Abstract
Proprotein Convertase Subtilisin/Kexin type 9 (PCSK9) promotes atherosclerosis by increasing low-density lipoprotein (LDL) cholesterol levels through degradation of hepatic LDL receptors (LDLR). Studies have described the systemic effects of PCSK9 on atherosclerosis, but whether PCSK9 has local and direct effects on the plaque in unknown. To study the local effect of human PCSK9 (hPCSK9) on atherosclerotic lesion composition independently of changes in serum cholesterol levels we generated chimeric mice expressing hPCSK9 exclusively from macrophages using marrow from hPCSK9 transgenic (hPCSK9tg) mice transplanted into apoE−/− and LDLR−/− mice, which were then placed on a high fat diet for 8 wk. We further characterized the effect of hPCSK9 expression on the inflammatory responses in the spleen and by mouse peritoneal macrophages (MPM) in vitro. We found that MPM from transgenic mice express both murine (m) Pcsk9 and hPCSK9 and that the latter reduces macrophage LDLR and LRP1 surface levels. hPCSK9 was detected in serum of mice transplanted with hPCSK9tg marrow, but did not influence lipid levels or atherosclerotic lesion size. However, marrow-derived PCSK9 progressively accumulated in lesions of apoE−/− recipient mice while increasing the infiltration of Ly6Chi inflammatory monocytes by 32% compared with controls. Expression of hPCSK9 also increased CD11b and Ly6Chi positive cell numbers in spleens of apoE−/− mice. In vitro, expression of hPCSK9 in LPS-stimulated macrophages increased mRNA levels of the pro-inflammatory markers Tnf and Il1b (40% and 45%, respectively) and suppressed those of the anti-inflammatory markers Il10 and Arg1 (30% and 44%, respectively). All PCSK9 effects were LDLR-dependent as PCSK9 protein was not detected in lesions of LDLR−/− recipient mice and did not affect macrophage or splenocyte inflammation. In conclusion, PCSK9 directly increases atherosclerotic lesion inflammation in an LDLR-dependent but cholesterol-independent mechanism, suggesting that therapeutic PCSK9 inhibition may have vascular benefits secondary to LDL reduction.
Keywords: Atherosclerotic lesion, Inflammation, Macrophages, Mouse models, LDLR, PCSK9
Introduction
Proprotein Convertase Subtilisin/Kexin type 9 was identified as a gene (PCSK9) whose gain-of-function mutants can cause autosomal dominant hypercholesterolemia [1]. PCSK9 is a circulating protein synthesized primarily by liver, intestine, and kidney. Produced as a 75 kDa precursor, it undergoes autocatalytic cleavage in the ER, thus releasing the 62 kDa mature PCSK9 protein [2]. Once in the circulation, PCSK9 regulates the concentration of low-density lipoprotein receptor (LDLR) [3–6]. Published data suggest that PCSK9 may target other members of the LDLR family, such as LRP1 [7, 8] which regulates the inflammatory responses within the atheroma through both apoE-dependent and independent pathways [9–11]. Besides its systemic effects, recent evidence suggests that PCSK9 is secreted by smooth muscle cells (SMC), and that SMC-derived PCSK9 causes macrophage LDLR degradation, introducing the notion that PCSK9 modulation of LDLR levels in the arterial wall may influence lesion biology [12].
Under hyperlipidaemic conditions, circulating monocytes are recruited into the lesion, mature into macrophages, and contribute to the progression of atherosclerosis [13, 14]. The Ly6Chi positive monocyte subpopulation is associated with acute inflammation and can be mobilized from the bone marrow in response to hypercholesterolaemia during the early stages of atherosclerosis [15]. Not only Ly6Chi monocytes infiltrate atherosclerotic lesions more easily than Ly6Clo, but also give rise to classically activated macrophages, responsible for the secretion of pro-inflammatory cytokines, such as Interleukin 1 beta (IL1b) and Tumor necrosis factor-α (Tnf) [13, 16]. Macrophages show a high degree of plasticity in response to different stimuli [17]. For example, Il-4 induces polarization toward an anti-inflammatory M2 phenotype, characterized by increased levels of Arginase 1 (Arg1) and secretion of Il10, whereas LPS induces a switch toward an M1 phenotype, characterized by secretion of pro-inflammatory cytokines Il1b and Tnf [18].
Recently a direct link was suggested between PCSK9 and inflammation, as PCSK9 levels were found to correlate with white blood cells count in patients with stable coronary artery disease [19]. Moreover, in vitro experiments of PCSK9 knock-down via small interfering RNA have shown a role for PCSK9 in the inflammatory response to oxLDL by macrophages [20]. In addition, anti-PCSK9 therapy with monoclonal antibodies has been shown to reduce inflammatory monocyte recruitment and improve lesion composition in hypercholesterolaemic mice [21].
Foam cell formation, the hallmark of atherosclerosis, is a consequence of lipoprotein uptake by lesion macrophages. LDLR mediates the uptake of native, unmodified LDL and should only exert a minor role in lesion development because of its tight and rapid feedback regulation compared with the massive lipid entry via scavenger receptors [22, 23]. However, we demonstrated that the absence of macrophage LDLR in C57BL/6 mice with diet-induced hyperlipidaemia reduces foam cell formation, suggesting a more active role for macrophage LDLR in the progression of the disease [24]. Thus, it is possible that PCSK9 action in the atheroma may influence disease progression via the loss of LDLR by lesion cells and aggravation of the inflammatory state. Moreover, published data suggest that PCSK9 interacts with other members of the LDLR family, such as LRP1 [7]. Since macrophage LRP1 deficiency in mice has been associated with increased atherosclerosis, it is also possible that PCSK9 interaction with LRP1 in the plaque influences the local inflammatory response.
The aim of our study was to determine the effect of PCSK9 on atherosclerotic lesion inflammation independently of systemic cholesterol changes. We generated apoE−/− and LDLR−/− mice expressing human PCSK9 (hPCSK9) from bone marrow-derived cells and placed them on a high fat diet (42% of calories from fat) for 8 wk. The experimental decision to drive overexpression of hPCSK9 was driven by the notoriously poor performance of antibodies against murine Pcsk9 in immuno-histological analyses. Additionally, since mPcsk9 and hPCSK9 may exhibit different functional characteristics, our model is more relevant to the human condition. It was previously shown that absence of LDLR in apoE−/− mice does not affect cholesterol levels [25]. Similarly, Pcsk9 expression or deletion alters LDLR levels but does not affect cholesterol levels in apoE−/− mice [26, 27]. Finally, Pcsk9 overexpression or deletion has no effect on cholesterol levels in the absence of LDLR [27]. We used local PCSK9 expression in apoE or LDLR null mice to study the direct effect of PCSK9 in the atheroma without systemic effects on plasma lipoproteins. Our results show that PCSK9 produced locally in the atheroma affects lesion composition by increasing plaque monocyte infiltration, and macrophage inflammation in an LDLR-dependent fashion. Taken together, our results suggest a direct role for PCSK9 in mediating the inflammation in atherogenesis, and imply that additional anti-inflammatory effects may be expected from pharmacological inhibition of PCSK9.
Methods
Mice
C57BL/6 (Wild type, WT), apoE−/−, LDLR−/− and mPCSK9−/− mice were purchased from the Jackson Laboratories (Bar Harbor, ME). Transgenic mice were generated to express a human PCSK9 construct containing a CMV promoter, as reported by us in a previous publication [28]. Detailed housing and breeding information appears in Supplementary information online.
Collection of macrophages from the peritoneal cavity
Murine peritoneal macrophages (MPM) were collected in PBS three to four d after peritoneal injection of 5% thioglycollate, as described in details in Supplementary information online.
