocycline selectively inhibits M1 polarization of microglia. Cell Death Dis. 2013;4:e525. doi: 10.1038/cddis.2013.54. https://dx.doi.org/10.1038/cddis.2013.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Potter EG, Cheng Y, Natale JE. Deleterious effects of minocycline after in vivo target deprivation of thalamocortical neurons in the immature, metallothionein-deficient mouse brain. J Neurosci Res. 2009;87(6):1356–1368. doi: 10.1002/jnr.21963. https://dx.doi.org/10.1002/jnr.21963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist. 2005;11:308–322. doi: 10.1177/1073858405275175. https://dx.doi.org/10.1177/1073858405275175. [DOI] [PubMed] [Google Scholar]
- 16.Tanaka T, Murakami K, Bando Y, Yoshida S. Minocycline reduces remyelination by suppressing ciliary neurotrophic factor expression after cuprizone-induced demyelination. J Neurochem. 2013;127(2):259–270. doi: 10.1111/jnc.12289. https://dx.doi.org/10.1111/jnc.12289. [DOI] [PubMed] [Google Scholar]
- 17.Yrjanheikki J, Keinanen R, Pellikka M, et al. Tetracyclines inhibit microglial activation and are neuroprotective in global brain ischemia. Proc Natl Acad Sci USA. 1998;95:15769–15774. doi: 10.1073/pnas.95.26.15769. https://dx.doi.org/10.1073/pnas.95.26.15769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yune TY, Lee JY, Jung GY, et al. Minocycline alleviates death of oligodendrocytes by inhibiting pro-nerve growth factor production in microglia after spinal cord injury. J Neurosci. 2007;27:7751–7761. doi: 10.1523/JNEUROSCI.1661-07.2007. https://dx.doi.org/10.1523/JNEUROSCI.1661-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faizul HG, Wutian W, Jafri Malin A. Histological analysis of motor neuron survival and microglial inhibition after nerve root avulsion treated with nerve graft implantation and minocycline: an experimental study. Sains Malays. 2016;45(11):1641–1648. [Google Scholar]
- 20.Sim SK, Tan YC, Tee JH, Yusoff AA, Abdullah JM. Paclitaxel inhibits expression of neuronal nitric oxide synthase and prevents mitochondrial dysfunction in spinal ventral horn in rats after C7 spinal root avulsion. Turk Neurosurg. 2015;25(4):617–624. doi: 10.5137/1019-5149.JTN.14035-15.1. htpps://dx.doi.org/10.5137/1019-5149. [DOI] [PubMed] [Google Scholar]
- 21.Animal Welfare, Otago University. http://www.otago.ac.nz/humanresources/health-and-safety/hazards/laboratory-safety/animal-welfare/index.html.