Abstract
Background and Objectives:
The well-known advantages of minimally invasive surgery make the approach well suited for hysterectomy and other gynecological procedures. The removal of specimens excised during surgery has been a challenge that has been answered by the use of power morcellation. With this study we sought to assess the feasibility of power morcellation within a specimen bag.
Methods:
This was a retrospective cohort study including patients from a private practice in suburban Chicago, Illinois, who underwent contained electromechanical power morcellation during a laparoscopic or robot-assisted hysterectomy or myomectomy from May 2014 through December 2015. Contained power morcellation was performed with the Espiner EcoSac 230 (Espiner Medical Ltd., North Somerset, United Kingdom) specimen bag. Descriptive statistics were performed for both categorical and continuous data.
Results:
Of the 187 procedures performed, 73.8% were myomectomies, and 26.2% were hysterectomies. The patients' mean age was 40 (range, 25–54) years and mean body mass index was 28.7 (range, 17.3–57.6). The average specimen weight was 300 g, with the largest weighing 2134 g. Estimated blood loss averaged 98.4 mL. The postoperative admission rate was 12.3%, most of which were due to nausea and urinary retention. Seventeen patients (9.1%) had postoperative complications, most of which were minor, and 4 (2.1%) were readmitted. There were no bag failures or complications that were due to the use of the specimen bag or to power morcellation.
Conclusions:
Performing electromechanical power morcellation within the Espiner EcoSac 230 specimen bag was successfully performed in 187 patients with no bag-related complications. This method of contained power morcellation is feasible, reliable, and reproducible, even for a large specimen.
Keywords: Morcellation, Contained morcellation, Uterine morcellation, Leiomyosarcoma
INTRODUCTION
Minimally invasive surgical techniques lead to decreased postoperative pain, reduced morbidity, and quicker recoveries when compared to open abdominal procedures,1,2 making it an ideal approach for hysterectomy or myomectomy.3 The problem of specimen removal during minimally invasive procedures led to the development of tissue morcellators by Kurt Semm in 1973.4–6 Electromechanical power morcellators were approved by the U. S. Food and Drug Administration (FDA) in 1995. However, the potential for tissue dissemination during power morcellation has led to increased concern and restrictions on their use. Benign sequelae including parasitic myoma, leiomyomatosis, and endometriosis are some of the potential complications.7–11 Of greater concern is the dissemination of unsuspected malignancy, which may result in upstaging and decreased survival.16–18
In April 2014, the FDA released a statement discouraging the use of laparoscopic power morcellation during hysterectomy or myomectomy.22 In November 2014, the FDA stated that power morcellation is contraindicated in peri- or postmenopausal women or women who are candidates for en bloc specimen removal. In addition, a black box warning on power morcellators was released.23 However, there is an inherent increased risk of morbidity and mortality in converting laparoscopic cases to laparotomy, as suggested.21 A decision analysis revealed that there would be more overall deaths with open hysterectomy, as compared to laparoscopic hysterectomy (103 vs 98 per 100,000), even incorporating an increase in deaths due to leiomyosarcoma in the laparoscopic group.24
In response to the FDA's statements, several reports of power morcellation in a contained isolation system (i.e., an insufflated bag), have demonstrated feasibility.26–38 The theory is that the contained system will prevent tissue from being disseminated throughout the abdomen. In our institutions, we have adapted a system for contained morcellation using the EcoSac230 bag developed by Espiner Medical Ltd. (North Somerset, United Kingdom). The purpose of this study was to prove the feasibility of a contained bag morcellation system.
MATERIALS AND METHODS
This study included a cohort of patients who underwent power morcellation within an insufflated contained bag after a laparoscopic hysterectomy or myomectomy from May 2014 through December 2015 at 3 hospitals in suburban Chicago, Illinois. The physicians who contributed to this study are high-volume fellowship-trained surgeons who specialize in minimally invasive gynecologic surgical techniques; most cases were performed by the senior author (CS). All patients were evaluated before surgery at the discretion of the primary surgeon with an updated Papanicolaou smear, endometrial sampling when appropriate, and imaging through at least one of the following imaging studies: pelvic ultrasonography, saline-infused sonohysterography, and magnetic resonance imaging (MRI). Patients who had known or suspected uterine malignancy or in whom specimen removal was possible without the use of power morcellation were excluded. Per practice and hospital guidelines, all patients gave informed consent before surgery after they were informed of the risks, benefits, and alternatives of power morcellation, including an understanding of the off-label use of the bag for contained morcellation.
Data were obtained through a retrospective chart review. Institutional Review Board approval was obtained from all institutions before data collection. Demographic data collected included age and body mass index (BMI; kilograms/square meter). Perioperative information included type of procedure performed (total laparoscopic or robot-assisted hysterectomy [TLH], laparoscopic or robot-assisted supracervical hysterectomy [LSH], and laparoscopic or robot-assisted myomectomy [LM]), specimen weight, number of fibroids removed (for myomectomies), estimated blood loss (EBL; in milliliters), intraoperative complications, bag rupture or bag failure, pathology, hospital admission, readmission, and postoperative complications. Operative time was not collected because of the high volume of concomitant procedures performed, and morcellation time was not recorded at the time of the procedures.
Because of the exploratory nature of the study, descriptive statistics for both categorical (n, %) and continuous data (mean, standard deviation, range) were calculated. All statistical analyses were performed with SPSS for Windows, version 22.0 (SPSS Inc., Chicago, Illinois, USA).