Analysis of hPCSK9 and mPcsk9 expression
MPM were collected as described above and incubated for 24h h in DMEM containing 10% FBS. Total RNA was isolated and the relative quantification of mRNA expression was analysed by reverse transcription and Quantitative Real Time-PCR (QRT-PCR) with the ABI Prism 7700 Sequence Detection System or standard PCR. Primers sequence and detailed methods appears in Supplementary information online. In addition, media were collected and ELISA was used to measure hPCSK9 and mPcsk9 secretion.
Immunoprecipitation
MPM from hPCSK9tg mice were lysed and a rat antibody specific for hPCSK9 was used to immunoprecipitate hPCSK9 with Protein G magnetic beads, according to the manufacturer’s instructions. Rat IgG was used as control. Detailed methods appear in Supplementary information online.
Bone marrow transplantation (BMT)
Recipient mice were lethally irradiated (9 Gy) using a γ137 source and transplanted with 5 million bone marrow cells from 8 wk transgenic or control mice, as described previously [30, 31]. After 4 wk on a regular diet, all mice were placed on a HFD (42% of calories from fat, Harlan laboratories, Indianapolis, IN) for 8 wk and then the extent of atherosclerosis was examined. Numbers of mice used in each experiment and detailed BMT information appears in Supplementary information online.
Aorta lesion analysis
Serial 10 μm thickness sections were taken in the region of the proximal aorta starting from the end of the aortic sinus and for 300 μm distally, as described previously [31]. Sections were stained with Oil Red O and counterstained with haematoxylin. Quantitative analysis of lipid-stained lesions was performed on 15 alternate cryosections. Cross-sections of the proximal aorta were analysed using the KS300 imaging system (Kontron Elektronik GmbH), as described by Linton and colleagues [31].
Serum lipoprotein and protein analysis
Serum was collected from overnight fasted animals and total cholesterol (TC) and triglyceride (TG) levels were determined by enzymatic colourimetric assay kits, as suggested by the manufacturer. Serum hPCSK9 levels were determined by ELISA, with a measured 5% cross reactivity between mPcsk9 and hPCSK9 within the linear range [6].
Immunohistochemistry and immunofluorescence
To detect hPCSK9 in tissues, 5 μm thick cryosections were incubated with anti-PCSK9 primary antibody followed by a goat anti-human secondary antibody. Sections were mounted in ProLong Gold with DAPI media and imaged on a confocal microscope, as described in detail in Supplementary information online.
Lesion content of the pro-inflammatory monocyte marker Ly6C was analysed using a rat anti-mouse Ly6C antibody conjugated to biotin and streptavidin-AlexaFluor 488. Sections were mounted with Vectashield containing DAPI to stain the nuclei. Additional details are presented in Supplementary information online.
Macrophage differentiation
MPM were washed, followed by incubation with LPS for 4 h as detailed in Supplementary information online. Total RNA was isolated using Trizol reagent and processed for reverse transcription. Relative quantification of mRNA levels was performed with the ABI Prism 7700 Sequence Detection System using TaqMan Gene expression assays. Expression levels were calculated using the ΔΔCT method and normalized to 18S ribosomal RNA as internal control [32, 33].
Flow Cytometry analyses
(1) surface LDLR and LRP1 levels: Single-cell suspensions of MPM were incubated with PE-labelled LDLR antibody or fluorescently labelled 5A6 LRP clone, as described in detail in Supplementary information online. Labelled cells were analysed on a MACS quant seven-colour flow cytometer (Miltenyi Biotech) and data were assessed with FlowJo software (Tree Star, Ashland, OR). (2) Splenic CD11b and Ly6Chi positive cells: Spleens were homogenized, and one million cells were stained with FITC rat anti-mouse CD90.2, B220, GR1 and NK cells, while monocytes were detected with rat anti-mouse CD11b-PE and rat anti-mouse Ly6C conjugated with biotin and streptavidin-linked AlexaFluor 647. Further detail is presented in Supplementary information online.
Statistical Analyses
Statistical analyses were carried out using GraphPad Prism Software. The Mann-Whitney test was used to compare atherosclerosis data between two groups, while student’s paired t-test was used for all the other analyses. Results are presented as means±SEM or as percentages ± CV. *p<0.05, **p<0.01, ***p<0.001.
Materials
A detailed list of all materials used in the described experiments can be found in Supplementary information online.
Results
PCSK9 is expressed by macrophages in its active form
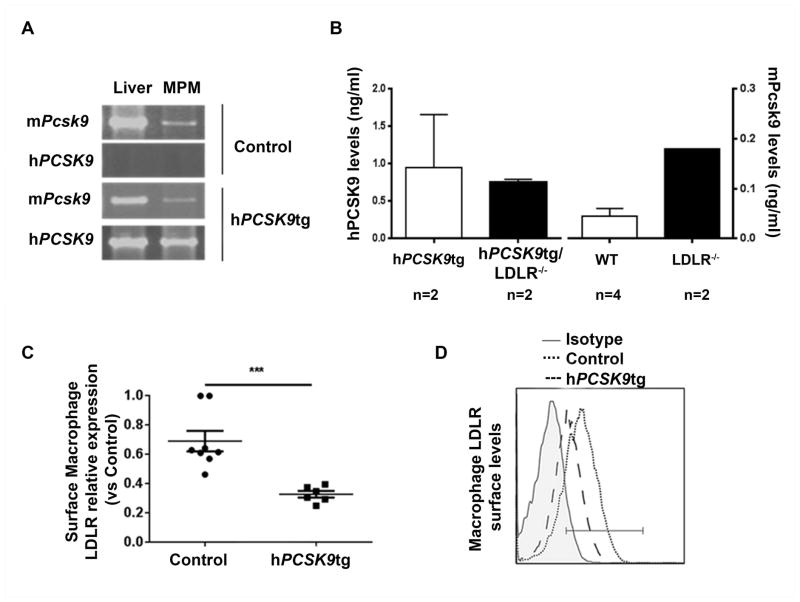
To test whether macrophages can express PCSK9 in the atherosclerotic lesion we first studied expression and function of hPCSK9 in MPM from the transgenic mice developed in our lab [6, 28]. In these mice, MPM synthesize hPCSK9 (Figure 1A) and secrete the protein into the culture medium (Figure 1B). We found that PCSK9 directly interacts with LDLR and LRP1 (Supplementary Figure 1A). Using flow cytometry analysis, we confirmed that macrophage hPCSK9 is active, becasue cell-surface LDLR levels in these MPM were significantly reduced (−52±8%) compared with control cells (Figure 1C/D). Using the same approach, we also found that macrophage hPCSK9 reduced LRP1 cell-surface levels; interestingly, this reduction was restricted to mice lacking apoE (Supplementary Figure 1B). Figure 1A/B and Supplementary Figure 2A show that mPcsk9 is also naturally expressed and secreted by MPM (though at levels lower than those of hPCSK9 in transgenic MPM), supporting the physiological relevance of studying PCSK9 expression in macrophages. As previously shown with other tissues [6], the presence of hPCSK9 did not alter mPcsk9 expression in MPM (Supplementary Figure 2A).
Figure 1. PCSK9 expression in murine peritoneal macrophages and LDLR degradation.
(A) mRNA levels of mPcsk9 and hPCSK9 in livers and MPM from WT and hPCSK9tg mice, as assessed by reverse transcription/PCR (B) hPCSK9 and mPcsk9 secretion in culture medium by MPM, as measured by ELISA (C) Flow cytometry analysis of surface LDLR levels in MPM from Control and hPCSK9tg mice. Student’s paired t-test was used for the analysis, ***p<0.0001. (D) Representative diagram of surface LDLR expression, as assessed by flow cytometry.
