Abstract
BACKGROUND & AIMS
The incidence and mortality of hepatocellular carcinoma (HCC) have been reported to be plateauing in the United States. The United States has large racial, ethnic, and regional variation; we collected data from all 50 states to better analyze changes in HCC incidence in the entire United States.
METHODS
We collected data from the US Cancer Statistics registry, which covers 97% of the population, and calculated adjusted incidence rates. We assessed annual trends among sociodemographic and geographic subgroups using joinpoint analysis.
RESULTS
HCC incidence increased from 4.4/100,000 in 2000 to 6.7/100,000 in 2012, increasing by 4.5% (95% confidence interval [CI], 4.3%–4.7%) annually between 2000 and 2009, but only by 0.7% annually (95% CI, −0.2% to 1.6%) from 2010 through 2012. The average annual percentage change (AAPC) between 2000 and 2012 was higher in men (increase, 3.7%) than in women (increase, 2.7%), and highest in 55- to 59-year-old individuals (AAPC, 8.9%; 95% CI, 7.1%–10.7%) and 60- to 64-year-old individuals (AAPC, 6.4%; 95% CI, 4.7%–8.2%). By 2012, rates in Hispanics surpassed those in Asians, and rates in Texas surpassed those in Hawaii (9.71/100,000 vs 9.68/100,000). Geographic variation within individual race and ethnic groups was observed, but rates were highest in all major race and ethnic groups in Texas.
CONCLUSIONS
In an analysis of the incidence of HCC in all 50 US states, we found the rate of increase in HCC to have slowed from 2010 through 2012. However, incidence is increasing in subgroups such as men ages 55- to 64 years old—especially those born in the peak era of hepatitis C virus infection and among whites/Caucasians. Rates in Hispanics have surpassed those in Asian Americans. We observed geographic differences, with Texas having the highest age-adjusted HCC rates nationwide.
Keywords: USCS, Epidemiology, NAFLD, HBV
The incidence and mortality of hepatocellular carcinoma (HCC) has been increasing in the United States for the past 3 decades, with hepatitis C virus (HCV)-related cirrhosis accounting for most new cases (50%–70%) of HCC. The other main risk factors for HCC are hepatitis B (HBV) infection, especially among recent immigrants from HBV-endemic areas, alcoholic cirrhosis, diabetes, and possibly nonalcoholic fatty liver disease (NAFLD).1 Based on assumptions related to the clinical course of the HCV-infected cohort in the United States, simulation models have projected a continued increase in HCV-related HCC through at least 2020. However, recent studies found slowing or even plateauing of the increase in incidence and mortality rates for HCC during 2009–2013,2 and a possible decrease in the next 2 decades among individuals younger than age 65 years and cohorts born after 1960.3 These studies used data from cancer registries participating in the Surveillance, Epidemiology, and End Results (SEER) program, which captures cancer cases from designated regions including statewide data for 10 states (California, Connecticut, Georgia, Hawaii, Iowa, Kentucky, Louisiana, New Jersey, New Mexico, and Utah), as well as data on select population subgroups within 4 additional states (Alaska: Alaska Natives; Arizona: Native American Indian; Michigan: Detroit; and Washington: Seattle/Puget Sound), and collectively represents 28% of the US population. Although the sampling of SEER is intended to capture a broadly representative sample of the US population, the generalizability of its findings may be limited for estimating HCC rates or evaluating secular trends, particularly if there are large underlying geographic differences in rates. For example, in their analysis of HCC incidence between 1998 and 2003 from cancer registries in 38 states and the District of Columbia (covering ~83% of the US population), Ahmed et al4 showed that HCC has strong racial/ethnic, age, and regional diversity in incidence trends. Therefore, solely evaluating overall HCC incidence rates may mask these important trends. Furthermore, although HCC incidence increased in all regions, the magnitude of this increase varied by up to 2-fold among regions.4 Therefore, examining data from all 50 states both individually and collectively may be required to obtain a more representative picture of recent HCC trends nationwide.
To better characterize recent HCC trends across the entire United States, we performed a comprehensive examination of the overall burden as well as secular trends in incident HCC rates at both a national and state level in all 50 US states between 2000 and 2012. This included a novel evaluation of race/ethnic differences in HCC rates across states and time periods as well as identification of states and major US urban areas with the greatest burden and most rapidly changing HCC incidence rates.
Materials and Methods
We obtained HCC incidence data from the United States Cancer Statistics (USCS) registry. The USCS registry is the official data source for federal government–reported cancer incidence statistics. It compiles data on all incident cancer cases reported in 2 primary sources, the Center for Disease Control and Prevention's National Program of Cancer Registries, which includes all population-based state cancer registries, and the SEER program, which includes 14 population-based cancer registries and 3 supplemental registries.5 The publically available USCS registry includes data only from cancer registries that meet all mandated standards for high quality, including ≥90% capture of all cases; ≤5% of cases identified solely from death certificates; ≤5% with missing age, sex or race; and ≥97% cases passing computerized validity and logic checks. In 2012, the USCS cancer registry data covered 97% of the US population. HCC is identified within USCS cancer registries by the International Classification of Diseases for Oncology, 3rd edition6: site code C220; ICD-O-3 histology type excluding 9050–9055, 9140, 9590–9992, or SEER recode 21071.
We accessed the USCS registry data via the Center for Disease Control and Prevention's Wide-ranging Online Data for Epidemiologic Research platform.7 Annual incidence rates for HCC were obtained using standard formula implemented within the Wide-ranging Online Data for Epidemiologic Research platform, which uses the number of cases as the numerator and the corresponding population size based on US Census Bureau data as the denominator. The corresponding 95% confidence intervals (CIs) were calculated using the Tiwari et al8 method. We present both age-group specific as well as age-adjusted rates, both overall and also by sex, race/ethnic group, geographic region, and birth cohort. The ethnic/racial groups included the following: Asian Pacific Islander (API), American Indian/Alaska Native, black/African American, Hispanic/Latino, and white/Caucasian; with birth cohorts defined as follows: the pre-peak HCV epidemic birth cohort (Pre-HCV) whose members were born between 1915 and 1944; the peak HCV epidemic cohort (Peak-HCV) whose members were born between 1945 and 1965; and the post-peak HCV epidemic cohort (Post-HCV) whose members were born between 1966 and 1992. All age-adjusted rates were standardized to the 2000 US population using the direct method.