Procedure
The procedure for contained power morcellation using the Espiner EcoSac230 (Figure 1) is similar to prior descriptions of contained morcellation. The main difference is the use of this particular bag.37 No funding or assistance was provided by Espiner Medical Ltd. for this study. The bag is an FDA-approved specimen-removal bag; however, insufflating the bag for the purpose of power morcellation is an off-label use. The bag is made of Espiner Medical Ltd.'s unique Superamide66 fabric, which is a polyurethane-coated “ripstop” nylon fabric. According to Espiner Medical, the strong ripstop nylon material prevents the bag from bursting or rupturing. The dimensions of the EcoSac230 are a mouth diameter of 15.3 cm, length of 34 cm, and volume of 3100 mL.
Figure 1.
The Espiner EcoSac 230 Bag.
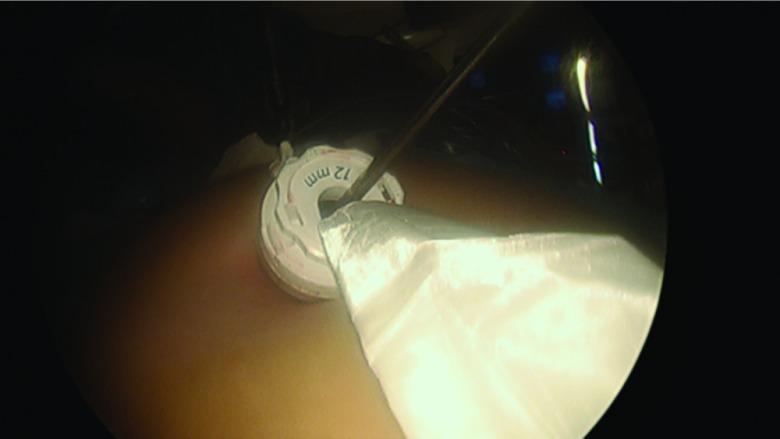
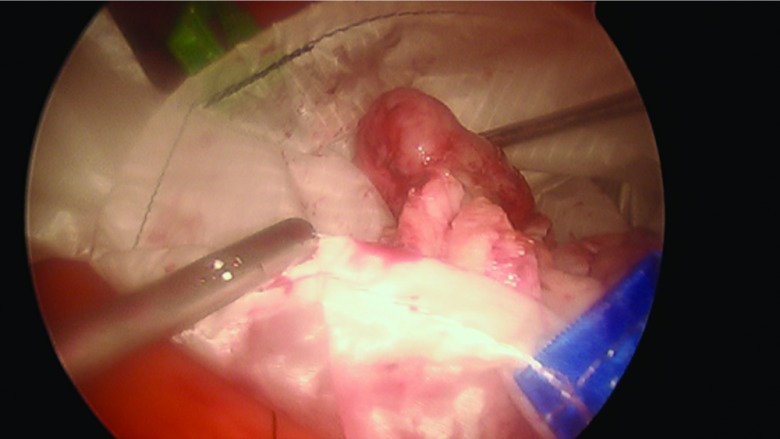
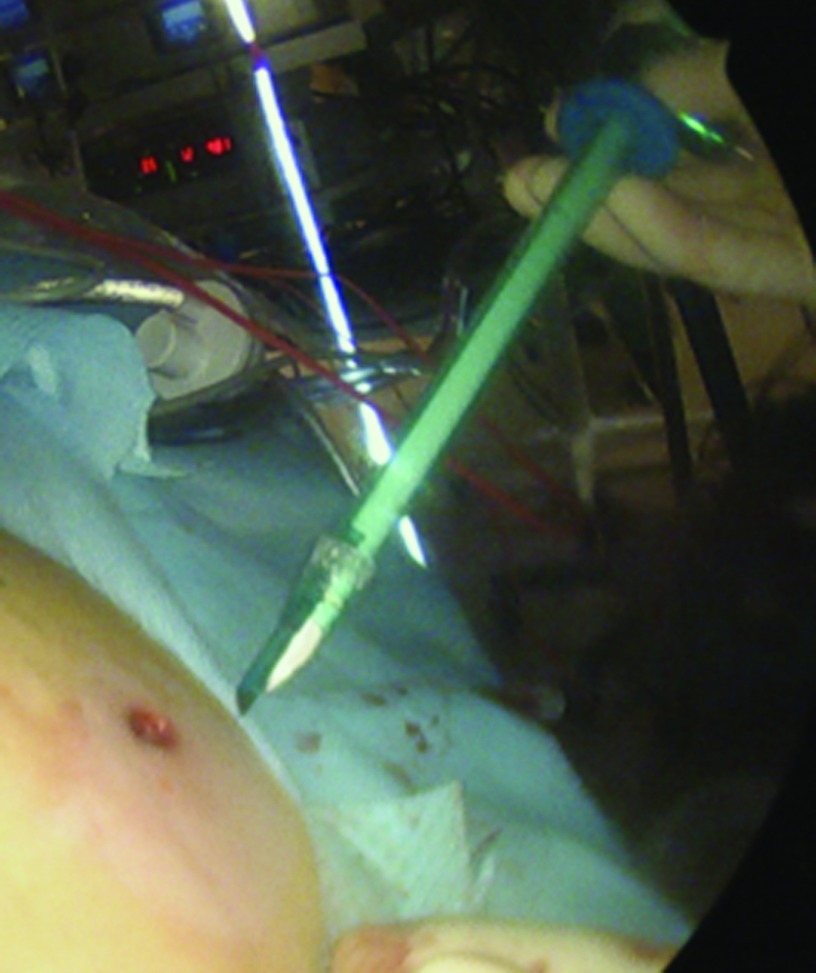
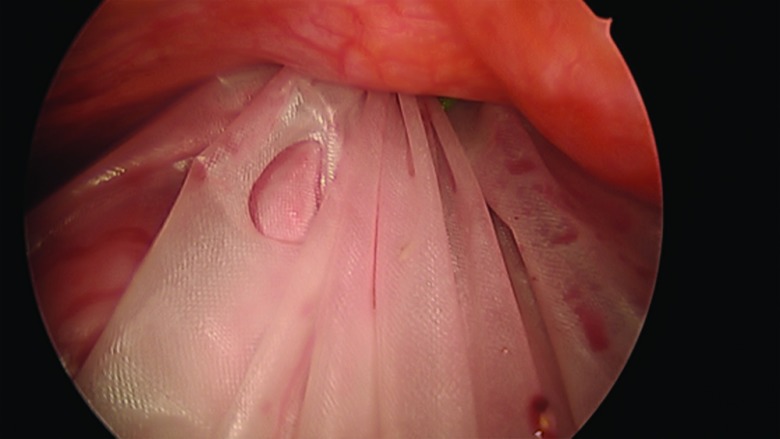
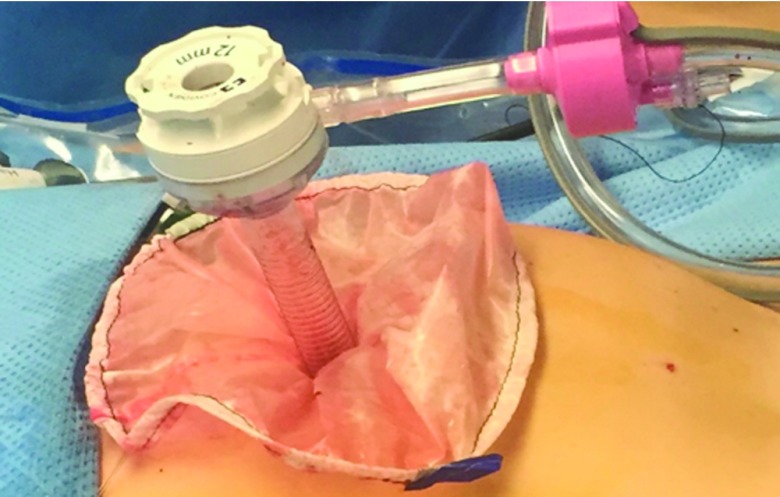
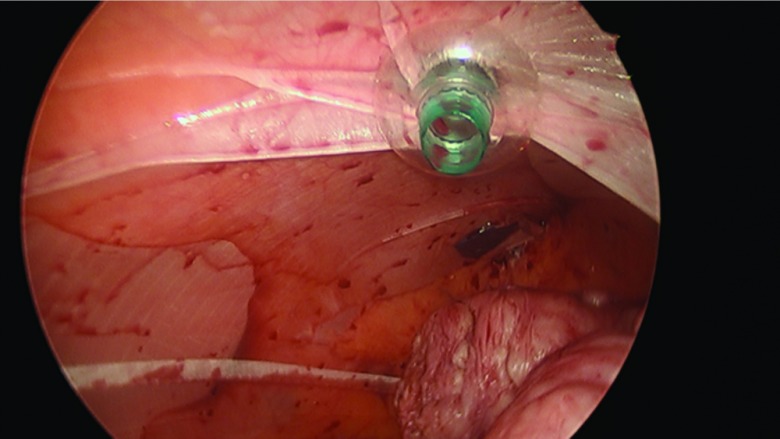
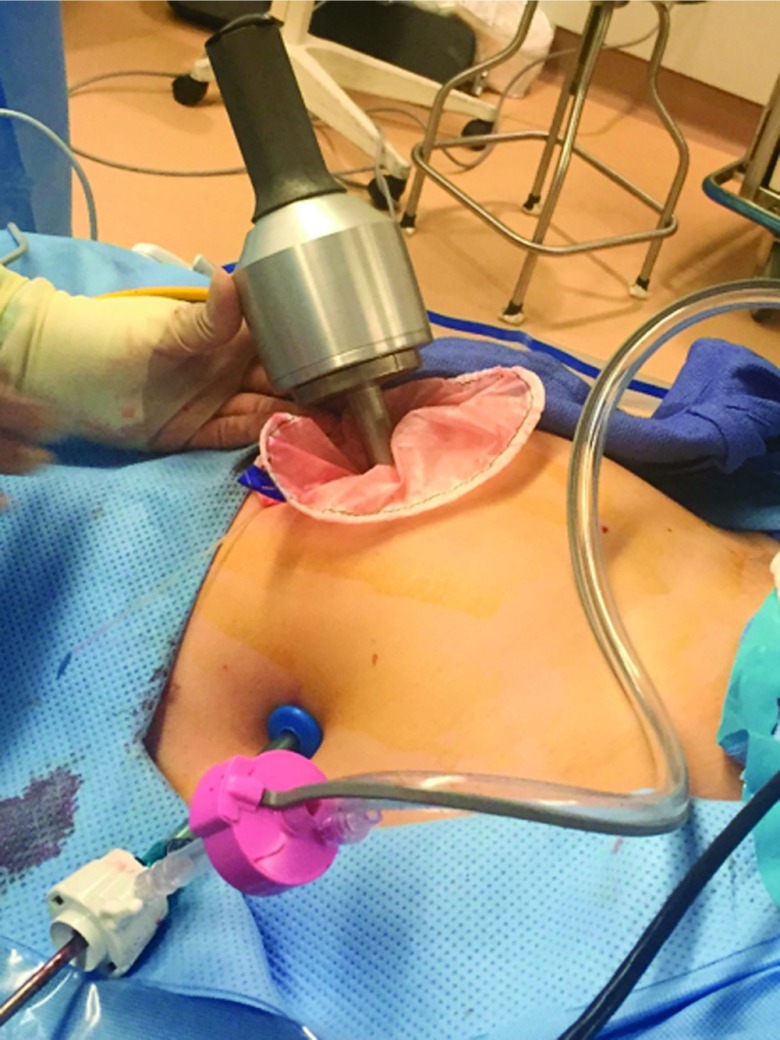
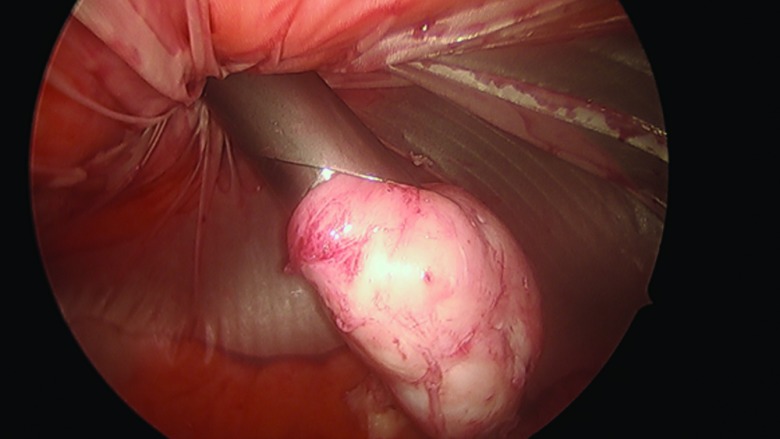
The procedure, either a laparoscopic hysterectomy or myomectomy, is completed using a standard multiport technique. After the specimen is isolated, the process of contained morcellation is begun. The inside of the EcoSac230 is first coated with a thin layer of sterile lubrication to prevent sticking on the inside of the bag. It is then inserted into the abdomen through a 12-mm umbilical trocar (Figure 2). Once inside the abdomen, the bag is opened by noting the easily identifiable colored tabs on the edge of the