PCSK9 increases Ly6Chi positive cells in the atheroma in apoE−/− mice
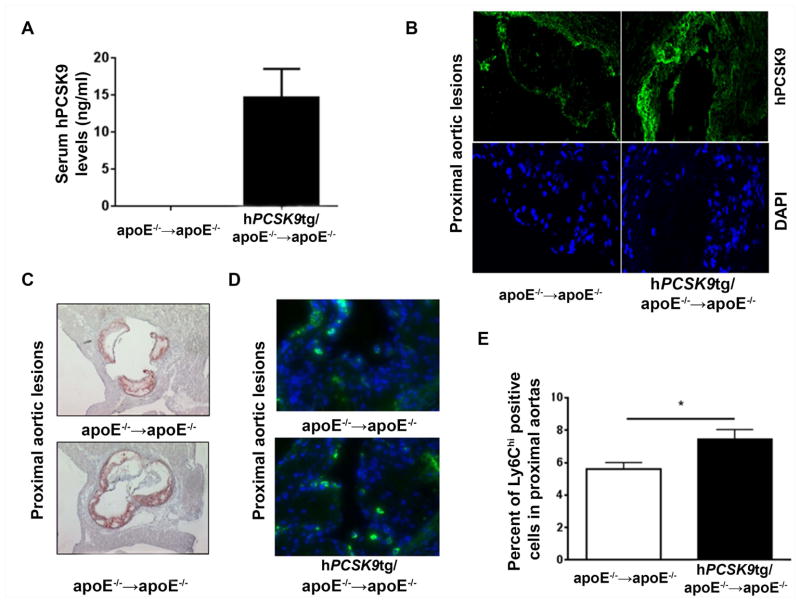
To study the direct effect of hPCSK9 on atherosclerotic lesion composition, bone marrow cells from hPCSK9tg/apoE−/− or apoE−/− mice were transplanted into apoE−/− recipients. After 8 wk on HFD, hPCSK9 was detected at low levels (14.6±3.9 ng/ml) in serum (Figure 2A) and in aortic lesions (Figure 2B and Supplementary Figure 3) of hPCSK9tg marrow recipient mice. This represents the first evidence that PCSK9 produced by bone marrow-derived cells reaches the circulation. It was previously reported that overexpression or knock-down of Pcsk9 in the absence of apoE does not affect serum lipid levels [26, 27]. We also found no differences in serum cholesterol (861±73 mg/dl vs. 881±52 mg/dl, respectively, Supplementary Figure 4A) or triglyceride levels (1431±36 mg/dl vs. 1441±26 mg/dl, respectively, Supplementary Figure 4B) between apoE−/−→apoE−/− and hPCSK9tg/apoE−/−→apoE−/− mice. In the absence of changes in lipid levels, no differences were found in lesion area at the aortic sinus (278 012±4 945 μm2 vs 238 505±34 621 μm2, Figure 2C and Supplementary Figure 4C) between the two groups.
Figure 2. Macrophage PCSK9 is detected in both serum and atherosclerotic lesions and increases infiltration of lesional Ly6Chi positive cells.
(A) Serum hPCSK9 levels measured in apoE−/− mice transplanted with bone marrow from apoE−/− or hPCSK9tg/apoE−/− mice (B) Representative immunofluorescence images showing hPCSK9 in lesions of proximal aortas in apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− mice. In green: hPCSK9; in blue: DAPI (C) Representative atherosclerotic lesions in cross-sections of proximal aortas of apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− mice stained with Oil red O at 12 wk post-BMT (8 wk on HFD) (D) Representative immunofluorescence images of Ly6C in atherosclerotic lesions of proximal aortas of apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− mice (E) Quantitation of the percentage of Ly6Chi positive cells compared to total number of cells in atherosclerotic lesions of proximal aortas of apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/−. Student’s paired t-test was used for the analysis, *p<0.05.
However, local accumulation of hPCSK9 (Figure 2B) induced significant changes in lesion composition, with a large and statistically significant 32% increase in inflammatory Ly6Chi positive cells in hPCSK9tg marrow recipients compared with control recipients (7.4±1.5% vs. 5.6±1.1%, respectively), (Figure 2D/E and Supplementary Figure 5). These results suggest that local PCSK9 drives infiltration of inflammatory monocytes into the lesion by mechanisms not related to cholesterol levels in serum or the aorta.
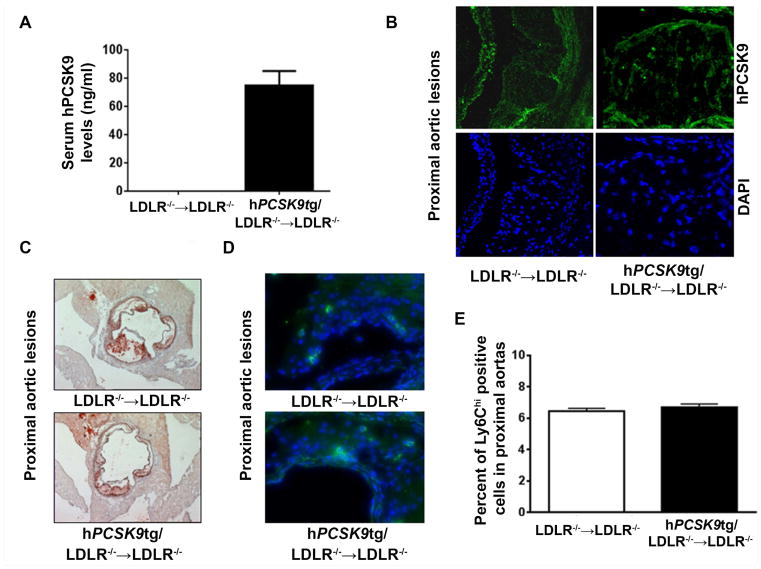
Next, we studied whether the lesion composition changes observed in mice with hPCSK9tg/apoE−/− macrophages are dependent on the presence of LDLR, the consensus target of PCSK9 action. Bone marrow cells from hPCSK9tg/LDLR−/− and LDLR−/− mice were transplanted into LDLR−/− recipients fed a HFD for 8 wk. Serum levels of hPCSK9 in LDLR−/− recipients were on average more than five times higher (74.9±10.1 ng/ml; Figure 3A) compared with apoE−/− recipients (14.6±3.9 ng/ml, Figure 2A), due to impaired hepatic clearance of secreted hPCSK9 in mice lacking LDLR [6]. Despite its high circulating levels, hPCSK9 was not detectable in the lesion of LDLR−/− mice transplanted with hPCSK9tg/LDLR−/− marrow (Figure 3B and Supplementary Figure 6). Interestingly, in vitro incubation of MPM lacking apoE−/− or LDLR−/− with recombinant hPCSK9 showed that PCSK9 internalization by MPM is LDLR-independent (Supplementary Figure 7).
Figure 3. PCSK9-mediated increase in lesional Ly6Chi positive cells is LDLR-dependent.
(A) Serum hPCSK9 levels measured in LDLR−/− mice transplanted with bone marrow from LDLR−/− or hPCSK9tg/LDLR−/− mice (B) Representative immunofluorescence images showing hPCSK9 in atherosclerotic lesions of proximal aortas in LDLR−/−→LDLR−/−or hPCSK9tg/LDLR−/−→LDLR−/− mice. In green: hPCSK9; in blue: DAPI (C) Representative atherosclerotic lesions in cross-sections of proximal aortas of LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− mice stained with Oil red O at 12 wk post-BMT (8 wk on HFD) (D) Representative immunofluorescence images of Ly6C in atherosclerotic lesions of proximal aortas of LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− mice (E) Quantitation of the percentage of Ly6Chi positive cells compared to total number of cells in atherosclerotic lesions of proximal aortas of LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− Student’s paired t-test was used for the analysis.