We evaluated trends in HCC incidence rates over time using the National Cancer Institute's Joinpoint program (version 3.5.2; available: http://surveillance.cancer.gov/joinpoint), which uses a piecewise linear regression approach to determine whether rates over time are best described by a single line or by multiple linear segments (ie, none or ≥1 joinpoints).9 We allowed a maximum of 3 joinpoints with a minimum of 4 observations per segment. The best joinpoint model10 (ie, in which addition of further joinpoints did not improve model fit) was identified using log-transformed data. We obtained the annual percentage change (APC) in incidence rates over a single linear segment as well as the average annual percentage change (AAPC) over the entire study period for each joinpoint model. The Monte Carlo permutation–based method was used to determine statistical significance. We considered trends to be statistically significant if the 2-sided P value was less than .05.
We created state heat-maps, highlighting the age-adjusted incidence rate in each state for 3 study years: 2003, 2010 (the first and the final years that all states reported high-quality data), and 2012, which was the most recent available year to assess further any geographic patterns in HCC incidence over time. Age-adjusted rates for each state were color-coded using quartile cut-off points determined based on overall national HCC incidence rates in 2010. We also identified the 5 states with the largest increases as well as the 5 states with the largest decreases in age-adjusted HCC incidence rates in the most recently reported 5-year period (2008–2012). We assessed for race/ethnic differences in incidence rates over the entire study period in the 5 most populous states (California, New York, Texas, Florida, and Illinois, and for API in Hawaii also because it had the fourth largest API population in the United States). Finally, we described age-adjusted incidence rates for the 15 metropolitan statistical areas with the highest HCC incidence rates nationally in 2012.
Results
There were 236,290 HCC cases diagnosed between 2000 and 2012 in the USCS registry (Table 1). In 2012 alone there were 24,696 new HCC cases, representing a 115% increase in the absolute numbers of cases reported in 2000 (n = 11,469). The age-adjusted incidence rate for HCC increased from 4.4/100,000 (95% CI, 4.3–4.5) in 2000 to 6.7/100,000 (95% CI, 6.6–6.8) in 2012, representing an AAPC of 3.5% (95% CI, 3.3%–3.8%; P < .001). Joinpoint regression identified 1 significant cut-off point (2009) and thus 2 distinct trends between 2000–2009 and 2010–2012, respectively. There was a statistically significant 4.5% (95% CI, 4.3–4.7) APC in HCC incidence between 2000 and 2009 (Table 2). Between 2009 and 2012, the increases in HCC incidence rates were lower (APC, 0.7%; 95% CI, −0.2% to 1.6%), with a no longer statistically significant trend (P = .09).
Table 1.
Annual Frequencies and Age-Adjusted Incidence Rates of HCC in the United States Between 2000 and 2012 Based on USCS Registry Data
| Year | Incident HCCs | Age-adjusted rate per 100,000a (95% CI) |
|---|---|---|
| 2000 | 11,469 | 4.4 (4.3–4.5) |
| 2001 | 12,283 | 4.6 (4.5–4.7) |
| 2002 | 13,178 | 4.8 (4.8–4.9) |
| 2003 | 14,654 | 5.0 (4.9–5.1) |
| 2004 | 15,896 | 5.3 (5.2–5.4) |
| 2005 | 16,891 | 5.5 (5.4–5.6) |
| 2006 | 17,999 | 5.7 (5.6–5.8) |
| 2007 | 19,590 | 6.1 (6.0–6.1) |
| 2008 | 20,704 | 6.2 (6.1–6.3) |
| 2009 | 22,553 | 6.6 (6.5–6.7) |
| 2010 | 22,712 | 6.5 (6.4–6.6) |
| 2011 | 23,665 | 6.6 (6.5–6.7) |
| 2012 | 24,696 | 6.7 (6.6–6.8) |
NOTE. The following have missing or suppressed data for specified years: Arkansas, 2000; District of Columbia, 2002; Mississippi, 2000–2002; Nevada, 2011–2012; South Dakota, 2000; Tennessee, 2000–2002; and Virginia, 2000–2002.
Direct adjustment performed using the 2000 US Standard Population.
Table 2.