mouth of the bag as well as the black stitching indicating the inside edge of the bag. The specimen is then placed inside the bag, and the bag is cinched closed by pulling on the monofilament drawstring (Figure 3). The 5-mm right lateral port is replaced with a Kii Advanced Fixation Sleeve (Applied Medical, Rancho Santa Margarita, CA, USA) that has a nonlatex balloon tip and a manually activated shielded blade (Figure 4). The mouth of the bag is then brought up through the umbilical incision by removing the umbilical trocar (Figure 5). This trocar is replaced inside the bag and an insufflator is attached (Figure 6). The bag is insufflated to a pressure of 25 mm Hg with CO2 gas. Simultaneously, existing gas is removed from the abdomen by opening the valves of the accessory trocars to allow the bag to contour against the abdominal wall. With the laparoscope inside the bag, the Kii Advanced Fixation trocar is now gently pressed against the side of the bag to confirm visually that there is nothing between the bag and the trocar. The blade is deployed, and the trocar pierces the side of the bag to gain access to its inside. The 5-mL balloon at the end of the trocar is now inflated and brought up to the bag entry site. Its location is stabilized by an external disc on the trocar that is pushed flush against the patient's skin, creating a seal at the entry point of the lateral trocar (Figure 7). The insufflation is hooked up to the lateral trocar, and the laparoscope is now placed through this trocar. Under direct visualization, the umbilical trocar is removed and the incision dilated to 15 mm with Hegar dilators (Medline