As expected [27], no significant changes were observed between the two mice groups in either serum cholesterol levels (996±26 mg/dl in LDLR−/−→LDLR−/− vs. 966±31 mg/dl in hPCSK9tg/LDLR−/−→LDLR−/−; Supplementary Figure 4D), triglyceride levels (1013±123 mg/dl vs in LDLR−/−→LDLR−/− vs. 859±141 mg/dl in hPCSK9tg/LDLR−/−→LDLR−/−, Supplementary Figure 4E), or lesion size (110 740± 20 436 μm2 in LDLR−/−→LDLR−/− vs. 104 355±15 948 μm2 in hPCSK9tg/LDLR−/−→LDLR−/−, Figure 3C and Supplementary Figure 4F).
Whereas, as described above, macrophage expression of hPCSK9 in the atheroma caused inflammation in apoE−/− mice (Figure 2D/E), it did not induce an increase in Ly6Chi positive cells in LDLR−/− mice (5.9±0.9% in LDLR−/−→LDLR−/− vs. 5.9±0.8% in hPCSK9tg/LDLR−/−→LDLR−/−; Figure 3D/E and Supplementary Figure 8), suggesting that PCSK9-mediated inflammation is LDR dependent.
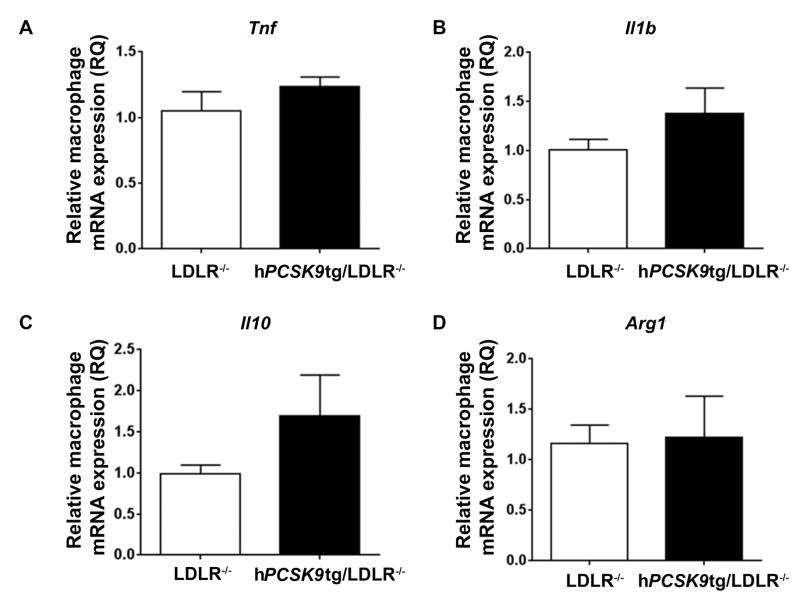
PCSK9 induces monocyte and macrophage inflammation
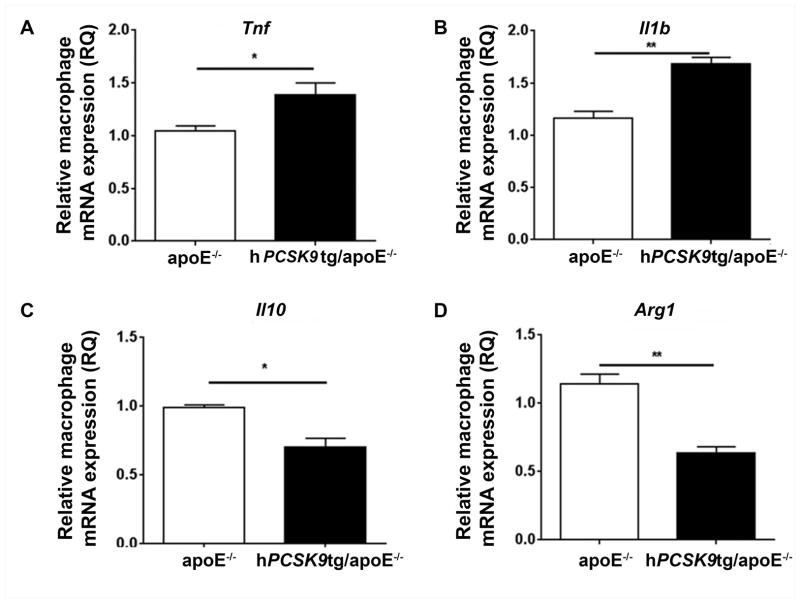
To investigate the inflammatory effects of hPCSK9 directly in macrophages, we collected MPM from both hPCSK9tg/apoE−/− and apoE−/− mice and exposed them to LPS, which is known to induce acute inflammatory responses [34]. In vitro analysis of cytokine expression revealed increased mRNA expression of pro-inflammatory Tnf and Il1b (40% and 45%, respectively; Figure 4A/B) and decreased expression of anti-inflammatory genes Il10 and Arg1 (30% and 44%, respectively; Figure 4C/D) in apoE−/− MPM expressing hPCSK9. The changes observed in the mRNA levels were measured after 4 h of incubation with LPS. This time is appropriate for detection of synthetic changes, but likely insufficient to show changes in the accumulation of inflammatory proteins [52, 53]. Similarly to our in vivo findings, the absence of LDLR abolished this effect, and expression of hPCSK9 was not associated with significant changes in mRNA levels of either pro-inflammatory (Figure 5A/B) or anti-inflammatory (Figures 5C/D) markers compared with LDLR−/− controls.
Figure 4. PCSK9 increases the expression of pro-inflammatory cytokines in LPS-stimulated MPM.
MPM were stimulated with LPS (50 ng/ml) for 4h. mRNA levels of Tnf (A), Il1b (B), Il10 (C) and Arg1 (D) in LPS-stimulated MPM from hPCSK9tg/apoE−/− and apoE−/− was analysed by RT-qPCR. Student’s paired t-test was used for the analysis, *p<0.05, **p<0.01.
Figure 5. PCSK9 increase in macrophage inflammation is LDLR-dependent.
MPM were stimulated with LPS (50 ng/ml) for 4h. mRNA levels of Tnf (A), Il1b (B), Il10 (C) and Arg1 (D) in LPS-stimulated MPM from hPCSK9tg/LDLR−/− and LDLR−/− was analysed by RT-qPCR. Student’s paired t-test was used for the analysis.
To test whether the contribution of PCSK9 to macrophage inflammation is specific to the human protein, we analysed the effect of mPcsk9 on MPM inflammation. After exposure to LPS, the expression of the pro-inflammatory markers Il1b and Tnf was significantly reduced by 50% and 44%, respectively, in MPM from mice lacking mPCSK9 compared to controls (Supplementary Figure 9A/B), suggesting that both mPcsk9 and hPCSK9 induce inflammation locally.