Annual and Average Annual Percentage Change in HCC Incidence Rates Over Time in the United States in Select Demographic Subgroups
| Joinpoint segment year start | Joinpoint segment year end | APC (95% CI) | P value | AAPC (95% CI) | P value | |
|---|---|---|---|---|---|---|
| Overall US population | 2000 | 2009 | 4.49 (4.28–4.70) | <.001 | 3.54 (3.30–3.78) | <.001 |
| 2009 | 2012 | 0.74 (−0.15 to 1.65) | .09 | |||
| Age-group at diagnosis, y | ||||||
| 20–24a | 2001 | 2012 | 0.99 (−2.84 to 4.97) | .58 | 0.99 (−2.84 to 4.97) | .58 |
| 25–29 | 2000 | 2002 | −7.92 (−48.81 to 65.64) | .61 | −0.01 (−8.77 to 9.59) | .998 |
| 25–29 | 2002 | 2007 | 7.38 (−10.33 to 28.60) | .23 | ||
| 25–29 | 2007 | 2010 | −11.06 (−47.40 to 50.39) | .44 | ||
| 25–29 | 2010 | 2012 | 8.27 (−36.76 to 85.37) | .59 | ||
| 30–34 | 2000 | 2004 | 8.25 (0.69–16.37) | .04 | 1.54 (−0.91 to 4.06) | .22 |
| 30–34 | 2004 | 2012 | −1.67 (−3.94 to 0.69) | .14 | ||
| 35–39 | 2000 | 2008 | 0.62 (−0.99 to 2.24) | .40 | −2.72 (−4.41 to −1.00) | .002 |
| 35–39 | 2008 | 2012 | −9.06 (−13.73 to −4.15) | .003 | ||
| 40–44 | 2000 | 2012 | −1.97 (−2.96 to −0.97) | .001 | −1.97 (−2.96 to −0.97) | .001 |
| 45–49 | 2000 | 2005 | 1.64 (−1.77 to 5.18) | .30 | −1.59 (−3.15 to −0.01) | .048 |
| 45–49 | 2005 | 2012 | −3.84 (−5.83 to −1.82) | .003 | ||
| 50–54 | 2000 | 2005 | 12.10 (8.91–15.40) | <.001 | 4.38 (2.46–6.23) | <.001 |
| 50–54 | 2005 | 2009 | 2.19 (−2.69 to 7.31) | .31 | ||
| 50–54 | 2009 | 2012 | −4.87 (−9.43 to −0.08) | .048 | ||
| 55–59 | 2000 | 2003 | 6.07 (−0.81 to 13.42) | .07 | 8.87 (7.06–10.72) | <.001 |
| 55–59 | 2003 | 2008 | 14.86 (11.53–18.30) | <.001 | ||
| 55–59 | 2008 | 2012 | 3.83 (1.55–6.17) | .007 | ||
| 60–64 | 2000 | 2003 | −0.56 (−7.40 to 6.80) | .86 | 6.44 (4.74–8.18) | <.001 |
| 60–64 | 2003 | 2012 | 8.89 (7.94–9.84) | <.001 | ||
| 65–69 | 2000 | 2012 | 3.11 (2.71–3.51) | <.001 | 3.11 (2.71–3.51) | <.001 |
| 70–74 | 2000 | 2012 | 2.20 (1.63–2.77) | <.001 | 2.20 (1.63–2.77) | <.001 |
| 75–79 | 2000 | 2008 | 3.60 (2.76–4.45) | <.001 | 2.12 (1.34–2.91) | <.001 |
| 75–79 | 2008 | 2012 | −0.79 (−2.92 to 1.39) | .43 | ||
| 80–84 | 2000 | 2012 | 2.63 (1.75–3.52) | <.001 | 2.63 (1.75–3.52) | <.001 |
| ≥85 | 2000 | 2012 | 1.83 (1.34–2.33) | <.001 | 1.83 (1.34–2.33) | <.001 |
| Sex | ||||||
| Female | 2000 | 2009 | 3.56 (3.14–3.99) | <.001 | 2.68 (2.18–3.17) | <.001 |
| Female | 2009 | 2012 | 0.05 (−1.82 to 1.96) | .95 | ||
| Male | 2000 | 2009 | 4.60 (4.26–4.93) | <.001 | 3.66 (3.29–4.03) | <.001 |
| Male | 2009 | 2012 | 0.91 (−0.48 to 2.31) | .17 | ||
| Race/ethnicity | ||||||
| AI/AN | 2000 | 2012 | 3.27 (0.97–5.63) | .009 | 3.27 (0.97–5.63) | .009 |
| API | 2000 | 2007 | 0.56 (−0.47 to 1.60) | .25 | −1.10 (−1.83 to −0.39) | .004 |
| API | 2007 | 2012 | −3.36 (−4.83 to −1.88) | .001 | ||
| Black/African American | 2000 | 2009 | 5.13 (4.25–6.03) | .000 | 3.92 (2.95–4.90) | <.001 |
| Black/African American | 2009 | 2012 | 0.37 (−3.19 to 4.07) | .82 | ||
| Latino/Hispanic | 2000 | 2010 | 3.04 (2.24–3.86) | <.001 | 2.00 (0.61–3.41) | .005 |
| Latino/Hispanic | 2010 | 2012 | −3.07 (−11.27 to 5.89) | .44 | ||
| White/Caucasian | 2000 | 2009 | 4.50 (4.29–4.71) | <.001 | 3.68 (3.45–3.92) | <.001 |
| White/Caucasian | 2009 | 2012 | 1.26 (0.36–2.17) | .01 |
NOTE. Results of joinpoint analyses.
AI/AN, American Indian or Alaska Native.
Data not available for 20- to 24-year-olds owing to sample size–related data privacy suppression.
Age
Most HCC cases (95%) diagnosed between 2000 and 2012 were among adults aged 45 years and older, with 54% occurring in those aged 50–69 years. HCC incidence showed significant decreases in persons aged 45–49 years (AAPC, −1.6%; 95% CI, −3.2% to −0.01%), with rapid decreases between 2005 and 2012 (Table 2). In contrast, age-specific incidence rates for HCC increased in all age groups aged 50 years and older. The largest increases were observed in persons aged 55–59 years (AAPC, 8.9%; 95% CI, 7.1%–10.7%) and 60–64 years (AAPC, 6.4%; 95% CI, 4.7%–8.2%) between 2000 and 2012 (both P < .001). Joinpoint regression analyses showed that the most rapid increases in rates occurred before 2009. In persons aged 55–59 years, average increases in incidence rates slowed but remained high and significant in subsequent years (between 2008 and 2012: APC, 3.8%; 95% CI, 1.6%–6.2%) (Table 2).
Among adults aged 35–39 years, HCC incidence rates began to significantly decrease later in the study period (APC, −9.1% between 2008 and 2012; P < .001); whereas rates in adults aged 40–44 years showed a smaller significant decreasing trend over the entire study (Table 2).