Industries, Inc., Danbury Connecticut, USA). The 15-mm Storz Rotocut G1 Morcellator (Karl Storz GmbH & Co., Tuttlingen, Germany) is inserted through the umbilical incision, and morcellation proceeds as usual (Figures 8 and 9). Once morcellation is complete, all small pieces of tissue are grasped and removed. The inside of the bag is irrigated, allowing for removal of any small particles from the sidewalls of the bag (Figure 10). The CO2 gas is removed from the bag, and the insufflator is attached to the left lateral trocar allowing insufflation of the abdomen again to a pressure of 15 mm Hg. The umbilical trocar is placed on the outside of the desufflated bag. Using the laparoscope in the left lateral port and a grasper in the umbilical port, the bag is grasped at the level of the right lateral trocar insertion site. The trocar balloon is deflated and the bag removed via the umbilical incision, leading at the location of the 5-mm hole that had been created by the trocar to prevent any tissue leakage through this hole. (This technique is presented in video 1 in Cholkeri-Singh and Miller38 for further reference, as well).
Figure 2.
The EcoSac230 bag is inserted through a 12-mm trocar.
Figure 3.
The specimen is placed inside the EcoSac230 bag. The inside of the bag is confirmed by the lack of stitching around the mouth of the bag.
Figure 4.
The Kii Advanced Fixation Sleeve.
Figure 5.
The mouth of the bag is brought up through the umbilical incision.
Figure 6.
The 12-mm trocar is placed into the bag through the umbilical incision. An insufflator is attached to this trocar and the bag is insufflated to a pressure of 25 mm Hg.
Figure 7.