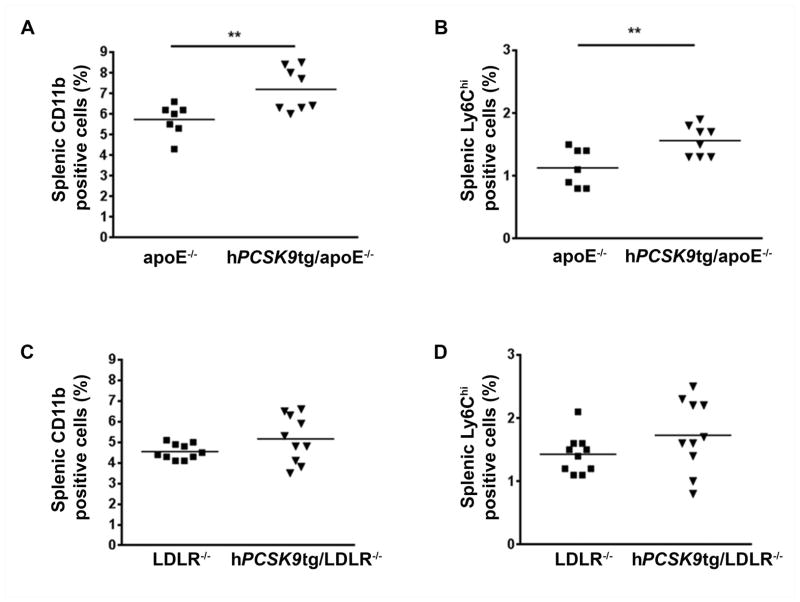
To further study the possible role for hPCSK9 in systemic inflammation under pro-atherogenic conditions, we examined the effects of hPCSK9 on the distribution of splenic monocyte subsets, by flow cytometry, in apoE−/− and LDLR−/− expressing hPCSK9 mice after 8 wk on HFD. Expression of hPCSK9 in apoE−/− mice significantly increased all splenic monocytes (defined as CD11bhiCD90loB220loNK1.1loLy-6Glo [13]) by 21% compared with apoE−/− controls (Figure 6A). The expression of hPCSK9 also increased the percentage of pro-inflammatory Ly6Chi positive monocytes by 38% compared with apoE−/− controls (Figure 6B), thus suggesting that an increase in splenic levels of pro-inflammatory monocytes might favour their accumulation in the lesion. Similarly to our in vivo and in vitro data, hPCSK9 expression in LDLR−/− mice did not affect levels of splenic CD11b (5.2±0.4% vs. 4.6±0.4% in LDLR−/− controls, Figure 6C) or Ly6Chi monocytes (1.7±0.2% vs. 1.4±0.1% in LDLR−/−, Figure 6D).
Figure 6. PCSK9 inflammatory effects in the spleens are LDLR dependent.
Flow cytometry analysis of CD11b (A) and Ly6Chi (B) positive cells in the spleens of apoE−/− and hPCSK9tg/apoE−/− mice fed a HFD for 8 wk. Flow cytometry analysis of CD11b (C) and Ly6Chi (D) positive cells in the spleens of LDLR−/− and hPCSK9tg/LDLR−/− mice fed a HFD for 8 wk. Student’s paired t-test was used for the analysis, **p<0.01.
Discussion
PCSK9 action increases serum cholesterol levels [1], and inhibition of PCSK9 is expected to produce cardiovascular benefits through cholesterol-lowering. We set out to study whether human PCSK9 has a direct effect on the atherosclerotic plaque in the absence of serum cholesterol changes. We found that PCSK9 is expressed and secreted by macrophages (both naturally and in the transgenic setting) and is active in reducing macrophage LDLR levels. We also showed that PCSK9 reduces LRP1 levels. However, surface LRP1 reduction was only observed in the absence of apoE, a ligand for LRP1 [9–11, 35]. To study the local effect of PCSK9 in the atheroma, apoE−/− and LDLR−/− mice were transplanted with bone marrow from hPCSK9tg mice of the same background (apoE−/− or LDLR−/−, respectively). Despite no changes in lipid levels or lesion size, lesion composition analysis showed LDLR-dependent increases in pro-inflammatory monocytes in the lesion. Similarly, expression of pro-inflammatory and anti-inflammatory cytokines was significantly altered by macrophage hPCSK9 in an LDLR-dependent fashion. The effect of macrophage PCSK9 was not dependent on whether the protein was human or murine.
PCSK9 targets the LDLR toward lysosomal degradation, leading to increased total and LDL cholesterol levels in humans and mice [1, 36, 37]. It was previously shown that the complete absence of both LDLR and apoE does not significantly increase serum cholesterol above the already elevated levels seen in apoE−/− mice [38]. It was previously shown that the over-expression of Pcsk9 in apoE−/− and LDLR−/− mice does not affect serum lipid levels [26, 27], whereas overexpression of murine Pcsk9 increases lesion size in apoE−/− but not in LDL−/− mice [27]. These observations suggest an LDLR-dependent, direct effect of Pcsk9 on atherosclerotic lesion development. Accordingly, we wanted to investigate the role of locally produced PCSK9 in the biology of the atheroma, independently of systemic lipid alterations. First, we demonstrated that hPCSK9 is expressed and secreted in the culture medium by macrophages (Figure 1A/B). Then, we showed that mPcsk9 is naturally expressed and secreted by macrophages (Figure 1A/B). However, due to the poor performance of antibodies against murine Pcsk9 in immuno-histological analyses, we used cells from hPCSK9tg mice as our model system to study the local effects of PCSK9 on plaque inflammation, with the working hypothesis that local PCSK9 accumulation has direct effect on lesion composition.
Ferri et al. have previously shown that SMC in the atheroma express Pcsk9 and exert paracrine effects on macrophage LDLR levels in culture [12]. The same group reported that other cell types populating the plaque, such as endothelial cells and macrophages, do not express Pcsk9. [12]. In contrast, another recent study suggests that PCSK9 is produced by macrophages [20]. We show that hPCSK9 expressed from macrophages accumulates in serum of apoE−/− and LDLR−/− recipient mice (14.6±3.9 and 74.9±10.1 ng/ml, respectively) (Figure 2A and 3A). These levels are between 0.5 and 1.5% of those seen in transgenic mice with systemic hPCSK9 expression, suggesting that macrophage contribution to plasma PCSK9 is small, and that the plaque environment may be enriched in macrophage-derived PCSK9. We also show that murine macrophages naturally secrete PCSK9. Although these results are in contrast with a previous study in which Pcsk9 expression in macrophages was not detected, we performed additional experiments yielding results that begin to explain the discrepancy. We now show that using RT-qPCR primers (as used by Ferri et al.), we are not able to detect mPcsk9 in macrophages (Supplementary Figure 2B). In contrast, standard reverse transcription/PCR clearly shows Pcsk9 in macrophages from WT mice, but not from mPcsk9−/− (Supplementary Figure 2A). Our hypothesis is that the lack of ability to detect Pcsk9 in macrophages, as described by Ferri et al. [12] was due to a technical issue related to the primers used. More importantly, we showed for the first time that macrophage-derived PCSK9 accumulates in the atherosclerotic lesion via a mechanism that is strictly LDLR dependent.
Our data show that in vitro MPM secrete low levels of PCSK9 (Figure 1B), and internalize PCSK9 regardless of LDLR expression (Supplementary Figure 7). However, hPCSK9 was barely detectable in lesions of mice lacking LDLR after transplantation with bone marrow-derived cells expressing hPCSK9 (Figure 3B). These results suggest that in vivo the local accumulation of PCSK9 in the atheroma depends on LDLR expression, whereas the in vitro setting allows phagocytotic processes to predominate. Besides the obvious differences between the in vivo and in vitro model, it is important to mention that MPM and bone marrow-derived cells differ in the expression of surface receptors and in internalization processes [39]. It is possible that the LDLR is required for PCSK9 internalization by arterial macrophages given the high outward flow pressure in the plaque [40, 41], a limiting condition that cannot be replicated in vitro.