Sex
Men comprised the majority (73%) of HCC cases, with the proportion of cases that were men increasing from 70% in 2000 to 75% in 2012. This gender disparity was largest in the 50- to 64-year-old group; for example, the incidence rate was 20.4 (19.9–20.9) per 100,000 in 50- to 54-year-old men compared with 4.3 per 100,000 (95% CI, 4.1–4.5) in 50- to 54-year-old women, with approximately 82% of cases within this age group in 2012 being men; and was greater in African Americans and whites than in APIs and Hispanics (with the proportion of cases who were female between 2000 and 2012 of 24%, 24%, 29%, and 28%, respectively) (data not shown). There were increases in HCC rates in both men and women over time (eg, age-adjusted rates increased between 2000 and 2012 from 6.9 [95% CI, 6.8–7.1] to 10.8 [95% CI, 10.6–10.9] in men, and from 2.3 [95% CI, 2.2–2.4] to 3.2 [95% CI, 3.1–3.3] per 100,000 in women, respectively) (Supplementary Figure 1). The AAPC in HCC incidence rates was higher in men (3.7%) than in women (2.7%) between 2000 and 2012, with both incidence rates significant (P < .001) (Table 2). The largest APC in rates occurred between 2000 and 2009 (3.6% and 4.6% in men and women, respectively; both P < .001), followed by a small although nonsignificant increase in subsequent years in men (APC, 0.91; P = .17) between 2010 and 2012.
Race and Ethnicity
Most men and women (62% and 59%, respectively) diagnosed with HCC were non-Hispanic white; however, the overall age-adjusted incidence rates were higher in non-whites than in whites (Figure 1). Age-adjusted incidence rates increased significantly over the study period in whites, African Americans, Hispanics, and American Indian/Alaska Natives, but decreased in APIs. The largest increases in rates occurred before 2010 among whites, African Americans, and Hispanics (eg, APCs of 5.1%, 3.0%, and 4.5% in African Americans, Hispanics, and whites, respectively) (Table 2). Only whites, however, still had significantly increasing incidence rates in subsequent years, with an APC of 1.3% between 2009 and 2012 (P = .01). Rates in APIs decreased during the overall study period (AAPC, −1.1; P = .004), particularly later in the study period (APC, −3.4 between 2007 and 2012; P = .001) (Table 2), and were surpassed by Hispanics in 2011 and 2012 (Figure 1).
Figure 1.
Age-adjusted HCC incidence rates in the United States between 2000 and 2012 shown for several race/ethnicity groups. AI/AN, American Indian or Alaska Native.
Birth Cohort
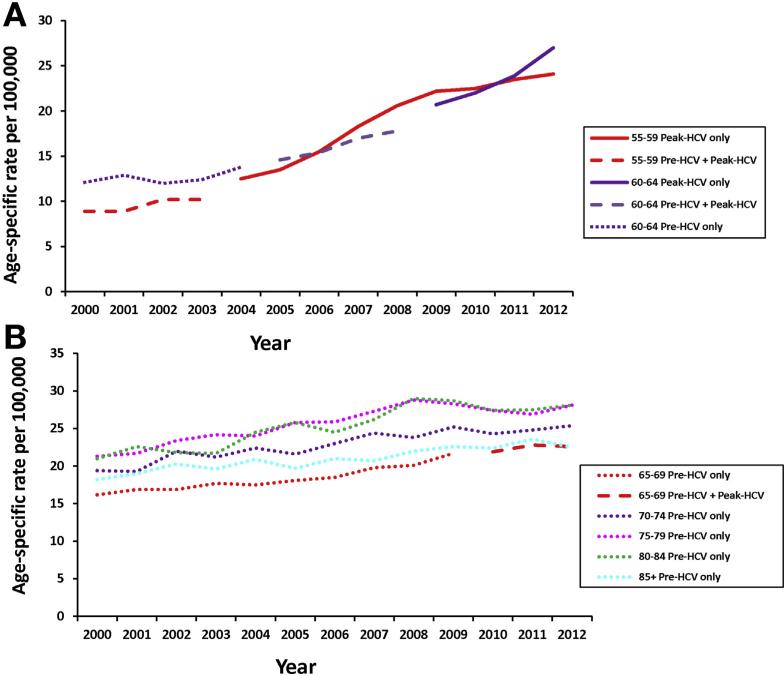
There was evidence for a birth cohort effect, in which the same age group as those born during the peak HCV era (1945–1965) had higher rates than those born before or after the peak HCV era. HCC rates in those age 40–44 years born in the Peak-HCV cohort era were higher than rates in the Post-HCV birth cohort from the same age group (range, 1.7–1.9/100,000 in Peak-HCV cohort members between 2000 and 2006 vs 1.3–1.5/100,000 in Post-HCV cohort members between 2010–2012, respectively); however, the incidence for both peak and post-peak HCV birth cohorts in this age group began decreasing in 2010 (data not shown). Among those aged 55–59 at HCC diagnosis, rates between 2000 and 2003, which reflect a mixture of both the Pre-HCV and Peak-HCV cohorts, increased from 8.9/100,000 in 2000 to 10.2/100,000 in 2003 (Figure 2A). Rates in those age 50–59 years after 2003 are comprised solely of cases from the Peak-HCV cohort and were higher than those reported in earlier years, including a rapid increase in rates from 12.5 in 2004 to 24.1 per 100,000 in 2012. Among those aged 60–64, HCC incidence rates increased in the Pre-HCV cohort from 12.1 to 13.8 per 100,000 between 2000 and 2004, with rates continuing to increase between 2005 and 2008 when comprising members of both the Pre-HCV and Peak-HCV cohorts, and with highest rates and greatest increases in rates seen between 2009 and 2012, which comprised Peak-HCV cohort members only. Similar trends were seen for those diagnosed with HCC between 65 and 69 years of age (Figure 2B).
Figure 2.
(A) Age- and birth cohort–specific HCC rates shown for ages 55–59 and 60–64 years, and peak (1945–1965) and pre-peak HCV (before 1945) birth cohorts. (B) Age- and birth cohort–specific HCC rates shown for 5-year age cohorts between 65 and ≥85 years and peak (1945-1965) and pre-peak HCV (before 1945) birth cohorts.