The Kii Advanced Fixation Sleeve is now inserted through the lateral aspect of the bag, and the balloon is inflated and pulled flush with the side of the bag.
Figure 8.
The 12-mm trocar is replaced with the morcellator placed in the bag under direct visualization (extra-abdominal view).
Figure 9.
The 12-mm trocar is replaced with the morcellator into the bag under direct visualization (intra-abdominal view), and morcellation takes place.
Figure 10.
All small morcellated pieces are removed from the bag.
RESULTS
A total of 187 cases involving contained power morcellation were performed during this time period. The mean patient age was 40 years (SD ±6.093; range, 25–54) and the mean BMI was 28.7 kg/m2 (SD ±7.42; range, 17.3–57.6). The mean specimen weight was 300 g (SD ±329) with the largest specimen successfully morcellated weighing 2134 g. The average EBL was 98.4 mL (SD ±136.2; range, 10–1000). For myomectomy, the average number of fibroids removed was 4.9 (SD ±4.57), with the maximum number removed being 25.
Most cases (73.8%) were myomectomies and the remaining cases were hysterectomies (26.2%). Of the hysterectomies, 46.9% were supracervical. Most pathology revealed benign leiomyoma (87.2%) with 2.1% revealing adenomyoma, and 9.1% revealing both leiomyoma and adenomyoma. No malignancy occurred in any of the cases. Complications, admissions and re-admission are listed in Table 1.
Table 1.
Summary of Observed Complications
| Complications | Number (%) | Reasons |
|---|---|---|
| Bag rupture or failure | 0 | n/a |
| Intraoperative complication | 0 | n/a |
| Reason for admission (n) | 23 (12.3) | Urinary retention (6), nausea/vomiting (5), high EBL (4), transfusion (1), pain (2), multiple medical problems (4), fluid overload (1), family request (1) |
| Postoperative complication (n) | 17 (9.1) | Wound cellulitis (8), postoperative fever (3), pulmonary embolism (1), labial hematoma (1), vomiting/constipation leading to readmission (1), corneal abrasion (1), UTI (1), bleeding requiring readmission (1) |
DISCUSSION
This study demonstrates the feasibility of an insufflated, contained power morcellation technique. There were no bag ruptures or bag failures in our 187-patient cohort. Objectively, there was no leakage from the bag in any of the cases. We were able to morcellate a wide range of specimens with the largest specimen weight of 2134 g. This is one of the first publications using the EcoSac 230. This bag is unique in that it is made of polyurethane coated ripstop nylon that prevents accidental ripping or tearing.
In the wake of the FDA statements discouraging power morcellation, there have been several case reports and case series demonstrating the technique of contained morcellation. Various bags have been used: The Lahey bag is a large (50 × 50-cm) isolation bag made of transparent plastic. It is produced by various companies, with the most popular being the 3M Steri-Drape isolation bag (3M, St. Paul, Minnesota, USA). The Lahey bag was first used for the laparoscopic removal of large spleens in 2001, with a report revealing successful removal of 2 spleens: one 2510 g and the other 1720 g.39 The LapSac Surgical Tissue Pouch (Cook Medical, Bloomington, Indiana, USA) is made of reinforced nylon with a polyurethane inner coating and the largest size is 8 × 10 inches with a volume of 1500 mL. The Anchor TRS-200 bag (Anchor Surgical, Addison, Illinois, USA) is made of ripstop nylon, has a volume of 3000 mL, and can hold specimen up to 1500 g.26 The EndoCatch bag (Covidien, Medtronic, Mansfield, Massachusetts, USA) comes in either a 10- or 15-mm long cylindrical tube. It is a deployable bag made of transparent polyurethane. The 10-mm size has a pouch with a 6.35-cm mouth that can hold 220 mL, whereas the 15 mm size has a 12.7-cm mouth that can hold 1000 mL. As mentioned before, the EcoSac 230 bag that was used in our study has a mouth diameter of 15.3 cm, a length of 34 cm, and volume of 3100 mL. A comparison of the various bags with information on the cost of each one is provided in Table 2.
Table 2.
Comparison of Different Containment Bags Used for Morcellation in the Literature
| Bag | Manufacturer | Size | Material | Volume | Cost per bag |
|---|---|---|---|---|---|
| EcoSac 230 | Espiner | mouth: 15.3 cm length: 34 cm | Rip-stop nylon | 3100 mL | $105–115 |
| Steri-DrapeTM isolation bag | 3MTM | width: 50 cm length: 50 cm | Transparent plastic | Not listed | $25–35 |
| LapSac Surgical Tissue Pouch | Cook Medical | width: 8 in length: 10 in | Reinforced nylon with a polyurethane inner coating | 1500 mL | $90–100 |
| Anchor TRS-200 | Anchor Surgical | mouth: 15.2 cm length: 32.3 cm | Rip-stop nylon | 3000 mL | $130–140 |
| EndoCatchTM 15 mm | Covidien | mouth: 12.7 cm | Transparent polyurethane | 1000 mL | $145–160 |
In the first publication in September 2014, Cohen et al26 reviewed 73 patients who had successful contained morcellation using a 50 × 50-cm Lahey Bag. The procedures were conducted in both single-site and multiport techniques. The largest specimen morcellated was 1481 g. In another study, Einarsson et al27 reported successfully contained morcellation in 15 patients. In their study, a 15-mm EndoCatch bag was used in 8 patients and the Anchor TRS-200 bag was used in 7. The largest specimen morcellated in this series was 1309 g. They also reported no complications and no bag ruptures.