As expected, the absence of serum lipid changes combined with the low serum PCSK9 levels did not have significant effects on the size of atherosclerotic lesions (Figure 2C). However, significant changes were found in lesion composition, with increased inflammatory Ly6Chi monocytes in the lesions of apoE−/− recipients of hPCSK9tg marrow compared with controls (Figure 2D/E). It has been demonstrated previously that lesion composition changes induced by pro-inflammatory leukocytes are a key determinant in atherosclerosis progression, even in the absence of changes in either plasma cholesterol levels or lesion size [42], and that lesion composition rather than size favours rupture and thrombosis [43]. It is thus possible to infer that, even in the absence of changes in lipid levels, hPCSK9 directly contributes to atherogenesis by altering plaque morphology and increasing inflammatory Ly6Chi monocyte infiltration into the lesion and their differentiation to macrophages. Since PCSK9 accumulation in tissues depends on LDLR [6], the absence of this receptor should mitigate the negative effect of PCSK9 on inflammation. However, we cannot rule out the contribution of other players such as LRP1, which we showed to interact directly with PCSK9 (Supplementary Figure 1A). An alternative interpretation can be proposed linking the inflammatory effect of PCSK9 to LRP1-mediated mechanisms evident only in macrophages lacking apoE, a competitive ligand for LRP1 (Supplementary Figure 1B), but not in those lacking LDLR (data not shown).
Prior studies conducted in mice show that the spleen contains large numbers of inflammatory and resident monocytes, and that in fat-fed apoE−/− mice monocytosis is caused by increased survival and proliferation of monocytes and by reduced conversion of Ly6Chi to Ly6Clo monocytes [13, 44]. Recently, a link between PCSK9 and inflammation was suggested in a report claiming that PCSK9 levels correlate with leukocyte count in patients affected by stable coronary artery disease [19]. Here, we show that hPCSK9 expression increases total and pro-inflammatory monocyte (Ly6Chi) numbers in the spleen (Figure 6A/B). An increase in this monocyte subset may cause increased accumulation of Ly6Chi cells in the lesion and thus favours its progression over time [13, 45]. Recent studies have suggested that the pro-inflammatory cytokine Il1b disrupts the cholesterol-mediated feedback regulation of LDLR in smooth muscle cells, thus causing massive uptake of native LDL and foam cell transformation through mechanisms involving mTOR activation [46, 47]. In addition, macrophage secretion of Tnf is induced by several stimuli, including oxLDL [48, 49]. Tang et al. reported that Pcsk9 knock-down decreases oxLDL-dependent cytokine expression by macrophages, highlighting the possibility of a direct effect of Pcsk9 on inflammation [20]. The hypothesis of PCSK9 as a mediator of inflammatory responses is also supported by a recent study by Walley et al. [50], who demonstrated that absence of PCSK9 protects against the septic shock caused by LPS administration in mice. We have now shown that expression of hPCSK9 induces (Figure 4A/B), and deletion of mPcsk9 suppresses (Supplementary Figure 9A/B), expression of Il1b and Tnf in LPS-stimulated macrophages. Moreover, we have shown that hPCSK9 reduces expression of the anti-inflammatory markers Arg1 and Il10 (Figure 4C/D), which were shown to have an anti-atherosclerotic effect [51]. Finally, we have shown that the effect of PCSK9 on inflammatory cytokine expression depends on the presence of its main target - the LDLR. In fact, in the absence of LDLR the effect of PCSK9 on cytokine expression (Figure 5A/B/C/D) and splenic monocytes level (Figure 6C/D) was completely lost.
In conclusion, PCSK9 secreted by macrophages reaches the plasma compartment and the atheroma, and its accumulation in the lesion directly affects plaque composition, independently of serum lipid levels, suggesting an additional cardiovascular benefit of anti-PCSK9 therapies. Limitations of our study include: 1. We employed human PCSK9 in the murine system; 2. We overexpressed PCSK9 in macrophages; 3. We used a construct not responsive to physiologic regulatory mechanisms; 4. A complete lack of apoE or LDLR are rarely encountered in patients.
Supplementary Material
Supplementary Figure 1. (A) Immunoprecipitation of hPCSK9 in liver, kidney, adrenals and MPM collected from hPCSK9tg mice and relative western blot analysis of LDLR and LRP1 (B) Flow cytometry analysis of surface LRP1 levels in MPM from apoE−/− or hPCSK9tg/apoE−/− mice.
Supplementary Figure 2. (A) Relative mRNA levels of mPcsk9 in MPM from mPcsk9−/−, WT and hPCSK9tg mice, as measured by reverse transcription-PCR. (B) Relative mRNA levels of mPcsk9 in MPM and livers from mPcsk9−/−, WT and hPCSK9tg mice, as measured by RT-qPCR.
Supplementary Figure 3. Representative images showing immunofluorescence negative controls for lesions of proximal aortas in apoE−/−→apoE−/−or hPCSK9tg/apoE−/−→apoE−/− mice. In green: anti-human secondary antibody; in blue: DAPI.
Supplementary Figure 4. (A) Total serum cholesterol levels measured in apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− (B) Serum triglyceride levels measured in apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− (C) Quantitation of proximal lesion area in Oil red O stained proximal aortas of apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− mice (D) Total serum cholesterol levels measured in LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− mice (E) Serum triglyceride levels measured in LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− mice (F) Quantitation of proximal lesion area in Oil red O stained proximal aortas of LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− mice.
Supplementary Figure 5. Representative images showing immunofluorescence negative controls for lesions of proximal aortas in apoE−/−→apoE−/−or hPCSK9tg/apoE−/−→apoE−/− mice. In green: streptavidin 488 antibody; in blue: DAPI.
Supplementary Figure 6. Representative images showing immunofluorescence negative controls for lesions of proximal aortas in LDLR−/−→LDLR−/−or hPCSK9tg/LDLR−/−→LDLR−/− mice. In green: anti-human secondary antibody; in blue: DAPI.
Supplementary figure 7. (A) (A) Western blot analysis of hPCSK9, LDLR and apoE in MPM from mPcsk9−/−/LDLR−/− or mPcsk9−/−/apoE−/− mice incubated overnight with recombinant hPCSK9 (rePCSK9) 1000ng/ml (B) Quantification of intracellular levels of hPCSK9 in MPM from mPcsk9−/−/LDLR−/− or mPcsk9−/−/apoE−/− mice incubated overnight with rePCSK9 1000ng/ml.
Supplementary Figure 8. Representative images showing negative control staining of lesions of proximal aortas in LDLR−/−→LDLR−/−or hPCSK9tg/LDLR−/−→LDLR−/− mice. In green: streptavidin 488 antibody; in blue: DAPI.
Supplementary Figure 9. mRNA levels of (A) Tnf and (B) Il1b in LPS-stimulated MPM from mPcsk9−/− and Control mice.
Acknowledgments
This study was supported by grant R01-HL106845 of the National Institutes of Health (NHLBI) to Sergio Fazio.
Footnotes
Conflict of interest: None of the authors has conflicts of interest to disclose.
Authors’ contribution statement: IG and HT designed and performed experiments, analysed data, and wrote the paper, RC, LD, YZ, RD, DF, SR and IP performed experiments, SR, AM, HL, ML interpreted the data, and SF designed research, interpreted the data and wrote the paper.