Geography
In 2003, 2010, and 2012, the states with the highest HCC age-adjusted incidence rates were located in southern and western regions of the United States (Figure 3 and also Supplementary Table 1 for incidence rates for all states in 2012). The highest age-adjusted rates in 2003 and 2010 were in Hawaii (9.0 and 9.7 per 100,000, respectively), whereas by 2012 they were in Texas and Hawaii (9.71 [95% CI, 9.33–10.33] and 9.68 [95% CI, 8.22–11.33] per 100,000, respectively). In 2003, 34 of the 50 states (68%) had age-adjusted incidence rates less than 5/100,000; this number decreased to 13 states by 2010 and decreased further to only 9 states by 2012. In contrast, the number of states with age-adjusted liver cancer rates greater than 7/100,000 increased from 1 state (Hawaii) in 2003 to 7 states by 2010, and 12 states by 2012. In 2012, 6 of the 15 metropolitan statistical areas (40%) with the highest age-adjusted incidence rates were in the state of Texas alone, with 5 (30%) located in California, the most populous state (Table 2). The 5 states with the greatest increase in overall age-adjusted HCC incidence rates during the latest 5-year period (2008–2012) were Kansas, Utah, Idaho, Arizona, and Georgia (AAPCs, 7.8%, 7.2%, 5.9%, 5.6%, and 5.4%, respectively), with trends significant or nearly significant in all but Utah (P = .15). The state with the lowest overall age-adjusted incidence rate in 2012 was North Dakota (2.4/100,000; 95% CI, 1.5–3.8) (Supplementary Table 1).
Figure 3.
Heat maps showing state-specific, age-adjusted HCC incidence rates in 2003, 2010, and 2012.
Considerable geographic differences in incidence rates within the same race/ethnic groups also were observed. Among the 5 most populous states (California, Texas, New York, Florida, and Illinois), the 2012 age-adjusted HCC incidence rates per 100,000 in Hispanics were highest in Texas (15.1), intermediate in California (11.8), and lowest in Florida (7.0) (Supplementary Figure 2). Age-adjusted rates in all other race/ethnic groups also were highest in Texas in 2012. Between 2000 and 2012, rates in APIs decreased in 4 (California, Texas, Florida, New York) of 5 states examined, with AAPCs between −1.9% and −1.0%, with a small nonsignificant increase in Hawaii (AAPC, 0.5%; P = .44) (Table 3 and Supplementary Figure 3). The largest increases in rates in Hispanics, whites, and African Americans between 2000 and 2012 also were found in Texas (AAPCs, 3.6%, 4.7%, and 5.0%, respectively; all P values < .001).
Table 3.
Age-Adjusted HCC Incidence Rates in Select US States and Subgroups
| Joinpoint segment year start | Joinpoint segment year end | APC (95% CI) | P value | AAPC (95% CI) | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Race/ethnicitya | |||||||||
| API | |||||||||
| Five most populous states in 2012 | |||||||||
| California | 2000 | 2007 | 1.13 (−1.40 to 3.72) | .34 | −1.61 (−3.40 to 0.21) | .08 | |||
| California | 2007 | 2012 | −5.33 (−8.85 to −1.67) | .01 | |||||
| Floridab | 2001 | 2012 | −1.86 (−4.19 to 0.54) | .11 | −1.89 (−4.19 to 0.54) | .11 | |||
| Hawaiic | 2000 | 2012 | 0.48 (−0.74 to 1.72) | .41 | 0.48 (−0.74 to 1.72) | .41 | |||
| New York | 2000 | 2012 | −1.44 (−2.71 to −0.16) | .03 | −1.44 (−2.71 to −0.16) | .03 | |||
| Texas | 2000 | 2012 | −1.04 (−2.70 to 0.66) | .20 | −1.04 (−2.70 to 0.66) | .20 | |||
| Black/African American | |||||||||
| California | 2000 | 2012 | 2.37 (1.18–3.57) | .001 | 2.37 (1.18–3.57) | .001 | |||
| Florida | 2000 | 2012 | 2.67 (0.67–4.70) | .01 | 2.67 (0.67–4.70) | .01 | |||
| Illinois | 2000 | 2012 | 1.49 (−0.14 to 3.14) | .07 | −0.88 (−2.69 to 0.93) | .34 | |||
| New York | 2000 | 2012 | 4.15 (2.80–5.51) | <.001 | 4.15 (2.80–5.51) | <.001 | |||
| Texas | 2000 | 2012 | 5.02 (3.44–6.63) | <.001 | 5.02 (3.44–6.63) | <.001 | |||
| Latino/Hispanic | |||||||||
| California | 2000 | 2012 | 2.66 (1.57–3.76) | <.001 | 2.66 (1.57–3.76) | <.001 | |||
| Florida | 2000 | 2012 | 1.30 (0.08–2.54) | .04 | 1.30 (0.08–2.54) | .04 | |||
| Illinois | 2000 | 2012 | 2.51 (0.02–5.07) | .048 | 2.51 (0.02–5.07) | .048 | |||
| New York | 2000 | 2006 | 8.59 (5.47–11.79) | <.001 | 1.66 (0.05–3.30) | .04 | |||
| New York | 2006 | 2012 | −4.82 (−7.08 to −2.51) | .001 | |||||
| Texas | 2000 | 2004 | 7.84 (1.32–14.78) | .02 | 3.60 (1.57–5.67) | <.001 | |||
| Texas | 2004 | 2012 | 1.54 (−0.06 to 3.17) | .057 | |||||
| White/Caucasian | |||||||||
| California | 2000 | 2012 | 3.94 (3.05–4.83) | <.001 | 3.94 (3.05–4.83) | <.001 | |||
| Florida | 2000 | 2012 | 3.46 (2.74–4.19) | <.001 | 3.46 (2.74–4.19) | <.001 | |||
| Illinois | 2000 | 2012 | 2.65 (1.79–3.52) | <.001 | 2.65 (1.79–3.52) | <.001 | |||
| New York | 2000 | 2012 | 1.91 (1.13–2.70) | <.001 | 1.91 (1.13–2.70) | <.001 | |||
| Texas | 2000 | 2012 | 4.73 (4.19–5.28) | <.001 | 4.73 (4.19–5.28) | <.001 | |||
| 5 states largest increases in 5-year age-adjusted incidence rates (2008–2012) |
5 states largest decreases increases in 5-year age-adjusted incidence rates (2008–2012) |
||||||||