In 2015, Vargas et al28 compared contained morcellation to noncontained morcellation in and concluded that the only statistically significant difference was an increase in operating room time of 26 minutes. In their study, 3 different bag systems were used: the 15-mm EndoCatch, the Anchor, and the Lahey bag (3M isolation bag). They had a small sample of only 36 patients in the contained morcellation group and 49 in the nonmorcellation group; however, theirs was the first study to compare 2 morcellation techniques. Winner et al29 then compared 51 operations with contained morcellation against 101 with noncontained morcellation in a retrospective review of a prospectively collected database. Their contained system used the 3M isolation bag described by Cohen et al.26 They noted that the operative time was longer for contained morcellation (164 vs 184 min) but there was no difference in EBL, length of stay, specimen weight, or complications. They concluded that contained morcellation is a feasible technique with similar short-term outcomes, and that operative time may improve as surgeons become more familiar with the technique.
Various other case reports, case series, and video articles have been reported in the literature for contained morcellation.31–34 We feel our procedure has 3 potential advantages over the previously reported procedures. First, the ripstop nylon provides extra security in the prevention of inadvertent ripping or tearing of the bag over the thinner plastic of the EndoCatch bag or the Lahey bag. Second, the size of the bag is smaller than the other bags in the literature, most notably the Anchor bag and the Lahey bag. The smaller size allows for potentially easier insertion into the abdomen and less cumbersome manipulation of the bag. And finally, despite the smaller size of the bag, we were able to morcellate very large specimens, with the largest being 2134 g. This specimen may be the largest to be morcellated with a contained morcellation system.
The limitation to this bag as well as all of the bags mentioned herein is the need for a puncture site to allow for a lateral trocar. The puncture can theoretically lead to microscopic leakage of cells, which could still lead to tissue spread. We attempt to avoid this risk by removing the bag directly from the puncture site. However, the risk remains. In a recent study, Cohen et al35 evaluated leakage of liquid or tissue outside the bag during contained morcellation using the EndoCatch, Lahey, Anchor, or EcoSac bag. They found a 9% leakage rate and concluded that the puncture site was the most common site for leaking.35
Therefore, the standard of practice should be to avoid creating a puncture site. There are 2 reports showcasing new bags developed specifically for multiport contained power morcellation without the need for a puncture site. One is with the MorSafe bag (Veol Technologies, Mumbai, India) which was successfully used in 10 patients,36 as well as another new bag system made by A.M.I. GmbH (Feldkirch, Austria), which has been shown to be comparable to no bag morcellation in 8 in vivo pig models. The results of this study show a prolonged surgery time in the bag group but peritoneal washings negative for muscle cells in all cases with bag use compared to positive cytology in 5 of 8 cases without the bag.37 In addition, Espiner Medical, Ltd. has created a new bag, called the Eco400 T-Sac, which has a lateral sleeve for the laparoscope, which the authors of this paper are currently evaluating (Figure 11).
Figure 11.
The Espiner Eco400 T-Sac.
Two other methods to avoid the puncture site are contained morcellation through a single-site incision and contained vaginal morcellation. We have developed this technique in our practice, and it has been reported in the literature as well.26,39 In the first technique, a single site platform, a morcellator with an extra-long cannula and an angled laparoscope are used. The downsides to this technique are instrument crowding and the need for a larger incision leading to potential increased postoperative pain, a slower return to normal activities, increased infection risk, and bleeding risk, as well as worse cosmesis.1 Contained vaginal morcellation is typically performed in a total laparoscopic hysterectomy with a manual morcellation technique if the uterus cannot be delivered intact. Once the specimen is in the bag and the bag is brought out through the vagina, an Alexis O Wound Protector/Retractor can be placed inside the bag to allow better visualization. Contained vaginal morcellation has been described using the LapSac and the EndoCatch in patients with known malignancy with no postoperative local or distant recurrence.41,42
Finally, the PneumoLiner (Olympus, Center Valley, Pennsylvania, USA) is the first contained morcellation bag that has been cleared by the FDA for marketing. This bag requires a 2–2.5-cm incision and an angled laparoscope and is marketed with the PlasmaSORD bipolar morcellator (Olympus America, Melville, NY, USA).
The strength of this study is that we are reporting on a large cohort of patients who underwent contained power morcellation. None of our complications, admissions, or readmissions are related to the use of the bag. The main limitation of this study is that, although there were no bag ruptures or failures, there is no measure of actual bag leakage. Although this study proves this technique to be feasible, future studies should focus on bags that avoid the need for a lateral puncture site. In addition, it would be beneficial in future studies to evaluate for physical or cellular leakage from a bag using a validated technique. Another limitation to our study is that there is no comparison group and that it is retrospective in nature. We recommend that more prospective comparative studies and studies evaluating bag leakage be conducted to validate the efficacy of a contained morcellation system.
CONCLUSION
Contained morcellation using the Espiner EcoSac 230 is a feasible, reliable, and reproducible method of contained power morcellation, even with large specimens. The technique was successfully performed in our group on 187 patients with no procedural complications and no bag failures or ruptures. The next step in contained morcellation is the creation of specimen bags developed specifically for power morcellation. The FDA has permitted the marketing of PneumoLiner (Olympus, Center Valley, Pennsylvania, USA), and has approved a study at our institution using the Espiner Eco400 T-Sac. Both of these bags are developed specifically for contained power morcellation and negate the need for a puncture hole in the bag. More studies are needed to prove the efficacy of these bags.