References
- 1.Abifadel M, Varret M, Rabes JP, et al. Mutations in PCSK9 cause autosomal dominant hypercholesterolemia. Nat Genet. 2003;34:154–156. doi: 10.1038/ng1161. [DOI] [PubMed] [Google Scholar]
- 2.Benjannet S, Rhainds D, Essalmani R, et al. NARC-1/PCSK9 and its natural mutants: zymogen cleavage and effects on the low density lipoprotein (LDL) receptor and LDL cholesterol. J Biol Chem. 2004;279:48865–48875. doi: 10.1074/jbc.M409699200. [DOI] [PubMed] [Google Scholar]
- 3.Lalanne F, Lambert G, Amar MJ, et al. Wild-type PCSK9 inhibits LDL clearance but does not affect apoB-containing lipoprotein production in mouse and cultured cells. J Lipid Res. 2005;46:1312–1319. doi: 10.1194/jlr.M400396-JLR200. [DOI] [PubMed] [Google Scholar]
- 4.Zaid A, Roubtsova A, Essalmani R, et al. Proprotein convertase subtilisin/kexin type 9 (PCSK9): hepatocyte-specific low-density lipoprotein receptor degradation and critical role in mouse liver regeneration. Hepatology. 2008;48:646–654. doi: 10.1002/hep.22354. [DOI] [PubMed] [Google Scholar]
- 5.Poirier S, Mayer G, Poupon V, et al. Dissection of the endogenous cellular pathways of PCSK9-induced low density lipoprotein receptor degradation: evidence for an intracellular route. J Biol Chem. 2009;284:28856–28864. doi: 10.1074/jbc.M109.037085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tavori H, Fan D, Blakemore JL, et al. Serum proprotein convertase subtilisin/kexin type 9 and cell surface low-density lipoprotein receptor: evidence for a reciprocal regulation. Circulation. 2013;127:2403–2413. doi: 10.1161/CIRCULATIONAHA.113.001592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Canuel M, Sun X, Asselin MC, et al. Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9) Can Mediate Degradation of the Low Density Lipoprotein Receptor-Related Protein 1 (LRP-1) PLoS One. 2013;8:e64145. doi: 10.1371/journal.pone.0064145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poirier S, Mayer G, Benjannet S, et al. The proprotein convertase PCSK9 induces the degradation of low density lipoprotein receptor (LDLR) and its closest family members VLDLR and ApoER2. J Biol Chem. 2008;283:2363–2372. doi: 10.1074/jbc.M708098200. [DOI] [PubMed] [Google Scholar]
- 9.Overton CD, Yancey PG, Major AS, et al. Deletion of macrophage LDL receptor-related protein increases atherogenesis in the mouse. Circ Res. 2007;100:670–677. doi: 10.1161/01.RES.0000260204.40510.aa. [DOI] [PubMed] [Google Scholar]
- 10.Yancey PG, Blakemore J, Ding L, et al. Macrophage LRP-1 controls plaque cellularity by regulating efferocytosis and Akt activation. Arterioscler Thromb Vasc Biol. 2010;30:787–795. doi: 10.1161/ATVBAHA.109.202051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yancey PG, Ding Y, Fan D, et al. Low-density lipoprotein receptor-related protein 1 prevents early atherosclerosis by limiting lesional apoptosis and inflammatory Ly-6Chigh monocytosis: evidence that the effects are not apolipoprotein E dependent. Circulation. 2011;124:454–464. doi: 10.1161/CIRCULATIONAHA.111.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ferri N, Tibolla G, Pirillo A, et al. Proprotein convertase subtilisin kexin type 9 (PCSK9) secreted by cultured smooth muscle cells reduces macrophages LDLR levels. Atherosclerosis. 2011;220:381–386. doi: 10.1016/j.atherosclerosis.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 13.Swirski FK, Libby P, Aikawa E, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Swirski FK, Pittet MJ, Kircher MF, et al. Monocyte accumulation in mouse atherogenesis is progressive and proportional to extent of disease. Proc Natl Acad Sci U S A. 2006;103:10340–10345. doi: 10.1073/pnas.0604260103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sunderkotter C, Nikolic T, Dillon MJ, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 16.Liu G, Yang H. Modulation of macrophage activation and programming in immunity. J Cell Physiol. 2013;228:502–512. doi: 10.1002/jcp.24157. [DOI] [PubMed] [Google Scholar]
- 17.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. J Leukoc Biol. 2004;76:509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wesemann DR, Benveniste EN. STAT-1 alpha and IFN-gamma as modulators of TNF-alpha signaling in macrophages: regulation and functional implications of the TNF receptor 1:STAT-1 alpha complex. J Immunol. 2003;171:5313–5319. doi: 10.4049/jimmunol.171.10.5313. [DOI] [PubMed] [Google Scholar]
- 19.Li S, Guo YL, Xu RX, et al. Association of plasma PCSK9 levels with white blood cell count and its subsets in patients with stable coronary artery disease. Atherosclerosis. 2014;234:441–445. doi: 10.1016/j.atherosclerosis.2014.04.001. [DOI] [PubMed] [Google Scholar]
- 20.Tang Z, Jiang L, Peng J, et al. PCSK9 siRNA suppresses the inflammatory response induced by oxLDL through inhibition of NF-kappaB activation in THP-1-derived macrophages. Int J Mol Med. 2012;30:931–938. doi: 10.3892/ijmm.2012.1072. [DOI] [PubMed] [Google Scholar]
- 21.Kuhnast S, van der Hoorn JW, Pieterman EJ, et al. Alirocumab inhibits atherosclerosis, improves the plaque morphology, and enhances the effects of a statin. J Lipid Res. 2014;55:2103–2112. doi: 10.1194/jlr.M051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fogelman AM, Haberland ME, Seager J, et al. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981;22:1131–1141. [PubMed] [Google Scholar]
- 23.Hiltunen TP, Yla-Herttuala S. Expression of lipoprotein receptors in atherosclerotic lesions. Atherosclerosis. 1998;137(Suppl):S81–88. doi: 10.1016/s0021-9150(97)00307-9. [DOI] [PubMed] [Google Scholar]
- 24.Linton MF, Babaev VR, Gleaves LA, et al. A direct role for the macrophage low density lipoprotein receptor in atherosclerotic lesion formation. J Biol Chem. 1999;274:19204–19210. doi: 10.1074/jbc.274.27.19204. [DOI] [PubMed] [Google Scholar]
- 25.Goldstein JL, Brown MS. Regulation of low-density lipoprotein receptors: implications for pathogenesis and therapy of hypercholesterolemia and atherosclerosis. Circulation. 1987;76:504–507. doi: 10.1161/01.cir.76.3.504. [DOI] [PubMed] [Google Scholar]
- 26.Ason B, van der Hoorn JW, Chan J, et al. PCSK9 inhibition fails to alter hepatic LDLR, circulating cholesterol, and atherosclerosis in the absence of ApoE. J Lipid Res. 2014;55:2370–2379. doi: 10.1194/jlr.M053207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Denis M, Marcinkiewicz J, Zaid A, et al. Gene inactivation of proprotein convertase subtilisin/kexin type 9 reduces atherosclerosis in mice. Circulation. 2012;125:894–901. doi: 10.1161/CIRCULATIONAHA.111.057406. [DOI] [PubMed] [Google Scholar]
- 28.Fan D, Yancey PG, Qiu S, et al. Self-association of human PCSK9 correlates with its LDLR-degrading activity. Biochemistry. 2008;47:1631–1639. doi: 10.1021/bi7016359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Du F, Hui Y, Zhang M, et al. Novel domain interaction regulates secretion of proprotein convertase subtilisin/kexin type 9 (PCSK9) protein. J Biol Chem. 2011;286:43054–43061. doi: 10.1074/jbc.M111.273474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazio S, Babaev VR, Murray AB, et al. Increased atherosclerosis in mice reconstituted with apolipoprotein E null macrophages. Proc Natl Acad Sci U S A. 1997;94:4647–4652. doi: 10.1073/pnas.94.9.4647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Linton MF, Atkinson JB, Fazio S. Prevention of atherosclerosis in apolipoprotein E-deficient mice by bone marrow transplantation. Science. 1995;267:1034–1037. doi: 10.1126/science.7863332. [DOI] [PubMed] [Google Scholar]