| State | Incident HCC cases in 2012, n | Population size in 2012 | 2012 Age-adjusted incidence/100,000a (95% CI) | AAPC between 2008 and 2012 (95% CI), P value | State | Incident HCC cases in 2012, n | Population size in 2012 | 2012 Age-adjusted incidence/100,000a (95% CI) | AAPC between 2008 and 2012 (95% CI), P value |
| Kansas | 206 | 2,885,398 | 6.13 (5.31–7.04) | 7.83 (1.41–14.66), .03 | Vermont | 43 | 625,953 | 5.25 (3.75–7.20) | −7.69 (−24.80 to 13.32), .30 |
| Utah | 116 | 2,854,871 | 4.71 (3.87–5.66) | 7.17 (−4.56 to 20.35), .15 | Virginia | 484 | 8,186,628 | 5.11 (4.66–5.60) | −4.68 (−10.56 to 1.58), .096 |
| Idaho | 95 | 1,595,590 | 5.20 (4.19–6.38) | 5.93 (1.27–10.80), .03 | Rhode Island | 75 | 1,050,304 | 5.71 (4.47–7.22) | −2.92 (12.44–7.64), .44 |
| Arizona | 565 | 6,551,149 | 7.22 (6.63–7.85) | 5.64 (−0.09 to 11.71), .05 | West Virginia | 111 | 1,856,680 | 4.42 (3.62–5.36) | −2.45 (−9.50 to 5.16), .37 |
| Georgia | 732 | 9,915,646 | 7.05 (6.53–7.59) | 5.40 (−0.89 to 12.10), .07 | Montana | 50 | 1,005,494 | 3.76 (2.77–5.03) | −1.16 (−11.355 to 10.21), .76 |
NOTE. Results of joinpoint analyses.
AI/AN, American Indian or Alaska Native.
Data for American Indian/Alaska Native not available at the state-level because the sample size required data suppression.
Data for 2000 not available for Florida in the United States Cancer Statistics registry.
Data from Hawaii included only for API.
Discussion
In the population-based data representing all 50 US states, we found that the overall age-adjusted incidence rates for HCC increased between 2000 and 2012 from 4.4/100,000 to 6.7/100,000. Most of the increase in incidence rates occurred by 2009, with a significant 4.5% average APC between 2000 and 2009, followed by a lower nonsignificant average change of 0.7% per year between 2010 and 2012. The small increases in annual age-adjusted incidence rates since 2010 (from 6.5, 6.6, and 6.7/100,000 in 2010, 2011, and 2012, respectively) suggest that a maximum peak for the overall age-adjusted HCC incidence rates in the United States may not have been reached yet. However, given the limited number of years of data since 2009 and the nonsignificant test for trend, we cannot exclude that rates have plateaued in recent years. The fastest increase in HCC incidence rates was seen among those aged 55–64 years, especially those born in the HCV peak era (ie, 1945–1965). HCC incidence increased in men, particularly those aged 50 years and older, and in certain ethnic/racial groups including Hispanics, African Americans, American Indians/Alaska Natives, and whites. Hispanics have constituted the group with the highest age-adjusted incidence rates, surpassing those of APIs, in whom the rates have decreased.
Geographic variations in HCC incidence were observed, with Texas closely followed by Hawaii having the highest rates in the nation, but the increase in HCC has affected most states; with only 9 states in 2012 still having overall age-adjusted rates of fewer than 5/100,000, the World Health Organization threshold defining a low HCC incidence region. By 2012, the highest age-adjusted rates for all major race/ethnic groups were found in Texas. The greatest differences in HCC rates within individual race/ethnic groups across the most populous states in the United States was found in Hispanics, with rates in Hispanics in Florida consistently lowest, intermediate in California, and consistently highest in Texas.
A notable strength of our study was that the data used are more representative of the entire US population than the SEER database that was used by recent studies reporting a decrease or plateau in HCC incidence rates. For example, Altekruse et al2 reported that HCC incidence rates in SEER registries did not increase during 2007–2010. Another article reported HCC incidence rates from 1973 through 2011 from the SEER 9 registries (Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco–Oakland, Seattle–Puget Sound, and Utah), and for 2000 through 2011 from the SEER 18 registries (SEER 9 plus Los Angeles, San Jose–Monterey, rural Georgia, Alaska Native Tumor Registry, greater California, Kentucky, Louisiana, and New Jersey). Although HCC incidence continued to increase in that report, a slowing of the rate of increase occurred around 2006, with no significant increase in incidence from 2009 to 2011.11 Finally, Ryerson et al12 examined liver cancer, including bile duct cancer, and not just HCC in SEER registries. An important limitation of our study was that the USCS registry used does not provide data on HCC risk factors (eg, viral hepatitis, alcohol use, metabolic syndrome) necessary to determine etiology, or on clinical data (eg, hisopathologic and radiologic findings) necessary to define HCC stage at diagnosis or whether a diagnosis was made in relation to surveillance or not. We therefore cannot determine specific causes for observed trends, for example, the extent to which observed differences in rates among Hispanics among the most populous states are owing to differences in a prevalence of risk factors such as HCV and obesity, differences in health care access, or the relative prevalence of Hispanic subgroups including of Mexican, Central and South American, and Caribbean origins.