Contributor Information
Courtney Steller, Department of Obstetrics and Gynecology.
Aarathi Cholkeri-Singh, Minimally Invasive Gynecology, Advocate Lutheran General Hospital, Park Ridge, Illinois, USA..
Kirsten Sasaki, Department of Obstetrics and Gynecology; Minimally Invasive Gynecology, Advocate Lutheran General Hospital, Park Ridge, Illinois, USA..
Charles E. Miller, Minimally Invasive Gynecology, Advocate Lutheran General Hospital, Park Ridge, Illinois, USA..
References:
- 1. Nieboer TE, Johnson N, Lethaby A, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database System Rev. 2009;3. [DOI] [PubMed] [Google Scholar]
- 2. Whiteman MK, Hillis SD, Jamieson DJ, et al. Inpatient hysterectomy surveillance in the United States, 2000–2004. Am J Obstet Gynecol. 2008;198:34.e1–34.e7. [DOI] [PubMed] [Google Scholar]
- 3. American College of Obstetrics and Gynecology. Choosing the route of hysterectomy for benign disease. ACOG Committee Opinion Number 444, November 2009 Albany, NY: ACOG. [DOI] [PubMed] [Google Scholar]
- 4. Semm K. Morcellement and suturing using pelviscopy: not a problem any more [in German]. Geburtshilfe Frauenheilkd. 1991;51:843–846. [DOI] [PubMed] [Google Scholar]
- 5. Steiner RA, Wight E, Tadir Y, Haller U. Electrical cutting device for laparoscopic removal of tissue from the abdominal cavity. Obstet Gynecol. 1993;81:471–474. [PubMed] [Google Scholar]
- 6. Carter JE, McCarus SD. Laparoscopic myomectomy: time and cost analysis of power vs. manual morcellation. J Reprod Med. 1997;42:383–388. [PubMed] [Google Scholar]
- 7. Milad MP, Milad EA. Laparoscopic morcellator-related complications. J Minim Invasive Gynecol. 2014;21:486–491. [DOI] [PubMed] [Google Scholar]
- 8. Takeda A, Mori M, Sakai K, et al. Parasitic peritoneal leiomyomatosis diagnosed 6 years after laparoscopic myomectomy with electric tissue morcellation: report of a case and review of the literature. J Minim Invasive Gynecol. 2007;14:770–775. [DOI] [PubMed] [Google Scholar]
- 9. Larrain D, Rabischong B, Khoo CK, et al. “Iatrogenic” parasitic myomas: unusual late complication of laparoscopic morcellation procedures. J Minim Invasive Gynecol. 2010;17:719–724. [DOI] [PubMed] [Google Scholar]
- 10. Cucinella G, Granese R, Calagna G, Somigliana E, Perino A. Parasitic myomas after laparoscopic surgery: an emerging complication in the use of morcellator? Description of four cases. Fertil Steril. 2011;96:e90–e96. [DOI] [PubMed] [Google Scholar]
- 11. Leren V, Langebrekke A, Qvigstad E. Parasitic leiomyomas after laparoscopic surgery with morcellation. Acta Obstet Gynecol Scand. 2012;91:1233–1236. [DOI] [PubMed] [Google Scholar]
- 12. Silverberg SG. Leiomyosarcoma of the uterus: a clinicopathologic study. Obstet Gynecol. 1971;38:613–628. [DOI] [PubMed] [Google Scholar]
- 13. Anupama R, Ahmad SZ, Kuriakose S, Vijaykumar DK, Pavithran K, Seethalekshmy NV. Disseminated peritoneal leiomyosarcomas after laparoscopic ‘myomectomy’ and morcellation. J Minim Invasive Gynecol 2011;18:386–389. [DOI] [PubMed] [Google Scholar]
- 14. Stine JE, Clarke-Pearson DL, Gehrig PA. Uterine morcellation at the time of hysterectomy: techniques, risks, and recommendations. Obstetr Gynecol Surv. 2014;69:415–425. [DOI] [PubMed] [Google Scholar]
- 15. Pritts EA, Vanness DJ, Berek JS, et al. The prevalence of occult leiomyosarcoma at surgery for presumed uterine fibroids: a meta-analysis. Gynecol Surg. 2015;12:165–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Park JY, Park SK, Kim DY, et al. The impact of tumor morcellation during surgery on the prognosis of patients with apparently early uterine leiomyosarcoma. Gynecol Oncol. 2011;122:255–259. [DOI] [PubMed] [Google Scholar]
- 17. Einstein MH, Barakat RR, Chi DS, et al. Management of uterine malignancy found incidentally after supracervical hysterectomy or uterine morcellation for presumed benign disease. Int J Gynecol Cancer. 2008;18:1065–1070. [DOI] [PubMed] [Google Scholar]
- 18. Mowers EL, Skinner B, Mclean K, Reynolds RK. Effects of morcellation of uterine smooth muscle tumor of uncertain malignant potential (STUMP) and endometrial stromal sarcoma (ESS): case series and recommendations for clinical practice. J Minim Invasive Gynecol. 2015;22:601–606. [DOI] [PubMed] [Google Scholar]
- 19. Pritts EA, Parker WH, Brown J, Ollive DL. Outcome of occult uterine leiomyosarcoma after surgery for presumed uterine fibroids: a systematic review. J Minim Invasive Gynecol. 2015;22:26–33. [DOI] [PubMed] [Google Scholar]