- 32.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 33.Forrest LM, Lough CM, Chung S, et al. Echium oil reduces plasma triglycerides by increasing intravascular lipolysis in apoB100-only low density lipoprotein (LDL) receptor knockout mice. Nutrients. 2013;5:2629–2645. doi: 10.3390/nu5072629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Benoit M, Desnues B, Mege JL. Macrophage polarization in bacterial infections. J Immunol. 2008;181:3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 35.Strickland DK, Kounnas MZ, Argraves WS. LDL receptor-related protein: a multiligand receptor for lipoprotein and proteinase catabolism. FASEB J. 1995;9:890–898. doi: 10.1096/fasebj.9.10.7615159. [DOI] [PubMed] [Google Scholar]
- 36.Maxwell KN, Breslow JL. Adenoviral-mediated expression of Pcsk9 in mice results in a low-density lipoprotein receptor knockout phenotype. Proc Natl Acad Sci U S A. 2004;101:7100–7105. doi: 10.1073/pnas.0402133101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park SW, Moon YA, Horton JD. Post-transcriptional Regulation of Low Density Lipoprotein Receptor Protein by Proprotein Convertase Subtilisin/Kexin Type 9a in Mouse Liver. JBiolChem. 2004;279:50630–50638. doi: 10.1074/jbc.M410077200. [DOI] [PubMed] [Google Scholar]
- 38.Ishibashi S, Herz J, Maeda N, et al. The two-receptor model of lipoprotein clearance: tests of the hypothesis in “knockout” mice lacking the low density lipoprotein receptor, apolipoprotein E, or both proteins. Proc Natl Acad Sci U S A. 1994;91:4431–4435. doi: 10.1073/pnas.91.10.4431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C, Yu X, Cao Q, et al. Characterization of murine macrophages from bone marrow, spleen and peritoneum. BMC Immunol. 2013;14:6. doi: 10.1186/1471-2172-14-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chistiakov DA, Bobryshev YV, Nikiforov NG, et al. Macrophage phenotypic plasticity in atherosclerosis: The associated features and the peculiarities of the expression of inflammatory genes. Int J Cardiol. 2015;184C:436–445. doi: 10.1016/j.ijcard.2015.03.055. [DOI] [PubMed] [Google Scholar]
- 41.Mallat Z. Macrophages. Arterioscler Thromb Vasc Biol. 2014;34:2509–2519. doi: 10.1161/ATVBAHA.114.304794. [DOI] [PubMed] [Google Scholar]
- 42.Guo J, Bot I, de Nooijer R, et al. Leucocyte cathepsin K affects atherosclerotic lesion composition and bone mineral density in low-density lipoprotein receptor deficient mice. Cardiovasc Res. 2009;81:278–285. doi: 10.1093/cvr/cvn311. [DOI] [PubMed] [Google Scholar]
- 43.Shah PK. Inflammation and plaque vulnerability. Cardiovasc Drugs Ther. 2009;23:31–40. doi: 10.1007/s10557-008-6147-2. [DOI] [PubMed] [Google Scholar]
- 44.Swirski FK, Nahrendorf M, Etzrodt M, et al. Identification of splenic reservoir monocytes and their deployment to inflammatory sites. Science. 2009;325:612–616. doi: 10.1126/science.1175202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tacke F, Alvarez D, Kaplan TJ, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ruan XZ, Moorhead JF, Tao JL, et al. Mechanisms of dysregulation of low-density lipoprotein receptor expression in vascular smooth muscle cells by inflammatory cytokines. Arterioscler Thromb Vasc Biol. 2006;26:1150–1155. doi: 10.1161/01.ATV.0000217957.93135.c2. [DOI] [PubMed] [Google Scholar]
- 47.Ma KL, Liu J, Wang CX, et al. Activation of mTOR modulates SREBP-2 to induce foam cell formation through increased retinoblastoma protein phosphorylation. Cardiovasc Res. 2013;100:450–460. doi: 10.1093/cvr/cvt203. [DOI] [PubMed] [Google Scholar]
- 48.Parameswaran N, Patial S. Tumor necrosis factor-alpha signaling in macrophages. Crit Rev Eukaryot Gene Expr. 2010;20:87–103. doi: 10.1615/critreveukargeneexpr.v20.i2.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stewart CR, Stuart LM, Wilkinson K, et al. CD36 ligands promote sterile inflammation through assembly of a Toll-like receptor 4 and 6 heterodimer. Nat Immunol. 2010;11:155–161. doi: 10.1038/ni.1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Walley KR, Thain KR, Russell JA, et al. PCSK9 is a critical regulator of the innate immune response and septic shock outcome. Sci Transl Med. 2014;6:258ra143. doi: 10.1126/scitranslmed.3008782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mallat Z, Besnard S, Duriez M, et al. Protective role of interleukin-10 in atherosclerosis. Circ Res. 1999;85:e17–24. doi: 10.1161/01.res.85.8.e17. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. (A) Immunoprecipitation of hPCSK9 in liver, kidney, adrenals and MPM collected from hPCSK9tg mice and relative western blot analysis of LDLR and LRP1 (B) Flow cytometry analysis of surface LRP1 levels in MPM from apoE−/− or hPCSK9tg/apoE−/− mice.
Supplementary Figure 2. (A) Relative mRNA levels of mPcsk9 in MPM from mPcsk9−/−, WT and hPCSK9tg mice, as measured by reverse transcription-PCR. (B) Relative mRNA levels of mPcsk9 in MPM and livers from mPcsk9−/−, WT and hPCSK9tg mice, as measured by RT-qPCR.
Supplementary Figure 3. Representative images showing immunofluorescence negative controls for lesions of proximal aortas in apoE−/−→apoE−/−or hPCSK9tg/apoE−/−→apoE−/− mice. In green: anti-human secondary antibody; in blue: DAPI.
Supplementary Figure 4. (A) Total serum cholesterol levels measured in apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− (B) Serum triglyceride levels measured in apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− (C) Quantitation of proximal lesion area in Oil red O stained proximal aortas of apoE−/−→apoE−/− or hPCSK9tg/apoE−/−→apoE−/− mice (D) Total serum cholesterol levels measured in LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− mice (E) Serum triglyceride levels measured in LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− mice (F) Quantitation of proximal lesion area in Oil red O stained proximal aortas of LDLR−/−→LDLR−/− or hPCSK9tg/LDLR−/−→LDLR−/− mice.
Supplementary Figure 5. Representative images showing immunofluorescence negative controls for lesions of proximal aortas in apoE−/−→apoE−/−or hPCSK9tg/apoE−/−→apoE−/− mice. In green: streptavidin 488 antibody; in blue: DAPI.
Supplementary Figure 6. Representative images showing immunofluorescence negative controls for lesions of proximal aortas in LDLR−/−→LDLR−/−or hPCSK9tg/LDLR−/−→LDLR−/− mice. In green: anti-human secondary antibody; in blue: DAPI.
Supplementary figure 7. (A) (A) Western blot analysis of hPCSK9, LDLR and apoE in MPM from mPcsk9−/−/LDLR−/− or mPcsk9−/−/apoE−/− mice incubated overnight with recombinant hPCSK9 (rePCSK9) 1000ng/ml (B) Quantification of intracellular levels of hPCSK9 in MPM from mPcsk9−/−/LDLR−/− or mPcsk9−/−/apoE−/− mice incubated overnight with rePCSK9 1000ng/ml.
Supplementary Figure 8. Representative images showing negative control staining of lesions of proximal aortas in LDLR−/−→LDLR−/−or hPCSK9tg/LDLR−/−→LDLR−/− mice. In green: streptavidin 488 antibody; in blue: DAPI.
Supplementary Figure 9. mRNA levels of (A) Tnf and (B) Il1b in LPS-stimulated MPM from mPcsk9−/− and Control mice.