Asian men in the United States used to be the group with the highest age-adjusted incidence rates attributed to chronic HBV, especially among immigrants from HBV-endemic areas. However, subsequent generations of Asians born in the United States have much lower rates of HBV infection, although recent immigrants from traditionally HBV-endemic areas also may be benefitting from reduced exposure to aflatoxin and an increase in HBV vaccinations. Improved antiviral treatment of chronic HBV with nucleoside analogs also has been associated with decreased HBV-associated HCC risk.13
For the first time in 2011, Hispanics surpassed Asians as the group most affected by HCC (ie, highest age-adjusted incidence rates). Our findings are consistent with earlier research suggesting that Hispanics in the United States continue to have the fastest sustained increase in HCC age-adjusted incidence rates,14 with rates higher in US-born Hispanics than foreign-born Hispanics. The reasons have not been examined directly, but likely are related to higher rates of HCV (particularly in Mexican Americans),15,16 alcoholic liver disease, NAFLD,17,18 and the metabolic syndrome including diabetes,19 which increases the risk of developing HCC either independently or through potentiating the effect of viral hepatitis and alcoholic liver disease. Furthermore, Hispanics with chronic HCV in the United States also have a risk of progression to cirrhosis and HCC, higher than any other ethnic or racial group.20 The high HCC rates in states with high proportions of Hispanic residents further highlight this point and the potential importance of the metabolic syndrome and nonalcoholic steatohepatitis in HCC.19,21
The consistently high and increasing HCC incidence rates among individuals born in the Peak-HCV cohort (1945–1965) irrespective of age or calendar year are supportive of a potential birth cohort effect related to HCV that has not decreased yet. The HCV treatment landscape changed in 2013 with the advent of highly efficacious directly acting antivirals that offer cure rates higher than 95% in most patient groups including those with cirrhosis. The directly acting antivirals may affect overall HCC incidence rates over the next 1–2 decades,22 but the magnitude and timing of anticipated decreases in HCC incidence rates will depend greatly on the availability and penetration of HCV treatment as well as increased detection, diagnosis, and linkage to care of individuals with chronic HCV infection. Patients with HCV are disproportionally minorities and of lower socioeconomic status, and are more likely to be uninsured or underinsured, thus likely to have less access to screening, diagnosis, and treatment.16 This is particularly relevant in that HCC rates are highest among some states that have not adopted Medicaid expansion (eg, Texas) as part of the Affordable Care Act. Furthermore, although HCC risk is reduced considerably among HCV patients who achieve a virologic cure, it remains significantly increased among patients who achieve cure at an older age or when they already have developed cirrhosis and diabetes.23,24
Although the increase in HCC has been observed in most states in the United States, the highest rates are seen in southern and western states, with Texas having the highest age-adjusted incidence rates overall and in all major/race ethnic groups by 2012. The reasons underlying these geographic differences in trends could not be examined directly but several characteristics may contribute to the high and increasing HCC burden in Texas. Established HCC risk factors including HCV, HBV, and alcoholic liver disease are disproportionately high in several subgroups of Texas residents.25 In addition, emerging obesity-associated HCC risk factors, specifically metabolic syndrome and NAFLD, are exceptionally common and increasing among Texas residents, especially among Hispanics who have 2- to 3-fold higher HCC incidence rates than those in the rest of the nation.26
In summary, this 50-state, population-based, descriptive study has confirmed the overall increasing HCC incidence rates. However, it is not clear whether the recent slowing down in the overall rates since 2009 represents a pending decrease in rates. Likely reflecting the shift in risk factors from HBV to HCV, alcohol and NAFLD, the largest overall increases in rates were seen among men between ages 55 and 64, especially those born in the HCV peak era, and among Hispanics, whom now for the first time have surpassed rates in Asians and Pacific Islanders, with Texas now surpassing Hawaii as the state most affected by HCC. States and large urban areas with high and/or increasing HCC incidence rates should address provisions for the detection and treatment of underlying risk factors.
Supplementary Material
Acknowledgment
Donna White, Jessica Davila, and Hashem El-Serag were responsible for research inception; Donna White, Hashem El-Serag, and Aaron Thrift performed the analysis; Donna White, Aaron Thrift, Fasiha Kanwal, and Hashem El-Serag were responsible for the interpretation of results; Donna White, Aaron Thrift, and Hashem El-Serag wrote the manuscript; and Donna White, Jessica Davila, Fasiha Kanwal, Aaron Thrift, and Hashem El-Serag were responsible for the critical review of the manuscript.
Funding
This research was supported in part by the Cancer Prevention and Research Institute of Texas (RP150587), the National Cancer Institute (CA190776 and CA125123), the National Diabetes Digestive and Kidney Disease Institute (DK 56338, DK078154A, and DK095082), and the Houston VA HSR&D Center of Innovations (CIN13-413). The National Institutes of Diabetes Digestive and Kidney Diseases, National Cancer Institute, the US Department of Veterans Affairs, and the Cancer Prevention Research Institute of Texas played no role in the design, analysis, interpretation, or publication of these results.
Abbreviations used in this paper
- AAPC
average annual percentage change
- APC
annual percentage change
- API
Asian Pacific Islander
- CI
confidence interval
- HBV
hepatitis B virus
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- NAFLD
nonalcoholic fatty liver disease
- Peak-HCV
peak hepatitis C virus epidemic cohort
- Post-HCV
post-peak hepatitis C virus epidemic cohort
- Pre-HCV
pre-peak hepatitis C virus epidemic birth cohort
- SEER
Surveillance, Epidemiology, and End Results
- USCS
United States Cancer Statistics
Footnotes
Supplementary Material
Note: To access the supplementary material accompanying this article, visit the online version of Gastroenterology at www.gastrojournal.org, and at http://dx.doi.org/10.1053/j.gastro.2016.11.020.
Conflicts of interest
The authors disclose no conflicts.