- 20. Brölmann H, Tanos V, Grimbizis G, et al. European Society of Gynaecological Endoscopy (ESGE) Steering Committee on Fibroid Morcellation: options on fibroid morcellation: a literature review. Gynecol Surg. 2015;12:3–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brown J. AAGL advancing minimally invasive gynecology worldwide: statement to the FDA on power morcellation. J Minim Invasive Gynecol. 2014;21:970–971. [DOI] [PubMed] [Google Scholar]
- 22. US Food and Drug Administration. Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy. FDA Safety Communication. Silver Spring, MD: USFDA, April 2014. [Google Scholar]
- 23. US Food and Drug Administration. UPDATED Laparoscopic Uterine Power Morcellation in Hysterectomy and Myomectomy: FDA Safety Communication. Silver Spring, MD: USFDA, November 2014. [Google Scholar]
- 24. Siedhoff MT, Wheeler SB, Rutstein SE, et al. Laparoscopic hysterectomy with morcellation vs abdominal hysterectomy for presumed fibroid tumors in premenopausal women: a decision analysis. Am J Obstet Gynecol. 2015;212:591.e1–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Brown J, regarding data by. Naumann RW, Herzog TJ, Coleman RL. Laparoscopy versus the risk of leiomyosarcoma morcellation: a decision analysis. Presented to the Obstetrics and Gynecology Devices Panel of the Medical Devices Advisory Committee Silver Spring, MD: US Food and Drug Administration; July 11, 2014. [Google Scholar]
- 26. Cohen SL, Einarsson JI, Wang KC, et al. Contained power morcellation within an insufflated isolation bag. Obstet Gynecol. 2014;124:491–497. [DOI] [PubMed] [Google Scholar]
- 27. Einarsson JI, Cohen SL, Fuchs N, Wang KC. In-bag morcellation. J Minim Invasive Gynecol. 2014;21:951–953. [DOI] [PubMed] [Google Scholar]
- 28. Vargas MV, Cohen SL, Fuchs-Weizman N, et al. Open power morcellation versus contained power morcellation within an insufflated isolation bag: comparison of perioperative outcomes. J Minim Invasive Gynecol. 2015;22:433–438. [DOI] [PubMed] [Google Scholar]
- 29. Winner B, Porter A, Velloze S, et al. Uncontained compared with contained power morcellation in total laparoscopic hysterectomy. Obstet Gynecol 2015;126:834–838. [DOI] [PubMed] [Google Scholar]
- 30. Kanade TT, McKenna JB, Choi S, et al. Sydney contained in bag morcellation for laparoscopic myomectomy. J Minim Invasive Gynecol. 2014;21:981. [DOI] [PubMed] [Google Scholar]
- 31. Srouji SS, Kaser DJ, Gargiulo AR. Techniques for contained morcellation in gynecologic surgery. Fertil Steril. 2015;103:e34. [DOI] [PubMed] [Google Scholar]
- 32. Kondrup JD, Anderson F, Sylvester B, Branning M. Laparoscopic morcellation and tissue spillage containment using the LI EndofieldTM Bag. Surg Technol Int. 2014;25:162–166. [PubMed] [Google Scholar]
- 33. McKenna JB, Kanade T, Choi S, et al. The Sydney Contained In Bag Morcellation Technique. J Minim Invasive Gyencol. 2014;21:984–985. [DOI] [PubMed] [Google Scholar]
- 34. Akdemir A, Taylan E, Zeybek B, Ergenoglu AM, Sendag F. Innovative technique for enclosed morcellation using a surgical glove. Obstet Gynecol. 2015;125:1145–1149. [DOI] [PubMed] [Google Scholar]
- 35. Cohen SL, Morris SN, Brown DN, et al. Contained tissue extraction using power morcellation: prospective evaluation of leakage parameters. Am J Obstet Gynecol. 2016;214:257.e1–e6. [DOI] [PubMed] [Google Scholar]
- 36. Paul PG, Thomas M, Das T, Patil S, Garg R. Contained morcellation for laparoscopic myomectomy within a specially designed bag. J Minim Invasive Gynecol. 2016;23;257–260. [DOI] [PubMed] [Google Scholar]
- 37. Rimbach S, Holzknecht A, Nemes C, Offner F, Craina M. A new in-bag system to reduce the risk of tissue morcellation: development and experimental evaluation during laparoscopic hysterectomy. Arch Gynecol Obstet. 2015;292:1311–1120. [DOI] [PubMed] [Google Scholar]
- 38. Cholkeri-Singh A, Miller CE. Power morcellation in a specimen bag. J Minim Invasive Gynecol. 2015;22:160. [DOI] [PubMed] [Google Scholar]
- 39. Kujansuu S, Salari BW, Galloway M, et al. Contained morcellation using the GelPOINT advance access platforms and 3M Steri-Drape endobag. Fertil Steril. 2015;103:e36. [DOI] [PubMed] [Google Scholar]
- 40. Green AK, Hodin RA. Laparoscopic splenectomy for massive splenomegaly using a Lahey bag. Am J Surg. 2001;181:534–536. [DOI] [PubMed] [Google Scholar]
- 41. Favero G, Anton C, Silva e Silva A, et al. Vaginal morcellation: a new strategy for large gynecological malignant tumor extraction, a pilot study. Gyencol Oncol. 2012;126:443–447. [DOI] [PubMed] [Google Scholar]
- 42. Gunthert AR, Christmann C, Kostov P, et al. Safe vaginal uterine morcellation following total laparoscopic hysterectomy. Am J Obstet Gynecol. 2015;212:546.e1–e4. [DOI] [PubMed] [Google Scholar]