References
- 1.Singal AG, El-Serag HB. Hepatocellular carcinoma from epidemiology to prevention: translating knowledge into practice. Clin Gastroenterol Hepatol. 2015;13:2140–2151. doi: 10.1016/j.cgh.2015.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altekruse SF, Henley SJ, Cucinelli JE, et al. Changing hepatocellular carcinoma incidence and liver cancer mortality rates in the United States. Am J Gastroenterol. 2014;109:542–553. doi: 10.1038/ajg.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Petrick JL, Kelly SP, Altekruse SF, et al. Future of hepatocellular carcinoma incidence in the United States forecast through 2030. J Clin Oncol. 2016;34:1787–1794. doi: 10.1200/JCO.2015.64.7412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ahmed F, Perz JF, Kwong S, et al. National trends and disparities in the incidence of hepatocellular carcinoma, 1998-2003. Prev Chronic Dis. 2008;5:A74. [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Cancer Statistics Working Group . United States Cancer Statistics 1999-2012 incidence and mortality web-based report. US Department of Health and Human Services; 2015. [July 1, 2016]. [Google Scholar]
- 6.Fritz, Percy, Jack, et al. International classification of diseases for oncology. (3rd ed.) 2001 [Google Scholar]
- 7.US Department of Health and Human Services . United States Cancer Statistics: 1999-2012. US Department of Health and Human Services; 2015. [DOI] [PubMed] [Google Scholar]
- 8.Tiwari RC, Clegg LX, Zou Z. Efficient interval estimation for age-adjusted cancer rates. Stat Methods Med Res. 2006;15:547–569. doi: 10.1177/0962280206070621. [DOI] [PubMed] [Google Scholar]
- 9.Yu B, Barrett MJ, Kim H- J, et al. Estimating joinpoints in continuous time scale for multiple change-point models. Bethesda: Statistical Research and Applications Branch. National Cancer Institute; 2006. [Google Scholar]
- 10.Kim HJ, Fay MP, Feuer EJ, et al. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med. 2000;19:335–351. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 11.Njei B, Rotman Y, Ditah I, et al. Emerging trends in hepatocellular carcinoma incidence and mortality. Hepatology. 2015;61:191–199. doi: 10.1002/hep.27388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ryerson AB, Eheman CR, Altekruse SF, et al. Annual report to the nation on the status of cancer, 1975-2012, featuring the increasing incidence of liver cancer. Cancer. 2016;122:1312–1337. doi: 10.1002/cncr.29936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gordon SC, Lamerato LE, Rupp LB, et al. Antiviral therapy for chronic hepatitis B virus infection and development of hepatocellular carcinoma in a US population. Clin Gastroenterol Hepatol. 2014;12:885–893. doi: 10.1016/j.cgh.2013.09.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.El-Serag HB, Lau M, Eschbach K, et al. Epidemiology of hepatocellular carcinoma in Hispanics in the United States. Arch Intern Med. 2007;167:1983–1989. doi: 10.1001/archinte.167.18.1983. [DOI] [PubMed] [Google Scholar]
- 15.Armstrong GL, Wasley A, Simard EP, et al. The prevalence of hepatitis C virus infection in the United States, 1999 through 2002. Ann Intern Med. 2006;144:705–714. doi: 10.7326/0003-4819-144-10-200605160-00004. [DOI] [PubMed] [Google Scholar]
- 16.Setiawan VW, Wei PC, Hernandez BY, et al. Disparity in liver cancer incidence and chronic liver disease mortality by nativity in Hispanics: the Multiethnic Cohort. Cancer. 2016;122:1444–1452. doi: 10.1002/cncr.29922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Browning JD, Szczepaniak LS, Dobbins R, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 18.Kallwitz ER, Daviglus ML, Allison MA, et al. Prevalence of suspected nonalcoholic fatty liver disease in Hispanic/ Latino individuals differs by heritage. Clin Gastroenterol Hepatol. 2015;13:569–576. doi: 10.1016/j.cgh.2014.08.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mittal S, Sada YH, El-Serag HB, et al. Temporal trends of nonalcoholic fatty liver disease-related hepatocellular carcinoma in the veteran affairs population. Clin Gastroenterol Hepatol. 2015;13:594–601. doi: 10.1016/j.cgh.2014.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.El-Serag HB, Kramer J, Duan Z, et al. Racial differences in the progression to cirrhosis and hepatocellular carcinoma in HCV-infected veterans. Am J Gastroenterol. 2014;109:1427–1435. doi: 10.1038/ajg.2014.214. [DOI] [PubMed] [Google Scholar]
- 21.Farrell G. Insulin resistance, obesity, and liver cancer. Clin Gastroenterol Hepatol. 2014;12:117–119. doi: 10.1016/j.cgh.2013.07.040. [DOI] [PubMed] [Google Scholar]
- 22.Chhatwal J, Kanwal F, Roberts MS, et al. Cost-effectiveness and budget impact of hepatitis C virus treatment with sofosbuvir and ledipasvir in the United States. Ann Intern Med. 2015;162:397–406. doi: 10.7326/M14-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.El-Serag HB, Kramer J, Duan Z, et al. Epidemiology and outcomes of hepatitis C infection in elderly US Veterans. J Viral Hepat. 2016;23:687–696. doi: 10.1111/jvh.12533. [DOI] [PubMed] [Google Scholar]
- 24.El-Serag HB, Kanwal F, Richardson P, et al. Risk of hepatocellular carcinoma after sustained virological response in veterans with hepatitis C virus infection. Hepatology. 2016;64:130–137. doi: 10.1002/hep.28535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yalamanchili K, Saadeh S, Lepe R, et al. The prevalence of hepatitis C virus infection in Texas: implications for future health care. Proc (Bayl Univ Med Cent) 2005;18:3–6. doi: 10.1080/08998280.2005.11928024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ramirez AG, Munoz E, Holden AE, et al. Incidence of hepatocellular carcinoma in Texas Latinos, 1995-2010: an update. PLoS One. 2014;9:e99365. doi: 10.1371/journal.pone.0099365. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.